Abstract
Purpose
The combination of chemotherapy and trastuzumab is the standard of care for adjuvant treatment of human epidermal growth factor receptor 2–positive breast cancer. Two regimens have been widely adopted in the United States: doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab (ACTH) and docetaxel, carboplatin, and trastuzumab (TCH). No head-to-head comparison of these regimens has been conducted in a clinical trial, and existing trial data have limited generalizability to older patients.
Methods
We used SEER-Medicare data from 2005 to 2013 to compare outcomes of ACTH versus TCH among patients age older than 65 years. Propensity score matching was used to balance cohort characteristics between treatment arms. Outcomes included toxicity-related hospitalization, survival, and trastuzumab completion. Data from 1,077 patients receiving ACTH or TCH were analyzed, and the propensity-matched subsample included 416 women.
Results
There was a significant shift toward TCH over time, with 88% of patients receiving ACTH in 2005 compared with 15% by 2011. Among propensity score–matched patients, we found no difference between regimens in health care use overall or for chemotherapy-related adverse events (ACTH, 34% v TCH, 36.5%; P = .46). Patients receiving TCH were significantly more likely to complete trastuzumab (89% v 77%; P = .001). There was no difference in 5-year breast cancer–specific survival (ACTH, 92% v TCH, 96%; hazard ratio, 2.08; 95% CI, 0.90 to 4.82) or overall survival.
Conclusion
Among a matched sample of older patients, ACTH compared with TCH was not associated with a higher rate of serious adverse events or hospitalizations, but it was associated with less completion of adjuvant trastuzumab. We did not detect a difference in 5-year survival outcomes for ACTH compared with TCH. In the context of limited evidence in older patients, selection between these two regimens on the basis of concerns about differential toxicity or efficacy may not be appropriate.
INTRODUCTION
Over the past two decades, the combination of chemotherapy and the monoclonal antibody trastuzumab has become the standard of care for systemic therapy of human epidermal growth factor receptor 2 (HER2) –positive breast cancer. Two adjuvant trastuzumab-based regimens have been widely adopted in the United States. The combination of doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab (ACTH) was approved for adjuvant use in 2006 on the basis of the NSABP (National Adjuvant Breast and Bowel Project) B31 and NCCTG (North Central Cancer Treatment Group) 9831 studies.1,2 Docetaxel combined with carboplatin and trastuzumab (TCH) was approved in 2008 on the basis of the BCIRG (Breast Cancer International Research Group) 006 study.3,4 Although less intensive regimens have subsequently emerged for lower-risk patients and are being evaluated for older patients,5,6 and dual biologic therapy including pertuzumab has been incorporated into neoadjuvant regimens,7,8 ACTH and TCH remain the standard of care for most women with HER2-positive breast cancer.
Most information available to clinicians choosing between ACTH and TCH comes from a single randomized trial, BCIRG 006, a three-arm study in which both trastuzumab-containing regimens were compared against chemotherapy alone. Although not designed to compare ACTH with TCH, the study demonstrated an absolute difference of 1.6% in 10-year breast cancer–specific survival between the trastuzumab-containing arms favoring ACTH, which was not statistically significant.9 Conversely, a difference in cardiac toxicity was noted favoring TCH, with 0.4% of patients receiving TCH and 2.0% of patients receiving ACTH developing congestive heart failure. No head-to-head clinical trial has compared ACTH and TCH in terms of efficacy or toxicity, nor is one anticipated. Moreover, patients represented in these pivotal studies had a median age of approximately 49 years, were mostly white, and had few comorbidities, limiting their relevance in the choice between ACTH and TCH for older and frailer women. Prior research has shown surprisingly low rates of adjuvant trastuzumab use among older, particularly minority women with HER2-positive breast cancer, possibly because of these data limitations.10,11 The impact of regimen choice on the likelihood of completing trastuzumab is also unknown. Given emerging evidence that shorter trastuzumab duration adversely affects outcomes,12,13 the effect of regimen choice on adherence may be an important factor to consider when selecting an initial regimen.
Comparative-effectiveness research as defined by the Institute of Medicine is “the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat and monitor a clinical condition, or to improve the delivery of care.”14(p203) Where prospective randomized data are unlikely to become available, rigorous observational studies can inform clinical care by offering information about the outcomes of treatment from large populations of community-dwelling patients receiving different treatments for clinically similar disease.
The linkage of the SEER cancer registry network to insurance claims from Medicare provides a national population-based sample of older patients for observational comparative-effectiveness studies of cancer treatments. We sought to compare the toxicities, completion rates, and disease outcomes of older women receiving ACTH and TCH in real-world settings, employing propensity-matching methods to mitigate selection bias. We also sought to characterize the patterns of use of ACTH and TCH over time and on the basis of patient characteristics.
METHODS
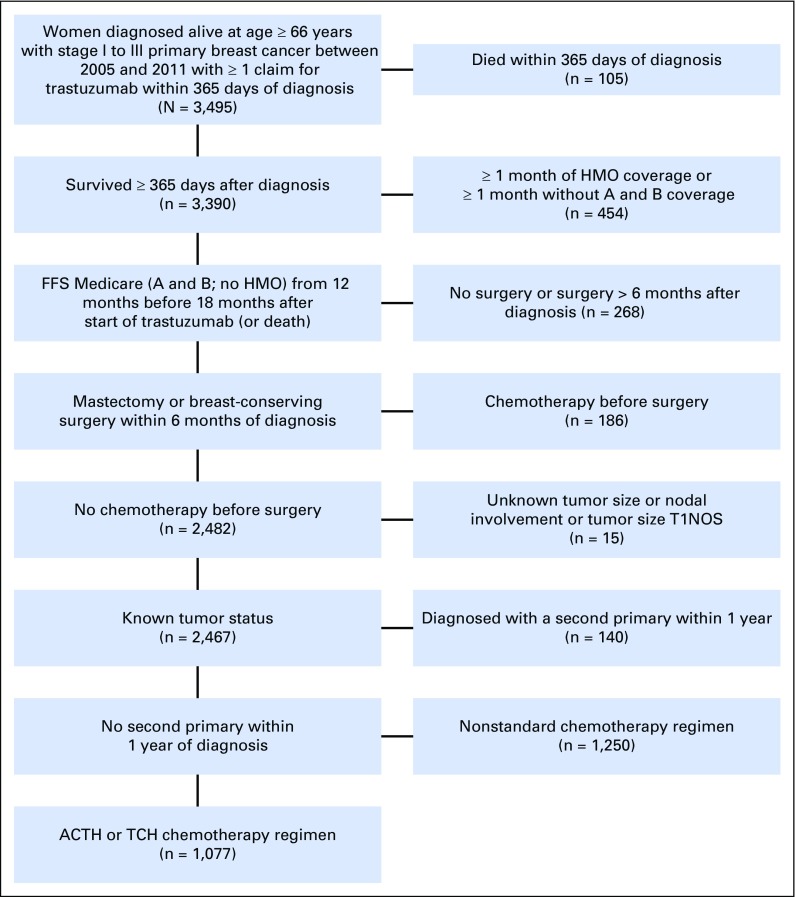
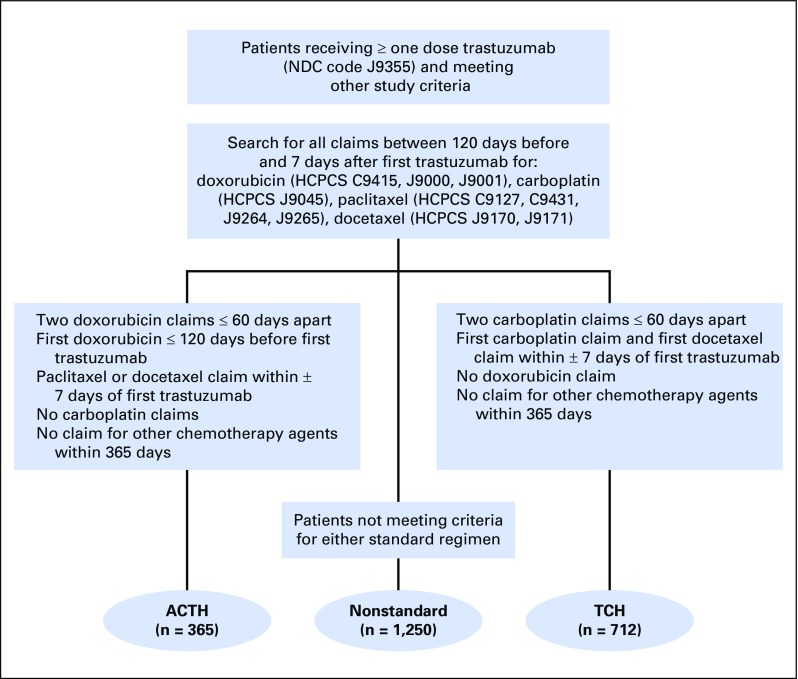
From the SEER-Medicare database,15 we identified women age 66 years or older with incident stage I to III breast cancer between 2005 and 2011 (Fig 1). Participants were required to have a claim for trastuzumab (Healthcare Common Procedure Coding System code J9355) within 1 year of diagnosis, a cancer surgery within 6 months of diagnosis, and no neoadjuvant chemotherapy. We excluded women with missing information on T or N stage or a second primary cancer within 1 year and those who were not continuously enrolled in fee-for-service Medicare (parts A and B; no health maintenance organizations) for 12 months before diagnosis. Participants were required to remain enrolled until completion of adjuvant trastuzumab or 18 months after trastuzumab initiation. Finally, we developed a claims-based algorithm to identify users of TCH or ACTH (Appendix Fig A1, online only). Following an intent-to-treat approach, patients were not required to complete all doses or to receive all doses on time. Patients who switched treatments were excluded.
Fig 1.
Patient distribution diagram. ACTH, doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab; FFS, fee for service; HMO, health maintenance organization; NOS, not otherwise specified; TCH, docetaxel, carboplatin, and trastuzumab.
Completion of Trastuzumab Therapy
We defined completion as having at least 270 days of trastuzumab within 540 days of the first claim.12 Women were considered ongoing trastuzumab users until they experienced a gap of more than 90 days between claims. Few women (4.4%) had more than 90-day gaps followed by resumption of therapy. Days covered by trastuzumab were defined as the time between treatment dates, with a maximum of 21 days of coverage for each claim.
Chemotherapy-Related Toxicities
We evaluated inpatient and outpatient claims within 6 months of beginning chemotherapy with primary or secondary diagnoses for the following chemotherapy-related adverse events16,17: nausea, emesis, diarrhea, mucositis, dehydration, malnutrition, fever, urinary tract infection, pneumonia, infections, anemia, neutropenia, headache, heart failure or cardiomyopathy, cerebrovascular accident or transient ischemic attack, deep-vein thrombosis or pulmonary embolism, neuropathy, liver or kidney failure, fractures, and delirium. For conditions likely to be present before chemotherapy (heart failure, cerebrovascular accident or transient ischemic attack, neuropathy, liver or kidney failure), the condition was only counted as a toxicity if the patient had no claims for the same condition within 365 days before chemotherapy. We separately evaluated toxicities recorded in the hospital or emergency department from those in outpatient visits. For the multivariable model, the primary outcome was any emergency or inpatient visit for a chemotherapy-related adverse event within 6 months of chemotherapy initiation. This model was performed on the propensity-matched subsample and further adjusted for age, race, marital status, poverty, geographic region, rurality, comorbidity, number of heart-related conditions, diagnosis year, and tumor characteristics. All evaluable patients had at least 6 months of claims after chemotherapy initiation.
Survival
We measured survival as the days alive from diagnosis through December 2013. To define overall survival (OS), we used the SEER vital status file. For recurrence-free survival, we used a claims-based proxy with high sensitivity and specificity for identifying secondary breast cancer events.18 For breast cancer–specific survival (BCSS), deaths were attributed to breast cancer if it was listed as the cause of death in the SEER record or if the patient had an algorithm-defined recurrence followed by death.19 Patients were censored at death resulting from other cause (for cancer end points), disenrollment, end of claims data in December 2013, or 5 years postdiagnosis. Because patients receiving TCH on average were diagnosed later than those receiving ACTH, we included diagnosis period as a matching variable.
Covariates
We measured age, race, marital status, SEER region, urban area, hormone receptor status, tumor size, tumor grade, nodal involvement, surgery, and radiation therapy. We also created census-tract poverty level measures. Using the Medicare Chronic Conditions Warehouse, we measured heart failure, hyperlipidemia, hypertension, ischemic heart disease, and myocardial infarction.20 Finally, we measured overall medical comorbidity using the Klabunde-adapted Charlson score.21
Analysis
We used propensity score matching to create comparable cohorts of women receiving ACTH and TCH regimens on the basis of pretreatment characteristics. Propensity score adjustment is a widely accepted approach to control for selection bias in observational studies.22,23 We considered alternative propensity-adjustment approaches, including inverse probability of treatment weighting (IPTW), but chose the matching approach for primary analysis because it produced the best balance between treatment arms regarding the measured covariates evaluated using standardized adjusted mean differences, with a standardized adjusted mean difference greater than 10% indicating acceptable balance. We performed sensitivity analyses using IPTW for all survival end points to take advantage of the larger size of the full sample. We created the propensity score using logistic regression, with regimen assignment as the dependent variable. The model was fit with covariates on the basis of clinical judgment and known confounders from prior literature, including age, race, marital status, geographic region, rurality, census tract–level poverty, Charlson comorbidity score, number of heart-related conditions, stage, hormone receptor status, grade, and diagnosis year. Patient cases were matched using the Greedy matching algorithm.
Among patients in the propensity score–matched sample, we used multivariable modified Poisson regression24,25 to assess the association between regimen (ACTH or TCH) and postchemotherapy inpatient, observation, and emergency room visits. We also used Poisson regression to examine the effect of regimen on likelihood of completing trastuzumab.
We compared Kaplan-Meier curves of 5-year survival rates for ACTH and TCH in the matched cohort. All surviving patients were censored at end of follow-up death, disenrollment, or 5 years postdiagnosis. We used the SAS LIFETEST procedure to produce the Kaplan-Meier curves and the phreg procedure to determine equality of ACTH and TCH strata (SAS Institute, Cary, NC).
RESULTS
Predictors of Regimen Choice
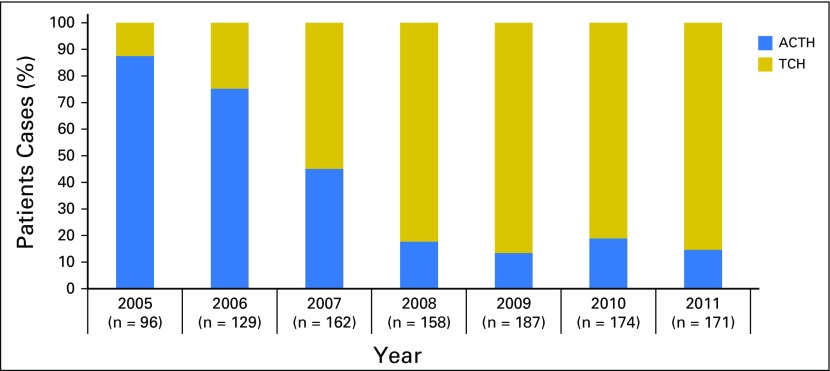
The full study cohort (Table 1) included 1,077 women. Before propensity score matching, patients receiving ACTH tended to be younger, reside in the North Central and Northeast SEER regions, and have less comorbidity. Patients receiving ACTH also had higher stage, higher rates of hormone receptor negativity, and more grade 3 tumors. Practice patterns changed significantly over time; ACTH use dropped from 88% of patients in 2005 to only 15% in 2011 (Fig 2). After matching, patient characteristics were similar between arms, except for slightly more breast-conserving surgeries without radiation therapy performed in the TCH arm. The proportionally small match rate was a function of the shift toward TCH over time and the necessary inclusion of diagnosis period as a matching variable. Detailed information regarding the distribution of patients among ACTH, TCH, and other chemotherapy regimens or single-agent trastuzumab is presented in Appendix Table A1 (online only) and in previously published work.10
Table 1.
Characteristics of Full and Propensity Score–Matched Cohorts
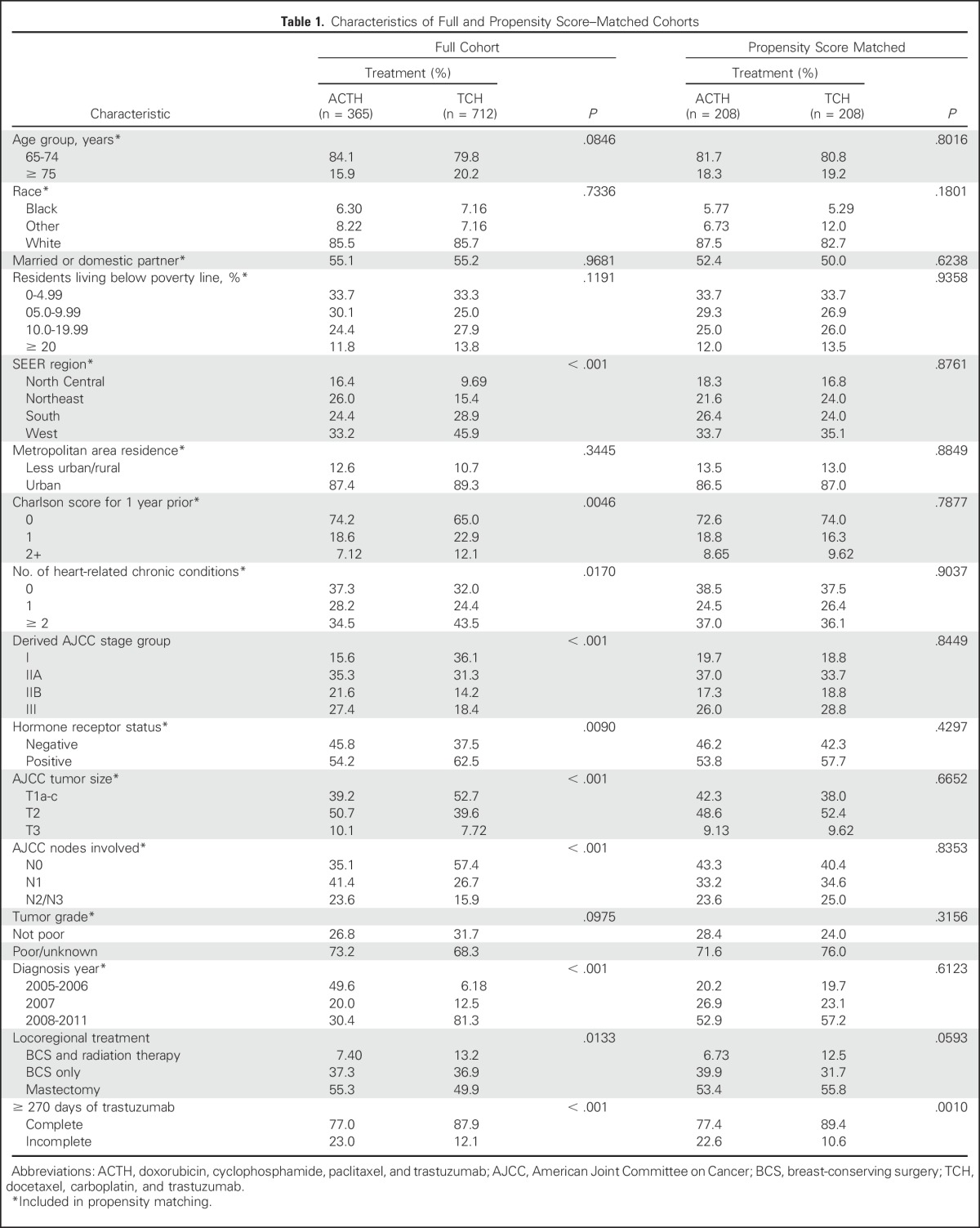
Fig 2.
Trends in use of trastuzumab-containing regimens by year; patient cases of breast cancer treated with doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab (ACTH) or docetaxel, carboplatin, and trastuzumab (TCH) from 2005 to 2011 (N = 1,077).
Toxicity of Therapy
In the full study cohort, claims for neutropenia and anemia were more common in patients receiving ACTH (ACTH: neutropenia, 57%; anemia, 59% v TCH: neutropenia, 45%; anemia, 46%; P < .01), whereas claims for dehydration were more common among those receiving TCH (36% v 24%; P < .01). After matching, there were no significant differences in the overall frequency of chemotherapy-related adverse events. Although claims for incident heart failure were more common in matched patients receiving ACTH (7.2% v 3.9%), this finding did not reach statistical significance. We found evidence of postchemotherapy acute myeloid leukemia in claims for fewer than 1% of patients, divided proportionally among those receiving ACTH and TCH. Because of small cell sizes, exact numbers of cases of acute myeloid leukemia cannot be presented.
Health care use related to adverse events was relatively common and similar between groups. Hospital stays related to adverse events within 6 months of beginning therapy occurred in 21% of ACTH users and 24% of TCH users in the overall cohort (P = .26). When emergency and observation visits were included, 34% of patients receiving ACTH and 36.5% of those receiving TCH used hospital services during the period (P = .46). Patterns of use among the propensity-matched sample were similar to those of the overall cohort (data not shown). In multivariable propensity score–matched analysis, there was no significant difference in the adjusted likelihood of the composite outcome of an emergency or inpatient visit within 6 months of chemotherapy initiation between patients receiving ACTH and TCH. We performed a sensitivity analysis limiting the outcome to visits with a chemotherapy-related event as the primary diagnosis; results were similar.
Completion of Therapy
In the overall cohort, 77% of patients receiving ACTH and 88% of patients receiving TCH completed adjuvant trastuzumab. In the matched subset, completion rates were significantly lower among ACTH users (ACTH, 77%; TCH, 89%; P = .001). Among patients completing more than 270 days, the median number of days covered was 371 for both ACTH and TCH. In multivariable propensity score–matched analysis, receiving ACTH was an independent predictor of noncompletion of maintenance trastuzumab (relative risk, 0.86; 95% CI, 0.80 to 0.93). Other predictors of noncompletion included age ≥ 75 years and Charlson comorbidity score of 2+. Race was not an independent predictor of completion.
Survival
In the overall cohort, 88% of ACTH users and 93% of TCH users were alive at 5 years (hazard ratio [HR], 1.41; 95% CI, 0.94 to 2.11); in the matched subset, 5-year OS was 90% for ACTH users and 92% for TCH users (HR, 1.22; 95% CI, 0.63 to 2.35). Five-year BCSS in the overall cohort was 90% in ACTH users and 96% in TCH users (HR, 2.14; 95% CI, 1.29 to 3.53). In the propensity score–matched sample, 5-year BCSS was 92% in the ACTH group and 96% in the TCH group (HR, 2.08; 95% CI, 0.90 to 4.82).
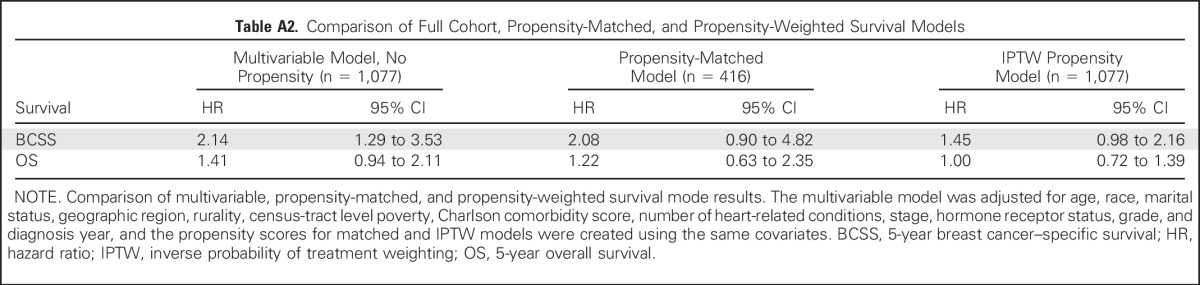
We performed a sensitivity analysis limited to matched patients who completed trastuzumab to explore the hypothesis that the lack of survival difference between treatment arms might be attributable to lower rates of trastuzumab completion among patients receiving ACTH. Among patients who completed trastuzumab, 5-year BCSS was 93% in the ACTH group and 97% in the TCH group (HR, 2.45; 95% CI, 0.85 to 7.04). We performed sensitivity analyses using an IPTW propensity approach for all survival end points to use data from the entire cohort (N = 1,077). This approach also found no significant difference between treatment arms in BCSS (HR, 1.45; 95% CI, 0.98 to 2.16) or OS (HR, 1.00; 95% CI, 0.72 to 1.39) but with greater precision in the effect estimates. A comparison of the effect estimates from the full cohort and propensity-matched and IPTW models is included in Appendix Table A2 (online only).
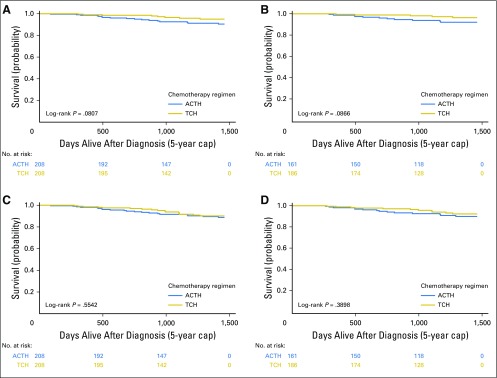
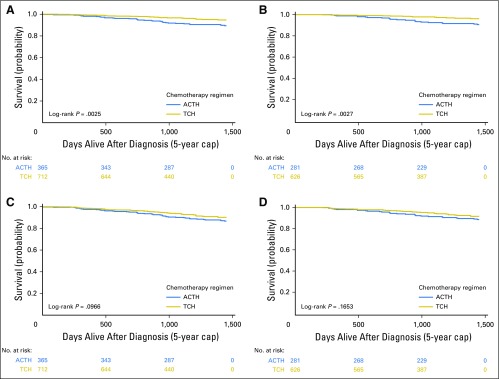
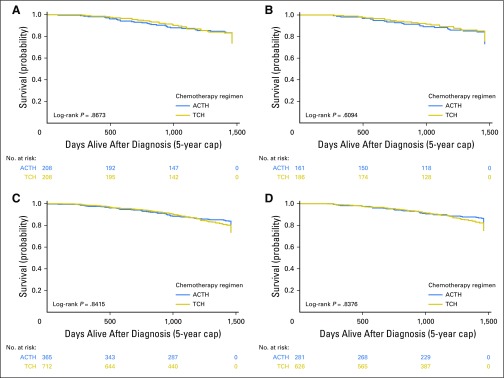
Survival curves for the propensity-matched sample are shown in Figure 3. Sensitivity analyses of the full cohort and matched subcohort using the alternative end points of OS and recurrence-free survival yielded similar results (Appendix Figs A2 and A3, online only).
Fig 3.
Kaplan-Meier curves for 5-year survival outcomes of propensity score–matched patients by regimen. (A, B) Breast cancer–specific and (C, D) overall survival in (A, C) all patients and (B, D) completers. ACTH, doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab; TCH, docetaxel, carboplatin, and trastuzumab.
DISCUSSION
In this national study of older women, we found no significant differences in health care use for chemotherapy-related adverse events between ACTH and TCH users. However, patients receiving TCH were significantly more likely to complete a full year of trastuzumab, even after limiting our sample to those who were well matched on measured characteristics. Among matched patients, BCSS and OS did not differ significantly between regimens. Surprisingly, there was a trend toward higher BCSS among matched patients receiving TCH for clinically similar disease, in contrast to the trend toward improved survival for ACTH observed in a previous clinical trial. However, the gap between TCH and ACTH was not statistically significant after propensity matching to account for measured differences in patient characteristics between treatment arms.
Previous research has reported underuse of trastuzumab in older women,10,11 widespread use of nonstandard regimens,16 noncompletion of therapy,26 and significant toxicities,26 but no previous study has compared the effectiveness of ACTH and TCH in older women. Comparative-effectiveness studies can fill a crucial knowledge gap when clinical trial data have limited applicability to a patient population or when a comparative trial is unlikely to be conducted. Clinical trials of adjuvant trastuzumab1,3,27 have included patients with a median age of 49 years. Women older than age 60 years made up approximately 15% of participants, and those older than age 70 years or with cardiac conditions were largely excluded. Although our study is constrained by the inherent limitations of observational research, we believe that it adds valuable information regarding tolerability and effectiveness of ACTH and TCH in older women.
There are several possible reasons why this study did not find differences in toxicity or efficacy between regimens. First, the null result may simply confirm the results of the BCIRG 006 trial, in which there was no significant survival advantage for the anthracycline-containing regimen. Alternative explanations must be considered. The lower completion rate of trastuzumab among ACTH users might decrease the effectiveness of this regimen. Although we did not detect a survival advantage for ACTH in the subset of patients who completed trastuzumab, this sensitivity analysis was underpowered to conclude whether ACTH was superior to TCH in patients who completed their full year of therapy. We were not able to examine the dose-intensity or dose density of the chemotherapy agents in detail. It is possible that more chemotherapy dose reductions or delays occurred in the ACTH arm, reducing effectiveness. Given that the marginal benefit of anthracyclines is greater in higher-risk patients,28 we might expect to see less difference between treatment groups if our study contained lower-risk patients compared with those in BCIRG 006. Our sample included a similar proportion of hormone receptor–positive patients (56% v 54%) but a slightly higher proportion of node-negative patients (42% v 29%) than BCIRG 006.3 Lastly, the analytic approach used cannot account for unmeasured confounders that may have been associated with treatment selection and that might affect survival. For instance, patients receiving ACTH may have been perceived as higher risk by their providers on the basis of characteristics not included in cancer registry data, such as the speed with which the cancer presented, lymphovascular invasion, extranodal extension, or inadequate surgical management.
This study has several limitations common to observational analyses. Insurance claims cannot measure the full extent of toxicity or quality-of-life effects of treatment. It is certainly possible that one regimen is superior to the other in terms of nonlethal long-term effects or quality of life. Similarly, retrospective observational studies cannot evaluate recurrence or adjudicate cause of death with the same level of detail as a clinical trial, introducing possible misclassification of survival events. Although we accounted for selection bias and potential confounders of the treatment-outcome relationships using statistical methods, residual confounding resulting from unmeasured factors may have remained and not been addressed by propensity score adjustment. Because pharmacy claims were not uniformly available, we were unable to control for the effect of antiestrogen therapy in hormone receptor–positive patients. Our sample size was limited, particularly among matched patients, and thus underpowered to determine whether the slight numeric advantage in survival among patients receiving TCH represented a true difference in efficacy. Finally, the assignment of treatment regimen in claims data necessarily relies on algorithms that may not correctly assign the intended treatment in all cases; however, the proportion of patients in our sample receiving ACTH and TCH, as well as other chemotherapy regimens or single-agent trastuzumab, is similar to that reported previously in a similar SEER-Medicare cohort by Freedman et al,16 using an independently developed algorithm. We did find a slightly lower proportion of patients receiving ACTH, likely because of our inclusion of 2 additional years of more recent data.
Among older women with HER2-positive breast cancer, ACTH compared with TCH was not associated with more chemotherapy-related adverse events, but it was associated with a lower rate of completion of adjuvant trastuzumab. We did not find a significant difference in survival outcomes between regimens. In the context of limited evidence in older patients, selection between these two regimens in older women on the basis of concerns for differential toxicity or efficacy may not be appropriate. As adjuvant therapy for HER2-positive breast cancer evolves to include de-escalated chemotherapy and dual-targeted therapy, it is important to continue to evaluate real-world toxicity and effectiveness, supplementing and extending the contributions of prospective clinical trials.
ACKNOWLEDGMENT
We acknowledge the efforts of the National Cancer Institute, the Office of Research, Development and Information, the Centers for Medicare and Medicaid Services, Information Management Services, and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Appendix
Fig A1.
Treatment assignment algorithm. ACTH, doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab; HCPCS, Healthcare Common Procedure Coding System; NDC, National Drug Code; TCH, docetaxel, carboplatin, and trastuzumab.
Fig A2.
Kaplan-Meier curves for 5-year survival outcomes of full unmatched cohort (N = 1,077). (A, B) Breast cancer–specific and (C, D) overall survival in (A, C) all patients and (B, D) completers. ACTH, doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab; TCH, docetaxel, carboplatin, and trastuzumab.
Fig A3.
Kaplan-Meier curves for 5-year recurrence-free survival outcomes of (A, B) propensity score–matched cohort (n = 416) and (C, D) full cohort (N = 1,077) in (A, C) all patients and (B, D) completers. ACTH, doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab; TCH, docetaxel, carboplatin, and trastuzumab.
Table A1.
Distribution of Chemotherapy Regimens
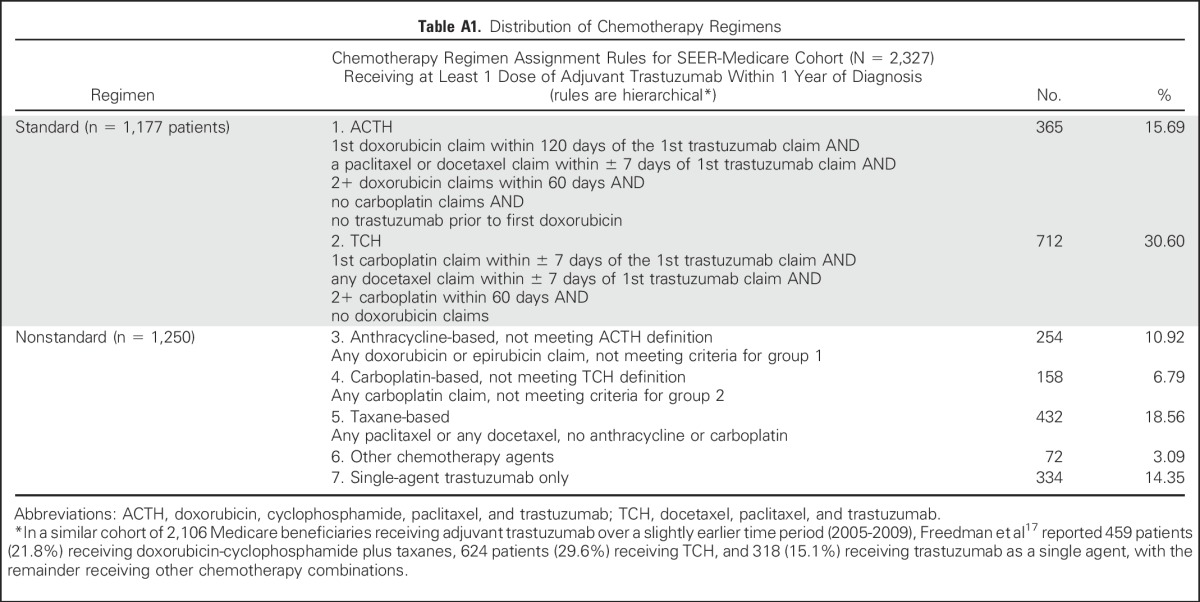
Table A2.
Comparison of Full Cohort, Propensity-Matched, and Propensity-Weighted Survival Models
Footnotes
Supported by the National Institutes of Health (NIH) National Center for Advancing Translational Sciences Grant No. UL1TR001111/CERR11402; in part by career development grants from the Conquer Cancer Foundation and the Susan G Komen Foundation and career development and project funds from the University of North Carolina Breast Cancer Specialized Programs of Research Excellence Grant No. P50CA058223-20 (K.R.-H.); in part by the NIH Building Interdisciplinary Research Careers in Women’s Health K12 Program (S.B.D.); and by the Integrated Cancer Information and Surveillance System of the University of Carolina Lineberger Comprehensive Cancer Center with funding provided by the University Cancer Research Fund via the State of North Carolina. Collection of cancer incidence data was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, the National Cancer Institute SEER Program under Contract No. HHSN261201000140C awarded to the Cancer Prevention Institute of California, Contract No. HHSN261201000035C awarded to the University of Southern California, and Contract No. HHSN261201000034C awarded to the Public Health Institute and the Centers for Disease Control and Prevention National Program of Cancer Registries under Agreement No. U58DP003862-01 awarded to the California Department of Public Health.
The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California Department of Public Health, the National Institutes of Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors or subcontractors is not intended, nor should it be inferred.
AUTHOR CONTRIBUTIONS
Conception and design: Katherine E. Reeder-Hayes, Anne Marie Meyer, Lisa A. Carey, Stacie B. Dusetzina
Collection and assembly of data: Katherine E. Reeder-Hayes, Sharon Peacock Hinton
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Comparative Toxicity and Effectiveness of Trastuzumab-Based Chemotherapy Regimens in Older Women With Early-Stage Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Katherine E. Reeder-Hayes
No relationship to disclose
Anne Marie Meyer
Honoraria: National Cancer Institute
Consulting or Advisory Role: Merck
Sharon Peacock Hinton
No relationship to disclose
Ke Meng
No relationship to disclose
Lisa A. Carey
Research Funding: GlaxoSmithKline, Genentech
Stacie B. Dusetzina
No relationship to disclose
REFERENCES
- 1.Romond EH, Perez EA, Bryant J, et al. : Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673-1684, 2005 [DOI] [PubMed] [Google Scholar]
- 2. US Food and Drug Administration: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279177.htm.
- 3.Slamon D, Eiermann W, Robert N, et al. : Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273-1283, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Genentech: Herceptin (trastuzumab) development timeline. https://www.gene.com/media/product-information/herceptin-development-timeline.
- 5.Tolaney SM, Barry WT, Dang CT, et al. : Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372:134-141, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Cancer Institute: Trastuzumab emtansine in treating older patients with human epidermal growth factor receptor 2-positive stage I-III breast cancer. https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/view?cdrid=771306.
- 7.Gianni L, Pienkowski T, Im YH, et al. : Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25-32, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Schneeweiss A, Chia S, Hickish T, et al. : Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278-2284, 2013 [DOI] [PubMed] [Google Scholar]
- 9. Slamon DJ, Eiermann W, Robert NJ, et al: Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→e) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→e) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. Presented at the San Antonio Breast Cancer Symposium, Houston, TX, December 10-13, 2015. [Google Scholar]
- 10.Reeder-Hayes K, Peacock Hinton S, Meng K, et al. : Disparities in use of human epidermal growth hormone receptor 2-targeted therapy for early-stage breast cancer. J Clin Oncol 34:2003-2009, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaz-Luis I, Lin NU, Keating NL, et al. : Treatment of early-stage human epidermal growth factor 2-positive cancers among medicare enrollees: Age and race strongly associated with non-use of trastuzumab. Breast Cancer Res Treat 159:151-162, 2016 [DOI] [PubMed] [Google Scholar]
- 12. doi: 10.1002/cncr.27831. Freedman RA, Hughes ME, Ottesen RA, et al: Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer 119:839-846, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pivot X, Romieu G, Debled M, et al. : 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): A randomised phase 3 trial. Lancet Oncol 14:741-748, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Sox HC, Greenfield S: Comparative effectiveness research: A report from the Institute of Medicine. Ann Intern Med 151:203-205, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Warren JL, Klabunde CN, Schrag D, et al. : Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care 40:IV-3-IV-18, 2002. (suppl) [DOI] [PubMed] [Google Scholar]
- 16.Freedman RA, Vaz-Luis I, Barry WT, et al. : Patterns of chemotherapy, toxicity, and short-term outcomes for older women receiving adjuvant trastuzumab-based therapy. Breast Cancer Res Treat 145:491-501, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Sanoff HK, Carpenter WR, Freburger J, et al. : Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: A population-based analysis. Cancer 118:4309-4320, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chubak J, Yu O, Pocobelli G, et al. : Administrative data algorithms to identify second breast cancer events following early-stage invasive breast cancer. J Natl Cancer Inst 104:931-940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dignam JJ, Huang L, Ries L, et al. : Estimating breast cancer-specific and other-cause mortality in clinical trial and population-based cancer registry cohorts. Cancer 115:5272-5283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Medicare and Medicaid Services: CMS Chronic Conditions Data Warehouse (CCW): CCW condition algorithms. https://www.ccwdata.org/cs/groups/public/documents/document/ccw_chronic_cond_algos.pdf.
- 21.Klabunde CN, Legler JM, Warren JL, et al. : A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol 17:584-590, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Brookhart MA, Wang PS, Solomon DH, et al. : Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology 17:268-275, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sox HC, Goodman SN: The methods of comparative effectiveness research. Annu Rev Public Health 33:425-445, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Zou G: A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159:702-706, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E: Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 162:199-200, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Vaz-Luis I, Keating NL, Lin NU, et al. : Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: A population-based study. J Clin Oncol 32:927-934, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. : Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659-1672, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Blum JL, Flynn PJ, Yothers G, et al: Interim joint analysis of the ABC (anthracyclines in early breast cancer) phase III trials (USOR 06-090, NSABP B-46I/USOR 07132, NSABP B-49 [NRG Oncology]) comparing docetaxel + cyclophosphamide (TC) v anthracycline/taxane-based chemotherapy regimens (TaxAC) in women with high-risk, HER2-negative breast cancer. J Clin Oncol 34, 2016 (suppl; abstr 1000)