Abstract
Background
Findings on the association between intake of red and processed meat with renal cell carcinoma (RCC) risk are mixed. We conducted a meta-analysis to investigate this association.
Materials and Methods
Eligible studies up to August 31, 2016, were identified and retrieved by searching the MEDLINE and Embase databases along with manual review of the reference lists from the retrieved studies. The quality of the included studies was evaluated using the Newcastle-Ottawa Quality Assessment Scale. The summary relative risk (SRR) and corresponding 95% confidence interval (CI) were calculated using a random-effects model.
Results
Twenty-three publications were included in this meta-analysis: four cohort studies, one pooled study, and 18 case-control studies. The SRR (95% CI) for the highest vs. lowest intake of red meat was 1.36 (1.16–1.58, Pheterogeneity < 0.001); that for processed meat was 1.13 (95% CI, 1.03–1.24, Pheterogeneity = 0.014). Linear dose-response analysis yielded similar results, i.e., the SRR for per 100 g/day increment of red meat and per 50 g/day increment of processed meat was 1.21 (95% CI, 1.08–1.36) and 1.16 (95% CI, 0.99–1.36), respectively. A non-linear association was observed only for red meat (Pnonlinearity = 0.002), and not for processed meat (Pnonlinearity = 0.231). Statistically significant positive associations were observed for intake of beef, salami/ham/bacon/sausage, and hamburger.
Conclusions
This meta-analysis indicates a significant positive association between red and processed meat intake and RCC risk.
Keywords: red and processed meat, renal cell carcinoma, meta-analysis, relative risk
INTRODUCTION
In the United States, the incidence of kidney cancer is the seventh and tenth highest in men and women, respectively [1]. Renal cell carcinoma (RCC) is the most common malignancy of the kidney [2]. Globally, RCC incidence demonstrates regional variations, with age-standardized incidence rates being about 11.9 per 100,000 in developed areas and 2.5 per 100,000 in less developed regions [3]. The incidence of RCC has increased in most countries over the past decade [4]. However, the reasons for the regional and historical variations in RCC incidence are unknown. The demonstrated risk factors for RCC development include age, smoking [5], obesity [6], hypertension [7], and acquired cystic kidney disease [8]. Although data are limited, a family history of kidney cancer [9], certain analgesics [10], history of diabetes [11], and occupational exposure (e.g., asbestos, silica, solder) have been linked to increased risk of RCC [12].
The consumption of red and processed meat has long been recognized as a risk factor of human cancer, as such meats are rich in well-established carcinogens, such as heterocyclic amines (HCAs), polycyclic aromatic hydrocarbons (PAHs), and N-nitroso compounds (NOCs) [13, 14]. Many epidemiological studies have investigated the association between the consumption of red and processed meat and the risk of RCC [15–28]. Recently, two meta-analyses [29, 30] of observational studies have been published on this issue. According to the former meta-analysis of 13 case-control studies, Mohammed et al. [30] concluded that there is evidence supporting an independent relation between high consumption of red and processed meat and the incidence of kidney cancer. Whereas findings of the latter one [29], which included 12 case-control, 3 cohort and 1 pooled analysis, were not supportive of an independent relation between red or processedmeat intake and kidney cancer. Since then, numerous epidemiological studies [31–40] evaluating the aforementioned associations have been published and have reported inconsistent results. In addition, the exact form of the dose-risk relationship of these associations has not been clearly defined. To better understand this issue, we carried out a comprehensive meta-analysis of observational studies according to Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [41].
MATERIALS AND METHODS
Data sources and searches
Two investigators (Z.S.J. and H.J.J.) conducted a computerized literature search independently in MEDLINE (from January 1, 1966) and Embase (from January 1, 1974) through to August 31, 2016. We searched the relevant studies using the following words and/or Medical Subject Heading (MeSH) terms: 1) intake OR consumption OR diet OR red meat OR processed meat OR preserved meat OR beef OR pork OR veal OR mutton OR lamb OR ham OR sausage OR bacon; 2) kidney OR renal; 3) carcinoma OR cancer OR neoplasm OR neoplasia; and 4) case-control OR cohort OR prospective OR retrospective. Furthermore, we reviewed the reference lists of the relevant articles to identify additional studies. Only studies published in English were included.
Study selection
In the present analysis, red meat was defined as beef, veal, pork, lamb, or a combination thereof [22]; processed meat was generally defined as meat products made largely from pork, veal, and beef that undergoes preservation such as curing, smoking, or drying [22]. We also assessed some specific red/processed meats, including beef, pork, hamburger, salami/ham/bacon/sausage, and barbecued/pan-fried/broiled meat. We attempted to evaluate other subcategories that were described as “lamb” and “liver”, but the number of included studies assessing these meats was too limited.
Studies were included if they
were published as an original article;
used a case-control or cohort design;
reported relative risk (RR) estimates with corresponding 95% CIs for the association between red and/or processed meat intake and the risk of RCC.
Non–peer-reviewed articles, abstracts, commentaries/letters, ecologic assessments, correlation studies, experimental animal studies, and mechanistic studies were excluded. When multiple reports on the same study were available, only the most informative one was considered.
Data collection and items
A standardized data collection sheet was designed before the extraction. Two investigators (Z.S.J. and H.J.J.) separately extracted the basic information (first author's last name, location, publication year, sample source, duration of follow-up, number of cases and non-cases), data of interest (methods of ascertainment of dietary variables, exposure type [total or individual meats], comparison groups, methods of outcome assessment, RR [95% CI] for the highest vs. lowest level), and adjustments. From each study, we extracted the risk estimates that reflected the greatest degree of control for potential confounders.
Quality assessment of individual studies
We used the NOS checklist to assess study quality [42], where the quality of case-control and cohort studies is assessed using three parameters: selection (four items, each awarded one star), comparability (one item, which can be awarded up to two stars), and exposure/outcome (three items, each awarded one star). A score of ≥ 7 stars is indicative of a high-quality study.
Statistical methods
We used a random-effects model to calculate the SRRs (95% CIs) for the high vs. low and dose-response analyses. This model accounts for heterogeneity among studies [43]. As outcomes were relatively rare, the ORs in the case-control studies were considered approximations of RRs. When sex-specific estimates were available, we analyzed for this separately. For studies [16, 18–20, 27, 28, 36, 37, 40] that presented results on meat subtypes separately, but not that for overall red/processed meat, we combined the results using a fixed-effects model, and then included the pooled RR estimates in the meta-analysis.
We used the χ2 test to assess heterogeneity among studies, defining significant heterogeneity as P < 0.10. We also used the I2 statistic to explore the extent of inconsistency, with I2 > 50% indicating high heterogeneity and I2 < 25% indicating no significant heterogeneity [44]. We performed subgroup and meta-regression analysis on location, study design (case-control vs. cohort), FFQ type (validated vs. non-validated), available exposure data, study quality score, number of cases, and confounders (smoking status, BMI, dietary energy intake, alcohol consumption, intake of vegetables and fruits, history of hypertension). We conducted sensitivity analysis by repeating the meta-analysis of remaining studies after omitting one study at a time.
When possible, we performed linear dose-response meta-analysis per 100 g/day increment of red meat intake and per 50 g/day increment of processed meat intake using generalized least squares trend estimation (GLST) [45, 46]. These methods require that the number of cases and person–time or controls for at least three quantitative exposure categories be known. GLST requires medians for categories of intake levels. For open-ended categories, we assumed that the range was the same as the adjacent interval. When the exposures were expressed as “times” or “servings”, we converted it into grams (g) using 120 g and 50 g as a standard portion size for red meat and processed meat, respectively, as described in the WCRF/AICR report [22]. For the study [34] reporting intakes as g/1000 kcal/day, the intake as g/day was estimated using the average energy intake reported in the article. We performed potential non-linear dose-response analysis using the best-fitting 2-term fractional polynomial regression model [47]. A likelihood ratio test was used to assess the difference between the non-linear and linear models to test for non-linearity [47]. All statistical analyses were performed using R-package (Version 2.11.0 beta, R Development Core Team, NJ, USA) and Stata version 11.0 (StataCorp, College Station, TX, USA). A 2-sided test with α = 0.05 was used to indicate the level of significance.
RESULTS
Search results and study characteristics
The search strategy generated 2,211 citations, of which 59 were considered of potential value and for which the full text was retrieved for detailed evaluation. An additional seven articles were identified from a review of the references. Forty-three of these 66 articles were subsequently excluded from the meta-analysis. The studies by Di Maso et al. [48] and Bravi et al. [17] were based on the same data. We included the latter [17] because it had the most informative data. The studies by De Stefani et al. [23] and De Stefani et al. [33] were based on the same setting, but in different time periods, i.e., from 1988 to 1995 and from 1996 to 2004. Therefore, we included both studies. We also included two studies with overlapping reports [19, 35]: one on overall processed meat intake [35] and the other on red meat intake [19]. One pooled study included 13 independent cohorts [15]; another four cohort studies included four different cohorts (the European Prospective Investigation into Cancer and Nutrition study [EPIC] [32]; the NIH-AARP Diet and Health Study [34], the Japan Collaborative Cohort Study for Evaluation of Cancer Risk [JACC] Study [18], and California Seventh-day Adventists [28]). An eventual total 23 publications were included in this meta-analysis (Figure 1).
Figure 1. Flow diagram of systematic literature search on red and processed meat intake and renal cell carcinoma risk.
The characteristics of these 23 publications are described in Tables 1 and 2. They comprised four prospective cohort studies [18, 28, 32, 34], one pooled study [15], and 18 case-control studies [16, 17, 20–27, 31, 33, 35, 37–40]. A total 14,285 patients with RCC and 1,821,615 controls/participants were included. The studies were conducted in North America (n = 11), Europe (n = 7), Asia (n = 1), and South America (n = 3). The pooled study was conducted in the United States and in Europe. The methods used in all studies for assessing meat consumption were based on the food items semiquantitative Food Frequency Questionnaire (FFQ). The Newcastle-Ottawa Scale (NOS) scores ranged 5–9; 19 studies were deemed to be of high quality (≥ 7 stars) (Supplementary Table 1).
Table 1. Characteristics of case-control studies of red and processed meat intake and renal cell carcinoma risk.
| Author/ year/ Country | number of subjects enrolled |
Outcome determined | Dietary assessments |
Exposure (Highest vs. lowest) |
RR (95% CI) (Highest vs. lowest) |
Adjustments | Score |
|---|---|---|---|---|---|---|---|
| Hospital-based | |||||||
| Melkonian et al. 2016, USA [31] | 659 RCC cases 699 controls |
Histological | Self-administered Validated FFQ |
Red meat T3 vs. T1 |
2.28 (1.67–3.10) | Age, sex, BMI, history of hypertension, smoking status, total energy intake, total fruit and vegetable intake | 7 |
| De Stefani et al. 2012 Uruguay [33] | 144 RCC 2,532 Controls |
Histological | Validated FFQ-64 Interview |
Processed meat: >28.3 vs. 11.4 g/d | 1.21 (0.65–2.25)M 2.15 (0.90–5.13)W |
Age, residence, BMI, smoking status, smoking, alcohol drinking, mate consumption, total energy, total vegetables and fruits, total white meat | 7 |
| Aune et al. 2009, Uruguay [40] | 114 RCC 2,032 Controls |
Histological | Validated FFQ-64 Interview |
Red meat: 300.2 vs. 85.5 g/d Beef: 300 vs. 85.5 g/d Lamb: 150 vs. 0g/d Processed meat: |
2.72 (1.22–6.07) 2.53 (1.14–5.59) 0.77 (0.22–2.67) 1.23 (0.68–2.22) |
Age, sex, residence, education, income, interviewer, smoking status, alcohol, dairy foods, grains, fatty foods, fruits and vegetables, fish, poultry, mate drinking, BMI and energy intake | 7 |
| Bravi et al. 2007, Italy [17] | 767 RCC 1,534 Controls |
Histological | Interview FFQ-78 validated |
Red meat: 5.9 vs. 2.4 serving/wk Processed meat:3.9 vs.0.9 serving/wk |
0.84 (0.62–1.14) 0.64 (0.45–0.90) |
Age, center, sex, period of interview, education, smoking, alcohol drinking, BMI, family history of kidney cancer, total energy intake |
7 |
| Hsu et al. 2007, Europe [16] | 1,065 RCC 1,509 Controls |
Histological | Interview FFQ-23 validated |
Red meat: ≥ 1 time/wk vs. < 1 time/month Ham, salami, sausages ≥ 1 time/wk vs. < 1 time/month |
2.01 (1.02–3.99) 1.03 (0.71–1.51) |
Age, country, sex, smoking, education, BMI, hypertension medication use, alcohol consumption, total white meat consumption |
7 |
| Tavani et al. 2000, Italy [21] | 190 RCC 7,990 Controls |
Histological | Self-administered FFQ, NA |
Red meat: > 6 vs. ≤ 3 servings/wk | 1.1 (0.8–1.6) | Age, year of recruitment, sex, education, smoking, alcohol, fat, fruit and vegetable intakes. | 5 |
| De Stefani et al. 1998, Uruguay [23] | 121 RCC 243 Controls |
Histological | Interview FFQ-23 validated |
Red meat: > 365 vs. ≤ 208 g/d Barbecued: > 53 vs. ≤ 12 g/d Processed meat: > 53 vs. ≤ 12 g/d |
3.42 (1.76–6.65) 2.07 1.03–4.19 0.78 (0.45–1.39) |
Age, sex, residence, urban-rural status, education, BMI, mate drinking. | 6 |
| Talamini et al. 1990, Italy [26] | 240 RCC 665 Controls |
Histological | Interview FFQ NA |
Salami: ≥ 3 serving/wk vs. the lowest | 1.01 (0.63–1.61) 1.25 (0.85–1.85) |
Age, sex, education, area of residence, BMI | 5 |
| Population-based | |||||||
| Hu et al. 2011, Canada [35] | 1,345 RCC 5,039 Controls |
Histological | Validated FFQ-69 Interview |
Processed meat: ≥ 5.42 vs.0.94 servings/wk | 1.3 (1.1–1.6) | Age, province, education, BMI, sex, alcohol use, smoking, total vegetable and fruit intake, and total energy intake | 9 |
| Daniel et al. 2011, USA [36] | 1,192 RCC 1,175 Controls |
Histological | Interviewer Diet History Questionnaire |
Red meat: 42.0 vs.11.7 g/1000kal/d Barbecued meat:16.7 vs.0 g/1000kal/d Pan-fried meat: 15.6 vs.0.3 g/1000kal/d Broiled meat:7.6 vs.0 g/1000kal/d |
1.11 (0.83–1.48) 1.35 (1.01–1.79) 1.05 (0.80–1.38) 0.75 (0.59–0.96) |
Age, race, sex, education, smoking status, BMI, history of hypertension, family history of cancer, alcohol, intake of fruit and vegetables, total energy intake, and other meat intake and/or cooking method offsets | 8 |
| Brock et al. 2009, USA [39] | 323 RCC 1,820 Controls |
Histological | Self-administered questionnaire NA |
Red meat: > 1.7 vs.0–0.8 servings/d Cured meat: > 0.6 vs.0–0.1servings/d |
1.5 (1·0–2.4) 1.6 (1·1–2.5) |
Age, sex, smoking, obesity, hypertension, physical activity, alcohol and vegetable intake and tea and coffee consumption |
9 |
| Grieb et al. 2009, USA [37] | 335 RCC 337 Controls |
Histological | Interview FFQ-70 Validated |
Red meat: > 5 vs. < 1 time/wk Bacon and sausage: > 5 vs. < 1 time/wk |
4.43 (2.02–9.75) 1.28 (0.63–2.62) |
Age, sex, race, income, BMI, smoking | 8 |
| Hu et al. 2003, Canada [19] | 1,279 RCC 5,380 Controls |
Histological | Self-administered FFQ-70 Validated |
Beef, pork or lam:T3 vs.T1 Hamburger:T3 vs.T1 Bacon:T3 vs.T1 Sausage:T3 vs.T1 |
1.3 (1.0–1.6) 1.4 (1.1–1.8) 1.3 (1.0–1.6) 1.5 (1.2–2.0) |
Age, sex, province, education, BMI, alcohol use, smoking and total energy | |
| Handa et al. 2002, Canada [20] | 461 RCC 672 Controls |
Histological | Self-administered FFQ-69 NA |
Beef: Q4 vs. Q1 | 1.2 (0.7–2.0) M | Age, smoking status, BMI | 7 |
| Yuan et al. 1998, USA [22] | 1204 RCC 1204 Controls |
Histological | Interview FFQ-40 NA |
Processed meat: Q4 vs. Q1 | 1.15 (0.86–1.54) | level of education, BMI, history of hypertension cigarettes, analgesics, use of amphetamines |
7 |
| Wolk et al. 1996, multi centers [24] | 1,185 RCC 1,526 Controls |
Histological | Self-administered and interview FFQ, NA |
Red meat: Q4 vs. Q1 Processed meat: Q4 vs. Q1 |
0.94(0.73–1.20) 0.94(0.73–1.22) |
Age, sex, stud center, BMI, smoking, total calories | 7 |
| Chow et al. 1994, USA [25] | 690 RCC 707 Controls |
Histological | Self-administered FFQ-65 validated |
Red meat: > 9.3 vs.4.3 servings/wk Processed meat:5.0 vs. 1.4 servings/wk |
1.3 (0.9–1.9) 1.0 (0.7–1.5) |
Age, sex, cigarette smoking, and BMI. | 8 |
| Maclure et al. 1990, USA [27] | 203 RCC 604 Controls |
Histological | Interview FFQ Validated |
Beef: Q4 vs. Q1 Pork: Q4 vs. Q1 Bacon: Q4 vs. Q1 Processed meat: Q4 vs. Q1 |
3.4(1.6–7.2) 0.74(0.4–1.4) 0.85(0.47–1.5) 1.3(0.86–2.0) |
Age, sex | 7 |
Abbreviation: BMI, body mass index; FFQ, food frequency questionnaire; NA, not available; M, men, W, women; RCC, renal cell carcinoma.
Table 2. Characteristics of cohort studies of red and processed meat intake and renal cell carcinoma.
| Author/year, Country | Study name and number of subjects FU, yr |
Case ascertainment Cases (n) |
Dietary assessments |
Exposure details | RR (95% CI) (Highest vs. lowest) |
Adjustments | Score |
|---|---|---|---|---|---|---|---|
| Rohrmann et al. 2015 [32], Europe | EPIC N = 375,851 FU, 11.6 yr |
cancer or mortality registries 691 RCC |
Self-administered Validated FFQ |
Red meat: > 80 vs. 0–9.9 g/d Processed meat: > 80 vs. 0–9.9 g/d |
1.46 (0.99–2.15) 1.23 (0.84–1.79) |
Age, center, sex, education, BMI, history of hypertension, smoking status, duration of smoking, energy intake,alcohol consumption, fruit and vegetable consumption | 9 |
| Daniel et al. USA2012 [34] | NIH-AARP Diet and Health Study N = 491,841 FU, 9 yr |
cancer registry 1,816 RCC |
Self-administered Validated FFQ-124 |
Red meat:48.1 vs. 6.8 g/1000k/d Processed meat:19.9 vs. 1.4 g/1000k/d |
1.08 (0.92–1.28) 1.12 (0.95–1.32) |
Age, sex, education, race, marital status, family history of any cancer, BMI, smoking status, hypertension, diabetes, alcohol, total energy, legumes, whole grains | 9 |
| Lee et al. 2008, Europe and USA [15] | 13 cohorts N = 774,952 FU, 7-20 yr |
medical records, cancer registries 1,478 RCC |
Self-administered Validated FFQ |
Red meat: > 80 vs. < 20 g/d Processed meat:12–27 vs. < 4 g/d |
0.99 (0.85–1.16) 1.06 (0.88–1.28) |
Age, history of hypertension, BMI, smoking, combination of parity and age at first birth, fruit and vegetable consumption, alcohol intake, and total energy intake |
9 |
| Washio et al. 2005, Japan [18] | JACC N = 114,517 FU, 10yr |
mortality registries 48 RCC |
Self-administered Validated questionnaire | Beef: 1–2 vs. seldom times/wk Pork: 1–2 vs. seldom times/wk Ham and sausage: 1–2 vs. seldom times/wk |
1.73(0.74–4.08) 0.92(0.34–2.27) 1.16(0.42–3.24) |
Age, sex | 7 |
| Fraser et al. 1990, USA [28] | California Seventh-day Adventists N = 34,198 FU, 6.2 yr |
mortality registries 14 RCC |
Self-administered Validated FFQ |
Beef: > 1 vs. < 1 serving/wk | 1.59 (0.49–5.01) | Age, sex | 6 |
Abbreviation: EPIC, the European Prospective Investigation into Cancer and Nutrition; JACC, the Japan Collaborative Cohort Study for Evaluation of Cancer Risk Study; BMI, body mass index; FFQ, food frequency questionnaire; NA, not available, RCC, renal cell carcinoma; FU, follow-up.
Red meat
High vs. low analysis
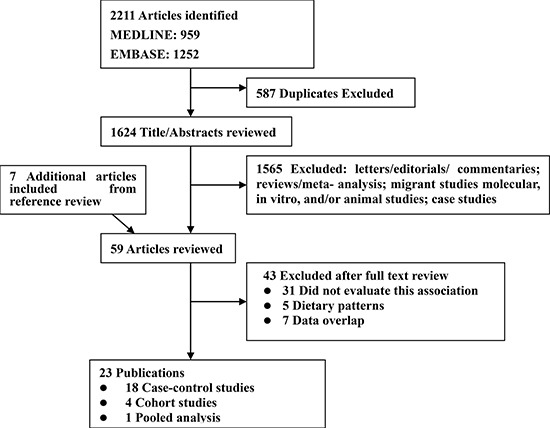
Nineteen studies reported on the highest vs. lowest levels of red meat intake and RCC risk. The summary relative risk (SRR) was 1.36 (95% confidence interval [CI], 1.16–1.58); there was evidence of high inter-study heterogeneity (Pheterogeneity < 0.001, I2 = 71.3%; Figure 2A).
Figure 2.
The summary risk association between red meat intake and risk of renal cell carcinoma according to (A) the highest vs. lowest analysis; (B) linear dose-response analysis (Per 100 g/day increment); (C) non-linear dose-response analysis. Studies are sub-grouped according to design.
Dose-response analysis
Thirteen studies were included in the dose-response analysis of red meat intake and RCC risk. The SRR per 100 g/day increment was 1.21 (95% CI, 1.08–1.36), with evidence of high heterogeneity (P heterogeneity < 0.001, I2 = 73.6%; Figure 2B). There was evidence of a non-linear association of red meat intake and RCC risk (P = 0.002). Visual inspection of the curve suggested that the risk increased linearly up to approximately 240 g/day red meat intake. Above that, the risk increase became even steeper (Figure 2C).
Processed meat
High vs. low analysis
Nineteen studies reported on the highest vs. lowest level of processed meat intake and RCC risk. The SRR was 1.13 (95% CI, 1.03–1.24), and there was evidence of moderate inter-study heterogeneity (Pheterogeneity = 0.014, I2 = 45.6%; Figure 3A).
Figure 3.

The summary risk association between processed meat intake and risk of renal cell carcinoma according to (A) the highest vs. lowest analysis; (B) linear dose-response analysis (Per 50 g/day increment); (C) non-linear dose-response analysis. Studies are sub-grouped according to design.
Dose-response analysis
Fourteen studies were included in the dose-response analysis of processed meat intake, and the SRR per 50 g/day increase was 1.16 (95% CI, 0.99–1.36), and there was high inter-study heterogeneity (Pheterogeneity < 0.001, I2 = 65.1%; Figure 3B). There was no evidence of a non-linear association between processed meat intake and RCC risk (P = 0.231; Figure 3C).
Subgroup, meta-regression, and sensitivity analyses
Table 3 shows the results of the stratified and meta-regression analyses. For high vs. low consumption of red meat, we observed an increased risk of RCC in case-control studies (SRR = 1.46; 95% CI, 1.18−1.81), but not in cohort studies (SRR = 1.07; 95% CI, 0.96−1.19). The SRRs were significant for studies conducted in North America (SRR = 1.44; 95% CI, 1.17–1.76; I2 = 68.6%) and South America (SRR = 3.12; 95% CI, 1.87–5.20; I2 = 72.3%), but not in those conducted in Europe (SRR = 1.04; 95% CI, 0.86–1.26) and Asia (SRR = 1.30; 95% CI, 0.69–2.46). There was significant between-subgroup heterogeneity in stratified analysis of location (P for difference = 0.038). Adjustments by body mass index (BMI), smoking, history of hypertension, total energy intake, intake of vegetables and fruits, and alcohol consumption did not significantly change the SRR for RCC risk.
Table 3. Subgroup analyses of red and processed meat intake and renal cell carcinoma risk, high vs. low.
| Sub-groups | Red meat | Processed meat | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies, n |
SRR (95% CI) | P for heterogeneity | I2 (%) | P for difference | Studies, n |
SRR (95% CI) | P for heterogeneity | I2 (%) | P for difference | |
| All | 19 | 1.36 (1.16–1.58) | < 0.001 | 71.3 | 19 | 1.13 (1.03–1.24) | 0.014 | 45.6 | ||
| Design | 0.751 | 0.956 | ||||||||
| Cohort | 5 | 1.07 (0.96–1.19) | 0.398 | 1.5 | 4 | 1.11 (0.99–1.25) | 0.797 | 0 | ||
| Case-control | 14 | 1.46 (1.18–1.81) | < 0.001 | 75.0 | 14 | 1.13 (1.00–1.27) | 0.004 | 55.4 | ||
| Sources of control | 0.470 | 0.152 | ||||||||
| Population-based | 8 | 1.29 (1.06–1.58) | 0.021 | 57.4 | 9 | 1.18 (1.04–1.34) | 0.024 | 54.5 | ||
| Hospital-based | 6 | 1.75 (1.10–2.78) | < 0.001 | 84.7 | 6 | 1.03 (0.79–1.34) | 0.059 | 50.6 | ||
| Geographic locations | 0.038 | 0.178 | ||||||||
| Europe | 6 | 1.04 (0.86–1.26) | 0.069 | 51.2 | 5 | 0.98 (0.78–1.23) | 0.065 | 54.7 | ||
| USA | 10 | 1.44 (1.17–1.76) | 0.001 | 68.6 | 9 | 1.20 (1.07–1.34) | 0.062 | 46.2 | ||
| South America | 2 | 3.12 (1.87–5.20) | < 0.001 | 72.3 | 3 | 1.16 (0.81–1.67) | 0.275 | 22.7 | ||
| Asia | 1 | 1.30 (0.69–2.460 | - | 1 | 1.60 (0.58–4.44) | - | ||||
| Data available | 0.189 | 0.424 | ||||||||
| Self-administered | 12 | 1.25 (1.07–1.45) | 0.002 | 63.5 | 8 | 1.17 (1.03–1.33) | 0.069 | 46.7 | ||
| Interview | 7 | 1.79 (1.16–2.75) | < 0.001 | 78.8 | 11 | 1.09 (0.95–1.26) | 0.052 | 43.7 | ||
| Type of FFQ | 0.294 | 0.857 | ||||||||
| Validated | 16 | 1.44 (1.20–1.75) | < 0.001 | 75.8 | 15 | 1.12 (1.00–1.25) | 0.012 | 49.9 | ||
| Not available | 4 | 1.10 (0.90–1.33) | 0.317 | 15.0 | 4 | 1.17 (0.94–1.44) | 0.172 | 40.0 | ||
| Study quality score | ||||||||||
| High (NOS score > 6) | 16 | 1.32 (1.30–1.54) | < 0.001 | 71.5 | 0.464 | 17 | 1.13 (1.03–1.25) | 0.011 | 48.4 | 0.713 |
| Low (NOS score ≤ 6) | 3 | 1.78 (0.79–4.00) | 0.012 | 77.4 | 2 | 1.03 (0.66–1.63) | 0.177 | 45.1 | ||
| Adjustments | ||||||||||
| BMI, yes | 15 | 1.39 (1.16–1.66) | < 0.001 | 77.4 | 0.705 | 17 | 1.12 (1.01–1.24) | 0.008 | 50.2 | 0.426 |
| no | 4 | 1.22 (0.95–1.57) | 0.842 | 0 | 2 | 1.34 (0.91–1.98) | 0.713 | 0 | ||
| Smoking, yes | 15 | 1.30 (1.11–1.52) | < 0.001 | 73.1 | 0.302 | 15 | 1.12 (1.01–1.25) | 0.006 | 53.4 | 0.979 |
| no | 4 | 1.75 (1.10–2.79) | 0.130 | 47.0 | 4 | 1.17 (0.92–1.50) | 0.441 | 0 | ||
| Energy intake, yes | 8 | 1.21 (1.01–1.46) | < 0.001 | 77.9 | 0.167 | 10 | 1.11 (1.01–1.26) | 0.001 | 65.2 | 0.716 |
| no | 11 | 1.49 (1.15–1.93) | 0.001 | 68.0 | 9 | 1.17 (1.01–1.35) | 0.637 | 0 | ||
| Hypertension, yes | 7 | 1.35 (1.07–1.70) | < 0.001 | 78.7 | 0.929 | 7 | 1.09 (1.00–1.18) | 0.457 | 0 | 0.947 |
| no | 12 | 1.38 (1.10–1.73) | < 0.001 | 68.2 | 12 | 1.13 (0.97–1.32) | 0.009 | 54.6 | ||
| Consumption of vegetables and fruits, yes | 7 | 1.40(1.07–1.85) | < 0.001 | 79.2 | 0.883 | 7 | 1.17 (1.03–1.34) | 0.152 | 34.6 | 0.368 |
| No | 12 | 1.33 (1.09–1.63) | < 0.001 | 67.4 | 12 | 1.08 (0.94–1.24) | 0.012 | 54.5 | ||
| Alcohol, yes | 10 | 1.17 (1.02–1.34) | 0.030 | 51.2 | 0.151 | 11 | 1.15 (1.01–1.30) | 0.002 | 62.4 | 0.635 |
| No | 9 | 1.65 (1.18–2.31) | < 0.001 | 77.2 | 8 | 1.08 (0.94–1.24) | 0.684 | 0 | ||
For high vs. low consumption of processed meat, we observed a borderline significant risk of RCC in both case-control (SRR = 1.13; 95% CI, 1.00−1.27; I2 = 55.4%) and cohort studies (SRR = 1.11; 95% CI, 0.99−1.25; I2 = 0). The SRR was significant for studies conducted in North America (SRR = 1.20; 95% CI, 1.07–1.34), but not for studies conducted in South America, Europe, and Asia.
In univariate meta-regression analysis, only location was a significant factor for the association between red meat intake and RCC risk; however, no variables were significant factors for processed meat intake.
The estimation of overall homogeneity and the effect of removing one study at a time from the analysis confirmed the stability of the relationship between intake of red and processed meat and RCC risk (data not shown). In addition, repeat analysis of high vs. low intake using the studies included in the linear dose-response analysis yielded results similar to that of the original analysis (red meat: SRR = 1.20; 95% CI, 1.07–1.34; processed meat: SRR = 1.13; 95% CI, 1.00–1.27).
Publication bias
For intake of red meat, visual inspection of the funnel plot, as well as Egger's test (P = 0.087) and Begg's test (P = 0.005), indicated publication bias. The trim-and-fill method indicated that eight additional risk estimates were needed to balance the funnel plot (Figure 4A), and the summary risk estimates were again not significant (SRR = 1.09; 95% CI, 0.92–1.29). For intake of processed meat, visual inspection of the funnel plot, as well as Egger's test (P = 0.145) and Begg's test (P = 0.183), did not indicate publication bias. The trim-and-fill method indicated that two additional risk estimates were needed to balance the funnel plot (Figure 4B), and the summary risk estimates were unchanged (SRR = 1.12; 95% CI, 1.02–1.23).
Figure 4.
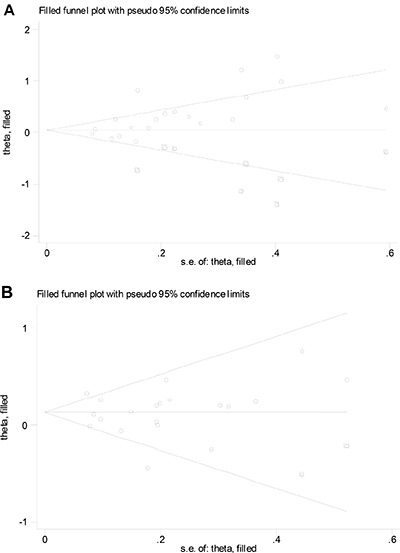
Filled funnel plot of log relative risk vs. standard error of log relative risks in studies that evaluated the effect of red meat (A) and processed meat (B) intake on the risk of renal cell carcinoma.
Individual meat items
There were positive associations between RCC risk with the consumption of beef (SRR = 1.89; 95% CI, 1.25–2.86), hamburger (SRR = 1.41; 95% CI, 1.12–1.78), and ham/salami/bacon/sausage (SRR = 1.30; 95% CI, 1.16–1.47). RCC risk was not positively associated with the intake of pork or barbecued/pan-fried/broiled meat (Supplementary Table 2).
DISCUSSION
The results of this comprehensive meta-analysis show that the consumption of red and processed meat is associated with increased RCC risk, as per the high vs. low and linear dose-response meta-analyses. There was significant heterogeneity across studies for both red and processed meat intake. In non-linear models, RCC risk appeared to increase approximately linearly with increased intake of processed meat, whereas there was evidence of non-linear increased risk with increased intake of red meat. Among individual red and processed meat types, there were statistically significant positive associations for the intake of beef, salami/ham/bacon/sausage, and hamburger.
Several mechanisms have been proposed to explain how the consumption of red and processed meat enhances cancer risk, and include the high intake of proteins and fats and intake of carcinogens (e.g., NOCs, HCAs, PAHs) [49, 50]. A large prospective cohort study observed increased risk of RCC with high consumption of nitrate and nitrite, the precursor of NOCs, and total RCC (hazard ratio = 1.28, 95% CI, 1.10–1.49) [51]. In animal studies, benzo (a) pyrene (BaP) and PhIP were two of the most potent PAHs [52]. Epidemiological studies have found a positive association between BaP and PhIP and RCC [34, 36]. The high saturated fat content of red and processed meat has also been proposed as a culprit for the increased risk of RCC in some studies [53], but not in other studies [54, 55].
In comparison with previous meta-analyses [29, 30], the present updated analysis included an additional 11 studies (two updated studies), and a total 14,285 patients with RCC and 1,821,615 controls/participants, which can provide sufficient power for detecting the putative moderate associations. In addition, we conducted comprehensive analyses based on high vs. low, linear, and non-linear dose-response models; importantly, we performed rigorous quality assessment. We also explored the association between specific subtypes of meat and RCC risk. Finally, by conducting a meta-regression analysis, we could explore the source of heterogeneity between studies.
We found that red and processed meat consumption was significantly associated with increased risk of RCC in the case-control studies, which might drive the overall epidemiological findings of the present study, but not in the cohort studies. Case-control studies are more susceptible to recall and selection bias than are cohort studies, as lifestyles and diet habits in retrospective case-control studies are determined after the diagnosis of cancer. Although the meta-regression results suggested that study design did not significantly alter the aforementioned associations, we observed that the positive association was weaker in the cohort studies than in the case-control studies. Therefore, the finding that red and processed meat consumption is associated with increased RCC risk should be received with caution.
The present meta-analysis has several limitations. First, inaccurate assessments of dietary intake could have led to overestimations of the range of intakes and consequent underestimation of the magnitude of the aforementioned relationship [56, 57]. Not all studies used validated semiquantitative FFQs for dietary assessment; however, subgroup analyses showed that the use of validated vs. non-validated FFQs did not significantly affect the association between the consumption of red and processed meat and RCC risk. Although some FFQs were not validated, its reproducibility has been confirmed, with the correlation coefficients between the two assessments being 0.77 and 0.55 for red meat and processed meat, respectively [58]. In addition, analyses of the highest vs. lowest intake are limited because they do not account for true differences among studies. For example, the definition of lowest intake of red meat ranged from 0 to < 1 time/month [16], and the highest intake ranged from 1 time/week [16] to > 365 g/day [23].
Second, there was great inter-study heterogeneity. Stratified and meta-regression analyses revealed a significant positive association between studies from North America (but not from Europe), and study location was the only significant factor in the association between intake of red meat and RCC risk. This might be attributed to the fact that different populations consume different types, levels of meat, and their cooking practices differ, which may partly explain the high heterogeneity among the included studies. Additionally, there was considerable heterogeneity in the dose-response analysis models, which might be ascribed to a consequence of the conversions of the intake units.
Third, the residual confounders inherent in primary observational studies are always of concern. Although most of the included studies reported adjusted risk estimates of RCC for confounders, some appeared to have failed to fully control for confounders. For example, only seven studies used adjustments for history of hypertension, which is one of the established risk factors of RCC [7]. High intake of red meat and processed meat is likely to be associated with other unhealthy lifestyle choices, for example, smoking, obesity, and lower intake of vegetables and fruits, all of which are indicated as risk factors for RCC [5, 6]. In addition, alcohol consumption is common in people with high intake of red and processed meat, and moderate alcohol consumption was identified as a protective factor against RCC [59]. When we limited the meta-analysis to studies controlled for BMI, smoking, alcohol use, and intake of vegetables and fruits, the aforementioned positive associations were not significantly modified.
Fourth, HCA and PAH formation increases with cooking temperature and duration; however, data on the degree of meat doneness in the included studies were not available. Additionally, the non-linear trend with intake of red meat should be interpreted with caution due to the low statistical power in the extremes of red meat intake distribution. This is an issue of the fractional polynomial method. Most of the included studies were based on data from Western populations; additional research in other populations is warranted to generalize these findings.
Lastly, we acknowledge the presence of significant publication bias in the results for red meat intake. The overall risk estimates for the association for red meat consumption were probably an overestimation, as small studies with null results tend not to be published. Indeed, the trim-and-fill method indicated that eight additional risk estimates were needed to balance the funnel plot, and the summary risk estimates were attenuated and not statistically significant.
In conclusion, our limited data suggest that high intake of red and processed meat may increase RCC risk. However, because the effect was only found in case-control studies and might be a consequence of bias, confounding factors, and importantly, publication bias, further prospective epidemiological studies that control for possible confounders and that examine the association between meat consumption and RCC risk are required.
SUPPLEMENTARY MATERIALS AND TABLES
Acknowledgments
Shaojing Zhang, Juanjuan He and Qingwei Wang participated in the design of this manuscript. Shaojing Zhang, Juanjuan He participated in abstracting the data and performing statistical analysis. All authors read and approved the final manuscript.
Footnotes
CONFLICTS OF INTEREST
None.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–21. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay JS, Bray F, Forman D, Mathers C, Parkin DM. Internation Agency for Research on Cancer. 10. Vol. Lyon, France: 2012. 2008. >GLOBOCAN 2008 v1.2 Cancer Incidence and Mortality Worldwide: IARC CancerBase No. [Google Scholar]
- 4.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–30. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114:101–8. doi: 10.1002/ijc.20618. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Xu Y. Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies. Int J Cancer. 2014;135:1673–86. doi: 10.1002/ijc.28813. [DOI] [PubMed] [Google Scholar]
- 7.Corrao G, Scotti L, Bagnardi V, Sega R. Hypertension, antihypertensive therapy and renal-cell cancer: a meta-analysis. Curr Drug Saf. 2007;2:125–33. doi: 10.2174/157488607780598296. [DOI] [PubMed] [Google Scholar]
- 8.Lee HH, Choi KH, Yang SC, Han WK. Renal cell carcinoma in kidney transplant recipients and dialysis patients. Korean J Urol. 2012;53:229–33. doi: 10.4111/kju.2012.53.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clague J, Lin J, Cassidy A, Matin S, Tannir NM, Tamboli P, Wood CG, Wu X. Family history and risk of renal cell carcinoma: results from a case-control study and systematic meta-analysis. Cancer Epidemiol Biomarkers Prev. 2009;18:801–7. doi: 10.1158/1055-9965.EPI-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choueiri TK, Je Y, Cho E. Analgesic use and the risk of kidney cancer: a meta-analysis of epidemiologic studies. Int J Cancer. 2016;134:384–96. doi: 10.1002/ijc.28093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia. 2011;54:1013–8. doi: 10.1007/s00125-011-2051-6. [DOI] [PubMed] [Google Scholar]
- 12.Kabaria R, Klaassen Z, Terris MK. Renal cell carcinoma: links and risks. Int J Nephrol Renovasc Dis. 2016;9:45–52. doi: 10.2147/IJNRD.S75916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghoshal A, Preisegger KH, Takayama S, Thorgeirsson SS, Snyderwine EG. Induction of mammary tumors in female Sprague-Dawley rats by the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and effect of dietary fat. Carcinogenesis. 1994;15:2429–33. doi: 10.1093/carcin/15.11.2429. [DOI] [PubMed] [Google Scholar]
- 14.Rohrmann S, Hermann S, Linseisen J. Heterocyclic aromatic amine intake increases colorectal adenoma risk: findings from a prospective European cohort study. Am J Clin Nutr. 2009;89:1418–24. doi: 10.3945/ajcn.2008.26658. [DOI] [PubMed] [Google Scholar]
- 15.Lee JE, Spiegelman D, Hunter DJ, Albanes D, Bernstein L, van den Brandt PA, Buring JE, Cho E, English DR, Freudenheim JL, Giles GG, Graham S, Horn-Ross PL, et al. Fat, protein, and meat consumption and renal cell cancer risk: a pooled analysis of 13 prospective studies. J Natl Cancer Inst. 2008;100:1695–706. doi: 10.1093/jnci/djn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu CC, Chow WH, Boffetta P, Moore L, Zaridze D, Moukeria A, Janout V, Kollarova H, Bencko V, Navratilova M, Szeszenia-Dabrowska N, Mates D, Brennan P. Dietary risk factors for kidney cancer in Eastern and Central Europe. Am J Epidemiol. 2007;166:62–70. doi: 10.1093/aje/kwm043. [DOI] [PubMed] [Google Scholar]
- 17.Bravi F, Bosetti C, Scotti L, Talamini R, Montella M, Ramazzotti V, Negri E, Franceschi S, La Vecchia C. Food groups and renal cell carcinoma: a case-control study from Italy. Int J Cancer. 2007;120:681–5. doi: 10.1002/ijc.22225. [DOI] [PubMed] [Google Scholar]
- 18.Washio M, Mori M, Sakauchi F, Watanabe Y, Ozasa K, Hayashi K, Miki T, Nakao M, Mikami K, Ito Y, Wakai K, Tamakoshi A, JACC Study Group Risk factors for kidney cancer in a Japanese population: findings from the JACC Study. J Epidemiol. 2005;15((Suppl 2)):S203–11. doi: 10.2188/jea.15.S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, Mao Y, White K, Canadian Cancer Registries Epidemiology Research Group Diet and vitamin or mineral supplements and risk of renal cell carcinoma in Canada. Cancer Causes Control. 2003;14:705–14. doi: 10.1023/a:1026310323882. [DOI] [PubMed] [Google Scholar]
- 20.Handa K, Kreiger N. Diet patterns and the risk of renal cell carcinoma. Public Health Nutr. 2002;5:757–67. doi: 10.1079/PHN2002347. [DOI] [PubMed] [Google Scholar]
- 21.Tavani A, La Vecchia C, Gallus S, Lagiou P, Trichopoulos D, Levi F, Negri E. Red meat intake and cancer risk: a study in Italy. Int J Cancer. 2000;86:425–8. doi: 10.1002/(sici)1097-0215(20000501)86:3<425::aid-ijc19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Yuan JM, Gago-Dominguez M, Castelao JE, Hankin JH, Ross RK, Yu MC. Cruciferous vegetables in relation to renal cell carcinoma. Int J Cancer. 1998;77:211–6. doi: 10.1002/(sici)1097-0215(19980717)77:2<211::aid-ijc7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.De Stefani E, Fierro L, Mendilaharsu M, Ronco A, Larrinaga MT, Balbi JC, Alonso S, Deneo-Pellegrini H. Meat intake, ‘mate’ drinking and renal cell cancer in Uruguay: a case-control study. Br J Cancer. 1998;78:1239–43. doi: 10.1038/bjc.1998.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolk A, Gridley G, Niwa S, Lindblad P, McCredie M, Mellemgaard A, Mandel JS, Wahrendorf J, McLaughlin JK, Adami HO. International renal cell cancer study. VII. Role of diet. Int J Cancer. 1996;65:67–73. doi: 10.1002/(SICI)1097-0215(19960103)65:1<67::AID-IJC12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.Chow WH, Gridley G, McLaughlin JK, Mandel JS, Wacholder S, Blot WJ, Niwa S, Fraumeni JF Jr. Protein intake and risk of renal cell cancer. J Natl Cancer Inst. 1994;86:1131–9. doi: 10.1093/jnci/86.15.1131. [DOI] [PubMed] [Google Scholar]
- 26.Talamini R, Baron AE, Barra S, Bidoli E, La Vecchia C, Negri E, Serraino D, Franceschi S. A case-control study of risk factor for renal cell cancer in northern Italy. Cancer Causes Control. 1990;1:125–31. doi: 10.1007/BF00053163. [DOI] [PubMed] [Google Scholar]
- 27.Maclure M, Willett W. A case-control study of diet and risk of renal adenocarcinoma. Epidemiology. 1990;1:430–40. doi: 10.1097/00001648-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Fraser GE, Phillips RL, Beeson WL. Hypertension, antihypertensive medication and risk of renal carcinoma in California Seventh-Day Adventists. Int J Epidemiol. 1990;19:832–8. doi: 10.1093/ije/19.4.832. [DOI] [PubMed] [Google Scholar]
- 29.Alexander DD, Cushing CA. Quantitative assessment of red meat or processed meat consumption and kidney cancer. Cancer Detect Prev. 2009;32:340–51. doi: 10.1016/j.cdp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Faramawi MF, Johnson E, Fry MW, Sall M, Zhou Y. Consumption of different types of meat and the risk of renal cancer: meta-analysis of case-control studies. Cancer Causes Control. 2007;18:125–33. doi: 10.1007/s10552-006-0104-9. [DOI] [PubMed] [Google Scholar]
- 31.Melkonian SC, Daniel CR, Ye Y, Tannir NM, Karam JA, Matin SF, Wood CG, Wu X. Gene-environment interaction of genome-wide association study-identified susceptibility loci and meat-cooking mutagens in the etiology of renal cell carcinoma. Cancer. 2016;122:108–15. doi: 10.1002/cncr.29543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohrmann S, Linseisen J, Overvad K, Lund Wurtz AM, Roswall N, Tjonneland A, Boutron-Ruault MC, Racine A, Bastide N, Palli D, Agnoli C, Panico S, Tumino R, et al. Meat and fish consumption and the risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Int J Cancer. 2015;136:E423–31. doi: 10.1002/ijc.29236. [DOI] [PubMed] [Google Scholar]
- 33.De Stefani E, Boffetta P, Ronco AL, Deneo-Pellegrini H, Correa P, Acosta G, Mendilaharsu M, Luaces ME, Silva C. Processed meat consumption and risk of cancer: a multisite case-control study in Uruguay. Br J Cancer. 2012;107:1584–8. doi: 10.1038/bjc.2012.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel CR, Cross AJ, Graubard BI, Park Y, Ward MH, Rothman N, Hollenbeck AR, Chow WH, Sinha R. Large prospective investigation of meat intake, related mutagens, and risk of renal cell carcinoma. Am J Clin Nutr. 2012;95:155–62. doi: 10.3945/ajcn.111.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu J, La Vecchia C, Morrison H, Negri E, Mery L, Canadian Cancer Registries Epidemiology Research Group Salt, processed meat and the risk of cancer. Eur J Cancer Prev. 2011;20:132–9. doi: 10.1097/CEJ.0b013e3283429e32. [DOI] [PubMed] [Google Scholar]
- 36.Daniel CR, Schwartz KL, Colt JS, Dong LM, Ruterbusch JJ, Purdue MP, Cross AJ, Rothman N, Davis FG, Wacholder S, Graubard BI, Chow WH, Sinha R. Meat-cooking mutagens and risk of renal cell carcinoma. Br J Cancer. 2011;105:1096–104. doi: 10.1038/bjc.2011.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grieb SM, Theis RP, Burr D, Benardot D, Siddiqui T, Asal NR. Food groups and renal cell carcinoma: results from a case-control study. J Am Diet Assoc. 2009;109:656–67. doi: 10.1016/j.jada.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 38.De Stefani E, Aune D, Boffetta P, Deneo-Pellegrini H, Ronco AL, Acosta G, Brennan P, Ferro G, Mendilaharsu M. Salted meat consumption and the risk of cancer: a multisite case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10:853–7. [PubMed] [Google Scholar]
- 39.Brock KE, Gridley G, Chiu BC, Ershow AG, Lynch CF, Cantor KP. Dietary fat and risk of renal cell carcinoma in the USA: a case-control study. Br J Nutr. 2009;101:1228–38. doi: 10.1017/S0007114508056043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aune D, De Stefani E, Ronco A, Boffetta P, Deneo-Pellegrini H, Acosta G, Mendilaharsu M. Meat consumption and cancer risk: a case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10:429–36. [PubMed] [Google Scholar]
- 41.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 42.Wells GA, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed June 15, 2012.
- 43.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 44.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 46.Orsini NB, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- 47.Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med. 2000;19:1831–47. doi: 10.1002/1097-0258(20000730)19:14<1831::aid-sim502>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Di Maso M, Talamini R, Bosetti C, Montella M, Zucchetto A, Libra M, Negri E, Levi F, La Vecchia C, Franceschi S, Serraino D, Polesel J. Red meat and cancer risk in a network of case-control studies focusing on cooking practices. Ann Oncol. 2013;24:3107–12. doi: 10.1093/annonc/mdt392. [DOI] [PubMed] [Google Scholar]
- 49.Whiteman DC, Wilson LF. The fractions of cancer attributable to modifiable factors: A global review. Cancer Epidemiol. 2016;44:203–21. doi: 10.1016/j.canep.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Ito N, Hasegawa R, Sano M, Tamano S, Esumi H, Takayama S, Sugimura T. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Carcinogenesis. 1991;12:1503–6. doi: 10.1093/carcin/12.8.1503. [DOI] [PubMed] [Google Scholar]
- 51.Dellavalle CT, Daniel CR, Aschebrook-Kilfoy B, Hollenbeck AR, Cross AJ, Sinha R, Ward MH. Dietary intake of nitrate and nitrite and risk of renal cell carcinoma in the NIH-AARP Diet and Health Study. Br J Cancer. 2013;108:205–12. doi: 10.1038/bjc.2012.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA. A comparison of the tumors induced by coal tar and benzo[a]pyrene in a 2-year bioassay. Carcinogenesis. 1998;19:117–24. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- 53.Hu J, La Vecchia C, DesMeules M, Negri E, Mery L, Canadian Cancer Registries Epidemiology Research Group Nutrient and fiber intake and risk of renal cell carcinoma. Nutr Cancer. 2008;60:720–8. doi: 10.1080/01635580802283335. [DOI] [PubMed] [Google Scholar]
- 54.Allen NE, Balkwill A, Beral V, Green J, Reeves G, Million Women Study Collaborators Fluid intake and incidence of renal cell carcinoma in UK women. Br J Cancer. 2011;104:1487–92. doi: 10.1038/bjc.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen NE, Roddam AW, Sieri S, Boeing H, Jakobsen MU, Overvad K, Tjonneland A, Halkjaer J, Vineis P, Contiero P, Palli D, Tumino R, Mattiello A, et al. A prospective analysis of the association between macronutrient intake and renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2009;125:982–7. doi: 10.1002/ijc.24447. [DOI] [PubMed] [Google Scholar]
- 56.Prentice RL. Dietary assessment and the reliability of nutritional epidemiology reports. Lancet. 2003;362:182–3. doi: 10.1016/S0140-6736(03)13950-5. [DOI] [PubMed] [Google Scholar]
- 57.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 58.Ronco AL, De Stefani E, Boffetta P, Deneo-Pellegrini H, Acosta G, Mendilaharsu M. Food patterns and risk of breast cancer: A factor analysis study in Uruguay. Int J Cancer. 2006;119:1672–8. doi: 10.1002/ijc.22021. [DOI] [PubMed] [Google Scholar]
- 59.Bellocco R, Pasquali E, Rota M, Bagnardi V, Tramacere I, Scotti L, Pelucchi C, Boffetta P, Corrao G, La Vecchia C. Alcohol drinking and risk of renal cell carcinoma: results of a meta-analysis. Ann Oncol. 2012;23:2235–44. doi: 10.1093/annonc/mds022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


