Abstract
Purpose
The low-income subsidy (LIS) substantially lowers out-of-pocket costs for qualifying Medicare Part D beneficiaries who receive orally administered chemotherapy. We examined the association of LIS with the use of novel oral immunomodulatory drugs (IMiDs; lenalidomide and thalidomide) among beneficiaries with myeloma, who can receive either orally administered or parenteral (bortezomib-based) therapy.
Methods
Using SEER-Medicare data, we identified Part D beneficiaries diagnosed with myeloma in 2007 to 2011. In multivariable models adjusted for sociodemographic and clinical characteristics, we analyzed associations between the LIS and use of IMiD-based therapy, delays between IMiD refills, and select health outcomes during the first year of therapy.
Results
Among 3,038 beneficiaries, 41% received first-line IMiDs. Median out-of-pocket cost for the first IMiD prescription was $3,178 for LIS nonrecipients and $3 for LIS recipients, whereas the respective median costs for the first year of therapy were $5,623 and $6, respectively. Receipt of the LIS was associated with a 32% higher (95% CI, 16% to 47%) probability of receiving IMiDs among beneficiaries age 75 to 84 years and a significantly lower risk of delays between refills in all age groups (adjusted relative risk, 0.54; 95% CI, 0.32 to 0.92). Duration of therapy did not significantly differ between LIS recipients and nonrecipients (median, 7.6 months). Patients treated with IMiDs had significantly fewer emergency department visits and hospitalizations compared with patients receiving bortezomib (without IMiDs), but 1-year overall survival and cumulative Medicare costs were similar.
Conclusion
Medicare beneficiaries with myeloma who do not receive LISs face a substantial financial barrier to accessing orally administered anticancer therapy, warranting urgent attention from policymakers. Limiting out-of-pocket costs for expensive anticancer drugs like the IMiDs may improve access to oral therapy for patients with myeloma.
INTRODUCTION
Plasma cell myeloma is a bone marrow cancer with median age at diagnosis of 69 years in the United States. Historically, most older patients with myeloma were treated using alkylating agents and corticosteroids, achieving response rates of 40% to 50% and 3-year overall survival rates of 50%.1-3 Prognosis markedly improved after introduction of two novel classes of agents, the proteasome inhibitors (eg, bortezomib, first approved by the US Food and Drug Administration in 2003) and oral immunomodulatory drugs (IMiDs; thalidomide and lenalidomide, first approved by the US Food and Drug Administration for myeloma in 2006).4 These drugs are characterized by high efficacy and relatively lower toxicity, increasing response rates to > 70% and 3-year survival to > 65% among older patients.2-7 By 2007, 75% of newly diagnosed patients in the United States received one of the new agents as part of their initial therapy, whereas the use of traditional chemotherapy decreased.8 Bortezomib and IMiDs differ in their mode of administration; bortezomib requires twice-weekly parenteral injections in the clinic, whereas IMiDs are administered orally. When parenteral and oral options have comparable efficacy, patients with cancer generally prefer orally administered regimens, which avoid painful injections and frequent medical visits.9 Because bortezomib and IMiDs have not been directly compared in randomized trials, either option (or their combination) is acceptable according to current guidelines for older patients.10
The availability of IMiDs coincided with a major change in Medicare coverage for oral anticancer drugs. Medicare has historically paid for parenteral chemotherapy drugs through the Part B benefit (with out-of-pocket costs covered by supplemental insurance for beneficiaries with such coverage). Before 2006, Medicare did not cover outpatient prescriptions, with few exceptions. As a result of the Medicare Modernization Act, in 2006, beneficiaries gained an option to purchase coverage for outpatient prescriptions (including oral anticancer drugs) by enrolling onto privately administered prescription drug plans or comprehensive managed care plans.11,12 Although this Part D coverage has had a positive impact on access to medications, it requires significant cost sharing by patients who use expensive specialty medications like the IMiDs. In 2017, the out-of-pocket costs include monthly premiums ($15 to $179); a $400 yearly deductible; a 25% coinsurance on the initial $3,700 of gross drug costs; a subsequent coverage gap, which requires $4,950 in out-of-pocket spending; and further 5% coinsurance in the catastrophic phase of coverage.13
Previous work has shown that Medicare beneficiaries face high out-of-pocket costs for orally administered anticancer medications and typically meet the threshold for catastrophic coverage with the first prescription.14 This means that patients who initiate IMiD therapy must pay thousands of dollars in immediate out-of-pocket expenses. Prior studies indicated a relatively lower use of IMiDs and bortezomib among Medicare beneficiaries compared with individuals with private or Medicaid insurance.8 Part D enrollees also underuse highly effective oral targeted agents in chronic myeloid leukemia.15,16 Antikickback statutes prohibit beneficiaries from receiving direct financial assistance from drug manufacturers in the form of copayment cards or coupons, although charity assistance is allowed. Enrollees with incomes < 150% of the federal poverty level and modest assets are eligible for the low-income subsidies (LISs), which largely eliminate all Part D–related out-of-pocket expenses. LIS recipients include most individuals who are also eligible for state-provided Medicaid assistance (based on poverty), who become automatically eligible for the subsidy. Because of potentially drastic differences in first-line out-of-pocket costs for IMiDs with and without the LIS, we hypothesized that IMiD use, and subsequent outcomes, would differ among Part D enrollees depending on whether they received the LIS.
METHODS
Data Source and Study Population
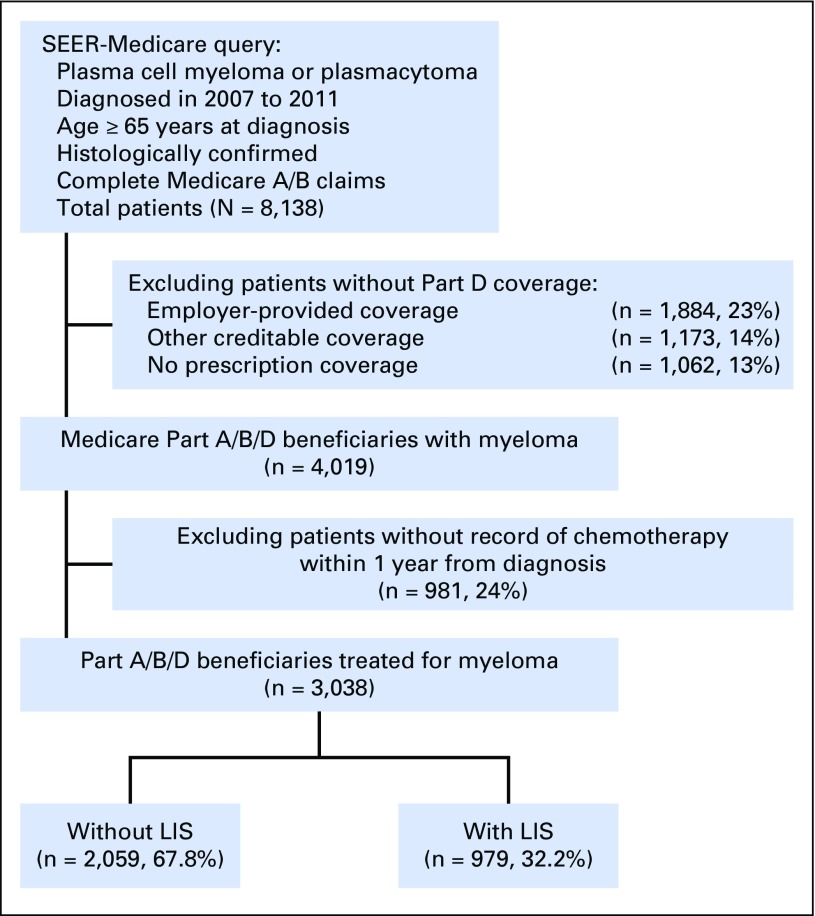
This research was approved by the Institutional Review Board at Rhode Island Hospital (Providence, RI). From the SEER-Medicare database, we selected patients with myeloma (or plasmacytoma, using the histology codes 9731/3, 9732/3, and 9734/3) diagnosed in 2007 to 2011 (Fig 1). Medicare claims until December 2013 were available. The SEER-Medicare data set provides cancer registry data from 18 geographic areas covering approximately 28% of the US population, linked to complete billing claims for Medicare beneficiaries (93% of records for persons ≥ 65 years old).17 Patients had to be continuously enrolled onto Medicare Parts A and B from 12 months before diagnosis onward, not be participating in a managed care plan (as their billing records would be unavailable), and have Part D coverage at diagnosis. We excluded patients who had prescription coverage provided by other sources (eg, employers, as recorded in Medicare files) or who had no creditable coverage. We additionally excluded patients without histologic confirmation of myeloma, those diagnosed by autopsy, and those who received no chemotherapy within 1 year from diagnosis.
Fig 1.
CONSORT diagram. Cohort selection from the SEER-Medicare database. LIS, low-income subsidy.
Measures
Receipt of the LIS was directly recorded by Medicare for every calendar month, and LIS receipt at myeloma diagnosis was the exposure of interest. Use of IMiDs as part of the initial antimyeloma chemotherapy was the primary outcome. We ascertained front-line regimens by identifying drugs administered during the first 60 days of treatment (with a sensitivity check using 30-day and 90-day time frames). Parenteral regimens were identified by outpatient administration of specific drugs.18,19 Orally administered agents were identified from Part D files. In addition, melphalan and cyclophosphamide, oral alkylating agents covered under Medicare Part B, were ascertained from other Medicare files. For each IMiD prescription, dispensation date, gross drug cost charged by the pharmacy, and dollar amount assigned as out-of-pocket responsibility were recorded. As a measure of adherence to therapy, we analyzed occurrence of a prolonged delay between any two prescriptions for IMiDs, defined as > 45 days from the day the refill was due, during the first 6 months of treatment. Duration of first-line IMiD therapy was defined as time from the first to the last filled prescription.
In addition, we evaluated 1-year health outcomes among beneficiaries treated with novel antimyeloma agents, grouped into the following three categories: those receiving first-line bortezomib (without IMiDs), those receiving an IMiD (without bortezomib), or those receiving the combination of bortezomib and IMiD. Outcomes included emergency department (ED) visits, hospitalizations, total Medicare spending, and death. Costs included actual Medicare payments for inpatient, outpatient, and Part D (prescription) services, inflation-adjusted to 2012 dollars using the Consumer Price Index. Costs did not include payments made by patients, supplementary insurance, or other sources.
For adjustments, apart from basic sociodemographic characteristics, we extracted claims-based measures of comorbidity,20 poor performance status,21 medical diagnoses corresponding to the toxicity profile of IMiDs, and health services used within the year before myeloma diagnosis (Data Supplement).19 Prevalence of poverty in the patient’s census tract of residence served as an additional indicator of socioeconomic status.
Statistical Analysis
Continuous variables were reported as medians and interquartile range (IQR) and compared using Wilcoxon rank sum test. Proportions were compared using the χ2 test. Multivariable models were adjusted for all available variables judged to be relevant based on clinical or economic rationale, regardless of statistical significance. For binary outcomes, we used log-linear models with robust SE, which provide direct estimation of relative risk, reported with 95% CIs.22 Because of a significant interaction between LIS receipt and age, we grouped age into three clinically relevant categories and expressed the main result as marginal semielasticity, accounting for the interaction. Marginal semielasticity indicates, for every age subgroup, the proportional change in the outcome (probability of using an IMiD) for a change in the independent variable (receipt of LIS). Duration of therapy and survival were compared in proportional hazards models, with proportional hazards assumption evaluated using the test by Grambsch and Therneau.23 Overdispersed count data (ED and hospital visits) were compared using negative binomial models (adjusting for exposure time), and costs were compared using a log-gamma model, without censoring, as all patients had either > 1 year of data or a terminal event.24 These models included interaction of chemotherapy regimen with LIS receipt, and main results were presented as marginal means with 95% CIs. Regression coefficients for groups were contrasted using Wald tests, without adjustments for multiple comparisons. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and Stata 14/MP (StataCorp LP, College Station, TX). We used α < .05 and two-sided tests of statistical significance.
RESULTS
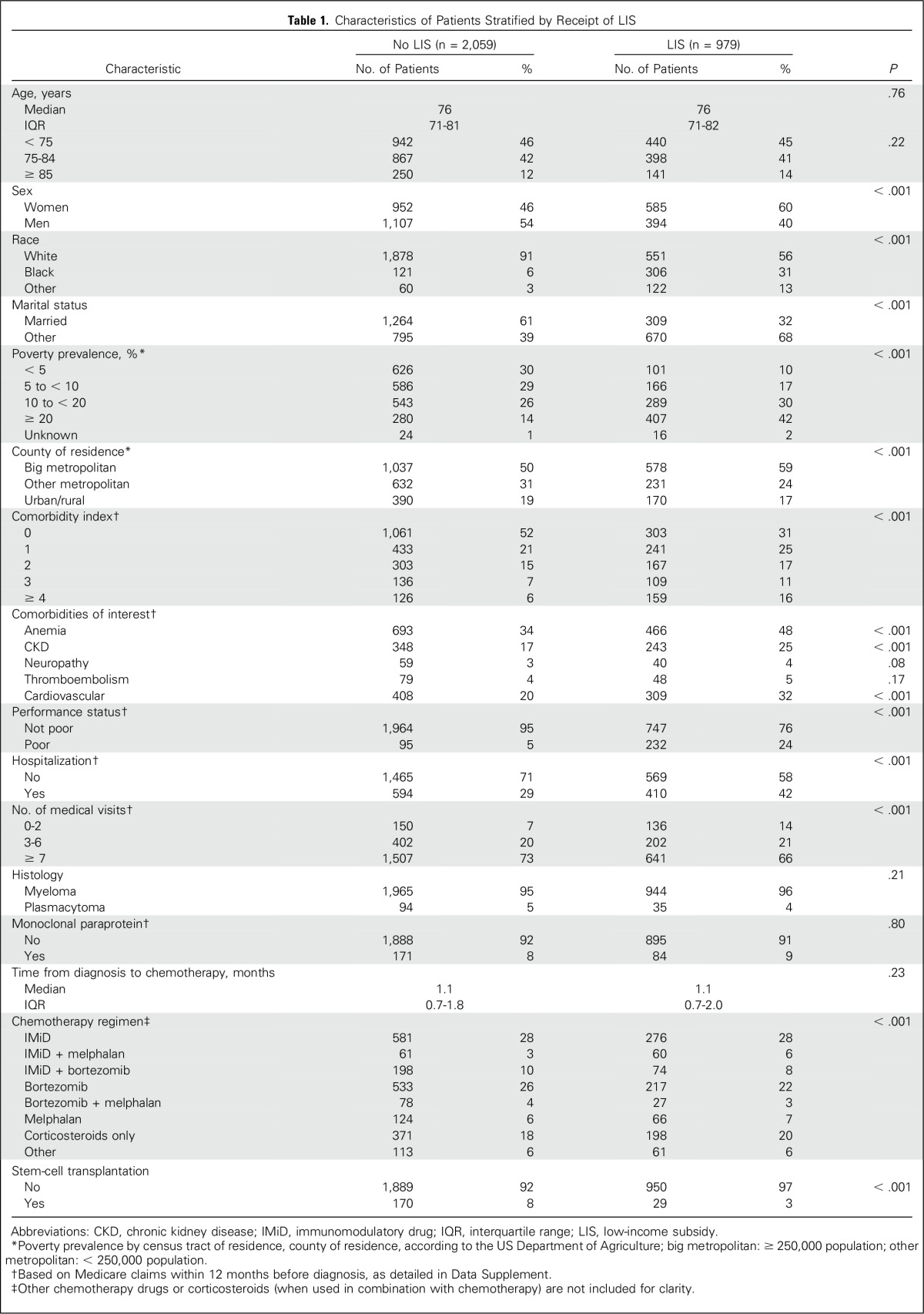
Patients initiated chemotherapy at a median of 1.1 months (IQR, 0.7 to 1.9 months) from their myeloma diagnosis. Median age was 76 years, 50.6% of patients were women, and 32.2% of patients were receiving the LIS at diagnosis (Table 1). Less than 0.5% of beneficiaries changed their LIS recipient status between the diagnosis and start of treatment. Overall, 1,250 patients (41.1%) received an IMiD as part of their first-line regimen, with lenalidomide gradually replacing thalidomide over time (Data Supplement). In univariable analysis, patients treated with IMiDs were younger, had a better performance status, and had fewer comorbidities, although they had a similar prevalence of plasmacytoma or prior monoclonal gammopathy (Data Supplement).
Table 1.
Characteristics of Patients Stratified by Receipt of LIS
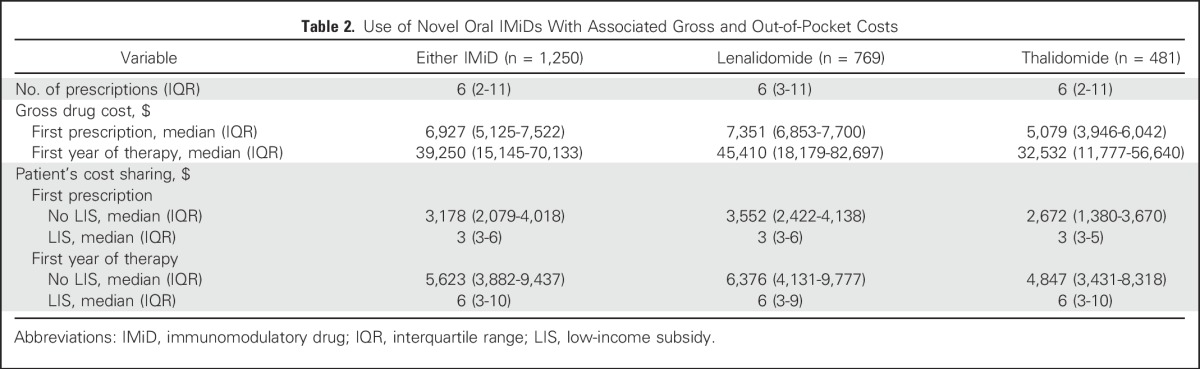
On average, patients received six IMiD prescriptions during the first year of therapy, at a median gross drug cost of $39,250 (Table 2). Median duration of first-line IMiD therapy was 7.6 months, with 38% of patients continuing therapy for > 12 months. Median out-of-pocket expense for the first prescription was $3,178 (IQR, $2,079 to $4,018) for beneficiaries without LIS and $3 (IQR, $3 to $6) for those with LIS. Median out-of-pocket expenses during the first year of therapy were $5,623 (IQR, $3,882 to $9,437) and $6 (IQR, $3 to $10) for those without and with LIS, respectively. Immediately before chemotherapy, 3.9% of all patients were in the catastrophic phase of their coverage, whereas 65.3% reached it with the first IMiD prescription and 79.4% with the second IMiD prescription.
Table 2.
Use of Novel Oral IMiDs With Associated Gross and Out-of-Pocket Costs
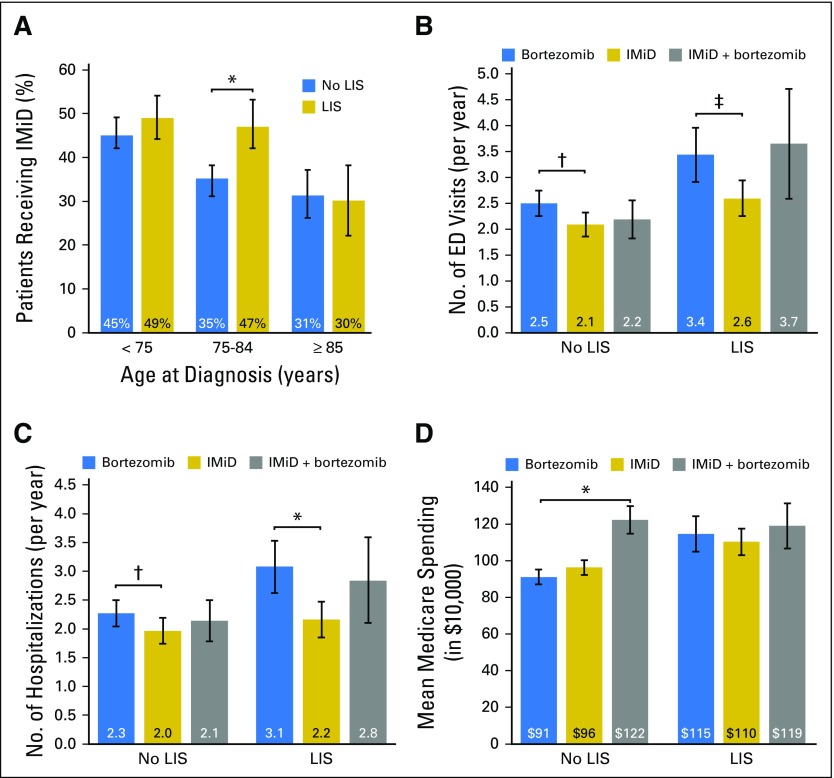
Although the crude proportions of patients receiving IMiDs were similar among beneficiaries with or without LIS (42% and 41%, respectively), LIS recipients had significantly more comorbidities, worse performance status, and less favorable socioeconomic characteristics. Adjusting for those multiple factors, IMiD use was significantly associated with the receipt of LIS (Table 3), but the association significantly differed by age group (P < .001 for interaction; Fig 2A). LIS recipients age 75 to 84 years had a 32% higher (95% CI, 16% to 47%) relative probability of being treated with an IMiD compared with nonrecipients, whereas the difference was not significant in the younger (semielasticity, 8%; 95% CI, −6% to 21%) and older subgroups (semielasticity, −4%; 95% CI, −37% to 28%). Poor performance status, chronic kidney disease, and plasmacytoma histology were negatively associated with the use of IMiDs.
Table 3.
Multivariable Model for Factors Associated With Use of IMiDs for First-Line Treatment of Myeloma Among Medicare Part D Enrollees Receiving Chemotherapy (N = 3,038)
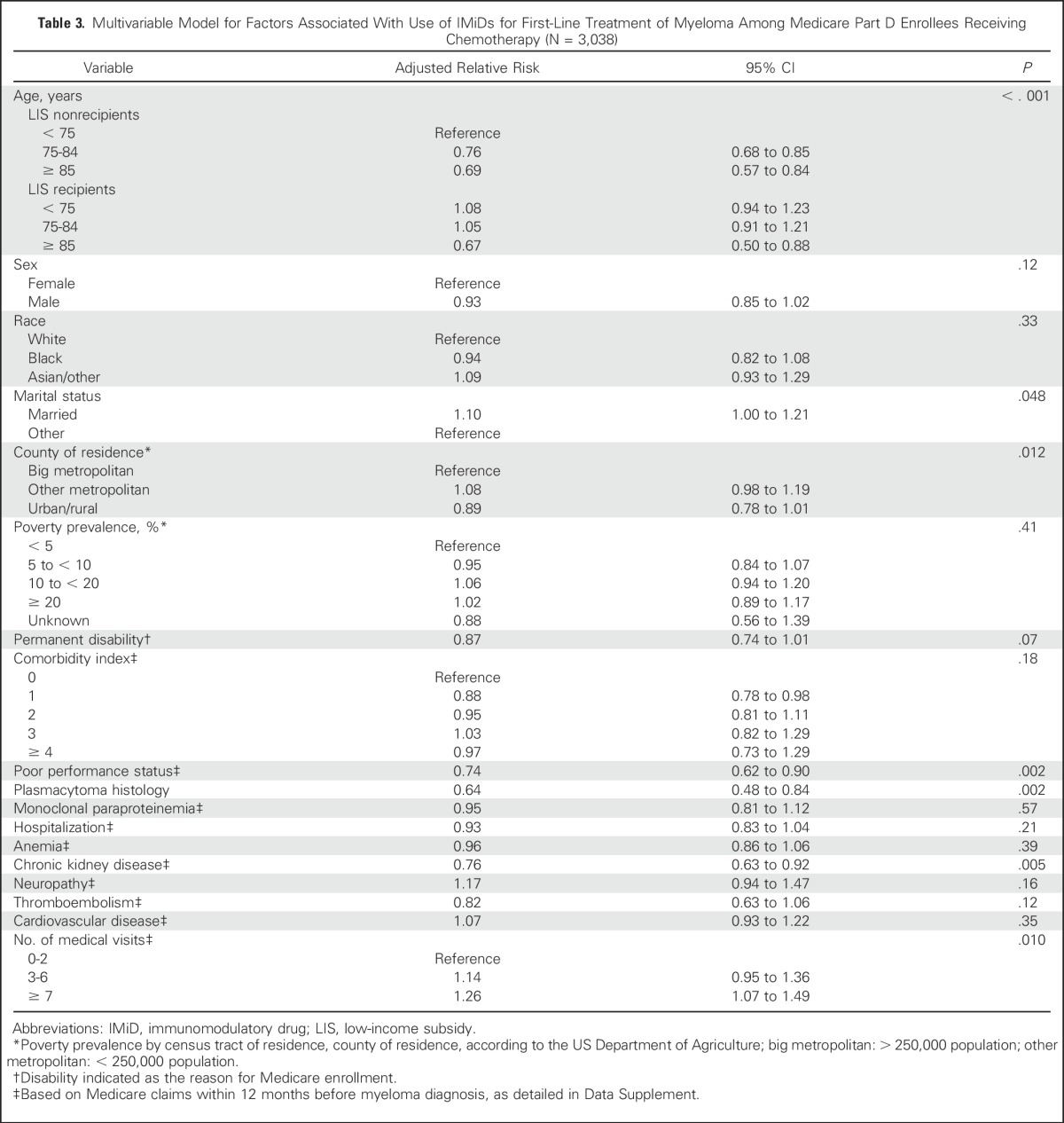
Fig 2.
(A) Probability of receiving an immunomodulatory drug (IMiD; lenalidomide or thalidomide), stratified by age group and receipt of the low-income subsidy (LIS). Average incidence of (B) emergency department (ED) visits and (C) hospitalizations during the first year of therapy for myeloma, and (D) cumulative Medicare costs during that year, stratified by type of regimen (bortezomib without IMiD, IMiD without bortezomib, or IMiD and bortezomib) and receipt of the LIS. All estimates are adjusted means derived from multivariable models, with error bars indicating 95% CIs. Horizontal bars with symbols indicate statistically significant contrasts between the groups of interest: (*) P < .001, (†) P < .05, and (‡) P < .01.
Among patients treated with IMiDs, the receipt of LIS was associated with a 46% lower probability of a prolonged (> 45 days) delay between any two consecutive IMiD prescriptions (adjusted relative risk, 0.54; 95% CI, 0.32 to 0.92; Table 4), without a significant interaction with age. Duration of IMiD therapy did not significantly differ between LIS recipients and nonrecipients in a multivariable model (adjusted hazard ratio, 1.02; 95% CI, 0.87 to 1.20; Data Supplement).
Table 4.
Multivariable Model for Prolonged (> 45 days) Delay in Refilling IMiD Prescriptions Among Medicare Part D Enrollees (n = 1,250)
LIS recipients had a higher incidence of ED visits or hospitalizations and higher costs of care during the first year of treatment (Data Supplement). These outcomes also differed according to type of first-line chemotherapy. Using the group treated with bortezomib as reference, in a multivariable model, patients treated with IMiDs had a significantly lower incidence of ED visits, regardless of the LIS recipient status (P = .012 for LIS nonrecipients and P = .004 for recipients), whereas the outcome did not significantly differ for the bortezomib plus IMiD combination (P = .17 for nonrecipients and P = .72 for recipients; Fig 2B). Similarly, in LIS nonrecipients and recipients, hospitalizations were significantly less frequent with an IMiD (P = .046 and P < .001, respectively) but not with the combination (P = .54 and P = .60, respectively; Fig 2C). Compared with bortezomib, Medicare spending was similar after first-line IMiD (P = .06 and P = .45 for nonrecipients and recipients, respectively). It was significantly higher with the IMiD plus bortezomib combination among LIS nonrecipients (P < .001), but not among the LIS recipients (P = .58; Fig 2D). Compared with patients using bortezomib, those receiving IMiDs had lower costs for inpatient and outpatient medical services, but higher prescription-related Part D spending (Data Supplement). Payments for novel drugs constituted 37% of all spending among beneficiaries receiving bortezomib, 50% among those receiving IMiDs, and 52% among those receiving both. Overall survival at 1 year was 71.4% (95% CI, 68.2% to 74.3%) with bortezomib, 75.1% (95% CI, 72.2% to 77.6%) with IMiDs, and 81.3% (95% CI, 76.1% to 85.4%) with the combination. There was no significant difference between these groups in a multivariable model (Data Supplement).
DISCUSSION
The escalating cost of novel anticancer medications has raised concerns about financial toxicity for patients and health care systems alike.25-28 Our study is the first, to our knowledge, to examine the association between the LIS, a Medicare policy alleviating patient cost sharing for orally administered chemotherapy, and the use of IMiDs in myeloma—a unique setting where highly efficacious parenteral and oral options became available in the mid-2000s. We found that for Part D beneficiaries without the LIS, the use of IMiDs entailed median out-of-pocket expenses of > $5,600 in the first year, corresponding to 23% of their median yearly income ($24,150 in 2014).29 These out-of-pocket costs were largely eliminated for LIS recipients, and we found a strong association between the LIS and use of IMiDs among patients age 75 to 84 years. There was also a significant association between the LIS and lower risk of delays in refilling IMiD prescriptions, but not with the overall duration of therapy. Finally, compared with patients treated with bortezomib, those who were treated with an IMiD instead had significantly lower rates of ED visits and hospitalizations but similar costs and survival within a 1-year time frame.
Patients with myeloma report high levels of financial distress.30,31 The LIS was intended to target financial assistance for the poorest beneficiaries, who are unable to afford Part D–related expenses. However, the resulting 1,000-fold disparity in the out-of-pocket burden for IMiDs between LIS recipients and nonrecipients may result in a differential use of those drugs. Median yearly income of Medicare enrollees decreases with age, from $29,700 for those age < 75 years to $18,850 for those age ≥ 85 years.29 Our results indicate that patients age 75 to 84 years may be particularly sensitive to financial barriers when choosing their antimyeloma therapy. These findings align with prior studies of associations between cost sharing and use of anticancer treatments. For example, Medicare beneficiaries without supplemental insurance had lower use of cancer chemotherapy overall32 and of home-based use of erythropoiesis-stimulating agents in myelodysplastic syndrome.33
Socioeconomically disadvantaged patients often have lower use of treatments or worse adherence. Conversely, we observed an increased use of IMiDs among LIS recipients with myeloma. Although it is possible that they preferred oral therapy for other reasons, such as inability to travel for injections, association after adjustment for other sociodemographic indicators suggests that the LIS may have facilitated access to IMiDs. This interpretation is corroborated by fewer delays between IMiD prescriptions among LIS recipients, consistent with better adherence to aromatase inhibitors or tyrosine kinase inhibitors in the absence of high out-of-pocket expenses.15,34 The LIS was not associated with duration of IMiD therapy, suggesting that treatment may have been discontinued because of planned fixed-duration therapy, adverse effects, or poor response, rather than as a result of financial toxicity. Alternatively, extreme high up-front costs may have selected patients with more motivation to continue their therapy, once initiated. The median 7-month duration of therapy seems consistent with contemporaneous clinical trial experience.2,6,35-37
The question of potential impact of the policies for oral chemotherapy coverage on health outcomes is complex. Our results suggest that access to IMiDs may be associated with a lower use of ED services and hospitalizations, although short-term survival and Medicare spending did not differ. When IMiDs were used instead of bortezomib, costs were largely shifted from the medical part of the Medicare program onto Part D plans. Even without an overall cost or survival advantage, fewer hospitalizations and ED visits may positively impact patients’ quality of life and decrease iatrogenic complications arising from acute care. However, confident assessment of such impact will require further research comparing groups that are more homogeneous with regard to chemotherapy regimens and clinical confounders.
Approaches to mitigate the skyrocketing costs of cancer therapy include value-oriented reimbursement, routine financial counseling, and negotiating prices of drugs by Medicare,25,38-40 but so far, simply deterring patients from treatment by increasing out-of-pocket costs has been commonly applied, despite a conflict with the ethical principle of equality in health care. Some policy observers have expressed concerns that high cost-sharing requirements ration care according to the ability to pay.41 Many US states have passed parity laws mandating equitable coverage for oral and parenteral anticancer drugs, but the federal Medicare program is exempt from these laws.42 A more functional solution would require addressing the differential out-of-pocket costs for oral and parenteral chemotherapy in the Medicare program, using input from patients, payers, clinicians, policymakers, and industry.27,28,31 Meanwhile, shifting from the percentage-based coinsurance to fixed copayments may assure a more predictable out-of-pocket burden, while maintaining patient financial responsibility.
Our analysis had several important limitations. We excluded beneficiaries enrolled onto managed care plans and those with alternative prescription coverage, thus narrowing the scope of the study, but also assuring a more homogeneous population. Individual plans could not be identified, precluding evaluation of plan-specific policies. We could not reliably discern reasons for prescription delays or whether out-of-pocket costs were actually paid by beneficiaries or tertiary sources. Apart from the LIS, some beneficiaries may qualify for financial help from state pharmaceutical assistance programs, although these were not operational in 12 of the 13 states covered by the SEER registries. Comparing out-of-pocket expenses for oral and parenteral chemotherapy would be important to assess true financial toxicity. This was not possible using Medicare data, as patients’ responsibility for Part B services was covered to an unknown degree by supplemental insurance held by an estimated 85% of beneficiaries. Our identification of comorbidities through claims may be insufficiently sensitive or specific in relation to actual clinical diagnoses, and we cannot rule out residual influence of additional confounding factors, like the extent or complications of myeloma itself. Finally, further research will need to analyze association of coverage policies with patient-reported or disease-related outcomes, including quality of life and disease-specific survival.
In summary, our analysis suggests that subsidies alleviating patients’ financial burden for orally administered chemotherapy may significantly influence treatment selection among certain beneficiaries with myeloma, and their subsequent health outcomes. Policymakers should recognize that the substantial out-of-pocket expenses may compromise access to cancer therapy and lead to catastrophic levels of spending, thereby undermining one of the purposes of health insurance. Although the future direction of the US health care system remains uncertain, coverage for novel oral anticancer agents like the IMiDs warrants reconsideration by Medicare administration to assure equitable access to those treatments for all Americans.
ACKNOWLEDGMENT
We acknowledge the efforts of the National Cancer Institute; the Office of Research, Development, and Information; the Centers for Medicare and Medicaid Services; Information Management Services; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Supported by Grant No. 128608-RSGI-15-211-01-CPHPS from the American Cancer Society and by an American Society of Hematology Research Scholar Grant (to A.J.O.). The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's SEER Program under Contract No. HHSN261201000140C awarded to the Cancer Prevention Institute of California, Contract No. HHSN261201000035C awarded to the University of Southern California, and Contract No. HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries under Agreement No. U58DP003862-01 awarded to the California Department of Public Health.
Presented in part at the 58th Annual Meeting and Exposition of the American Society of Hematology, San Diego, CA; December 3-6, 2016.
The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Listen to the podcast by Dr Shih at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Adam J. Olszewski, Stacie B. Dusetzina, Amy J. Davidoff
Collection and assembly of data: Adam J. Olszewski
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Subsidies for Oral Chemotherapy and Use of Immunomodulatory Drugs Among Medicare Beneficiaries With Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Adam J. Olszewski
Research Funding: Incyte (Inst), Genentech (Inst), TG Therapeutics (Inst)
Stacie B. Dusetzina
No relationship to disclose
Charles B. Eaton
No relationship to disclose
Amy J. Davidoff
Honoraria: Celgene (I)
Consulting or Advisory Role: Celgene (I)
Research Funding: Celgene (I), Boehringer Ingelheim (I)
Travel, Accommodations, Expenses: Celgene (I)
Other Relationship: Pharmaceutical Research and Manufacturers Association Foundation
Amal N. Trivedi
Consulting or Advisory Role: Merck Sharp & Dohme
REFERENCES
- 1.Facon T, Mary JY, Hulin C, et al. : Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): A randomised trial. Lancet 370:1209-1218, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Palumbo A, Bringhen S, Liberati AM, et al. : Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: Updated results of a randomized controlled trial. Blood 112:3107-3114, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Mateos MV, Richardson PG, Schlag R, et al. : Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: Updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol 28:2259-2266, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Palumbo A, Rajkumar SV: Treatment of newly diagnosed myeloma. Leukemia 23:449-456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zonder JA, Crowley J, Hussein MA, et al. : Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: A randomized Southwest Oncology Group trial (S0232). Blood 116:5838-5841, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Rosiñol L, Hussein M, et al. : Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol 26:2171-2177, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Jacobus S, Callander NS, et al. : Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol 11:29-37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren JL, Harlan LC, Stevens J, et al. : Multiple myeloma treatment transformed: A population-based study of changes in initial management approaches in the United States. J Clin Oncol 31:1984-1989, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu G, Franssen E, Fitch MI, et al. : Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol 15:110-115, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Palumbo A, Rajkumar SV, San Miguel JF, et al. : International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol 32:587-600, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Medicare & Medicaid Services, Department of Health and Human Services : Medicare program; Medicare prescription drug benefit. Final rule. Fed Regist 70:4193-4585, 2005 [PubMed] [Google Scholar]
- 12.Bowman J, Rousseau A, Silk D, et al. : Access to cancer drugs in Medicare Part D: Formulary placement and beneficiary cost sharing in 2006. Health Aff (Millwood) 25:1240-1248, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kaiser Family Foundation : The Medicare Part D Prescription Drug Benefit. http://kff.org/medicare/fact-sheet/the-medicare-prescription-drug-benefit-fact-sheet/
- 14.Dusetzina SB, Keating NL: Mind the gap: Why closing the doughnut hole is insufficient for increasing Medicare beneficiary access to oral chemotherapy. J Clin Oncol 34:375-380, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doshi JA, Li P, Huo H, et al. : High cost sharing and specialty drug initiation under Medicare Part D: A case study in patients with newly diagnosed chronic myeloid leukemia. Am J Manag Care 22:s78-s86, 2016. (suppl 4) [PubMed] [Google Scholar]
- 16.Winn AN, Keating NL, Dusetzina SB: Factors associated with tyrosine kinase inhibitor initiation and adherence among Medicare beneficiaries with chronic myeloid leukemia. J Clin Oncol 34:4323-4328, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Schrag D, et al. : Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care 40:IV-3-IV-18, 2002. (suppl 8) [DOI] [PubMed] [Google Scholar]
- 18.Warren JL, Harlan LC, Fahey A, et al. : Utility of the SEER-Medicare data to identify chemotherapy use. Med Care 40:IV-55-IV-61, 2002. (suppl 8) [DOI] [PubMed] [Google Scholar]
- 19.Olszewski AJ, Treon SP, Castillo JJ: Evolution of management and outcomes in Waldenström macroglobulinemia: A population-based analysis. Oncologist 21:1377-1386, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klabunde CN, Potosky AL, Legler JM, et al. : Development of a comorbidity index using physician claims data. J Clin Epidemiol 53:1258-1267, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Davidoff AJ, Zuckerman IH, Pandya N, et al. : A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol 4:157-165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenland S: Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol 160:301-305, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Grambsch PM, Therneau TM: Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81:515-526, 1994 [Google Scholar]
- 24. Hardin JW, Hilbe JM: Generalized Linear Models and Extensions (ed 3). College Station, TX, Stata Press, 2012. [Google Scholar]
- 25.Bach PB: Costs of cancer care: A view from the centers for Medicare and Medicaid services. J Clin Oncol 25:187-190, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Sullivan R, Peppercorn J, Sikora K, et al. : Delivering affordable cancer care in high-income countries. Lancet Oncol 12:933-980, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Experts in Chronic Myeloid Leukemia : The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: From the perspective of a large group of CML experts. Blood 121:4439-4442, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantarjian H, Steensma D, Rius Sanjuan J, et al. : High cancer drug prices in the United States: Reasons and proposed solutions. J Oncol Pract 10:e208-e211, 2014 [DOI] [PubMed] [Google Scholar]
- 29. Jacobson G, Swoope C, Neuman T: Kaiser Family Foundation: Income and assets of Medicare Beneficiaries, 2014–2030. http://kff.org/medicare/issue-brief/income-and-assets-of-medicare-beneficiaries-2014-2030/
- 30.Huntington SF, Weiss BM, Vogl DT, et al. : Financial toxicity in insured patients with multiple myeloma: A cross-sectional pilot study. Lancet Haematol 2:e408-e416, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Rajkumar SV, Harousseau JL: Next-generation multiple myeloma treatment: A pharmacoeconomic perspective. Blood 128:2757-2764, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidoff AJ, Shaffer T, Erten MZ, et al. : Use and spending on antineoplastic therapy for Medicare beneficiaries with cancer. Med Care 51:351-360, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Davidoff AJ, Hendrick FB, Zeidan AM, et al. : Patient cost sharing and receipt of erythropoiesis-stimulating agents through Medicare part D. J Oncol Pract 11:e190-e198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuner JM, Kamaraju S, Charlson JA, et al. : The introduction of generic aromatase inhibitors and treatment adherence among Medicare D enrollees. J Natl Cancer Inst 107:djv130, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palumbo A, Hajek R, Delforge M, et al. : Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med 366:1759-1769, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Waage A, Gimsing P, Fayers P, et al. : Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood 116:1405-1412, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Wijermans P, Schaafsma M, Termorshuizen F, et al. : Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: The HOVON 49 Study. J Clin Oncol 28:3160-3166, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Bach PB: Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med 360:626-633, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Shankaran V, Ramsey S: Addressing the financial burden of cancer treatment: From copay to can’t pay. JAMA Oncol 1:273-274, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Lotvin AM, Shrank WH, Singh SC, et al. : Specialty medications: Traditional and novel tools can address rising spending on these costly drugs. Health Aff (Millwood) 33:1736-1744, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Mason A, Drummond M, Ramsey S, et al. : Comparison of anticancer drug coverage decisions in the United States and United Kingdom: Does the evidence support the rhetoric? J Clin Oncol 28:3234-3238, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Kircher SM, Meeker CR, Nimeiri H, et al. : The parity paradigm: Can legislation help reduce the cost burden of oral anticancer medications? Value Health 19:88-98, 2016 [DOI] [PubMed] [Google Scholar]