Abstract
The aim of this study was to evaluate circulating tumor cell (CTC) detection in the differential diagnosis of adnexal masses. A total of 87 preoperative women with an indeterminate adnexal mass were prospectively enrolled. Preoperative diagnostic modalities including CTC detection, risk of ovarian malignancy algorithm, risk of malignancy index, and computed tomography or magnetic resonance imaging were compared. Forty-three (49.4%) benign tumors, 13 (14.9%) borderline malignant masses, and 31 (35.7%) cancers were pathologically confirmed. Forty-nine (56.3%) cases were positive for CTCs: 19/43 (44.2%) benign, 10/10 (100%) early-stage, and 14/21 (66.7%) advanced-stage cancer. CTC detection had sensitivities of 77.4%, 100%, and 100% for benign vs. all stage cancer (n = 74), benign vs. stage I–II cancer (n = 53), and benign vs. stage I cancer (n = 49), respectively. CTC detection had a specificity of 55.8% across all comparisons. The sensitivities of the other modalities assayed were decreased in stage I–II cancer and stage I cancer vs. benign masses. Receiver operating characteristic curves showed that CTCs, of which the area under the curve was modest in all stage cancer (0.655), had the widest area under the curve among the evaluated modalities in stage I–II cancer and stage I cancer (0.768 for both). In conclusion, our study findings suggest that preoperative CTCs could have a substantial role in differentiating early stage cancer from benign tumors for adnexal masses.
Keywords: ovarian neoplasm, circulating tumor cell, preoperative period, differential diagnosis, early stage cancer
INTRODUCTION
About 22,440 cases of ovarian cancer will be newly diagnosed in the United States in 2017 [1]. The incidence of ovarian cancer in Korea has increased gradually from 3.2 to 4.8 per 100,000 females between 2004 and 2014 [2]. Intraoperative rupture of the ovary-confined stage IA cancer results in an upstaging to IC1. This iatrogenic upstaging makes the patient receive otherwise unnecessary chemotherapy in order to minimize the risk of tumor recurrence [3]. Upstaging often happens when ovarian cancer is preoperatively misdiagnosed as a benign tumor and tumor cells migrate during surgery.
Many studies have examined the modalities currently used for preoperatively discriminating adnexal tumors, such as serum biomarkers (cancer antigen-125 [CA-125] and the risk of ovarian malignancy algorithm [ROMA]), imaging studies (computed tomography [CT], magnetic resonance imaging [MRI], and positron emission tomography [PET]), or modalities combining the two (risk of malignancy index [RMI]). However, as these methods provide an inadequate level of sensitivity and specificity, there remains an unmet medical need for a more convenient and non-invasive method with better diagnostic performance.
Circulating tumor cells (CTCs) are viable tumor cells disseminated from the site of disease in metastatic and/or primary neoplasms that can be isolated from the peripheral blood [4]. CTC detection is convenient and minimally invasive. There are multiple lines of literature reporting prognostic significance of CTC as well as the clinical usefulness of CTC as a therapy monitoring tool in various kinds of cancer including breast, colorectal, lung, and prostate cancers [5–9]. Although there has been a growing interest in evaluating the clinical value of CTCs in ovarian cancer, almost all of the relevant studies have explored CTC detection as a prognostic biomarker for tumor burden, risk of residual disease after debulking surgery, and treatment response [4]. To the best of our knowledge, there are no studies evaluating the presence of CTCs prior to surgery in the differential diagnosis of indeterminate adnexal masses. Therefore, we performed this study to evaluate the detection of CTCs by a newly developed platform in the differential diagnosis of adnexal masses.
RESULTS
Patient characteristics
Of 87 patients who presented with an adnexal mass, 43 (49.4 %), 13 (14.9 %), and 31 (35.7 %) were pathologically diagnosed with a benign mass, borderline malignant mass, and cancer, respectively. The median age of the population was 47 years (21–78 years). Patients with cancer were older than those with benign tumors (median [range]: 45 years [21–74 years] vs. 57 years [24–77 years]; p = 0.002). Preoperative diagnostic markers had higher mean values in cancer than in benign tumors, including serum CA-125 levels (176.7 U/mL ± 429.3 U/mL vs. 1539.4 U/mL ± 2278.0 U/mL; p = 0.002), ROMA (10.8 % ± 17.4 % vs. 63.0 % ± 36.5 %; p < 0.001), and RMI (642.7 ± 1807.5 vs. 4630.7 ± 7632.6; p = 0.008) (Table 1). These significant differences were maintained when dichotomized into ‘within normal range’ and ‘abnormal’ based on designated cut-off values. On preoperative CT/MRI, findings suspicious of cancer were more significantly associated with a pathologic diagnosis of malignancy (p < 0.001). Tumor size >10 cm (p = 0.024) and moderate to severe ascites on preoperative CT/MRI (p <0.001) were also significantly associated with cancer. For 22 healthy normal controls, the median age was 51.5 years ranging from 46 to 55 years. Mean values of ROMA and serum CA-125 levels were 6.7% ± 2.9% and 13.8 U/mL ± 5.3 U/mL, respectively (data not shown).
Table 1. Patient characteristics of study population with adnexal tumor.
| Characteristic | Total (n=87) | Benign (n=43) | BOT (n=13) | Cancer (n=31) | P* |
|---|---|---|---|---|---|
| Age (yr), median (range) | 47 (21-78) | 45 (21-74) | 47 (41-78) | 57 (24-77) | 0.002 |
| ≤47 | 46 (52.9) | 28 (65.1) | 7 (53.8) | 11 (35.5) | 0.012 |
| >47 | 41 (47.1) | 15 (34.9) | 6 (46.2) | 20 (64.5) | |
| Preoperative serum CA-125 (U/ml) | 646.9±1533.1 | 176.7±429.3 | 73.9±144.4 | 1539.4±2278.0 | 0.002 |
| ≤35 | 31 (35.6) | 17 (39.5) | 9 (69.2) | 5 (16.1) | 0.030 |
| >35 | 56 (64.4) | 26 (60.5) | 4 (30.8) | 26 (83.9) | |
| Preoperative ROMA (%) | 30.6±35.5 | 10.8±17.4 | 17.1±17.6 | 63.0±36.5 | <0.001 |
| Within normal range | 37 (43.0) | 27 (64.3) | 5 (38.5) | 5 (16.1) | <0.001 |
| Abnormal† | 49 (57.0) | 15 (35.7) | 8 (61.5) | 26 (83.9) | |
| Preoperative RMI | 2019.0±5106.4 | 642.7±1807.5 | 237.6±454.9 | 4630.7±7632.6 | 0.008 |
| ≤200 | 49 (57.0) | 27 (64.3) | 10 (76.9) | 12 (38.7) | 0.030 |
| >200 | 37 (43.0) | 15 (35.7) | 3 (23.1) | 19 (61.3) | |
| Preoperative CT or MRI | <0.001 | ||||
| Benign | 26 (29.9) | 23 (53.5) | 2 (15.4) | 1 (3.2) | |
| r/o borderline malignancy | 15 (17.2) | 9 (20.9) | 6 (46.2) | 0 | |
| r/o cancer | 46 (52.9) | 11 (25.6) | 5 (38.5) | 30 (96.8) | |
| Laparoscopic operation | <0.001 | ||||
| No | 58 (67.4) | 21 (48.8) | 9 (69.2) | 28 (93.3) | |
| Yes | 28 (32.6) | 22 (51.2) | 4 (30.8) | 2 (6.7) | |
| Tumor size (cm) | 15.0±7.1 | 8.4±5.1 | 15.0±7.1 | 10.5±5.8 | 0.118 |
| ≤10 | 54 (63.5) | 33 (78.6) | 5 (38.5) | 16 (53.3) | 0.024 |
| >10 | 31 (36.5) | 9 (21.4) | 8 (61.5) | 14 (46.7) | |
| Ascitesǂ | <0.001 | ||||
| Absence | 69 (79.3) | 39 (90.7) | 13 (100) | 17 (54.8) | |
| Presence | 18 (20.7) | 4 (9.3) | 0 | 14 (45.2) | |
| Tumor histology | <0.001 | ||||
| Serous | 24 (27.6) | 4 (9.3) | 3 (23.1) | 17 (54.8) | |
| Non-serous | 63 (72.4) | 39 (90.7) | 10 (76.9) | 14 (45.2) | |
| FIGO stage | - | ||||
| I | 19 (43.2) | NA | 13 (100)§ | 6 (19.4) | |
| II | 4 (9.1) | NA | 0 | 4 (12.9) | |
| III, IV | 21 (47.7) | NA | 0 | 21 (67.7) |
Values are presented as number (%) or mean±standard deviation.
*Benign vs. cancer.
†Abnormal ROMA criteria: ≥7.4% (premenopause) and ≥25.3% (postmenopause).
ǂModerate to severe ascites on preoperative CT or MRI.
§Incomplete staging.
BOT, borderline ovarian tumor; CT, computed tomography; CTC, circulating tumor cell; FIGO, International Federation of Gynecology and Obstetrics; MRI, magnetic resonance imaging; NA, not available; RMI, risk of malignancy index; ROMA, risk of ovarian malignancy algorithm; r/o rule out.
In a subgroup analysis of stage I and II cancers vs. benign tumors, the significant differences in age, preoperative serum CA-125 level, and RMI disappeared. The mean difference in preoperative ROMA was significant (10.8 % ± 17.4 % vs. 43.1 % ± 40.0 %; p = 0.032), but patients with abnormal ROMA > reference value were not different between benign vs. stage I and II cancer (Table 2). Although abnormal preoperative CT/MRI findings (p = 0.001) and tumor size >10 cm (p = 0.001) showed significant associations with early stage cancers, there was no significant difference in ascites between benign masses and early stage cancers. Further analysis of stage I vs. benign tumors showed similar findings; however, in this comparison, laparoscopic operation (p=0.194) and mean preoperative ROMA (10.8% ± 17.4% vs. 19.1% ± 21.9%; p = 0.299) were no longer significantly associated with cancer (Table 3).
Table 2. Characteristics for patients with benign vs. early-stage ovarian cancer (n=53).
| Characteristic | Benign (n=43) | Cancer, stage I and II (n=10) | P |
|---|---|---|---|
| Age (yr), median (range) | 45 (21-74) | 52.5 (24-73) | 0.910 |
| ≤46 | 25 (58.1) | 5 (50.0) | 0.730 |
| >46 | 18 (41.9) | 5 (50.0) | |
| Preoperative serum CA-125 (U/ml) | 176.7±429.3 | 652.3±1199.3 | 0.246 |
| ≤35 | 17 (39.5) | 3 (30.0) | 0.725 |
| >35 | 26 (60.5) | 7 (70.0) | |
| Preoperative ROMA (%) | 10.8±17.4 | 43.1±40.0 | 0.032 |
| Within normal range | 27 (64.3) | 3 (30.0) | 0.075 |
| Abnormal† | 15 (35.7) | 7 (70.0) | |
| Preoperative RMI | 642.7±1807.5 | 4860.7±10919.9 | 0.254 |
| ≤200 | 27 (64.3) | 5 (50.0) | 0.480 |
| >200 | 15 (35.7) | 5 (50.0) | |
| Preoperative CT or MRI | 0.001 | ||
| Benign | 23 (53.5) | 1 (10.0) | |
| r/o borderline malignancy | 9 (20.9) | 0 | |
| r/o cancer | 11 (25.6) | 9 (90.0) | |
| Laparoscopic operation | 0.031 | ||
| No | 21 (48.8) | 9 (90.0) | |
| Yes | 22 (51.2) | 1 (10.0) | |
| Tumor size (cm) | 8.4±5.1 | 13.1±5.6 | 0.013 |
| ≤10 | 33 (78.6) | 2 (20.0) | 0.001 |
| >10 | 9 (21.4) | 8 (80.0) | |
| Ascitesǂ | 0.114 | ||
| Absence | 39 (90.7) | 7 (70.0) | |
| Presence | 4 (9.3) | 3 (30.0) |
Values are presented as number (%) or mean±standard deviation.
*International Federation of Gynecology and Obstetrics (FIGO) stage I and II.
†Abnormal ROMA criteria: ≥7.4% (premenopause) and ≥25.3% (postmenopause).
ǂModerate to severe ascites on preoperative CT or MRI.
CT, computed tomography; MRI, magnetic resonance imaging; RMI, risk of malignancy index; ROMA, risk of ovarian malignancy algorithm; r/o rule out.
Table 3. Characteristics for patients with benign vs. stage I ovarian cancer (n=49).
| Characteristic | Benign (n=43) | Cancer, stage I (n=6) | p |
|---|---|---|---|
| Age (yr), median (range) | 45 (21-74) | 52.5 (24-73) | 0.713 |
| ≤46 | 25 (58.1) | 4 (66.7) | >0.999 |
| >46 | 18 (41.9) | 2 (33.3) | |
| Preoperative serum CA-125 (U/ml) | 176.7±429.3 | 53.1±65.7 | 0.677 |
| ≤35 | 17 (39.5) | 3 (50.0) | |
| >35 | 26 (60.5) | 3 (50.0) | |
| Preoperative ROMA (%) | 10.8±17.4 | 19.1±21.9 | 0.299 |
| Within normal range | 27 (64.3) | 3 (50.0) | 0.658 |
| Abnormal† | 15 (35.7) | 3 (50.0) | |
| Preoperative RMI | 642.7±1807.5 | 642.7±1807.5 | 0.510 |
| ≤200 | 27 (64.3) | 5 (83.3) | 0.648 |
| >200 | 15 (35.7) | 1 (16.7) | |
| Preoperative CT or MRI | 0.016 | ||
| Benign | 23 (53.5) | 1 (16.7) | |
| r/o borderline malignancy | 9 (20.9) | 0 | |
| r/o cancer | 11 (25.6) | 5 (83.3) | |
| Laparoscopic operation | 0.194 | ||
| No | 21 (48.8) | 5 (83.3) | |
| Yes | 22 (51.2) | 1 (16.7) | |
| Tumor size (cm) | 8.4±5.1 | 8.4±5.1 | 0.015 |
| ≤10 | 33 (78.6) | 1 (16.7) | 0.006 |
| >10 | 9 (21.4) | 5 (83.3) | |
| Ascitesǂ | >0.999 | ||
| Absence | 39 (90.7) | 6 (100) | |
| Presence | 4 (9.3) | 0 |
Values are presented as number (%) or mean±standard deviation.
†Abnormal ROMA criteria: ≥7.4% (premenopause) and ≥25.3% (postmenopause).
ǂModerate to severe ascites on preoperative CT or MRI.
CT, computed tomography; MRI, magnetic resonance imaging; RMI, risk of malignancy index; ROMA, risk of ovarian malignancy algorithm; r/o rule out.
Diagnostic performance of circulating tumor cells in differentiating cancer from benign tumor: all stage vs. early-stage cancer
Median CTC count was 1 ranging from 0 to 23. Forty-nine (56.3%) cases had at least one CTC found in preoperative peripheral blood: 19/43 (44.2%) benign, 10/10 (100%) early-stage, and 14/21 (66.7%) advanced-stage cancer. Of 22 normal controls, there was only one (4.5%) who had CTC in her blood sample (data not shown). Mean CTC counts of cancer patients were not significantly different from those of patients with benign tumors irrespective of stage (benign vs. early-stage cancer vs. advanced-stage cancer, 1.5 ± 3.6 vs. 2.0 ± 0.7 vs. 1.8 ± 1.8) (Table 4).
Table 4. Preoperative circulating tumor cells of study population with adnexal tumor.
| Characteristic | Total (n=87) | Benign (n=43) | Borderline malignancy (n=13) | Cancer, early-stage* (n=10) | Cancer, advanced- stage (n=21) | P† |
|---|---|---|---|---|---|---|
| Preoperative CTC count | 1.6±2.8 | 1.5±3.6 | 1.2±2.0 | 2.0±0.7 | 1.8±1.8 | 0.647/0.725 |
| Preoperative CTC | 0.001/0.091 | |||||
| Absence | 38 (43.7) | 24 (55.8) | 7 (53.8) | 0 | 7 (33.3) | |
| Presence | 49 (56.3) | 19 (44.2) | 6 (46.2) | 10 (100) | 14 (66.7) |
Values are presented as number (%) or mean±standard deviation.
*International Federation of Gynecology and Obstetrics (FIGO) stage I and II.
†Benign vs. cancer, early-stage/advanced-stage.
CTC, circulating tumor cell.
Table 5 and Figure 1 show the diagnostic performance of the various modalities of preoperative differential diagnosis of adnexal masses (benign vs. cancer, excluding borderline malignancy). For benign vs. all stage cancer (n = 74), the sensitivity and specificity of CTC detection were 77.4% and 55.8%, respectively (i.e., false negative rate, 22.6%; false positive rate, 44.2%). McNemar's test showed that the sensitivities and specificities of ROMA (83.9% and 64.3%, respectively), CA-125 (83.9% and 39.5%, respectively), RMI (61.3% and 64.3%, respectively), and CT/MRI (96.8% and 74.4%, respectively) were not significantly different from those obtained by CTC detection. The diagnostic accuracy of CTC detection, ROMA, CA-125, RMI, and CT/MRI was 64.9%, 71.6%, 58.1%, 62.2%, and 83.8%, respectively. Figure 1A shows the receiver operating characteristic (ROC) curves for various preoperative diagnostic methods in the differential diagnosis of adnexal masses (benign vs. all stage cancer). The area under the curve (AUC) (95% CI; p value) for CTC detection, ROMA, CA-125, RMI, and CT/MRI was 0.655 (0.53–0.78; 0.025), 0.736 (0.62–0.85; 0.001), 0.614 (0.48–0.74; 0.098), 0.636 (0.51–0.77; 0.050), and 0.752 (0.64–0.86; < 0.001), respectively.
Table 5. Diagnostic performance of preoperative modalities evaluating adnexal mass (benign vs. cancer excluding borderline malignancy).
| Sensitivity | Specificity | Accuracy | Psens/Pspec* | |
|---|---|---|---|---|
| Benign vs. all stage cancer | ||||
| CTC | 77.4% | 55.8% | 64.9% | |
| ROMA | 83.9% | 64.3% | 71.6% | 0.727/0.541 |
| CA-125 | 83.9% | 39.5% | 58.1% | 0.754/0.210 |
| RMI | 61.3% | 64.3% | 62.2% | 0.267/0.503 |
| CT or MRI | 96.8% | 74.4% | 83.8% | 0.070/0.152 |
| Benign vs. stage I and II cancer | ||||
| CTC | 100% | 55.8% | 64.2% | |
| ROMA | 70.0% | 63.4% | 65.4% | - /0.405 |
| CA-125 | 70.0% | 39.0% | 45.3% | - /0.286 |
| RMI | 50.0% | 65.9% | 61.5% | - /0.503 |
| CT or MRI | 90.0% | 53.7% | 60.4% | - /0.189 |
| Benign vs. stage I cancer | ||||
| CTC | 100% | 55.8% | 61.2% | |
| ROMA | 50.0% | 63.4% | 62.5% | - /0.405 |
| CA-125 | 50.0% | 39.0% | 40.8% | - /0.286 |
| RMI | 16.7% | 65.9% | 58.3% | - /0.503 |
| CT or MRI | 83.3% | 53.7% | 57.1% | - /0.189 |
*McNemar test for comparison of sensitivity and specificity with those of CTC, respectively. P value for the comparison of sensitivity in benign vs. early stage cancer could not be calculated because all cancer cases had CTC positive results, that is, sensitivity of CTC 100%.
RMI=U x M x CA-125, U, ultrasound score, is scored 1 point for each of the following characteristics: multilocular cyst, solid areas, metastases, ascites and bilateral lesions. U=0 (for score of 0), U=1 (for score of 1), and U=3 (for score of 2-5). M, menopausal status, is scored as 1 (premenopause) and 3 (postmenopause), which was defined as no period for more than 1 year or age >50 who has had a hysterectomy.
Abnormal criteria: ≥1 for CTC; ≥7.4% (menopause) and ≥25.3% (postmenopause) for ROMA, >35 U/ml for serum CA-125 level; >200 for RMI; report as rule out borderline malignancy or cancer for CT.
CT, computed tomography; CTC, circulating tumor cell; MRI, magnetic resonance imaging; RMI, risk of malignancy index; ROMA, risk of ovarian malignancy algorithm.
Figure 1. Receiver operating characteristic curves of preoperative diagnostic methods including circulating tumor cell (CTC) detection in the differential diagnosis of adnexal mass.
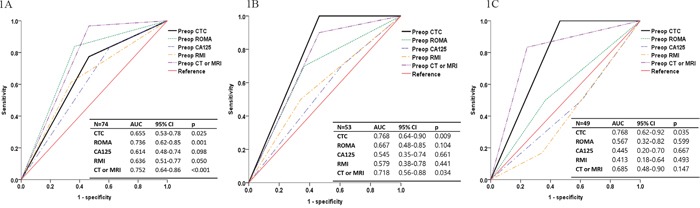
(A) Benign vs. all stage cancer (n = 74). (B) Benign vs. stage I–II cancer (n = 53). (C) Benign vs. stage I cancer (n = 49). AUC, area under the curve; CA-125, cancer antigen-125; CT, computed tomography; RMI, risk of malignancy index; ROMA, risk of ovarian malignancy algorithm.
For benign vs. stage I and II cancer (n = 53), the sensitivity of CTC detection was 100% (zero false negative rate), bettering the sensitivities of the other modalities (ROMA, 70.0%; CA-125, 70.0%; RMI, 50.0%; and CT/MRI, 90.0%). P values for the comparison of sensitivities of the other modalities with that of CTC detection could not be calculated as all of the cancer cases had positive CTC results. The specificities of ROMA (64.3%), CA-125 (39.5%), RMI (64.3%), and CT/MRI (53.5%) were not significantly different from that of CTC detection (55.8%). Diagnostic accuracy of CTC detection, ROMA, CA-125, RMI, and CT/MRI was 64.2%, 65.4%, 45.3%, 61.5%, and 60.4%, respectively. Figure 1B shows ROC curves for the various preoperative diagnostic methods in the differential diagnosis of adnexal masses (benign vs. stage I and II cancer). The AUC (95% CI; p value) for CTC detection, ROMA, CA-125, RMI, and CT/MRI was 0.768 (0.64–0.90; 0.009), 0.667 (0.48–0.85; 0.104), 0.545 (0.35–0.74; 0.661), 0.579 (0.38–0.78; 0.441), and 0.718 (0.56–0.88; 0.034), respectively.
For benign vs. stage I cancer (n = 49), the sensitivity and specificity of CTC detection did not change from stage I and II cancer (100% and 55.8%, respectively). However, the sensitivities of the other modalities were decreased, although this was not statistically significant. There was no change in specificity for the other modalities. ROC curves showed that the AUC of the modalities other than CTC were decreased, and the curves for preoperative CA-125 and RMI reversed (Figure 1C). The AUC (95% CI; p value) for CTC detection, ROMA, CA-125, RMI, and CT/MRI was 0.768 (0.62–0.92; 0.035), 0.567 (0.32–0.82; 0.599), 0.445 (0.20–0.70; 0.667), 0.413 (0.18–0.64; 0.493), and 0.685 (0.48–0.90; 0.147), respectively. Preoperative CTC detection was the only modality that had a significant difference between the curve and reference line (p = 0.035).
Including borderline ovarian tumors (BOT) in the comparisons decreased the sensitivity of CTC detection (benign vs. BOT and stage I and II cancer, 69.6%; benign vs. BOT and stage I cancer, 63.2%). The AUC of ROC curves of CTC detection also decreased to 0.616 (p = 0.125) for early stage and 0.584 (p = 0.298) for stage I cancer. In both comparisons including BOT, CT/MRI imaging was the only preoperative diagnostic modality showing a significant AUC of ROC curves (CT, 0.703, p = 0.007; MRI, 0.689 p = 0.019; data not shown). These findings suggest that preoperative CTC detection might have the best cancer-detecting performance among the evaluated modalities in distinguishing between benign and stage I ovarian tumors, but this did not extend to benign vs. early stage malignancy including BOT.
Association of circulating tumor cells with tumor risk factors
Associations between the presence of preoperative CTCs and tumor risk factors were evaluated (Supplementary Table 1). Age >47 years (p = 0.034) and moderate to severe ascites on preoperative CT/MRI (p = 0.009) were significantly associated with presence of CTCs. However, high serum CA-125 level (>35 U/mL), ROMA >reference value, tumor size >10 cm, and CT/MRI findings suspicious of malignancy were not significantly associated with CTCs. Multivariate analysis revealed no independent risk factors for the presence of preoperative CTC among the variables (age >47 years, high serum CA-125 level, and moderate to severe ascites on preoperative CT/MRI), none of which showed a significant (p < 0.2) association with the presence of CTCs in univariate analysis (data not shown).
DISCUSSION
When an adnexal mass is found in routine ultrasonography without definitive evidence of tumor spreading or distant metastasis and is thought to be borderline or stage I ovarian cancer, it could be challenging for a physician to determine treatment, as an otherwise minimally invasive surgery could be safely performed without significant operation-related complications. Considering practice guidelines which recommend to obtain family history for workup of suspicious pelvic mass and significant proportion (16%) of Korean ovarian cancer patients with a strong family history as well as high prevalence (33%) of BRCA mutations in such patients [3, 10], genetic analysis based on the genetic test and family history might be one of the clues favorable for diagnosis of ovarian cancer. Although studies have tried to determine the best method or combination of methods to differentiate ovarian cancer from benign tumors [11, 12], there remains an unmet medical need for differential tools to accurately diagnose early stage ovarian cancer.
Our study demonstrated for the first time that CTCs could be used as a useful diagnostic marker for differentiating ovarian cancer from benign adnexal tumors. Notably, preoperative CTC detection was more sensitive in benign vs. early stage cancer (stage I and II) compared with benign vs. all stage cancer. This improvement remained even in benign vs. stage I cancer. However, serum CA-125, ROMA, RMI, and CT/MRI showed the reverse pattern of diagnostic performance: modest performance in early stage cancer and significantly better performance in all stage cancer excluding BOT. These findings suggest that CTCs might reflect early stage hematogenous metastasis, in contrast to serum CA-125, which reflects advanced-stage peritoneal tumor spread. No significant associations were found between CTCs and serum CA-125 level or ROMA, which supports this hypothesis. Several studies have demonstrated that early hematogenous metastasis in ovarian cancer can occur before peritoneal tumor spread, suggesting that CTCs, so called “liquid tumor biopsies,” could be a feasible method of detection [13–16]. Many relevant studies have reported the presence of CTCs in disease predominantly confined to the abdomen [13]. Fehm et al. observed hematogenous dissemination of isolated tumor cells in stage I ovarian cancer, which implies that single tumor cells might acquire the potential to disseminate to extraperitoneal sites very early in ovarian carcinogenesis [14, 15]. Another report showed that ovarian CTCs implanted and grew in the omentum preferentially and subsequently spread to other peritoneal surfaces using a parabiosis model [16], suggesting that hematogenous metastasis could be an important mode of ovarian cancer metastasis, including intraperitoneal seeding.
Serum CA-125, the best performing single tumor marker so far, is known to be normal or only marginally elevated in approximately 20% of ovarian cancers, especially in early stage disease [12]. Moreover, CA-125 is also elevated in several benign gynecologic and non-gynecologic diseases including endometriosis, adenomyosis, and pelvic inflammatory disease. Recently, Richards et al., in a prospective study, reported that women with stage I ovarian cancer had a higher human epididymis protein 4 (HE4) level compared with those with benign pathology (p = 0.025) [17]. They also showed that the AUC of ROC curves of HE4 was higher than that of CA-125 in all women, with better specificity (p = 0.045). RMI, the most widely used tool for the detection of ovarian cancer, is currently the most accurate tool for stratifying patients into high and low risk groups, with 81% to 92% sensitivity and 82% to 85% specificity [12]. However, some authors insist that ROMA, by combining CA-125 and HE4 together, has better diagnostic performance than RMI [11], whereas others have failed to show an additional benefit of ROMA compared with HE4 or CA-125 alone [18, 19]. Because there is no specific marker uniformly expressed by all cancer types [20] and CTCs are outnumbered by white blood cells by a factor of at least 106 [21], enrichment and purification of CTCs are critical for CTC detection in collected blood. There are various feasible methods, largely biochemical and physical, for isolating CTCs. Biochemical methods, such as the CellSearch system (Veridex), which was approved by the Food and Drug Administration, use CTC-specific antibody-antigen interactions, including epithelial cell adhesion molecule (EpCAM) [22]. Most studies evaluating the prognostic value of CTCs in ovarian cancer using CellSearch have reported negative results, probably owing to the low number of EpCAM-positive CTCs in ovarian cancer or the downregulation of EpCAM during the epithelial-mesenchymal transition [23, 24]. While biochemical methods show unstable capture efficiencies because of varying expression levels by cancer type, physical methods have shown stable capture efficiency regardless of surface marker expression. Therefore, our study team created a new platform using both physical and biochemical methods [22]. Physically, tapered-slit membrane filters (TSF) with vertical slits with a tapered angle of 2° were primarily used for viable CTC isolation, based on CTC size and deformability. Using this TSF platform, about 90% of the cancer cells were captured at a sample flow rate of 5ml/hour, which was 33.3 times faster than previous filters. TSF with a gap that was wide at the entrance and gradually decreased with depth was shown to provide minimal cell stress and reduce 82.14% of the stress generated in conventional straight-hole filters [22]. Biochemically, our criteria included the expression of EpCAM and/or cytokeratin (CK). Finally, morphologic criteria were used for confirming genuine CTCs. With these criteria, we minimized the possibility of missing CTCs, a common problem in EpCAM-only methods.
There are a few limitations to our study. First, the small sample size lowers the power of statistical analysis. Second, the specificities of the evaluated methods were lower than expected, which might be associated with factors related to the study population, because low specificities were observed for all evaluated methods. Therefore, we mainly focused on the sensitivity at a fixed specificity level for evaluating diagnostic performance. However, the low specificity of preoperative CTC detection, that is, its high false positive rate, was not likely owing to our CTC detection method. Lastly, not including a family history in the case report form could be a disadvantage of our study given that the practice guidelines for the management of ovarian cancer address that the initial step is to investigate the family history.
In conclusion, our study findings suggest that preoperative CTC detection could have a substantial role in differentiating early stage cancer from benign adnexal masses, where other commonly used diagnostic methods are not as competent as expected. Nonetheless, the definitive role of CTC in the clinical settings is to be determined, particularly in the field of differential diagnosis of pelvic masses. Diagnostic performance of the CTC detection method using a combination of TSF platform and surface marker expression with confirmatory morphologic criteria should be validated in further studies with a larger sample size.
MATERIALS AND METHODS
Patient data and blood sample collection
A total of 87 women with an indeterminate adnexal mass who were scheduled to undergo surgery at Seoul National University Bundang Hospital between May 2015 and April 2016 were prospectively enrolled after getting informed consent. Twenty-two healthy women without no demonstrable adnexal cyst were additionally enrolled for normal control. All of the enrolled patients had received preoperative ROMA, CT, and RMI as standard of care. ROMA was calculated using the following algorithms proposed by Moore et al. [25]:
Premenopausal: PI (predictive index) = -12 + 2.38 × LN(HE4) + 0.0626 × LN(CA-125)
Postmenopausal: PI = -8.09 +1.04 × LN(HE4) + 0.732 × LN(CA-125)
The ROMA-value (predictive value) was then calculated using the following equation:
ROMA (%) = ePI/(1+eP) × 100
Postmenopausal status was defined as absence of periods for more than 1 yr.
RMI was calculated using the following equation:
RMI = US × menopausal status × serum CA-125 level
US is a quantitative measure of the results of ultrasound score and ranges from 0 to 3. One point is given for each of the following characteristics: multilocular cysts, solid areas, metastases, ascites, and bilateral lesions. US is 0, 1, or 3 for an ultrasound score of 0, 1, or 2–5 points, respectively. Menopausal status is 1 or 3 for premenopause or postmenopause, respectively. MRI was an alternative to CT for the patients who were unable to undergo CT for any reason, such as an allergy to contrast media. Ascites was evaluated on CT/MRI, and a moderate to severe amount of fluid in the abdominal and pelvic cavities was counted as positive for ascites. Patients with a prior malignancy less than 5 years from enrollment were excluded.
While the patient was under general anesthesia, 5 mL of peripheral blood for isolating CTCs was withdrawn from the antecubital vein before the start of surgery. All blood samples were collected in BD Vacutainer® tube and transferred to the Korea Advanced Institute of Science and Technology for identifying and counting CTCs in the blood sample. To avoid cell lysis and destruction during delivery, collection tubes were packed with ice packs in a foam plastic box and delivered within 6 hours after sampling. One week after surgery, diagnosis of the adnexal mass (cancer or benign) and tumor size were confirmed in the final pathological report.
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-1408/263-003).
Identification and counting of circulating tumor cells
CTC isolation and counting were performed using the previously reported TSF platform with optimizations for this work [22]. The TSF isolates CTCs based on their physical properties, such as size and deformability, regardless of their surface protein expression. In addition, its unique design, having a wider cell entrance and gradually narrower slit exits, increases sample flow rate with minimal cell stress, thus achieving rapid, viable CTC isolation from clinical samples. Five milliliters of patient blood was diluted in 10 mL of phosphate-buffered saline (PBS) without any pretreatment and directly processed into the TSF platform under syringe pump. After sample processing, the captured cells were gently released by applying a reverse flow of PBS, and the released cells were mounted onto glass slides by cytocentrifuge (Shandon Cytospin III, Thermo Scientific, Wilmington, DE, USA). The immunostaining protocol was optimized for TSF, and described previously [26]. Briefly, the cell-mounted glass slides were immunostained by fixation, permeabilization, blocking, and immunofluorescent staining. Then, fluorescent images were acquired by fluorescence microscope system (Eclipse Ti, Nikon) and quantified using MetaMorph® software (Molecular Devices, Sunnyvale, CA, USA). All immunofluorescent cells were carefully examined and counted as CTCs considering both staining criteria (4′,6-diamidino-2-phenylindole [DAPI]+, cluster of differentiation 45 [CD45]-, and CK+ or EpCAM+) and morphological criteria, such as bigger size, higher nucleus-to-cytoplasm ratio, and higher degree of irregularity than background blood cells (Figure 2). Staining intensity for positive cases was graded from mild (1+) to severe (3+). The case with intensity 3+ was counted as a positive control. The case was counted as a negative control when no staining intensity was perceived at all.
Figure 2.
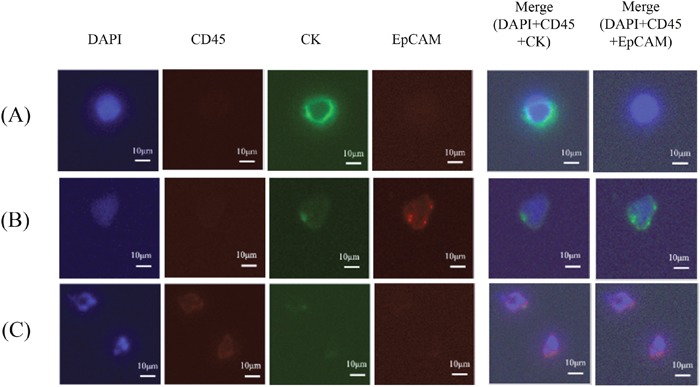
Immunostaining of filtered blood for isolating circulating tumor cells (A, DAPI+/CD45-/CK+/EpCAM-; B, DAPI+/CD45-/CK+/EpCAM+) and excluding white blood cells (C, DAPI+/CD45+/CK±/EpCAM-). The bar represents 10 μm. CD45, cluster of differentiation 45; CK9, cytokeratin 9; DAPI, 4′,6-diamidino-2-phenylindole; EpCAM, epithelial cell adhesion molecule.
CTCs were identified and counted by two independent researchers (J Bu, YT Kang), both of whom were blinded to the results of final pathology.
Assessment of diagnostic performance of CTCs
All of the variables, including presence and mean number of CTCs, were compared between benign and cancerous masses. The association of presence of CTCs with other variables was evaluated for statistical significance. The cut-off values of the preoperative diagnostic tools were as follows: 35 U/mL for serum CA-125 level; 7.4% (premenopause) and 25.3% (postmenopause) for ROMA; and 200 for RMI. By creating ROC curves, sensitivity and specificity of CTC detection for the differential diagnosis of adnexal masses were compared with those of other tools. McNemar's test was used for calculating the statistical significance of each comparison. Otherwise, chi-square test and Student's t-test were used for comparing categorical and numeric variables, respectively. A two-sided p-value of <0.05 was considered statistically significant. SPSS software (version 19.0; SPSS Inc., Chicago, IL) was used for statistical analyses.
SUPPLEMENTARY MATERIALS TABLE
Acknowledgments
This research was supported by the Converging Research Center Program funded by the Ministry of Science, ICT and Future Planning of Korea (Project No. NRF-2014M3C1A8048780).
Abbreviations
- AUC
area under the curve
- BOT
borderline ovarian tumor
- CD45
cluster of differentiation 45
- CK
cytokeratin
- CT
computed tomography
- CTC
circulating tumor cell
- DAPI
4′,6-diamidino-2-phenylindole
- EpCAM
epithelial cell adhesion molecule
- HE4
human epididymis protein 4
- MRI
magnetic resonance imaging
- PBS
phosphate-buffered saline
- PET
positron emission tomography
- PI
predictive index
- RMI
risk of malignancy index
- ROC
receiver operating characteristic
- ROMA
risk of ovarian malignancy algorithm
- TSF
tapered-slit membrane filters.
Footnotes
Author contributions
DHS wrote the manuscript and made significant contributions to conception, design, and acquisition, interpretation, and analysis of data. MK, JYC, BL, and BSK were involved in acquisition of the samples and data collection. JB, YTK, and YHC took charge of experimentation. KK, JHN, and YBK made substantial contributions to conception, acquisition, and revising and editing the manuscript. All authors have read and approved the final manuscript.
CONFLICTS OF INTEREST
All authors declare no potential conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. https://doi.org/10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Korean Statistical Information Service KOSIS. Statistical Database: cancer incident canses and incidence rates by site (24 items) and sex. Daejeon, Korea: Statistics Korea; cited 2017 Jun 21 Available from: http://kosis.kr/eng/statisticsList/statisticsList_01List.jsp?vwcd=MT_ETITLE&parmTabId=M_01_01#SubCont.
- 3.National Comprehensive Cancer Network (US) Fort. Washington (PA): National Comprehensive Cancer Network; 2016. 2016. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): Ovarian Cancer version I. cited 2016 Dec 15 Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. [Google Scholar]
- 4.Romero-Laorden N, Olmos D, Fehm T, Garcia-Donas J, Diaz-Padilla I. Circulating and disseminated tumor cells in ovarian cancer: a systematic review. Gynecol Oncol. 2014;133:632–9. doi: 10.1016/j.ygyno.2014.03.016. https://doi.org/10.1016/j.ygyno.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Banys-Paluchowski M, Krawczyk N, Fehm T. Potential role of circulating tumor cell detection and monitoring in breast cancer: a review of current evidence. Front Oncol. 2016;6:255. doi: 10.3389/fonc.2016.00255. https://doi.org/10.3389/fonc.2016.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. https://doi.org/10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–21. doi: 10.1200/JCO.2007.15.8923. https://doi.org/10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 8.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. https://doi.org/10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton G, Rath B, Ulsperger E. A review of the role of surgery for small cell lung cancer and the potential prognostic value of enumeration of circulating tumor cells. Eur J Surg Oncol. 2016;42:1296–302. doi: 10.1016/j.ejso.2016.04.063. https://doi.org/10.1016/j.ejso.2016.04.063. [DOI] [PubMed] [Google Scholar]
- 10.Lim MC, Kang S, Seo SS, Kong SY, Lee BY, Lee SK, Park SY. BRCA1 and BRCA2 germline mutations in Korean ovarian cancer patients. J Cancer Res Clin Oncol. 2009;135:1593–9. doi: 10.1007/s00432-009-0607-3. https://doi.org/10.1007/s00432-009-0607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, Kurman RJ, Bast RC, Skates SJ. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol. 2010;203:228–1-6. doi: 10.1016/j.ajog.2010.03.043. https://doi.org/10.1016/j.ajog.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsen MA, Sandhu N, Hogdall C, Christensen IJ, Nedergaard L, Lundvall L, Engelholm SA, Pedersen AT, Hartwell D, Lydolph M, Laursen IA, Hogdall EV. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2012;127:379–83. doi: 10.1016/j.ygyno.2012.07.106. https://doi.org/10.1016/j.ygyno.2012.07.106. [DOI] [PubMed] [Google Scholar]
- 13.Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B. Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res. 1984;44:3584–92. [PubMed] [Google Scholar]
- 14.Braun S, Schindlbeck C, Hepp F, Janni W, Kentenich C, Riethmuller G, Pantel K. Occult tumor cells in bone marrow of patients with locoregionally restricted ovarian cancer predict early distant metastatic relapse. J Clin Oncol. 2001;19:368–75. doi: 10.1200/JCO.2001.19.2.368. https://doi.org/10.1200/jco.2001.19.2.368. [DOI] [PubMed] [Google Scholar]
- 15.Fehm T, Banys M, Rack B, Janni W, Marth C, Blassl C, Hartkopf A, Trope C, Kimmig R, Krawczyk N, Wallwiener D, Wimberger P, Kasimir-Bauer S. Pooled analysis of the prognostic relevance of disseminated tumor cells in the bone marrow of patients with ovarian cancer. Int J Gynecol Cancer. 2013;23:839–45. doi: 10.1097/IGC.0b013e3182907109. https://doi.org/10.1097/IGC.0b013e3182907109. [DOI] [PubMed] [Google Scholar]
- 16.Pradeep S, Kim SW, Wu SY, Nishimura M, Chaluvally-Raghavan P, Miyake T, Pecot CV, Kim SJ, Choi HJ, Bischoff FZ, Mayer JA, Huang L, Nick AM, et al. Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell. 2014;26:77–91. doi: 10.1016/j.ccr.2014.05.002. https://doi.org/10.1016/j.ccr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards A, Herbst U, Manalang J, Pather S, Saidi S, Tejada-Berges T, Tan K, Williams P, Carter J. HE4, CA125, the Risk of Malignancy Algorithm and the Risk of Malignancy Index and complex pelvic masses - a prospective comparison in the pre-operative evaluation of pelvic masses in an Australian population. Aust N Z J Obstet Gynaecol. 2015;55:493–7. doi: 10.1111/ajo.12363. https://doi.org/10.1111/ajo.12363. [DOI] [PubMed] [Google Scholar]
- 18.Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, Timmerman D, De Moor B, Vergote I. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer. 2011;104:863–70. doi: 10.1038/sj.bjc.6606092. https://doi.org/10.1038/sj.bjc.6606092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob F, Meier M, Caduff R, Goldstein D, Pochechueva T, Hacker N, Fink D, Heinzelmann-Schwarz V. No benefit from combining HE4 and CA125 as ovarian tumor markers in a clinical setting. Gynecol Oncol. 2011;121:487–91. doi: 10.1016/j.ygyno.2011.02.022. https://doi.org/10.1016/j.ygyno.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35:122–8. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Mostert B, Sleijfer S, Foekens JA, Gratama JW. Circulating tumor cells (CTCs): detection methods and their clinical relevance in breast cancer. Cancer Treat Rev. 2009;35:463–74. doi: 10.1016/j.ctrv.2009.03.004. https://doi.org/10.1016/j.ctrv.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Kang YT, Doh I, Cho YH. Tapered-slit membrane filters for high-throughput viable circulating tumor cell isolation. Biomed Microdevices. 2015;17:45. doi: 10.1007/s10544-015-9949-6. https://doi.org/10.1007/s10544-015-9949-6. [DOI] [PubMed] [Google Scholar]
- 23.Obermayr E, Castillo-Tong DC, Pils D, Speiser P, Braicu I, Van Gorp T, Mahner S, Sehouli J, Vergote I, Zeillinger R. Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance -- a study of the OVCAD consortium. Gynecol Oncol. 2013;128:15–21. doi: 10.1016/j.ygyno.2012.09.021. https://doi.org/10.1016/j.ygyno.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Pearl ML, Zhao Q, Yang J, Dong H, Tulley S, Zhang Q, Golightly M, Zucker S, Chen WT. Prognostic analysis of invasive circulating tumor cells (iCTCs) in epithelial ovarian cancer. Gynecol Oncol. 2014;134:581–90. doi: 10.1016/j.ygyno.2014.06.013. https://doi.org/10.1016/j.ygyno.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, Gajewski W, Kurman R, Bast RC, Jr, Skates SJ. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112:40–6. doi: 10.1016/j.ygyno.2008.08.031. https://doi.org/10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang YT, Doh I, Cho YH. 2015 Transducers-2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCER) Anchorage, AK, USA: IEEE; 2015. A handheld device for rapid viable curculating tumor cell isolation using microfabricated tapered-slit filters; pp. 1557–60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


