Abstract
Young Indigenous children in North America suffer from a higher degree of severe early childhood caries (S-ECC) than the general population, leading to speculation that the etiology and characteristics of the disease may be distinct in this population. To address this knowledge gap, we conducted the first microbiome analysis of an Indigenous population using modern molecular techniques. We investigated the caries-associated microbiome among Canadian First Nations children with S-ECC. Thirty First Nations children <72 mo of age with S-ECC and 20 caries-free children were recruited in Winnipeg, Canada. Parents or caregivers completed a questionnaire on general and dental health, diet, and demographics. The plaque microbiome was investigated by sequencing the 16S rRNA gene. Sequences were clustered into operational taxonomic units and taxonomy assigned via the Human Oral Microbiome Database, then analyzed at the community level with alpha and beta diversity measures. Compared with those who were caries free, children with S-ECC came from households with lower income; they were more likely to live in First Nations communities and were more likely to be bottle-fed; and they were weaned from the bottle at a later age. The microbial communities of the S-ECC and caries-free groups did not differ in terms of species richness or phylogenetic diversity. Beta diversity analysis showed that the samples significantly clustered into groups based on caries status. Twenty-eight species-level operational taxonomic units were significantly different between the groups, including Veillonella HOT 780 and Porphyromonas HOT 284, which were 4.6- and 9-fold higher, respectively, in the S-ECC group, and Streptococcus gordonii and Streptococcus sanguinis, which were 5- and 2-fold higher, respectively, in the caries-free group. Extremely high levels of Streptococcus mutans were detected in the S-ECC group. Overall, First Nations children with S-ECC have a significantly different plaque microbiome than their caries-free counterparts, with the S-ECC group containing higher levels of known cariogenic organisms.
Keywords: oral health, dental health survey, preschool child, healthcare disparities, Indigenous population, Streptococcus mutans
Introduction
Early childhood caries (ECC), defined as decay involving the primary dentition in children <72 mo of age, is the most common chronic disease of childhood (American Academy of Pediatrics 2016). ECC is a critical public health concern due to its high prevalence, high treatment costs, negative effect on quality of life, and potential long-term complications (Schroth et al. 2009; Martins-Júnior et al. 2013; Schroth et al. 2016). Severe ECC (S-ECC) is an aggressive form of decay that is overrepresented among Indigenous children in North America, including Canadian First Nations, Métis and Inuit, and American Indian and Alaska Natives, and it reflects an underlying extreme oral health disparity in these populations (American Academy of Pediatrics and Canadian Paediatric Society 2011; Irvine et al. 2011). In some Canadian First Nations on-reserve communities, the prevalence of decay in the primary dentition can exceed 90% (Schroth et al. 2005). S-ECC is a major cause of hospital visits for young children (Sheller et al. 1997), and it frequently requires rehabilitative dental surgery under general anesthesia due to the extent of decay and the young age of the children affected (Schroth and Smith 2007; American Academy of Pediatrics 2016). Alarmingly, children living in communities with a high proportion of Aboriginal residents have pediatric dental surgery rates nearly 8 times higher than those living in communities with a low Aboriginal population among children 1 to 5 y old (Canadian Institute for Health Information 2013; Schroth et al. 2016)
In addition to the well-known microbial and host-related causal factors of caries, the etiology of ECC includes many additional factors, such as socioeconomic status, nutrition, and education (Reisine and Douglass 1998; Fisher-Owens et al. 2013). The early presentation and rapid progression in young Canadian First Nations, Métis, American Indian and Alaska Native children suggests that ECC in these populations may have distinct attributes and etiology (Schroth et al. 2009; QUEST 2015), which warrants further study.
In the current study, we utilized next-generation sequencing to analyze the plaque microbiome from Canadian First Nations and Métis children, with and without S-ECC, to investigate the role of the oral microbiome and to identify microbial characteristics that may account for the aggressive presentation. Defining the etiologic microbiota for S-ECC in these populations will potentially facilitate improvements in care and caries prevention policies, important steps for reducing the extent of S-ECC and improving overall quality of life.
Materials and Methods
Study Population and Design
The study protocol was approved by the University of Manitoba’s Health Research Ethics Board and reviewed by the Assembly of Manitoba Chiefs’ Health Information Research Governance Committee. Children who were <72 mo of age and identified by their parent or legal caregiver as being Canadian First Nations or Métis were included in the study. Thirty children with S-ECC had severe tooth decay involving multiple primary teeth and were recruited from the Misericordia Health Centre in Winnipeg, Canada, on the day of their scheduled dental rehabilitative surgery under general anesthesia.Twenty caries-free children were recruited from the community and assessed to ensure that there was no evidence of caries (dmft = 0, no cavitations or white spot lesions). Dental examinations and caries assessment were performed by R.J.S. Children who had taken antibiotics within the last 3 mo were excluded. All parents or caregivers of participating children provided written informed consent.
Health-Related Questionnaire
All parents and caregivers completed an interviewed questionnaire proctored by members of the study team. Information was collected on nutritional habits, oral hygiene habits, socioeconomic and demographic characteristics, and history of previous dental visits.
Sample Collection and Sequencing Analysis
Plaque samples were collected from each subject by swabbing a sterile interdental brush on all available tooth surfaces, and samples were immediately frozen at −80 °C in 15% glycerol until used for analysis. Extracted DNA was sent to the Forsyth Institute for library preparation and Illumina sequencing of the amplified V3-V4 16S region. Sequencing data were analyzed with QIIME 1.9.1 (Quantitative Insights into Microbial Ecology; Caporaso et al. 2010). Detailed DNA extraction, sequencing, and analysis methods are supplied in the Appendix.
Statistical Analysis
Questionnaire and microbiological data were linked in an Excel spreadsheet (Microsoft Office) and analyzed with Number Cruncher Statistical Software 10 and GraphPad Prism 7. Bivariate analyses, such as chi-square, Fisher’s exact, and t tests (Aspin-Welch t test for unequal variance), were performed where appropriate. For sequencing data, differences in the relative abundances of taxa between the groups were determined with the Kruskal-Wallis test, controlling the false discovery rate to correct for multiple comparisons (Hochberg and Benjamini 1990). A corrected P value ≤0.05 was considered significant. Differences in weighted and unweighted Unifrac distances between the groups were analyzed with analysis of similarity. A P value ≤0.05 was considered statistically significant.
Results
Demographics and Health-Related Questionnaire Data
A total of 50 children were recruited: 30 with S-ECC and 20 caries free. The mean age of all children was 40.7 ± 11.6 mo. Results from the health-related questionnaire are presented in Table 1. A considerable proportion (56.7%) of children with S-ECC resided in First Nations communities, while all of the caries-free children lived in the Winnipeg region. We found a significant difference in household income (P = 0.032) between the groups, with S-ECC children coming from households with lower incomes.
Table 1.
Demographics and Health Characteristics of Study Population.
| Caries Status |
|||
|---|---|---|---|
| Variable | Caries Free | S-ECC | P Value |
| Age, moa | 37.4 ± 10.3 | 42.8 ± 12.2 | 0.11 |
| Sexb | |||
| Male | 9 (32.1) | 19 (67.9) | 0.20 |
| Female | 11 (50.0) | 11 (50.0) | |
| Weight at birth, ga | 3,529.9 ± 699.0 | 3,421.3 ± 573.2 | 0.57 |
| Prematureb | |||
| Yes | 2 (16.7) | 10 (83.3) | 0.073c |
| No | 18 (48.7) | 19 (51.4) | |
| Feeding habitsb | |||
| Child was breastfed | |||
| Yes | 13 (52.0) | 12 (48.0) | 0.083 |
| No | 7 (28.0) | 18 (72.0) | |
| Child was exclusively breastfed | |||
| Yes | 12 (70.6) | 5 (29.4) | 0.0015 |
| No | 8 (24.2) | 25 (75.8) | |
| Child was bottle-fed | |||
| Yes | 16 (34.8) | 30 (65.2) | 0.021 c |
| No | 4 (100.0) | 0 (0.0) | |
| Age the child was weaned | |||
| From the breasta | 12.9 ± 11.4 | 3.3 ± 5.4 | 0.014 |
| From the bottlea | 17.9 ± 8.9 | 25.8 ± 12.0 | 0.028 |
| Times per day the child snacksa | 3.7 ± 1.7 | 3.9 ± 1.4 | 0.71 |
| Oral hygiene habitsb | |||
| Child brushes ≥ daily | 17 (51.2) | 16 (48.5) | 0.032 c |
| Child brushes < daily | 3 (17.7) | 14 (82.4) | |
| Yearly household income, $b | |||
| ≥28,000 | 7 (70.0) | 3 (30.0) | 0.032 c |
| <28,000 | 12 (32.4) | 25 (67.6) | |
| Family sizeb | |||
| Other children | 2 (50.0) | 2 (50.0) | 1.00c |
| Only child | 18 (39.1) | 28 (60.9) | |
| Receives social assistanceb | |||
| Yes | 13 (37.1) | 22 (62.9) | 0.41 |
| No | 7 (50.0) | 7 (50.0) | |
| Lives in a First Nations communityb | |||
| Yes | 0 (0.0) | 17 (100.0) | 0.000010 c |
| No | 20 (60.6) | 13 (39.4) | |
| Age of child at first dental visit, moa | 20.8 ± 16.0 | 27.8 ± 14.6 | 0.11 |
Values are presented as mean ± SD or n (%). Bold value indicates P ≤ .05.
S-ECC, severe early childhood caries.
T test.
Chi-square analysis.
Fisher’s exact test.
There were significant differences in the proportion of children with S-ECC who were bottle-fed in comparison with caries-free children (P = 0.021). Children with S-ECC were also bottle-fed for a significantly longer duration (P = 0.028), and the age in which the child was weaned from the breast was significantly lower among S-ECC children (3.3 ± 5.4 mo vs. 12.9 ± 11.4; P = 0.015). Children with S-ECC were also less likely to be exclusively breastfed at any point in their infancy (P = 0.0015).
Plaque Microbial Community
Plaque samples were obtained from 20 caries-free subjects and 30 subjects with S-ECC. Sequencing generated a total of 3,502,879 sequences after quality filtering, with an average of 66,855 (range, 34,190 to 89,179) sequences per sample and a median length of 421.
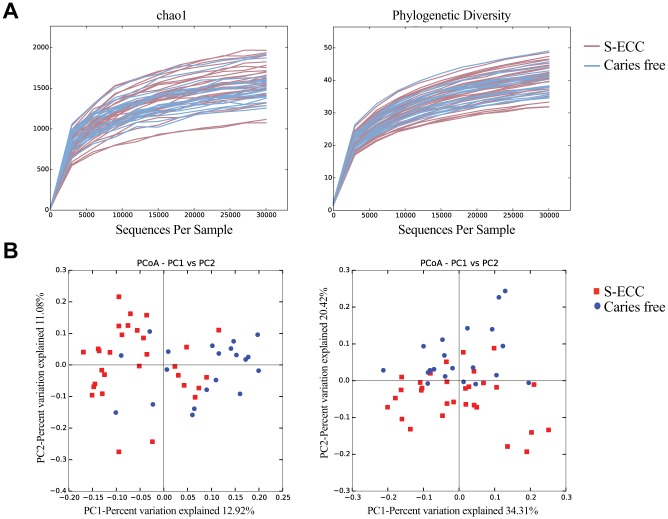
Alpha (within-sample) diversity was calculated at a maximum depth of 30,000 sequences per sample, with the rarefaction curves shown in Figure 1A. On average, the samples from caries-free and S-ECC subjects did not differ in terms of species richness or phylogenetic diversity.
Figure 1.
Diversity analyses. (A) Rarefaction curves of alpha diversity indices. Left: chao1 (species richness); right, Faith’s phylogenetic diversity index. (B) Beta diversity shown by principal coordinates analysis (PCoA) of unweighted Unifrac distances (left) and weighted Unifrac distances (right). The plaque microbial communities significantly clustered by caries status (P < 0.05, analysis of similarity). S-ECC, severe early childhood caries.
Principal coordinates analysis was used on weighted and unweighted Unifrac distances to examine clustering of samples between the S-ECC and caries-free groups (beta diversity; Fig. 1B). Weighted Unifrac distances take into account abundance of each taxon, while unweighted distances are based only on presence/absence data (Lozupone and Knight 2005). The samples significantly cluster according to caries status (caries free vs. S-ECC) for both weighted and unweighted distance measures (P < 0.05, analysis of similarity).
Taxonomic Identification and Relative Abundance
Taxonomy assignment revealed 10 phyla, 4 of which were differentially represented in the caries-free versus S-ECC groups: Firmicutes (39.4% vs. 47.2%, P = 0.01), Actinobacteria (14.4% vs. 6.8%, P = 0.002), Fusobacteria (16.8% vs. 11.3%, P = 0.008), and TM7 (0.5% vs. 0.24%, P = 0.008). A total of 95 genera and 290 species were detected, and those with the highest relative abundances are listed in Table 2. Twenty-eight species-level operational taxonomic units were significantly different (P < 0.05) in the S-ECC versus caries-free groups. Most of these species have been associated with either health or caries; for example, the caries-free group had 5- and 2-fold higher abundances of Streptococcus gordonii and Streptococcus sanguinis, respectively, than the S-ECC group, while the S-ECC group had 7- and 9-fold higher levels of an Haemophilus species (HOT 036) and a Porphyromonas species (HOT 284), respectively. In addition, a Veillonella species (HOT 780) was 4.6-fold higher in the S-ECC group, although the relative abundances were low.
Table 2.
Relative Abundance of the Top 25 Species- and Genus-Level OTUs Detected in Plaque of Caries-Free Children and Children with S-ECC.
| Median Relative Abundance, % (Range) |
||
|---|---|---|
| OTU | Caries Free (n = 20) | S-ECC (n = 30) |
| Species level | ||
| Streptococcus HOT 058 | 23.7 (12.3 to 42.0) | 26.7 (11.1 to 42.3) |
| Leptotrichia shahii | 3.4 (0 to 16.7) | 2.1 (0.2 to 11.7) |
| Lautropia mirabilis | 3.2 (0.2 to 11.6) | 2.2 (0.05 to 10.0) |
| Haemophilus parainfluenzae | 2.0 (0.01 to 7.2) | 3.1 (0.12 to 12.6) |
| Veillonella dispar | 2.2 (0.15 to 9.4) | 3.0 (0.33 to 19.0) |
| Rothia aeria a | 2.4 (0.37 to 7.6) | 0.52 (0.004 to 1.7) |
| Corynebacterium matruchotii a | 2.0 (0.66 to 5.4) | 0.85 (0.13 to 5.4) |
| Actinomyces naeslundii a | 1.8 (0.68 to 6.2) | 0.67 (0.15 to 5.7) |
| Rothia dentocariosa | 1.7 (0.12 to 24.5) | 0.70 (0.16 to 5.5) |
| Abiotrophia defectiva | 1.1 (0.07 to 6.3) | 1.3 (0.003 to 5.7) |
| Gemella haemolysans | 0.87 (0.09 to 3.8) | 1.2 (0.15 to 5.3) |
| Granulicatella adiacens | 0.82 (0.14 to 2.3) | 1.1 (0.02 to 2.4) |
| Porphyromonas HOT 279 | 0.63 (0.03 to 3.5) | 1.1 (0.02 to 8.4) |
| Granulicatella elegans a | 0.32 (0.03 to 1.2) | 1.0 (0.003 to 4.0) |
| Leptotrichia HOT 225 | 1.0 (0.03 to 4.5) | 0.45 (0.008 to 5.9) |
| Fusobacterium nucleatum ss. vincentii | 0.54 (0.04 to 3.8) | 0.88 (0.09 to 4.1) |
| Corynebacterium durum | 0.80 (0.14 to 9.4) | 0.41 (0 to 4.3) |
| Streptococcus mutans | 0.15 (0.006 to 10.4) | 0.73 (0.02 to 22.9) |
| Prevotella melaninogenica a | 0.10 (0.002 to 3.6) | 0.71 (0.03 to 11.8) |
| Alloprevotella HOT 473a | 0.04 (0 to 1.7) | 0.69 (0.001 to 9.3) |
| Gemella morbillorum | 0.58 (0.11 to 3.3) | 0.69 (0.07 to 2.0) |
| Haemophilus HOT 036a | 0.07 (0.003 to 0.3) | 0.56 (0.001 to 3.5) |
| Streptococcus sanguinis a | 0.56 (0.19 to 0.8) | 0.28 (0.13 to 0.7) |
| Neisseria mucosa | 0.44 (0.09 to 1.2) | 0.34 (0.02 to 1.2) |
| Aggregatibacter HOT 458 | 0.25 (0.003 to 2.3) | 0.43 (0.05 to 2.8) |
| Genera level | ||
| Streptococcus | 28.3 (16.8 to 49.6) | 31.3 (12.8 to 50.0) |
| Leptotrichia a | 10.5 (4.2 to 23.7) | 5.7 (0.61 to 30.4) |
| Neisseria | 7.5 (0.70 to 27.9) | 9.0 (0.22 to 26.4) |
| Rothia a | 4.8 (0.72 to 29.9) | 1.7 (0.04 to 10.3) |
| Fusobacterium | 4.8 (0.65 to 12.3) | 3.7 (1.1 to 9.5) |
| Haemophilus | 2.1 (0.01 to 7.5) | 4.6 (0.13 to 12.8) |
| Veillonella | 2.4 (0.18 to 10.1) | 4.1 (0.39 to 19.8) |
| Corynebacterium a | 3.3 (1.4 to 14.8) | 1.6 (0.01 to 8.1) |
| Actinomyces a | 3.2 (1.4 to 9.4) | 1.8 (0.25 to 7.4) |
| Lautropia | 3.2 (0.19 to 11.6) | 2.2 (0.05 to 10.2) |
| Prevotella | 0.93 (0.17 to 9.1) | 2.5 (0.20 to 26.5) |
| Granulicatella | 1.3 (0.22 to 2.6) | 2.3 (0.06 to 5.7) |
| Gemella | 1.6 (0.20 to 4.9) | 2.0 (0.29 to 7.0) |
| Porphyromonas | 1.3 (0.08 to 5.1) | 1.8 (0.023 to 9.2) |
| Capnocytophaga | 1.5 (0.48 to 5.1) | 0.94 (0.19 to 2.7) |
| Abiotrophia | 1.1 (0.07 to 6.3) | 1.3 (0.003 to 5.7) |
| Kingella | 1.2 (0.61 to 2.5) | 0.80 (0.065 to 2.1) |
| Alloprevotella a | 0.13 (0.003 to 1.7) | 1.0 (0.006 to 9.5) |
| Aggregatibacter | 0.88 (0.01 to 3.3) | 0.99 (0.14 to 4.1) |
| Campylobacter | 0.59 (0.14 to 2.5) | 0.46 (0.08 to 3.9) |
| Selenomonas | 0.29 (0.02 to 3.7) | 0.54 (0.02 to 4.2) |
| Cardiobacterium a | 0.45 (0.04 to 2.2) | 0.17 (0.002 to 0.7) |
| Lachnoanaerobaculum | 0.43 (0.16 to 2.6) | 0.32 (0.03 to 1.6) |
| TM7 [G-1]a | 0.33 (0.004 to 1.4) | 0.11 (0.005 to 1.8) |
| Bergeyella | 0.33 (0.03 to 0.8) | 0.27 (0.05 to 1.2) |
HOT, Human Oral Taxon; OTU, operational taxonomic unit; S-ECC, severe early childhood caries.
P ≤ 0.05, Kruskal-Wallis test, corrected for multiple comparisons by the false discovery rate method.
Streptococcus mutans was detected in all samples, with a 3-fold higher amount detected in the S-ECC group as compared with the caries-free group. However, the S-ECC group contained subjects with extremely high values. Six subjects in the S-ECC group were carrying >5% S. mutans, 2 subjects with >10%, and 1 subject with an extraordinarily high level of almost 23% of the total species detected. For comparison, in the caries-free group, there was only 1 subject with >5% S. mutans (Fig. 2). Scardovia wiggsiae has been recently characterized as a possible important factor in ECC (Tanner, Mathney, et al. 2011); in the current study, S-ECC children had on average 7-fold-higher levels of this organism than the caries-free children, although the relative abundances were low (0.007% vs. 0.001%).
Figure 2.
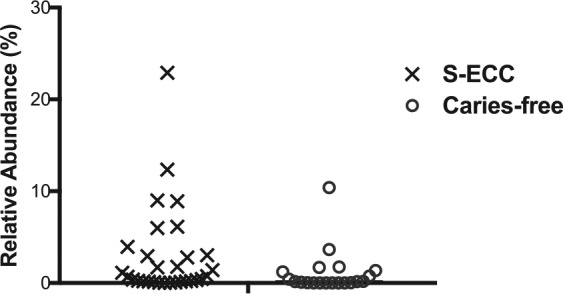
Relative abundance of Streptococcus mutans in all subjects. Percentage relative abundance of S. mutans is plotted for each subject. S-ECC, severe early childhood caries.
S-ECC Subgroup Analysis
Since the majority of children with S-ECC (17 of 30) resided in First Nations communities, we further investigated the microbiome of this group according to place of residency (First Nations community vs. non–First Nations urban community). Beta diversity analysis based on weighted Unifrac distances revealed that the subgroups were significantly different (Fig. 3A). The average species richness (chao1) of the samples in each subgroup was not different (data not shown). At the species level, Fusobacterium nucleatum subsp. vincentii was significantly higher in the children who did not reside in a First Nations community versus those from First Nations communities (0.5% vs. 1.7%, P < 0.05). While the average relative abundance of S. mutans was higher in the subjects from non–First Nations communities, this was not statistically significant (4.9% vs. 1.4%, P = 0.3). At the phylum level, Fusobacteria was also significantly higher in the children with S-ECC who did not reside in a First Nations community (14.9% vs. 8.5%, P < 0.05). The top 100 species-level taxa identified in each subgroup are shown in Figure 3B.
Figure 3.
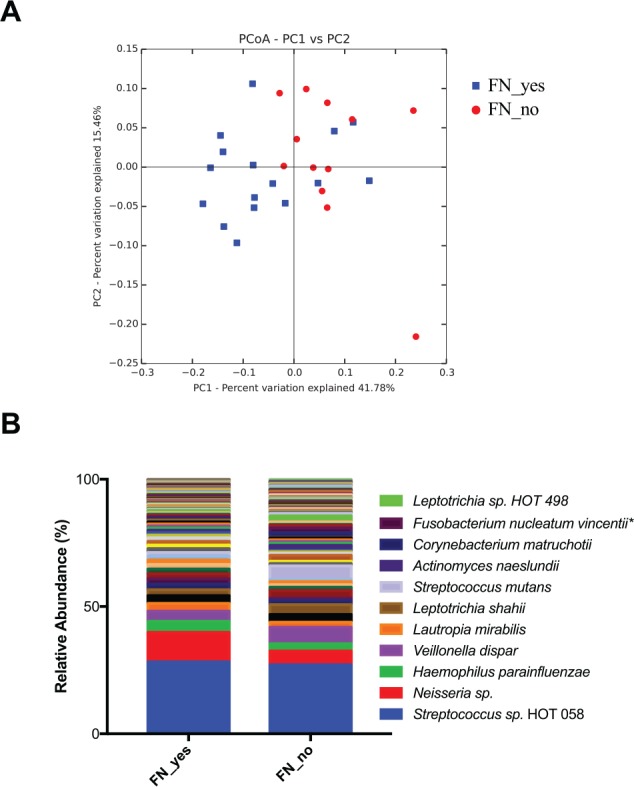
S-ECC subgroup analysis. Subjects from the S-ECC group were further divided per their residency in a First Nations community. (A) Beta diversity shown by principal coordinates analysis (PCoA) of weighted Unifrac distances. The plaque microbial communities significantly clustered by place of residency (P < 0.05, analysis of similarity). Blue, resides in First Nations community; red, does not reside in a First Nations community. (B) Average of the top 100 most abundant species identified in each group. FN_no = does not reside in a First Nations community (n = 13); FN_yes = resides in a First Nations community (n = 17). *P < 0.05 (Kruskal-Wallis with false discovery rate correction). S-ECC, severe early childhood caries.
Additionally, we investigated if species richness correlated with caries severity based on dmft (mean 10.8 ± 3.3) and dmfs (45.3 ± 19.1) scores among those with S-ECC, and we found no correlation in either instance (Pearson’s r = 0.20 and r = 0.23, respectively).
Discussion
To the best of our knowledge, this study is the first to use advanced microbial analyses to investigate the oral microbiome of North American Indigenous children, specifically, Canadian First Nations and Métis children, affected by S-ECC. Despite recent advances in understanding the role of the oral microbiome in health and disease, knowledge of its importance in the etiology of ECC is lacking, especially in Indigenous populations. The literature clearly reveals that Indigenous children suffer considerable oral health disparities when compared with other children of the same age. Much of this discrepancy stems from the historical and ongoing effects of colonialism and racism that have resulted in major socioeconomic and health care inequities (American Academy of Pediatrics and Canadian Paediatric Society 2011). With rates of S-ECC in these populations drastically higher than rates in the general population and with the potential for S-ECC to negatively affect systemic health and quality of life, it is critical to investigate the underlying causes and identify any potential unique risk factors that may exist (Schroth et al. 2009; Schroth et al. 2016).
The results of the health questionnaire confirm previously reported behavioral and socioeconomic risk factors, including less frequent brushing, bottle-feeding, later age at weaning, and lower household income (Reisine and Douglass 1998; American Academy of Pediatrics and Canadian Paediatric Society 2011). It has become apparent that S-ECC is a complex, multifactorial disease and that there is a major microbiological component (Irvine et al. 2011; QUEST 2015).
We analyzed the plaque microbiome in each group by sequencing a region of the 16S rRNA gene. The results of the beta diversity analysis revealed a statistically significant separation between the microbiomes of children with S-ECC and those caries free, indicated in Figure 1 by clustering of the samples into their groups. Overall, this result signifies that plaque microbial communities from the S-ECC subjects were more similar to others within the S-ECC group than they were to the communities from caries-free subjects. This finding demonstrates that the composition of the entire microbial community is a determining factor in S-ECC for this population.
Alpha diversity describes the number of different types of sequences within a sample. In the current study, we calculated species richness and phylogenetic diversity of each sample, and on average there was no difference between the S-ECC and caries-free groups (Fig. 1). Some studies have shown that increased alpha diversity is associated with health (Gross et al. 2012; Belstrøm et al. 2014; Xiao et al. 2016); however, other studies report the opposite (Griffen et al. 2012; Xu et al. 2014; Johansson et al. 2016). For example, in a study of young children, Xu et al. (2014) found no significant difference in the species diversity of those with caries and those without. Interestingly, Johansson et al. (2016) showed that in a population of Swedish adolescents with and without caries, the groups did not differ in terms of alpha diversity, but when compared with a caries-active population of Romanian adolescents, the Swedish subjects had much lower alpha diversity. This finding suggests that the extent to which species richness correlates to caries status is not the same in all populations, with the environment potentially playing a major role.
Four phyla were significantly differentially represented in each group. The S-ECC group had a higher abundance of Firmicutes, while Actinobacteria and Fusobacteria were higher in the caries-free group. This result supports a recent longitudinal study in young children, in which Actinobacteria decreased and Firmicutes increased as caries progression proceeded (Gross et al. 2012) and a study that reported a significantly higher relative abundance of Firmicutes in children with S-ECC versus caries-free controls (Jiang et al. 2013). Regarding the most abundant taxa, 7 of the top 25 genera detected were significantly different between the groups. Alloprevotella was significantly increased in the S-ECC group; this genus was also reported to be increased in a study of adult subjects with caries (Xiao et al. 2016). The genera Leptrotrichia, Rothia, Corynebacterium, Actinomyces, Cardiobacterium, and TM7 [G-1] were significantly higher in the caries-free group. These genera have been frequently identified in plaque and associated with health (Tanner, Kent, et al. 2011; Xu et al. 2014; Johansson et al. 2016; Xiao et al. 2016).
Of the top 25 most abundant species detected, Granulicatella elegans, Prevotella melaninogenica, and a Haemophilus species (HOT 036) were significantly more abundant in the S-ECC group. G. elegans and Prevotella melaninogenica have been reported to be increased in children with S-ECC when compared with those caries free (Kanasi et al. 2010; Ling et al. 2010; Tanner, Kent, et al. 2011). Conversely, we found that Rothia aeria, Corynebacterium matruchotii, Actinomyces naeslundii, and Streptococcus sanguinis were significantly increased in the caries-free group. Both C. matruchotii and A. naeslundii have been associated with health and caries-free status (Marchant et al. 2001; Gross et al. 2010; Tanner, Kent, et al. 2011; Ma et al. 2015). S. sanguinis is a known health-related species that has been shown to have an inverse and antagonistic relationship with S. mutans (Caufield et al. 2000; Kreth et al. 2008). Rothia spp. are commonly detected in plaque (Aas et al. 2008; Bik et al. 2010; Kanasi et al. 2010; Ling et al. 2010), but R. aeria has not been previously well associated with health. Caries-free subjects in our study had almost 5 times the amount of R. aeria on average as compared with the S-ECC children. Interestingly, another Rothia species, R. dentocariosa, has been associated with S-ECC (Jiang et al. 2016), but in our study, the caries-free group had >2-fold-higher levels. This discrepancy may be one example of the uniqueness of this particular population, and it reinforces the need for further study of dental health in Indigenous children.
The relative abundance of S. mutans, the quintessential cariogenic organism, was 3 times higher in the S-ECC group than in the caries-free group, but the average value masks the extremely high levels of some children in the S-ECC group (Fig. 2) of up to 23% of the total taxa detected. Interestingly, a recent study comparing the microbiomes of European adolescents with and without caries from 2 countries showed that the relative importance of S. mutans in determining caries status was different according to where the populations resided; the role of S. mutans as an important etiologic factor was more pronounced in the population lacking access to caries prevention and treatment strategies, as opposed to one in which there was adequate dental care (Johansson et al. 2016). This finding supports the idea that the cariogenic etiology of certain populations may be unique on the basis of socioeconomic or geographic factors, and the high levels of S. mutans in some subjects in our study may be a reflection of that.
Notably, 2 subjects in the caries-free group (of 20 total) had a high relative abundance (>2%) of S. mutans; these 2 subjects also had high levels (>2%) of R. aeria and C. matruchotii, 2 species significantly associated with caries-free status in this study. In the S-ECC group, 10 subjects (of 30 total) had high abundances of S. mutans, with none exhibiting high levels of R. aeria and C. matruchotii. This result suggests that certain health-related species may protect against the risk of carrying a high abundance of S. mutans, and it indicates that the balance and structure of the microbial community as a whole may be the most important factor in determining caries risk. The ecologic plaque hypothesis describes plaque as a dynamic microbial community in which pathogenic and protective species exist in a delicate balance, and the development of caries is the consequence of a shift in the population toward a virulent state, as opposed to the consequence of the virulence of a single pathogen (Takahashi and Nyvad 2008, 2011). Our results fit in with this ecologic perspective.
Interestingly, subgroup analysis of the S-ECC group based on residency in a First Nations community revealed that the plaque microbiomes of the 2 subgroups are overall significantly different, with the phylum Fusobacteria significantly higher in the children who did not live in a First Nations community (Fig. 3). Previous studies have found the genus Fusobacterium associated with healthy tooth surfaces (Jiang et al. 2013; Xu et al. 2014). This observation generates questions regarding environment as a risk factor, and it paves the way for further investigation.
This study is not without limitations. Due to budgetary constraints, we relied on a convenience sample of children with S-ECC on the day of their dental surgery. All controls were from Winnipeg, and those with S-ECC were from different First Nations communities or off-reserve communities, including Winnipeg. Some questions were retrospective, which might have resulted in recall bias, and the potential for response bias on the part of parents and caregivers is noted. The primary goal of this pilot study was to generate data on this understudied population to provide the foundation for future larger studies.
Overall, this study yielded important information on the microbiome of First Nations and Métis children with S-ECC and those free from caries. The only previous study to investigate the microbiology of Canadian First Nations children with S-ECC was a longitudinal observation in 1985 (Milnes and Bowden 1985). Therefore, this study is the first to investigate the microbiome of this population using modern molecular techniques. We confirmed previous reports that implicate behavioral as well as microbiological factors in the development of S-ECC, with S. mutans as the major cariogenic factor, along with many other species. Furthermore, we found that the S-ECC and caries-free groups represent disparate plaque microbial communities, supporting the notion that there is potentially a distinct caries-causing community that can eventually be identified and used for diagnosis and prognosis. It is clear that socioeconomics, cultural factors, and microbiology all play a role in the high rates of S-ECC experienced by Canadian Indigenous populations, but the finer details are still very much unknown. Therefore, it is imperative to continue to study the underlying causes (including the microbiome) of the extreme oral health disparities that these populations face to provide prevention and treatment services that accurately reflect the underlying etiology.
Author Contributions
M. Agnello, L. Cen, contributed to data analysis and interpretation, drafted and critically revised the manuscript; J. Marques, contributed to data acquisition, analysis, interpretation, drafted and critically revised the manuscript; B. Mittermuller, A. Huang, N. Chaichanasakul Tran, contributed to data acquisition, drafted and critically revised the manuscript; W. Shi, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; X. He, contributed to design, data analysis, and interpretation, drafted and critically revised the manuscript; R.J. Schroth, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
The authors thank the Misericordia Health Centre (Winnipeg); participating pediatric dentists, parents, and children; and Ms. Eleonore Kliewer. Additional thanks to Drs. Dee Robertson and Fred Eichmiller and QUEST in AI/AN Children, which is a 501(c)(3) organization whose mission is to convene and focus the expertise and resources necessary to elucidate the etiology of rampant caries in the primary dentition of American Indian/Alaska Native children and to identify optimal strategies to prevent and control it. The authors acknowledge additional present and past QUEST in AI/AN Children microbiology group members: Drs. Don Marianos, David Drake, and Anne Tanner.
Footnotes
A supplemental appendix to this article is available online.
Operating funds were provided by Delta Dental of Wisconsin. BScDent studentship funding for J. Marques was provided by the Children’s Hospital Research Institute of Manitoba. R.J. Schroth holds a Canadian Institutes of Health Research Embedded Clinician Salary Award in improving access to oral health care and oral health care delivery for at-risk young children in Manitoba. This work was also supported in part by grants from the National Institutes of Health (NIH-1-R01-DE020102, NIH-1-R01-DE023810, and NIH-1-R01-DE026186-01) and National Institute of Dental and Craniofacial Research (T90 DE022734) training award to M. Agnello.
W. Shi is the founding scientist for C3 Jian, Inc., which has licensed anti–S. mutans technology from the Regents of the University of California that could be indirectly related to this research project. The other authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ. 2008. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 46(4):1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics. 2016. Policy on early childhood caries (ECC): classifications, consequences, and preventive strategies. Pediatr Dent. 38(6):52–54. [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Native American Child Health and Canadian Paediatric Society First Nations, Inuit and Métis Committee. 2011. Early childhood caries in Indigenous communities. Pediatrics. 127(6):1190–1198. [DOI] [PubMed] [Google Scholar]
- Belstrøm D, Fiehn NE, Nielsen CH, Holmstrup P, Kirkby N, Klepac-Ceraj V, Paster BJ, Twetman S. 2014. Altered bacterial profiles in saliva from adults with caries lesions: a case-cohort study. Caries Res. 48(5):368–375. [DOI] [PubMed] [Google Scholar]
- Bik EM, Long CD, Armitage GC, Loomer P, Emerson J, Mongodin EF, Nelson KE, Gill SR, Fraser-Liggett CM, Relman DA. 2010. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 4(8):962–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Institute for Health Information. 2013. Treatment of preventable dental cavities in preschoolers: a focus on day surgery under general anesthesia. Ottowa (Canada): Canadian Institute for Health Information. [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 7(5):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caufield PW, Dasanayake AP, Li Y, Pan Y, Hsu J, Hardin JM. 2000. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun. 68(7):4018–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Owens SA, Isong IA, Soobader MJ, Gansky SA, Weintraub JA, Platt LJ, Newacheck PW. 2013. An examination of racial/ethnic disparities in children’s oral health in the United States. J Public Health Dent. 73(2):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, Podar M, Leys EJ. 2012. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 6(6):1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL. 2012. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One. 7(10):e47722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross EL, Leys EJ, Gasparovich SR, Firestone ND, Schwartzbaum JA, Janies DA, Asnani K, Griffen AL. 2010. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J Clin Microbiol. 48(11):4121–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. 1990. More powerful procedures for multiple significance testing. Stat Med. 9(7):811–818. [DOI] [PubMed] [Google Scholar]
- Irvine J, Holve S, Krol D, Schroth R. 2011. Early childhood caries in Indigenous communities: a joint statement with the American Academy of Pediatrics. Paediatr Child Health. 16(6):351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Gao X, Jin L, Lo EC. 2016. Salivary microbiome diversity in caries-free and caries-affected children. Int J Mol Sci. 17(12):E1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhang J, Chen H. 2013. Pyrosequencing analysis of oral microbiota in children with severe early childhood dental caries. Curr Microbiol. 67(5):537–542. [DOI] [PubMed] [Google Scholar]
- Johansson I, Witkowska E, Kaveh B, Lif Holgerson P, Tanner AC. 2016. The microbiome in populations with a low and high prevalence of caries. J Dent Res. 95(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasi E, Dewhirst FE, Chalmers NI, Kent R, Moore A, Hughes CV, Pradhan N, Loo CY, Tanner AC. 2010. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 44(5):485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Zhang Y, Herzberg MC. 2008. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol. 190(13):4632–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Kong J, Jia P, Wei C, Wang Y, Pan Z, Huang W, Li L, Chen H, Xiang C. 2010. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microb Ecol. 60(3):677–690. [DOI] [PubMed] [Google Scholar]
- Lozupone C, Knight R. 2005. Unifrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 71(12):8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Chen F, Zhang Y, Sun X, Tong P, Si Y, Zheng S. 2015. Comparison of oral microbial profiles between children with severe early childhood caries and caries-free children using the Human Oral Microbe Identification Microarray. PLoS One. 10(3):e0122075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant S, Brailsford SR, Twomey AC, Roberts GJ, Beighton D. 2001. The predominant microflora of nursing caries lesions. Caries Res. 35(6):397–406. [DOI] [PubMed] [Google Scholar]
- Martins-Júnior PA, Vieira-Andrade RG, Corrêa-Faria P, Oliveira-Ferreira F, Marques LS, Ramos-Jorge ML. 2013. Impact of early childhood caries on the oral health-related quality of life of preschool children and their parents. Caries Res. 47(3):211–218. [DOI] [PubMed] [Google Scholar]
- Milnes AR, Bowden GH. 1985. The microflora associated with developing lesions of nursing caries. Caries Res. 19(4):289–297. [DOI] [PubMed] [Google Scholar]
- QUEST. 2015. Symposium on caries in American Indian and Alaska Native children. Hood River (OR) [accessed 2017 Jun 12]. http://www.ada.org/~/media/ADA/Education%20and%20Careers/Files/QUEST%202015%20Symposium%20FINAL%20REPORT20151202t154929.ashx.
- Reisine S, Douglass JM. 1998. Psychosocial and behavioral issues in early childhood caries. Community Dent Oral Epidemiol. 26 Suppl 1:32–44. [DOI] [PubMed] [Google Scholar]
- Schroth RJ, Harrison RL, Moffatt ME. 2009. Oral health of Indigenous children and the influence of early childhood caries on childhood health and well-being. Pediatr Clin North Am. 56(6):1481–1499. [DOI] [PubMed] [Google Scholar]
- Schroth RJ, Quiñonez C, Shwart L, Wagar B. 2016. Treating early childhood caries under general anesthesia: a national review of Canadian data. J Can Dent Assoc. 82:g20. [PubMed] [Google Scholar]
- Schroth RJ, Smith PJ, Whalen JC, Lekic C, Moffatt ME. 2005. Prevalence of caries among preschool-aged children in a northern Manitoba community. J Can Dent Assoc. 71(1):27. [PubMed] [Google Scholar]
- Schroth RJ, Smith WF. 2007. A review of repeat general anesthesia for pediatric dental surgery in Alberta, Canada. Pediatr Dent. 29(6):480–487. [PubMed] [Google Scholar]
- Sheller B, Williams BJ, Lombardi SM. 1997. Diagnosis and treatment of dental caries-related emergencies in a children’s hospital. Pediatr Dent. 19(8):470–475. [PubMed] [Google Scholar]
- Takahashi N, Nyvad B. 2008. Caries ecology revisited: microbial dynamics and the caries process. Caries Res. 42(6):409–418. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Nyvad B. 2011. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 90(3):294–303. [DOI] [PubMed] [Google Scholar]
- Tanner AC, Kent RL, Holgerson PL, Hughes CV, Loo CY, Kanasi E, Chalmers NI, Johansson I. 2011. Microbiota of severe early childhood caries before and after therapy. J Dent Res. 90(11):1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner AC, Mathney JM, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, et al. 2011. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 49(4):1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Ran S, Huang Z, Liang J. 2016. Bacterial diversity and community structure of supragingival plaques in adults with dental health or caries revealed by 16s pyrosequencing. Front Microbiol. 7:1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Hao W, Zhou Q, Wang W, Xia Z, Liu C, Chen X, Qin M, Chen F. 2014. Plaque bacterial microbiome diversity in children younger than 30 months with or without caries prior to eruption of second primary molars. PLoS One. 9(2):e89269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.