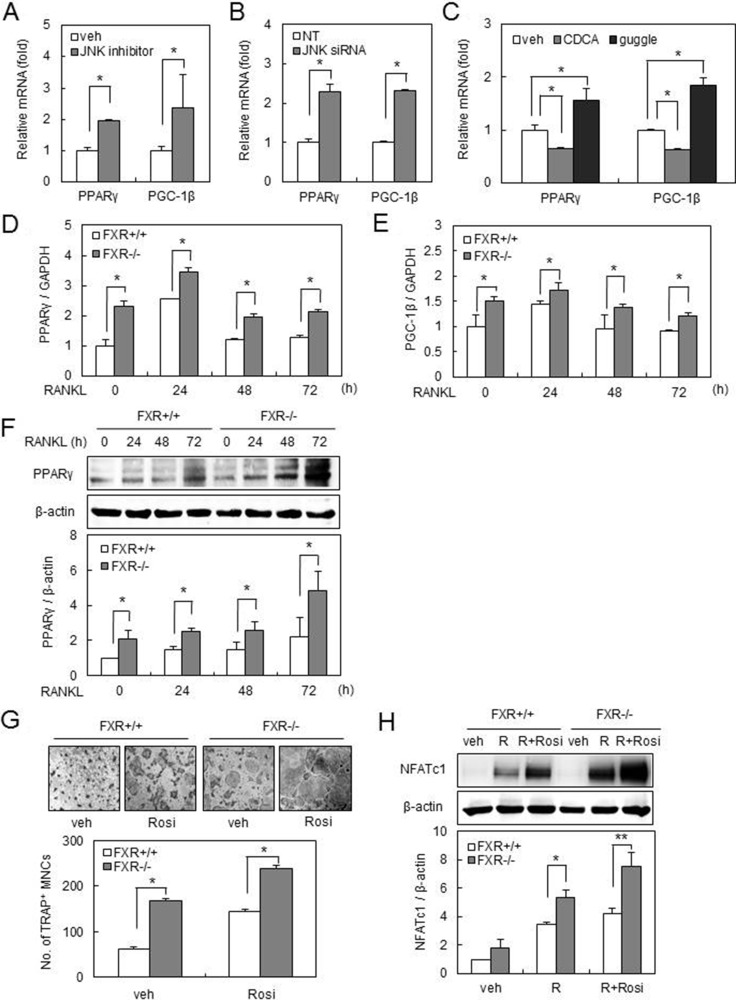
Figure 3. FXR deficiency up-regulates PPARγ and PGC-1β expression via downregulation of JNK.
(A) FXR+/+ BMMs were cultured in the presence or absence of SP600125 (0.3 μM) for 24 h. The mRNA expression of PPARγ or PGC-1β was analyzed by real-time PCR. (B) FXR+/+ BMMs were transfected with 40 nM siRNA. The mRNA expression was analyzed by real-time PCR using PPARγ or PGC-1β primer. (C) FXR+/+ BMMs were cultured with CDCA (75 μM) or guggulsterone (0.3 μM) for 24 h. The mRNA expression of PPARγ or PGC-1β was analyzed by real-time PCR. (D–E) BMMs from FXR+/+ and FXR−/− mice were cultured with RANKL (200 ng/ml) for the indicated time. The mRNA level was analyzed by real-time PCR with PPARγ (D) or PGC-1β (E) primer. (F) FXR+/+ and FXR−/− BMMs were cultured with RANKL (200 ng/ml) for the indicated time. Cell lysates were then subjected to western blot analysis with anti-PPARγ antibody. (G) FXR+/+ and FXR−/− BMMs were cultured with RANKL (100 ng/ml) in the presence or absence of rosiglitazone (0.3 μM) for 3 days. Scale bar, 200 μm. (H) BMMs from FXR+/+ and FXR−/− mice were cultured with RANKL (200 ng/ml) in presence or absence of rosiglitazone (0.3 μM) for 48 h. Cell lysates were then subjected to western blot analysis with anti-NFATc1 antibody. Data are expressed as means ± SD from at least three independent experiments. *p < 0.05, **p < 0.01.