Abstract
Standardized guidelines for the oral health management of patients with rare diseases exhibiting morphologic anomalies are currently lacking. This review considers Bardet-Biedl syndrome (BBS), a monogenic autosomal recessive nonmotile ciliopathy, as an archetypal condition. Dental anomalies are present in a majority of individuals affected by BBS due to abnormal embryonic orofacial and tooth development. Genetically encoded intrinsic oral structural anomalies and heterogeneous BBS clinical phenotypes and consequent oral comorbidities confound oral health management. Since the comorbid spectrum of BBS phenotypes spans diabetes, renal disease, obesity, sleep apnea, cardiovascular disease, and cognitive disorders, a broad spectrum of collateral oral disease may be encountered. The genetic impact of BBS on the anatomic development of oral components and oral pathology encountered in the context of various BBS phenotypes and their associated comorbidities are reviewed herein. Challenges encountered in managing patients with BBS are highlighted, emphasizing the spectrum of oral pathology associated with heterogeneous clinical phenotypic expression. Guidelines for provision of care across the spectrum of BBS clinical phenotypes are considered. Establishment of integrated medical-dental delivery models of oral care in the context of rare diseases is emphasized, including involvement of caregivers in the context of managing these patients with special needs.
Keywords: ciliopathies, cilia, mutation, registries, Wnt signalling pathway, maxillofacial abnormalities
Introduction
Bardet-Biedl syndrome (BBS) is a pleiotropic autosomal recessive genetic disorder with vast genetic heterogeneity (Forsythe et al. 2003). To date, causal mutations of 21 genes (BBS1 to BBS21; see Fig. 1A; Forsythe et al. 2003; Heon et al. 2016; Schaefer et al. 2016) have been identified, accounting for approximately 80% of individuals meeting diagnostic criteria for BBS. Relative frequency of genetic mutations seen in BBS was summarized in a recent review by Haws et al. (2015), with BBS1 and BBS10 showing the highest prevalence and approximately 20% remaining undefined to date (see Fig. 1B). The clinical diagnosis is established if 4 primary features, or 3 primary and 2 secondary features, are evident. Table 1 summarizes primary and secondary features of BBS (Beales et al. 1999; Forsythe and Beales 2013). Improved understanding of dental anomalies associated with BBS is essential to informing proper dental treatment planning and providing supportive oral care for affected patients, which is often complicated by coexisting renal, cardiac, metabolic, and developmental abnormalities. The focus of this review is as follows: 1) to summarize current knowledge on primary craniofacial abnormalities and oral manifestations associated with BBS and secondary oral manifestations that may arise from other clinical manifestations associated with BBS; 2) expand on current understanding of pathophysiology of dental abnormalities encountered in BBS; and 3), in the absence of clinical practice guidelines for management of patients with ciliopathies, highlight considerations surrounding care planning and oral health management of patients with BBS presenting with primary and secondary oral manifestations.
Figure 1.
Summary and prevalence of genes implicated in BBS. (A) List of BBS genes with chromosomal location (locus) and number of significant mutations identified in each gene. Source: Online Mendelian Inheritance in Man. *HUGO Gene Nomenclature Committee–approved gene symbol. #Alternative gene symbol. (B) Relative frequency of BBS gene mutations reported within the population known to be affected by Bardet-Biedl syndrome. Source: Reprinted with permission from Haws et al. (2015).
Table 1.
Major and Minor Diagnostic Features of Bardet-Biedl Syndrome.
| Feature Type | Diagnostic Features |
|---|---|
| Major criteria | Renal anomalies |
| Rod-cone dystrophy | |
| Polydactyly | |
| Obesity | |
| Learning disabilities, cognitive impairment | |
| Hypogonadism in males | |
| Minor criteria | Speech disorder |
| Ocular defects | |
| Brachydactyly, syndactyly | |
| Developmental delay | |
| Polyuria, polydipsia | |
| Ataxia | |
| Mild spasticity | |
| Diabetes mellitus | |
| Dental crowding, hypodontia, small roots, high-arched palate | |
| Congenital heart disease | |
| Hepatic fibrosis |
Modified from Beales et al. (1999) and Forsythe and Beales (2013).
Epidemiology
First described by Georges Louis Bardet and Artur Biedl in the early 1920s, BBS is characterized as a condition with an array of clinical characteristics (Forsythe et al. 2003). Incidence of BBS is very rare, and few studies have assessed its prevalence. The male:female ratio is estimated at 1.3:1 with high variability. The frequency of BBS is very low in North America and Europe. Among the nonconsanguineous populations of northern Europe and America, the prevalence ranges from 1:100,000 (North America) to 1:160,000 (Switzerland) (Forsythe et al. 2003). Prevalence is high in consanguineous populations of Newfoundland, Canada (1:18,000), and Bedouin communities in the Middle East (1:13,500) (Beales et al. 1999; Forsythe et al. 2003; Forsythe and Beales 2013).
Clinical Registry Investigating Bardet-Biedl Syndrome
The Clinical Registry Investigating Bardet-Biedl Syndrome (CRIBBS; 2017), a web-based database that comprehensively captures data on patients with BBS across all features of the condition, is currently the largest international registry, longitudinally tracking 290 participants from around the world. It was developed at Marshfield Clinic, Wisconsin, for the purpose of gathering comprehensive health information on patients with BBS in a single repository. De-identified data from this database are shared with the Global Rare Diseases Registry Data Repository, maintained by the National Center for Advancing Translational Sciences at the National Institutes of Health, to centralize availability of complex genetic and clinical information to researchers worldwide for accelerating research on BBS (Haws et al. 2015). The Appendix Table summarizes information on various oral manifestations in individuals with BBS from the CRIBBS. Other registries, including the EURO-WABB registry (Farmer et al. 2013) supported by the European Union, collect information on BBS and other rare conditions.
Role of Primary Cilium in Craniofacial Development
BBS is a nonmotile ciliopathy attributable to defects in structure and functioning of the primary cilium or nonmotile cilium (Mitchison and Valente 2017). The primary cilium has a range of functions, such as modulation of developmental pathways, transduction of sensory signals, alteration of energy homeostasis, and regulation of stem cell proliferation and growth (Hisamoto et al. 2016; Mitchison and Valente 2017). Most craniofacial phenotypes in BBS are due to mutations in genes required for encoding ciliary proteins, including the intraflagellar transport (IFT) proteins responsible for axonemal extensions in cilia (Schock et al. 2017). The IFT mechanism supports bidirectional movement along the microtubules of the cilia and consists of protein complexes called IFT particles and 2 microtubule-based motor systems—namely, heterotrimeric kinesin 2 and cytoplasmic dynein 2—which, with IFT particles, carry out anterograde and retrograde transport in the ciliary microtubule (Scholey 2008; Mitchison and Valente 2017).
The development of the craniofacial complex requires intricate interaction among various tissues, including the neural crest cells, neural ectoderm, and surface ectoderm (Schock et al. 2017). Murine studies indicate that loss of primary cilium and ciliary proteins in each of the aforementioned tissues during embryonic development results in distinct phenotypes and alterations in cellular differentiation, proliferation, and apoptosis, suggesting that primary cilium and ciliary proteins have a unique role in individual craniofacial tissues (tissue-specific role) (Schock et al. 2017). Facial structures largely originate from neural crest cells, a multipotent cell type arising from the junction of the neural plate and surface ectoderm during early developmental stages. The controlled migration of neural crest cells along predefined paths in the neural tube during embryonic development is central to craniofacial morphogenesis, and disturbances give rise to various craniofacial anomalies (Tobin et al. 2008). During embryonic development, craniofacial structural abnormalities may be noted in the presence of cilial defects consequential to BBS mutations. For example, zebrafish studies showed a decreased ratio of neurocranial length to width in BBS4, BBS6, and BBS8 mutants, supporting involvement of protein products encoded by these genes in migration processes during embryonic development (Tobin et al. 2008). Furthermore, BBS3 gene knockout in mouse embryos gave rise to cleft lip/palate defects; presphenoid and basisphenoid bone defects; fusion of premaxillary bones, vomer wings, and central upper incisors; and hypomorphic premaxillary shelves during embryonic developmental stages (Kawasaki et al. 2016).
The craniofacial defects and aberrant cellular behavior during embryonic development in mice with defects in Shh and Wnt signaling pathways underline the role of these pathways in the development of the craniofacial complex (Tobin et al. 2008; Kawasaki et al. 2016). Perturbation of these pathways in BBS mutant zebrafish further suggests involvement of BBS proteins (ciliary proteins) in regulating these pathways during embryonic development (Tobin et al. 2008).
Role of Primary Cilium in Tooth Development
Dental anomalies in BBS patients have been attributed to structural and functional defects in primary cilium. Murine studies have established the presence of primary cilia in fetal tooth germs and dental follicle, neonate tooth germs, and adult molar teeth and periodontal ligament (Hisamoto et al. 2016).
The integration of Shh and Wnt pathways plays an important role in tooth development. These pathways exhibit synergistic and antagonistic relationships that are context dependent. For example, studies have shown that loss of the IFT ciliary protein kif3a resulted in a gain in Wnt signaling and loss of the Shh pathway, resulting in disruption of amelogenesis and dentinogenesis during tooth formation due to aberrant signaling of dental epithelium (Liu et al. 2014). Primary cilium plays a crucial role in tooth development by regulating epithelial-mesenchymal interaction. Studies of human dental follicle cells and human dental pulp cells showed that selective knockout of the kif3a gene suppressed the osteoblastic differentiation of dental mesenchyme during tooth development by suppressing the Wnt signaling pathway, which plays a critical role in cell differentiation of dental mesenchyme (Hisamoto et al. 2016; Jiang et al. 2016). Hypodontia, present in a subset of BBS patients, has been attributed to defective ciliary structure. Studies in animal models have provided evidence that defects in primary cilium resulted in aberrant Shh signaling leading to defects in tooth number during fetal development (Ohazama et al. 2009). BBS4 and BBS6 gene expression is important for ciliogenesis in odontoblasts during tooth development, and gene mutations may underlie tooth abnormalities in BBS patients with mutations in these genes (Thivichon-Prince et al. 2009). These studies suggest that cilia and ciliary proteins regulate these signaling pathways, which are vital for tooth development.
Clinical Manifestations
Diagnosis of BBS is primarily based on clinical features. Early detection of BBS is uncommon, since most features emerge during the first and second decades of life, with most affected individuals diagnosed in late childhood or early adulthood (Forsythe and Beales 2013). Fetal nephromegaly and increased renal echogenicity combined with polydactyly may alert the astute clinician to a prenatal diagnosis of BBS (Beales et al. 1999; Dar et al. 2001). Diagnosis of BBS is challenging due to phenotypic overlap of clinical symptoms with other ciliopathies, giving rise to an array of potential differential diagnoses. Genetic testing is commercially available, providing clinicians a valuable diagnostic tool to define the correct syndrome or confirm clinical diagnosis. Commercial laboratories employ a combination of next-generation sequencing (NGS) and Sanger sequencing technology to identify pathogenic gene variants. Mutations in BBS1, BBS2, and BBS10 account for >50% of all individuals meeting diagnostic criteria for BBS. However, 20% of individuals with BBS may not have a confirmed diagnosis based on the NGS and Sanger sequencing protocols (see Fig. 1B). Undefined deletions and duplications may account for inconclusive NGS and Sanger sequencing results encountered with the genotyping of some individuals with BBS. Employing an array of comparative genomic hybridization enables the detection of deletions and duplications of single and multiple exons within a given gene reference and can be especially useful when a clinical and genetic diagnosis remains uncertain (Tayeh et al. 2009).
Primary Features of BBS
Impaired vision and obesity pose significant and common problems in BBS. Rod-cone dystrophy, a typical feature of BBS, is seen in 90% to 93% of patients (Beales et al. 1999; Forsythe et al. 2003; Forsythe and Beales 2013). Retinal rod-cone photoreceptors are destroyed, gradually leading to night blindness, constricted visual fields, and eventually complete blindness. Night blindness is typically evident by age 5 to 6 y, while the median age of legal blindness is 15.5 y. Incidence of obesity, a second common major primary feature, occurs in an estimated 72% to 92% of patients with BBS (Beales et al. 1999; Forsythe and Beales 2013). A majority of patients exhibit normal weight at birth but develop truncal obesity during childhood.
Genitourinary abnormalities are present in a majority of individuals (Forsythe and Beales 2013). The urogenital malformations include urogenital sinus, hydrometrocolpos, vesicoureteral reflux, calyceal and parenchymal cysts, fetal lobulation, unilateral renal agenesis, and horseshoe kidney. Chronic kidney disease (CKD) is a leading cause of morbidity and premature mortality in BBS (Moore et al. 2005). The long-term renal impairment seen in most BBS patients may lead to CKD, progressing to end-stage renal disease, dialysis, and renal transplantation. Chronic tubulointerstitial nephritis, cystic tubular disease, and renal malformations are the most common causes for renal impairment apart from glomerular defects and urinary concentrating defects (Beales et al. 1999; Forsythe and Beales 2013). The presence of other comorbidities, including diabetes mellitus, hypertension, and hyperlipidemia, in BBS patients further affects renal function (Beales et al. 1999; Forsythe et al. 2003; Imhoff et al. 2011). Renal ultrasonography often reveals fetal lobulation and loss of corticomedullary differentiation. Chronic tubulointerstitial nephritis with varying degrees of fibrosis is found on renal biopsy.
Cognitive impairment manifests in 61% of cases, with the majority of affected individuals exhibiting learning difficulties and with a smaller percentage having IQ impairment, attention deficits, and delayed thought processes (Beales et al. 1999; Forsythe et al. 2003; Majumdar et al. 2012; Hassona et al. 2017). Postaxial polydactyly is present in 63% to 81% of cases (Beales et al. 1999). Genital abnormalities are noted in 59% to 98% of patients (Beales et al. 1999; Forsythe et al. 2003).
Secondary Features of BBS
Secondary features include speech delay/disorder due to failure of coordination between pharyngeal and laryngeal muscles (Forsythe et al. 2003; Forsythe and Beales 2013). Developmental delay, behavioral abnormalities, brachydactyly, eye abnormalities (including strabismus, cataract, and astigmatism), ataxia, diabetes mellitus, dental anomalies, craniofacial dysmorphism, congenital cardiovascular anomalies, hepatic involvement, anosmia, hypertension, and hyperlipidemia represent other frequently encountered secondary features (Beales et al. 1999; Forsythe et al. 2003; Imhoff et al. 2011; Forsythe and Beales 2013).
Oral Manifestations of BBS
Primary Oral Manifestations
Dentofacial anomalies are present in >50% of affected individuals in concert with other clinical features, including intra- and extraoral manifestations (see Table 2). Common extraoral manifestations are orbital hypertelorism, strabismus, philtrum discrepancies, hypotonic upper lip mouth breathing, retrognathia, incompetent lips, and bitemporal narrowing (Moore et al. 2005; Drugowick et al. 2007; Majumdar et al. 2012; Ferreira et al. 2014; Hassona et al. 2017). Ptosis, palpebral fissure discrepancies, down-turned mouth, brachycephaly, macrocephaly, long ears, frontal balding in males, flat nasal bridge, and maxillary atresia were reported by Moore et al. (2005). Prominence of nasolabial folds were reported by Beales et al. (1999).
Table 2.
Oral Manifestations of Bardet-Biedl Syndrome.
| Manifestation | |
|---|---|
| Extraoral | Intraoral |
| Hypertelorism | Hypodontia |
| Strabismus | Microdontia |
| Ptosis | High-arched palate |
| Long ears | Crowding and spacing problems |
| Short palpebral fissures | Short roots |
| Narrow palpebral fissures | Taurodontism |
| Shallow philtrum | Posterior crossbites |
| Long philtrum | Thick mandible |
| Hypotonic upper lip | |
| Incompetent lips | |
| Mouth breathing | |
| Brachycephaly | |
| Macrocephaly | |
| Flat nasal bridge | |
| Prominent nasolabial folds | |
| Maxillary atresia | |
| Bitemporal narrowing | |
| Retrognathia | |
| Downturned mouth | |
The intraoral manifestations reported to date include hypodontia, predominantly with absent maxillary and mandibular premolars, followed by lower lateral incisors, high-arched palate, microdontia, crowding and spacing problems, and short dental roots in anterior and posterior teeth with obliterated pulp chambers (Borgström et al. 1996; Urben et al. 1999; Moore et al. 2005; Drugowick et al. 2007; Majumdar et al. 2012; Andersson et al. 2013; Ferreira et al. 2014; Hassona et al. 2017). Taurodontism (Andersson et al. 2013), unilateral and bilateral posterior crossbite (Ferreira et al. 2014; Hassona et al. 2017), and thicker mandible (Borgström et al. 1996) are less common. Enamel hypoplasia was also reported by Beales et al. (1999).
Secondary Oral Manifestations
Oral and clinical manifestations of patients with BBS may converge and lead to additional oral complications affecting oral health–related quality of life. Oral manifestations associated with genetic and clinical phenotypic expression in BBS are summarized in Table 3.
Table 3.
Potential Oral Complications Due to the Clinical Manifestations in Patients with Bardet-Biedl Syndrome.
| Clinical Manifestations | Potential Oral Manifestations and Barriers in Care Delivery |
|---|---|
| Rod-cone dystrophy, retinal degeneration | Accessibility issues |
| Fear, apprehension, and anxiety during dental treatment | |
| Poor oral hygiene | |
| Hard and soft tissue injuries in mouth | |
| Obesity | Accessibility issues |
| Periodontal disease | |
| Dental caries | |
| Peri- and postoperative complications | |
| Cognitive defects, learning disabilities | Poor oral hygiene |
| Noncooperative and defiance behavior during treatment | |
| Dental pain is poorly perceived and expressed | |
| Drug-induced gingival hyperplasia | |
| Renal abnormalities | Periodontal diseases |
| Drug-induced gingival hyperplasia | |
| Xerostomia, candidiasis, uremic fetor, altered taste, petechiae, ecchymosis, enamel hypoplasia, uremic stomatitis, erosions on lingual tooth surfaces | |
| Increased risk of bleeding during invasive dental procedures due to anticoagulant use during dialysis | |
| Mouth breathing | Xerostomia |
| Increased anterior facial height | |
| Malocclusions | |
| Sleep disturbances and nocturia in children | |
| Incompetent lips | Gummy smile |
| Difficulty in swallowing | |
| Increased activity of perioral musculature leading to malocclusions | |
| Hypodontia | Problems in speech mastication and phonation |
| Decreased lower facial height | |
| Increased overbite | |
| Dental crowding | Poor oral hygiene and oral halitosis |
| High-arched palate | Breast-feeding problems in infants |
Mouth breathing (Drugowick et al. 2007; Hassona et al. 2017) in BBS patients may cause xerostomia and decreased salivary production and lead to increased anterior facial height and malocclusions (Malhotra et al. 2012; El Aouame et al. 2016). Approximately 60% of affected patients (172 of 290) in the CRIBBS were mouth breathers. Hypoxic conditions in mouth breathing may cause sleep disturbances and nocturia in children. Incompetent lips occurring in these patients may result in increased occlusal vertical dimension causing difficulty in swallowing (MacAvoy et al. 2016). Establishing a lip seal in affected patients may lead to overactive perioral musculature, further leading to malocclusion of anterior teeth (Tosello et al. 1999).
In most cases, drug-induced gingival hyperplasia is consequential to prescribed pharmaceutical exposures, including calcium channel blockers, anticonvulsants, and immune suppressants (Drugowick et al. 2007; Ferreira et al. 2014; Hassona et al. 2017). Drug-induced gingival hyperplasia is mostly seen in patients suffering from renal and neurologic problems and may cause tooth malposition and delayed tooth eruptions.
Hypodontia is seen in some affected individuals and may lead to problems in speech, mastication, and phonation, giving rise to craniofacial dysmorphism with decreased lower facial height and increased overbite (Nodal et al. 1994; Ogaard et al. 1995; Kreczi et al. 2011). However, dental crowding is more common than hypodontia in these individuals. Approximately 46% (134 of 290) of patients in the CRIBBS reported dental crowding, and 19% (56 of 290) underwent tooth extractions due to spacing problems. Individuals with dental crowding are at increased risk for poor oral hygiene and plaque accumulation leading to oral halitosis and periodontitis, which may be exacerbated in mouth breathers (Ferreira et al. 2014; Hassona et al. 2017).
Approximately 35% (101 of 290) of individuals in the CRIBBS exhibited high-arched palate. Parents and caregivers of infants with high-arched palate may face feeding difficulties. The poor sucking reflex in infants due to difficulty in achieving required negative pressure leads to inadequate intake of breast milk, affecting proper growth and functioning (Kudiyirickal and Pappachan 2015). Compromised airway protection during swallowing may lead to other serious respiratory complications (Kudiyirickal and Pappachan 2015).
Obesity is a frequently encountered clinical phenotype in patients with BBS, which may further contribute to periodontal disease susceptibility, influencing the oral health–related quality of life of these patients. Notably, approximately 75% (199 of 264) of individuals with BBS in the CRIBBS were obese, and 14% (37 of 264) were overweight. Truncal obesity observed in adult individuals with BBS (Forsythe et al. 2003; Forsythe and Beales 2013) has been attributed to leptin resistance, adipocyte alterations, and hyperleptinemia (Feuillan et al. 2011). A growing evidence base suggests that inflammatory processes associated with obesity and overweight status may exacerbate periodontal disease and its progression (Pischon et al. 2007; Chaffee et al. 2010; Costacurta et al. 2011). Greater clinical attachment loss and increased periodontal pocket depth were also reported in obese patients (Saito et al. 2001; Linden et al. 2007). Obese individuals with periodontal disease may exhibit greater periodontal destruction, as higher abdominal fat and higher waist-to-hip ratio will further increase periodontium susceptibility to inflammatory destruction (Saito et al. 2001). Moreover, incidence of caries is higher in obese individuals, and the decayed-missing-filled teeth index is higher (Mathus-Vliegen et al. 2007; Vázquez-Nava et al. 2010; Costacurta et al. 2011). Thus, regularly monitoring the periodontal health of patients with BBS is paramount, and periodicity of oral examinations needs to be defined based on the oral health status of individual patients and the capacity of the patient to regularly engage in effective oral hygiene practices.
Diabetes mellitus is a secondary feature in BBS individuals. Approximately 8% (24 of 290) of the BBS patients in the CRIBBS carry a diabetes mellitus diagnosis. The bidirectional relationship between diabetes mellitus and periodontitis is well documented. In some BBS patients, the combined presence of diabetes mellitus and CKD may synergize, thereby increasing the susceptibility of these individuals to periodontal destruction. Dissemination of periodontal pathogens may further contribute to poor glycemic control.
An association between periodontitis and CKD negatively affects vitamin D metabolism, which is important for synthesis of bone matrix proteins and leads to periodontal tissue destruction (Klassen and Krasko 2002; Craig 2008; Fisher et al. 2011). The majority of individuals with BBS have CKD, with approximately 8% (24 of 290) having end-stage CKD. Inflammatory mediators commonly elevated in BBS, including high sensitivity C-reactive protein, exacerbate periodontal inflammation. The inflamed periodontal tissue may concomitantly exacerbate CKD and coronary heart disease due to increased inflammatory response. Notably, high-sensitivity C-reactive protein levels may increase with increased periodontal inflammation (Craig 2008; Fisher et al. 2011). Dialysis therapy has been utilized in 6% (17 of 290) of individuals in the CRIBBS, and 7% (19 of 290) who had undergone renal transplantation were undergoing some form of dialysis. The most common oral manifestations in patients undergoing hemodialysis and peritoneal dialysis are xerostomia, candidiasis, decreased salivary flow, uremic fetor, altered taste, gingival bleeding, petechiae, ecchymosis, enamel hypoplasia, mucosal lesions including uremic stomatitis, pulp obliterations, bone demineralization, plaque accumulation, tooth mobility, erosions on lingual tooth surfaces, and periodontitis exhibiting increased attachment loss and periodontal pocket depths (Klassen and Krasko 2002; Bayraktar et al. 2007; Bayraktar et al. 2008; Jover Cerveró et al. 2008). Gingival inflammation is another common oral manifestation for renal transplantation patients being treated with immunosuppressive drugs such as cyclosporine (Drugowick et al. 2007; Majumdar et al. 2012).
Patient Management
Current dental management approaches in the context of BBS address symptomology. The presence of cardiac, renal, cognitive, and other comorbidities complicates the dental management of affected individuals. In context of the BBS patient with obesity, dentists must be knowledgeable about perioperative considerations and postoperative complications (Marciani et al. 2004; Krishnan 2009). Appropriate perioperative planning is crucial to avoid mechanical, cardiovascular, and respiratory injuries to these patients. Issues surrounding accessibility, including recalibration of the standard chair and other operatory equipment, which may not be suitable for obese patients, must be addressed. Using armless dental and waiting room chairs is suggested. Providing wheelchairs for patients with mobility issues must be considered (Marciani et al. 2004; Krishnan 2009).
Individuals affected with BBS may present across a range of cognitive impairment and exhibit varying degrees of learning disabilities (Beales et al. 1999; Forsythe et al. 2003; Majumdar et al. 2012; Hassona et al. 2017). Autistic-like behavior in these patients may require fixed daily routines to promote function (Forsythe et al. 2003; Majumdar et al. 2012; Hassona et al. 2017). Patients with attention deficit may be slow to comply with verbal cues or may exhibit noncooperative or defiant behavior during dental treatment (Ferreira et al. 2014; Hassona et al. 2017). Oral hygiene may be compromised in some patients due to fear and anxiety regarding placement of foreign objects into their mouths (Shanmugam et al. 2014). Dental pain may be poorly perceived and expressed by patients with cognitive difficulties. Pain expression in these individuals may differ from that of unaffected individuals. Dental pain–associated behaviors in patients with BBS may include crying, reaching for cheek while eating, difficulty in chewing or brushing, excessive salivation, putting hands inside the mouth while eating, and complaints of nocturnal earaches (Alaki and Bakry 2012; Shanmugam et al. 2014). Caregivers must understand these behaviors and seek immediate attention. A busy waiting room, dental operatory lights, noisy diagnostic and treatment equipment, use of water spray and suction, and the taste of mouthwash may create anxiety, resulting in challenging and undesirable behavior (Dougall and Fiske 2008; Majumdar et al. 2012). A functional behavioral assessment during a practice visit to a dental clinic to familiarize with the dental environment, equipment, and staff; teaching skills required for dental examination; and establishing a routine that includes taking the same route to the dental office and seeing the same staff in the same uniforms in the same operatory are recommended (Dougall and Fiske 2008). Reinforcement techniques include understanding the influence of a certain stimulus or action on a child’s behavior. For example, if an appraisal for a good child’s action (“You are being a good boy today”) increases the compliance to dental procedures, then that stimulus can act as positive reinforcement. A positive reinforcement along with the “tell-show-do” technique is helpful (Delli et al. 2013). Caregivers must actively participate during treatment to achieve the BBS patient’s compliance with treatment. Using symbols and photographs should be considered. Minimizing the waiting time of these patients by giving them the first or last appointments of the day, using music or earplugs to mask the background noise, using protective eye gear, and other interventions can help reduce patients’ anxiety and increase coping mechanisms (Dougall and Fiske 2008).
Dental management of BBS patients undergoing dialysis or renal transplantation is challenging. The use of anticoagulants during dialysis increases the bleeding risk during invasive dental procedures (Klassen and Krasko 2002; Jover Cerveró et al. 2008; Mitchison and Valente 2017). Requesting a complete hematologic assessment with coagulation testing is advisable before invasive dental procedures in patients with BBS under renal management (Jover Cerveró et al. 2008), and consultation with a nephrologist to withhold the use of anticoagulants for a certain period is advisable when dental procedures are planned (Klassen and Krasko 2002; Georgakopoulou et al. 2011). Dental procedures must be performed on nondialysis days to avoid the potential presence of residual anticoagulants in the system (Klassen and Krasko 2002; Jover Cerveró et al. 2008). Antibiotic prophylaxis is recommended before invasive procedures to prevent bacterial endocarditis. The drugs to be prescribed must be carefully selected, with their dosage and frequency modified, and certain nephrotoxic drugs must be avoided (including aminoglycoside antibiotics, vancomycin, and nonsteroidal anti-inflammatory drugs) (Klassen and Krasko 2002; Jover Cerveró et al. 2008; Georgakopoulou et al. 2011). Completing all necessary dental care should occur before renal transplantation, since use of immunosuppressive agents after transplantation reduces the body’s ability to cope with systemic infections (Klassen and Krasko 2002; Georgakopoulou et al. 2011). Invasive procedures, including gingivectomy, which is usually the treatment of choice for gingival hyperplasia, must be carefully planned in consultation with the nephrologist. Using local anesthetic agents without vasoconstrictors, additional corticosteroid doses to prevent adrenal crisis for patients on long-term corticosteroids, and proper prescription medication selection is recommended.
Future Directions
Genetic and clinical heterogeneity surrounding BBS makes diagnosis of the syndrome challenging. Dental providers must maximize their knowledgeability surrounding individual patients to enhance accurate diagnoses and project clinical and dental manifestations of these patients, including prediction of potential oral complications that may arise as a consequence of the genetic and associated clinical phenotype. Gathering as much clinical and genetic data as possible will inform timely provision of preventive care and interventions. Educating and involving caregivers and clinical consultation is important and highly recommended. An interdisciplinary team (medical specialists, psychologists, speech pathologists, and dentists) and a holistic approach are necessary to improve the quality of life of these patients.
To assess structural manifestations, cephalometric analysis by an orthodontist or oral surgeon may currently be used to analyze bone and soft tissue landmarks of the face to identify craniofacial anomalies (Fig. 2), which may be of value in establishing an early differential diagnosis. Future directions include opportunities for use of predictive big data analytics to identify the facial and skeletal discrepancies in these patients, which may provide early diagnostic clues and focus the list of potential differential diagnoses. For example, creating a database of facial photographs of BBS patients with manually annotated facial points can be used to train and test a machine learning algorithm that might inform early diagnosis. This database could also aid in identifying various mutations associated with a given facial phenotype. Using 3-dimensional dense surface modeling will be an effective method to study the craniofacial defects in BBS individuals. The sonographic imaging can be used to gather the information of any fetal facial anomalies associated with this syndrome and incorporate this information into a database capturing fetal face profiling, which may inform prenatal diagnosis of this syndrome. These data can also be used to develop applications, such as Face2Gene, that use deep learning algorithms to build syndrome-specific classifier models. Further research based on NGS to identify additional mutational variants of BBS genes responsible for dentofacial anomalies is needed in the context of BBS and other ciliopathies to assist with accurate syndromic diagnosis. Collectively, these data will inform creation of clinical decision support tools and definitive clinical practice guidelines to inform optimal clinical management across the broad spectrum of oral and systemic manifestations encountered in patients affected by BBS.
Figure 2.
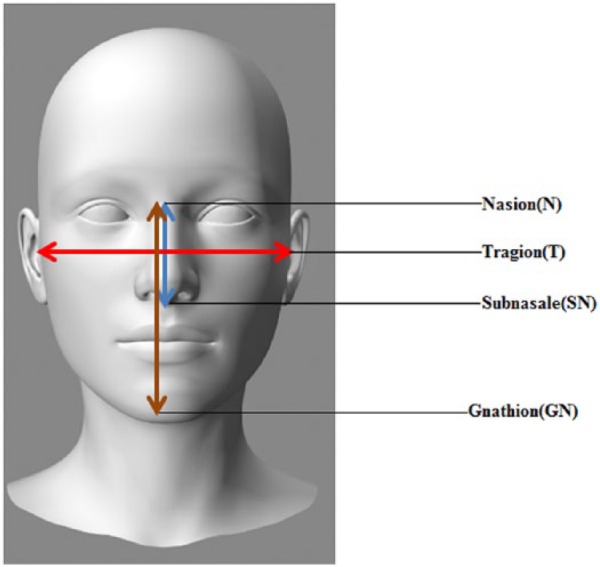
Cephalometric analysis of patients with Bardet-Biedl syndrome (BBS) for early diagnosis. Modified from Tobin et al. (2008). Ratio of T-T/N-SN is greater in patients with BBS than nonaffected individuals. Ratio of N-GN/N-SN is greater in patients with BBS than nonaffected individuals. Ratio of N-GN/T-T is greater in patients with BBS than nonaffected individuals.
Author Contributions
A. Panny, I. Glurich, R.M. Haws, A. Acharya, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Ms. Deborah Johnson from the Center for Clinical Research at Marshfield Clinic Research Foundation for her help with providing aggregate data from the CRIBBS at Marshfield Clinic. The authors also thank Ms. Marie Fleisner from the Office of Scientific Writing at Marshfield Clinic Research Foundation for editorial assistance and uploading of this manuscript.
Footnotes
This study was supported in part by funds from Marshfield Clinic Research Foundation, Family Health Center of Marshfield, Inc., Delta Dental of Wisconsin, and Bardet Biedl Syndrome Foundation. Additional funding was provided through the Clinical and Translational Science Award program, through the National Institutes of Health National Center for Advancing Translational Sciences, grants UL1TR000427 and1UL1RR025011.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alaki SM, Bakry NS. 2012. Dental pain in children with intellectual disabilities: caregivers’ perspective. Int J Dent. 2012:701608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson EM, Axelsson S, Gjølstad LF, Storhaug K. 2013. Taurodontism: a minor diagnostic criterion in Laurence-Moon/Bardet-Biedl syndromes. Acta Odontol Scand. 71(6):1671–1674. [DOI] [PubMed] [Google Scholar]
- Bayraktar G, Kurtulus I, Duraduryan A, Cintan S, Kazancioglu R, Yildiz A, Bural C, Bozfakioglu S, Besler M, Trablus S, et al. 2007. Dental and periodontal findings in hemodialysis patients. Oral Dis. 13(4):393–397. [DOI] [PubMed] [Google Scholar]
- Bayraktar G, Kurtulus I, Kazancioglu R, Bayramgurler I, Cintan S, Bural C, Bozfakioglu S, Besler M, Trablus S, Issever H, et al. 2008. Evaluation of periodontal parameters in patients undergoing peritoneal dialysis or hemodialysis. Oral Dis. 14(2):185–189. [DOI] [PubMed] [Google Scholar]
- Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. 1999. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet. 36(6):437–446. [PMC free article] [PubMed] [Google Scholar]
- Borgström MK, Riise R, Tornqvist K, Granath L. 1996. Anomalies in the permanent dentition and other oral findings in 29 individuals with Laurence-Moon-Bardet-Biedl syndrome. J Oral Pathol Med. 25(2):86–89. [DOI] [PubMed] [Google Scholar]
- Chaffee BW, Weston SJ. 2010. Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol. 81(12):1708–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Registry Investigating Bardet-Biedl Syndrome. 2017. Marshfield Clinic Research Foundation [accessed 2017 June 1]. https://www.bbs-registry.org/
- Costacurta M, Di Renzo L, Bianchi A, Fabiocchi F, De Lorenzo A, Docimo R. 2011. Obesity and dental caries in paediatric patients: a cross-sectional study. Eur J Paediatr Dent. 12(2):112–116. [PubMed] [Google Scholar]
- Craig RG. 2008. Interactions between chronic renal disease and periodontal disease. Oral Dis. 14(1):1–7. [DOI] [PubMed] [Google Scholar]
- Dar P, Sachs GS, Carter SM, Ferreira JC, Nitowsky HM, Gross SJ. 2001. Prenatal diagnosis of Bardet-Biedl syndrome by targeted second-trimester sonography. Ultrasound Obstet Gynecol. 17(4):354–356. [DOI] [PubMed] [Google Scholar]
- Delli K, Reichart PA, Bornstein MM, Livas C. 2013. Management of children with autism spectrum disorder in the dental setting: concerns, behavioural approaches and recommendations. Med Oral Patol Oral Cir Bucal. 18(6):e862–e868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall A, Fiske J. 2008. Access to special care dentistry, part 2. Communication. Br Dent J. 205(1):11–21. [DOI] [PubMed] [Google Scholar]
- Drugowick RM, Da Rós Gonçalves L, Barrôso AS, Feres-Filho EJ, Maia LC. 2007. Treatment of gingival overgrowth in a child with Bardet-Biedl syndrome. J Periodontol. 78(6):1159–1163. [DOI] [PubMed] [Google Scholar]
- El Aouame A, Daoui A, El Quars F. 2016. Nasal breathing and the vertical dimension: a cephalometric study. Int Orthod. 14(4):491–502. [DOI] [PubMed] [Google Scholar]
- Farmer A, Ayme S, de Heredia ML, Maffei P, McCafferty S, Młynarski W, Nunes V, Parkinson K, Paquis-Flucklinger V, Rohayem J, et al. 2013. EURO-WABB: an EU rare diseases registry for Wolfram syndrome, Alstrom syndrome and Bardet-Biedl syndrome. BMC Pediatr. 13:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira do, Amaral CO, Logar Gde A, Parisi AG, Takahashi K, Straioto FG. 2014. General and stomatologic aspects of Bardet-Biedl syndrome. J Craniofac Surg. 25(6):e575–e578. [DOI] [PubMed] [Google Scholar]
- Feuillan PP, Ng D, Han JC, Sapp JC, Wetsch K, Spaulding E, Zheng YC, Caruso CR, Brian BP, Johnston JJ, et al. 2011. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. J Clin Endocrinol Metab. 96(3):E528–E535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MA, Taylor GW, West BT, McCarthy ET. 2011. Bidirectional relationship between chronic kidney and periodontal disease: a study using structural equation modeling. Kidney Int. 79(3):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe E, Beales PL. 2013. Bardet-Biedl syndrome. Eur J Hum Genet. 21(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe E, Beales PL, Ross AJ, Waters MA. 2003. Bardet-Biedl syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews [accessed 2017 June 1]. https://www.ncbi.nlm.nih.gov/books/NBK1363/
- Georgakopoulou EA, Achtari MD, Afentoulide N. 2011. Dental management of patients before and after renal transplantation. Stomatologija. 13(4):107–112. [PubMed] [Google Scholar]
- Hassona Y, Kasabreh N, Hammoudeh H, Scully C. 2017. Oral healthcare management in Bardet Biedl syndrome. Spec Care Dentist. 37(1):47–50. [DOI] [PubMed] [Google Scholar]
- Haws RM, Krentz AD, Stankowski RV, Steiner RD. 2015. Bardet-Biedl syndrome: a model for translational research in rare diseases. New Horiz Transl Med. 2(4–5):102–109. [Google Scholar]
- Heon E, Kim G, Qin S, Garrison JE, Tavares E, Vincent A, Nuangchamnong N, Scott CA, Slusarski DE, Sheffield VC. 2016. Mutations in C8ORF37 cause Bardet Biedl syndrome (BBS21). Hum Mol Genet. 25(11):2283–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamoto M, Goto M, Muto M, Nio-kobayashi J, Iwanaga T, Yokoyama A. 2016. Developmental changes in primary cilia in the mouse tooth germ and oral cavity. Biomed Res. 37(3):207–214. [DOI] [PubMed] [Google Scholar]
- Imhoff O, Marion V, Stoetzel C, Durand M, Holder M, Sigaudy S, Sarda P, Hamel CP, Brandt C, Dollfus H, et al. 2011. Bardet-Biedl syndrome: a study of the renal and cardiovascular phenotypes in a French cohort. Clin J Am Soc Nephrol. 6(1):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Chen G, Feng L, Jiang Z, Yu M, Bao J, Tian W. 2016. Disruption of kif3a results in defective osteoblastic differentiation in dental mesenchymal stem/precursor cells via the Wnt signaling pathway. Mol Med Rep. 14(3):1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jover Cerveró A, Bagán JV, Jiménez Soriano Y, Poveda Roda R. 2008. Dental management in renal failure: patients on dialysis. Med Oral Patol Oral Cir Bucal. 13(7):E419–E426. [PubMed] [Google Scholar]
- Kawasaki M, Izu Y, Hayata T, Ideno H, Nifuji A, Sheffield VC, Ezura Y, Noda M. 2016. Bardet-Biedl Syndrome 3 regulates development of cranial base midline structures. Bone. 101:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen JT, Krasko BM. 2002. The dental health status of dialysis patients. J Can Dent Assoc. 68(1):34–38. [PubMed] [Google Scholar]
- Kreczi A, Proff P, Reicheneder C, Faltermeier A. 2011. Effects of hypodontia on craniofacial structures and mandibular growth pattern. Head Face Med. 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B. 2009. Obese oral and maxillofacial surgical patient. J Craniofac Surg. 20(1):53–57. [DOI] [PubMed] [Google Scholar]
- Kudiyirickal MG, Pappachan JM. 2015. Diabetes mellitus and oral health. Endocrine. 49(1):27–34. [DOI] [PubMed] [Google Scholar]
- Linden G, Patterson C, Evans A, Kee F. 2007. Obesity and periodontitis in 60-70-year-old men. J Clin Periodontol. 34(6):461–466. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen S, Cheng D, Jing W, Helms JA. 2014. Primary cilia integrate hedgehog and Wnt signaling during tooth development. J Dent Res. 93(5):475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAvoy SK, Jack HC, Kieser J, Farella M. 2016. Effect of occlusal vertical dimension on swallowing patterns and perioral electromyographic activity. J Oral Rehabil. 43(7):481–487. [DOI] [PubMed] [Google Scholar]
- Majumdar U, Arya G, Singh S, Pillai A, Nair PP. 2012. Oro-dental findings in Bardet-Biedl syndrome. BMJ Case Rep. 2012:bcr1220115320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S, Pandey RK, Nagar A, Agarwal SP, Gupta VK. 2012. The effect of mouth breathing on dentofacial morphology of growing child. J Indian Soc Pedod Prev Dent. 30(1):27–31. [DOI] [PubMed] [Google Scholar]
- Marciani RD, Raezer BF, Marciani HL. 2004. Obesity and the practice of oral and maxillofacial surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 98(1):10–15. [DOI] [PubMed] [Google Scholar]
- Mathus-Vliegen EM, Nikkel D, Brand HS. 2007. Oral aspects of obesity. Int Dent J. 57(4):249–256. [DOI] [PubMed] [Google Scholar]
- Mitchison HM, Valente EM. 2017. Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol. 241(2):294–309. [DOI] [PubMed] [Google Scholar]
- Moore SJ, Green JS, Fan Y, Bhogal AK, Dicks E, Fernandez BA, Stefanelli M, Murphy C, Cramer BC, Dean JC, et al. 2005. Clinical and genetic epidemiology of Bardet-Biedl syndrome in Newfoundland: a 22-year prospective, population-based, cohort study. Am J Med Genet A. 132(4):352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodal M, Kjaer I, Solow B. 1994. Craniofacial morphology in patients with multiple congenitally missing permanent teeth. Eur J Orthod. 16(2):104–109. [DOI] [PubMed] [Google Scholar]
- Ogaard B, Krogstad O. 1995. Craniofacial structure and soft tissue profile in patients with severe hypodontia. Am J Orthod Dentofacial Orthop. 108(5):472–477. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Haycraft CJ, Seppala M, Blackburn J, Ghafoor S, Cobourne M, Martinelli DC, Fan CM, Peterkova R, Lesot H, et al. 2009. Primary cilia regulate Shh activity in the control of molar tooth number. Development. 136(6):897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischon N, Heng N, Bernimoulin JP, Kleber BM, Willich SN, Pischon T. 2007. Obesity, inflammation, and periodontal disease. J Dent Res. 86(5):400–409. [DOI] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y, Koga T, Tsuzuki M, Ohshima A. 2001. Relationship between upper body obesity and periodontitis. J Dent Res. 80(7):1631–1636. [DOI] [PubMed] [Google Scholar]
- Schaefer E, Stoetzel C, Scheidecker S, Geoffroy V, Prasad MK, Redin C, Missotte I, Lacombe D, Mandel JL, Muller J, et al. 2016. Identification of a novel mutation confirms the implication of IFT172 (BBS20) in Bardet-Biedl syndrome. J Hum Genet. 61(5):447–450. [DOI] [PubMed] [Google Scholar]
- Scholey JM. 2008. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 180(1):23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam M, Shivakumar V, Anitha V, Meenapriya BP, Aishwarya S, Anitha R. 2014. Behavioral pattern during dental pain in intellectually disabled children: a comparative study. Int Sch Res Notices. 2014:824125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock EN, Struve JN, Chang CF, Williams TJ, Snedeker J, Attia AC, Stottmann RW, Brugmann SA. 2017. A tissue-specific role for intraflagellar transport genes during craniofacial development. PLoS ONE. 12(3):e0174206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayeh MK, Chin EL, Miller VR, Bean LJ, Coffee B, Hegde M. 2009. Targeted comparative genomic hybridization array for the detection of single- and multiexon gene deletions and duplications. Genet Med. 11(4):232–240. [DOI] [PubMed] [Google Scholar]
- Thivichon-Prince B, Couble ML, Giamarchi A, Delmas P, Franco B, Romio L, Struys T, Lambrichts I, Ressnikoff D, Magloire H, et al. 2009. Primary cilia of odontoblasts: possible role in molar morphogenesis. J Dent Res. 88(10):910–915. [DOI] [PubMed] [Google Scholar]
- Tobin JL, Di Franco M, Eichers E, May-Simera H, Garcia M, Yan J, Quinlan R, Justice MJ, Hennekam RC, Briscoe J, et al. 2008. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung’s disease in Bardet-Biedl syndrome. Proc Natl Acad Sci U S A. 105(18):6714–6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosello DO, Vitti M, Berzin F. 1999. EMG activity of the orbicularis oris and mentalis muscles in children with malocclusion, incompetent lips and atypical swallowing—part II. J Oral Rehab. 26(8):644–649. [DOI] [PubMed] [Google Scholar]
- Urben SL, Baugh RF. 1999. Otolaryngologic features of Laurence-Moon-Bardet-Biedl syndrome. Otolaryngol Head Neck Surg. 120(4):571–574. [DOI] [PubMed] [Google Scholar]
- Vázquez-Nava F, Vázquez-Rodríguez EM, Saldívar-González AH, Lin-Ochoa D, Martínez-Perales GM, Joffre-Velázquez VM. 2010. Association between obesity and dental caries in a group of preschool children in Mexico. J Public Health Dent. 70(2):124–130. [DOI] [PubMed] [Google Scholar]



