Abstract
Purpose
Lenalidomide maintenance therapy after autologous stem-cell transplantation (ASCT) demonstrated prolonged progression-free survival (PFS) versus placebo or observation in several randomized controlled trials (RCTs) of patients with newly diagnosed multiple myeloma (NDMM). All studies had PFS as the primary end point, and none were powered for overall survival (OS) as a primary end point. Thus, a meta-analysis was conducted to better understand the impact of lenalidomide maintenance in this setting.
Patients and Methods
The meta-analysis was conducted using primary-source patient-level data and documentation from three RCTs (Cancer and Leukemia Group B 100104, Gruppo Italiano Malattie Ematologiche dell'Adulto RV-MM-PI-209, and Intergroupe Francophone du Myélome 2005-02) that met the following prespecified inclusion criteria: an RCT in patients with NDMM receiving ASCT followed by lenalidomide maintenance versus placebo or observation with patient-level data available and achieved database lock for primary efficacy analysis.
Results
Overall, 1,208 patients were included in the meta-analysis (605 patients in the lenalidomide maintenance group and 603 in the placebo or observation group). The median PFS was 52.8 months for the lenalidomide group and 23.5 months for the placebo or observation group (hazard ratio, 0.48; 95% CI, 0.41 to 0.55). At a median follow-up time of 79.5 months for all surviving patients, the median OS had not been reached for the lenalidomide maintenance group, whereas it was 86.0 months for the placebo or observation group (hazard ratio, 0.75; 95% CI, 0.63 to 0.90; P = .001). The cumulative incidence rate of a second primary malignancy before disease progression was higher with lenalidomide maintenance versus placebo or observation, whereas the cumulative incidence rates of progression, death, or death as a result of myeloma were all higher with placebo or observation versus lenalidomide maintenance.
Conclusion
This meta-analysis demonstrates a significant OS benefit and confirms the PFS benefit with lenalidomide maintenance after ASCT in patients with NDMM when compared with placebo or observation.
INTRODUCTION
After induction therapy, patients with newly diagnosed multiple myeloma (NDMM) may be treated with high-dose melphalan (HDM) and autologous stem-cell transplantation (ASCT).1-4 ASCT is not curative, and most patients will experience progressive disease (PD),5-8 even patients who attain a complete response.9-11 Strategies to delay PD include achieving a deep response after ASCT12-15 and sustaining response with maintenance therapy to provide long-term disease control.9,16,17
Effective maintenance therapy should be convenient for the patient, extend remission, and have a tolerable safety profile.3,18 The immunomodulatory drug lenalidomide has been shown to improve progression-free survival (PFS) and overall survival (OS) in patients with NDMM and relapsed or refractory multiple myeloma (MM).2,19-24 Lenalidomide modulates the immune response, inhibits MM growth, and is suitable for maintenance therapy after ASCT.1,3,18,25 In three studies,2,19,20 lenalidomide maintenance after ASCT reduced the risk of progression or death by 50% to 58% versus placebo or observation, and one of these studies showed a significant OS improvement.19 The primary end point of these studies was PFS, and the studies were not powered for a primary OS end point. In addition, second primary malignancies (SPMs) were reported in patients receiving lenalidomide maintenance after HDM and ASCT.19,20 A meta-analysis was conducted at the request of the US Food and Drug Administration to evaluate the effect of post-ASCT lenalidomide maintenance on outcomes, including OS, in patients with NDMM.
PATIENTS AND METHODS
Study Identification and Selection
We examined all randomized controlled trials (RCTs) evaluating maintenance treatment with lenalidomide after HDM and ASCT. Inclusion criteria included RCT in patients with NDMM receiving post-ASCT lenalidomide before progression; maintenance comparing a lenalidomide arm to a placebo or observation arm; achieved database lock for the primary efficacy analysis; and primary-source patient-level data or available documentation. A search of the PubMed database using the keywords “lenalidomide,” “maintenance,” and “myeloma” found no other studies. We identified 17 studies (Data Supplement) and the following three studies met the prespecified inclusion criteria: Cancer and Leukemia Group B (CALGB) 100104 (A Phase III Randomized, Double-Blind Study of Maintenance Therapy With Lenalidomide or Placebo Following Autologous Stem Cell Transplantation for Multiple Myeloma)19; Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) RV-MM-PI-209 (A Phase III, Multicenter, Randomized, Controlled Study to Determine the Efficacy and Safety of Lenalidomide, Melphalan, and Prednisone Versus Melphalan (200 mg/m2) Followed by Stem Cell Transplant in Patients with Newly Diagnosed Multiple Myeloma)2; and Intergroupe Francophone du Myélome (IFM) 2005-02 (Relevance of Maintenance Therapy Using Lenalidomide After Autologous Stem Cell Transplantation Patients Under the Age of 65)20. The Myeloma XI trial (Use of Thalidomide, Lenalidomide, Carfilzomib, Bortezomib and Vorinostat in the Initial Treatment of Newly Diagnosed Multiple Myeloma Patients)26 had not completed accrual at the data cutoff date, and the other 13 studies did not compare lenalidomide maintenance with placebo or observation. Additional study details are provided in the Data Supplement.
The human investigations for the CALGB study were performed after approval by a local or central human investigations committee and in accordance with an assurance filed with and approved by the Department of Health and Human Services. The IFM and GIMEMA studies were performed after approval by a local or central human investigations committee in accordance with the European Medicines Agency. All data were anonymized to protect the identities of patients involved in the research. All investigators obtained informed consent from each participant or each participant's guardian.
Outcome Measures
The primary end point for the meta-analysis was OS. Other analyses included PFS, PFS after next therapy (PFS2), duration of maintenance treatment, time to second antimyeloma treatment, and safety. Individual analyses of OS, PFS, and PFS2 for each study were performed. All analyses used patient-level data from each study. Additional end point analytic details are provided in the Data Supplement.
Statistical Analysis
A 20-month improvement in median OS from 70 months for the placebo or observation control group to 90 months in the lenalidomide maintenance group, representing a hazard ratio (HR) of 0.78, was considered clinically relevant. March 1, 2015 was the cutoff date for the meta-analysis on the basis of number of events required. All patients were evaluated on an intent-to-treat (ITT) basis after ASCT. Additional methods, including sensitivity, heterogeneity, and exploratory analytic details, are presented in the Data Supplement.
RESULTS
Patient Demographics
A total of 1,208 patients composed the ITT population; 605 patients were in the lenalidomide maintenance group, and 603 patients were in the placebo or observation group (Data Supplement). The ITT population included all patients who were randomly assigned to lenalidomide versus placebo or observation and who received HDM or ASCT, regardless of whether they received maintenance therapy.
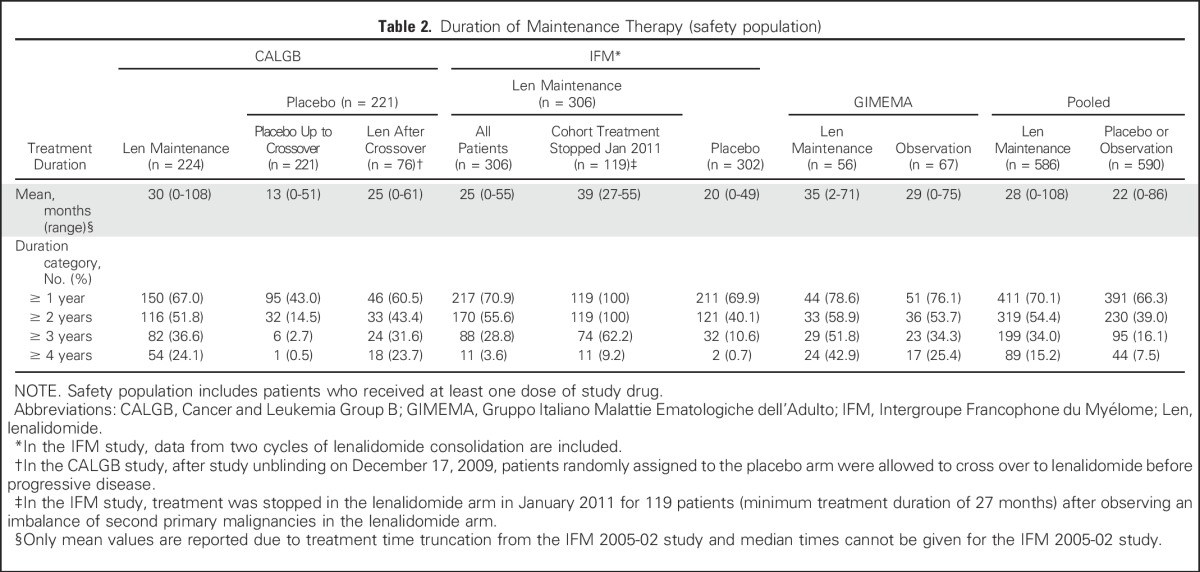
As of the March 1, 2015, data cutoff, the median follow-up time for all surviving patients was 79.5 months (range, 0.0 to 114.3 months). Patient demographics and disease-related characteristics were generally balanced based on available data, except for International Staging System (ISS) disease stage, cytogenetics, and renal function, which favored the placebo or observation group (P < .1; Table 1). The median age was approximately 58 years. Additional details on patient demographics and disease evaluation after ASCT and before maintenance therapy are provided in the Data Supplement. Maintenance treatment duration was affected by study changes within the CALGB study (allowing crossover from placebo to lenalidomide at unblinding before PD) and the IFM study (stopping maintenance in 119 patients without PD). Maintenance therapy durations are listed in Table 2. The percentages of patients receiving lenalidomide for ≥ 4 years were 24.1% in CALGB, 3.6% in IFM (which includes 2 cycles of lenalidomide consolidation), and 42.9% in the GIMEMA study. The mean treatment duration was 28 months with lenalidomide maintenance and 22 months with placebo or observation (Table 2).
Table 1.
Demographics and Disease-Related Characteristics at Diagnosis (intent-to-treat population)

Table 2.
Duration of Maintenance Therapy (safety population)
PFS
In this meta-analysis, the significant PFS improvement observed with lenalidomide maintenance versus placebo or observation was confirmed. The median PFS time was 52.8 months for the lenalidomide group and 23.5 months for the placebo or observation group. The risk of progression or death was reduced by 52% with lenalidomide maintenance versus placebo or observation (HR, 0.48; 95% CI, 0.41 to 0.55; Fig 1A). Results were consistent when alternative censoring rules were applied (Data Supplement). The PFS improvement with lenalidomide maintenance versus placebo or observation was confirmed in each study (Fig 1B) and was seen in all subgroups that had data available from all three studies (Fig 1C). Subgroup analysis of data not available from all three studies is presented in the Data Supplement. Lenalidomide maintenance improved PFS in all subgroups, including patients with high-risk cytogenetics; however, the majority of patients did not have available cytogenetic data.
Fig 1.
Progression-free survival (PFS) analysis (intent-to-treat population). For analyses of PFS, patients who started another antimyeloma therapy before documented disease progression or patients with missing assessments are censored. (A) Kaplan-Meier estimates of PFS. (B) Hazard ratios (HRs) for PFS by individual study. (C) HRs for PFS by subgroup. (*) The size of each blue box corresponds to the size of the individual study. The CI is a function of the overall sample size. (†) Age at random assignment was available and used for the Cancer and Leukemia Group B (CALGB) 100104 study19 and the Intergroupe Francophone du Myélome (IFM) 2005-02 study20. Only age at diagnosis was available for the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) RV-MM-PI-209 study2. (‡) The International Staging System (ISS) stage was based on β2-microglobulin and albumin at diagnosis for the GIMEMA and IFM studies and at registration for the CALGB study. (§) Upon central review, four patients did not meet the criteria for stable disease (SD). ASCT, autologous stem-cell transplantation; CR, complete response; Len, lenalidomide; PR, partial response; VGPR, very good partial response.
OS
As of the March 1, 2015, data cutoff, 490 deaths occurred in both cohorts. Median OS was not reached in the lenalidomide maintenance group and was 86.0 months in the placebo or observation group (HR, 0.75; 95% CI, 0.63 to 0.90; P = .001), representing a 25% reduction in the risk of death with lenalidomide maintenance versus placebo or observation (Fig 2A). Fisher’s combination test confirmed the significant OS benefit with lenalidomide maintenance versus placebo or observation (P = .001). The 7-year survival rate was 62% with lenalidomide maintenance and 50% with placebo or observation. At the median follow-up time of 79.5 months, 64% and 54% of patients were alive in the lenalidomide maintenance and placebo or observation groups, respectively. The proportional hazards (PH) assumption was tested using the time-dependent covariate in the PH Cox model; the proportionality was not significant (P = .21). The graphical check of the PH assumption using the −log (−log) plot of the estimated survival function for each treatment group revealed the same results. The OS subgroup analyses are shown in Figure 2B. Except for patients with ISS disease stage III, the HRs for the subgroup analyses favor lenalidomide maintenance. Patients with better responses (≥ very good partial response) after HDM or ASCT seemed to have more favorable outcomes with lenalidomide maintenance. The most favorable OS benefit with lenalidomide maintenance was observed in patients who received a lenalidomide-based induction treatment (Fig 2C). Subgroup analysis of data not available for all three studies is presented in the Data Supplement. An OS improvement with lenalidomide maintenance was not seen in the following diagnostic subgroups: elevated lactate dehydrogenase, low creatinine clearance, and adverse-risk cytogenetics; however, not all patients had available information.
Fig 2.
Overall survival (OS) analysis (intent-to-treat population). (A) Kaplan-Meier estimates of OS. (B) Hazard ratios (HRs) for OS by subgroup. (C) HRs for OS by prior induction subgroup. (D) HRs for OS by individual study. (*) Age at random assignment was available or used for the Cancer and Leukemia Group B (CALGB) 100104 study19 and the Intergroupe Francophone du Myélome (IFM) 2005-02 study20. Only age at diagnosis was available for the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) RV-MM-PI-209 study2. (†) The International Staging System (ISS) stage was based on β2-microglobulin and albumin at diagnosis for the GIMEMA and IFM studies and at registration for the CALGB study. (‡) Upon central review, four patients did not meet the criteria for stable disease (SD). (§) All patients in the GIMEMA study received lenalidomide-based induction, which could be used in combination with any other drug. The size of each blue box corresponds to the size of the individual study. The CI is a function of the overall sample size. ASCT, autologous stem-cell transplantation; CR, complete response; Len, lenalidomide; NR, not reached; PR, partial response; TD, thalidomide and dexamethasone; VAD, vincristine, doxorubicin, and dexamethasone; VD, bortezomib and dexamethasone; VGPR, very good partial response.
Heterogeneity and Exploratory Analyses
The Pignon heterogeneity test indicated a significant difference in quantitative treatment effect across studies (P = .047). No qualitative heterogeneity in OS was found using the Gail-Simon test (P = .75), indicating no directional difference in treatment effect. Thus, the HRs of the individual studies all favored lenalidomide maintenance treatment (Fig 2D). The heterogeneity observed is driven by the difference in magnitude of the treatment effect among the studies. A simplified multivariate analysis with treatment, study, and treatment by study interaction in the model revealed similar results; the heterogeneity in OS was mainly a result of differences between the CALGB and IFM studies (P = .015 for treatment by study [CALGB v IFM] interaction). There was no significant heterogeneity in OS between CALGB and GIMEMA (P = .525 for treatment by study [CALGB v GIMEMA] interaction). There were differences between the three studies regarding pre- and post-ASCT patient characteristics and treatment. These differences include prestudy patient characteristics, induction regimens, consolidation therapies (Data Supplement), and second-line therapies (Data Supplement), as well as changes in study conduct such as post-ASCT lenalidomide consolidation in the IFM trial, crossover from placebo to lenalidomide after unblinding in the CALGB trial, and stopping of lenalidomide maintenance therapy in the IFM trial (Data Supplement). With extended follow-up (February 1, 2016), lenalidomide maintenance reduced the risk of death by 23% compared with placebo or observation (HR, 0.77; 95% CI, 0.65 to 0.91; P = .002). The median OS time was 111.0 months with lenalidomide maintenance compared with 86.9 months with placebo or observation. The median duration of follow-up among all surviving patients was 88.8 months (range, 0 to 119.8 months). Heterogeneity in OS across studies was not detected by the Pignon test with the longer follow-up (P = .10).
PFS2
Median PFS2 was 73.3 months with lenalidomide maintenance compared with 56.7 months with placebo or observation (HR, 0.72; 95% CI, 0.62 to 0.84; Data Supplement). Lenalidomide maintenance reduced the risk of a PFS2 event (progression on second-line treatment or death) by 28% compared with placebo or observation. The PFS2 HRs in all studies favored lenalidomide maintenance versus placebo or observation (Data Supplement).
Second-Line Antimyeloma Treatment
Time to second-line antimyeloma treatment was prolonged with lenalidomide maintenance versus placebo or observation (HR, 0.57; 95% CI, 0.49 to 0.66; Data Supplement). Fewer patients in the lenalidomide maintenance group (52.6%) started second-line antimyeloma therapy compared with patients in the placebo or observation group (70.8%). The most common second-line antimyeloma treatment was lenalidomide based (27.9%) in the placebo or observation group and bortezomib based (19.5%) in the lenalidomide maintenance group.
Safety
Treatment-emergent adverse events (TEAEs) were analyzed for patients in the CALGB and IFM trials. Data were not available from GIMEMA. Rates of treatment discontinuation as a result of TEAEs were 29.1% in the lenalidomide maintenance group and 12.2% in the placebo or observation group (Table 3). The most common TEAEs leading to treatment discontinuation in the lenalidomide maintenance and placebo or observation groups were blood and lymphatic system disorders (4.3% v 2.1%, respectively) and general disorders and administration site conditions (4.7% v 1.5%, respectively). Consistent with previous CALGB and IFM reports, this analysis showed a higher frequency of SPMs with lenalidomide maintenance versus placebo or observation. Frequencies of hematologic SPMs occurring before PD (5.3% and 0.8% for lenalidomide and placebo or observation, respectively) and before and after PD (6.1% and 2.8% for lenalidomide and placebo or observation, respectively) were reported for both groups. In the lenalidomide maintenance and placebo or observation groups, frequencies of solid tumor SPMs occurring before PD (5.8% and 2.0%, respectively) and before and after PD (7.3% and 4.2%, respectively) were also reported. The cumulative incidence curves for hematologic and solid tumor SPMs are shown in Figures 3A and 3B. Time to invasive SPMs occurring before PD or start of second-line therapy was shorter in the lenalidomide maintenance group versus the placebo or observation group (HR, 2.67; 95% CI, 1.54 to 4.62; P < .001), whereas time to PD or second-line therapy was longer with lenalidomide maintenance versus placebo or observation (HR, 0.51; 95% CI, 0.45 to 0.59; P < .001; Fig 3C). The risk of developing PD was higher than the risk of developing an invasive SPM in both groups. In the lenalidomide maintenance and placebo or observation groups, the cumulative incidence rates of PD or second-line therapy were consistently higher than that of invasive SPM over time (Data Supplement). The time to death as a result of MM was significantly longer in the lenalidomide maintenance group versus the placebo or observation group (HR, 0.66; 95% CI, 0.53 to 0.81; P < .001; Fig 3D). For time to death as a result of SPM or adverse event, there were no differences between the two groups.
Table 3.
Discontinuations as a Result of TEAEs (safety population)

Fig 3.
Second primary malignancy and mortality analyses. (A) Cumulative incidence curve of time to hematologic second primary malignancy (SPM) onset (as-treated population). Patients who were randomly assigned but not treated with lenalidomide maintenance are included in the control group. (B) Cumulative incidence of time to solid tumor SPM onset (as-treated population). Patients who were randomly assigned but not treated with lenalidomide maintenance are included in the control group. (C) Cumulative incidence curves of time to disease progression and time to invasive SPM onset before disease progression. (D) Kaplan-Meier (KM) curve of time to death by cause of death. AE, adverse event; Len, lenalidomide; MM, multiple myeloma; PD, progressive disease.
DISCUSSION
An improved PFS with lenalidomide maintenance versus placebo or observation after HDM and ASCT in patients with NDMM was previously reported in the three studies analyzed in this report.2,19,20 The studies were not powered for OS, and OS data were not mature at the time of their initial publications. One trial, the CALGB trial, showed an early significant improvement in OS with lenalidomide maintenance. As a result of the increased SPM incidence with lenalidomide maintenance versus placebo reported in two of the trials, the CALGB and IFM trials, it was important to understand the risks and benefits of long-term lenalidomide maintenance after HDM and ASCT for patients with NDMM. The meta-analysis was planned to demonstrate a 20-month improvement in median OS based on an estimated median OS of 70 months with placebo or observation, approximating an HR of 0.78. The OS analysis was planned before identifying studies for data collection. Primary-source patient data from the three studies meeting the prespecified inclusion criteria were analyzed. The three studies consisted of generally similar patient populations, study designs, and maintenance approaches. The aggregate patient population provided sufficient power for the OS end point. A limitation of this analysis is the small number of studies included. Nevertheless, the meta-analysis was adequately powered to detect a treatment effect and demonstrated that lenalidomide maintenance versus placebo or observation after HDM and ASCT significantly prolonged OS and reduced the risk of death by 25%.
A 29.3-month PFS improvement was observed with lenalidomide maintenance versus placebo or observation on an ITT basis; these results are not adjusted for the crossover of placebo arm patients without PD in the CALGB study. The PFS2 benefit with lenalidomide maintenance versus placebo or observation indicated that lenalidomide maintenance did not induce PD resistant to salvage treatment. The PFS improvements observed with lenalidomide maintenance in each study were similar despite different dosing schedules (days 1 to 28 of a 28-day schedule for CALGB and IFM and days 1 to 21 of a 28-day schedule for GIMEMA). The recently presented results from the Myeloma XI trial demonstrated a similar PFS improvement with lenalidomide maintenance versus observation (HR, 0.47; 95% CI, 0.38 to 0.60) after ASCT using a treatment schedule of 21 days of treatment in a 28-day schedule.26
The PFS subgroup analyses consistently favored lenalidomide maintenance versus placebo or observation, with differences in the magnitude of benefit. For the OS subgroup analyses, the benefit with lenalidomide maintenance was heterogeneous. The OS benefit with lenalidomide maintenance was less pronounced for patients older than age 60 years and for women. Patients with ISS disease stage III did not experience an OS benefit. The risk of death was reduced by 30% and 37% with lenalidomide maintenance versus placebo or observation in patients achieving very good partial response or complete response, respectively. The induction subgroup analyses for OS favored lenalidomide maintenance versus placebo or observation. The effect was less favorable in patients who received a thalidomide-containing induction regimen or a vincristine, doxorubicin, and dexamethasone induction regimen. A PFS benefit, but not an OS benefit was seen with lenalidomide maintenance versus placebo or observation in patients with high-risk cytogenetics. Only a small number of patients had adverse-risk cytogenetics (n = 92). The Myeloma XI trial demonstrated improved PFS with lenalidomide maintenance versus observation in patients with high-risk cytogenetics, which included patients who had and had not received ASCT as a result of eligibility. The data are not yet mature enough for an OS analysis.26
The heterogeneity analysis demonstrated that all three studies contributed to the positive results of the meta-analysis. The HRs for each study are consistent with an OS improvement with lenalidomide maintenance versus placebo or observation. The quantitative heterogeneity results from the differences in magnitude of the treatment effect among the studies, particularly between the CALGB and IFM studies. Possible reasons for this heterogeneity include differences in and incomplete data for some patient demographics, different induction regimens, number of ASCTs, consolidation therapy after ASCT in the IFM study, discontinuation of lenalidomide maintenance before PD in the IFM study, crossover from placebo to lenalidomide maintenance in the CALGB study for patients without progression, and differences in frequency and type of second-line therapy.
There were differences in the length of maintenance therapy in the three studies. The IFM study stopped lenalidomide maintenance at a mean duration of approximately 2 years. For the IFM study patients who stopped lenalidomide maintenance without progression (n = 119) the mean duration of maintenance was 3.3 years. The mean durations of maintenance in the CALGB and GIMEMA studies were 2.5 and 3 years, respectively. The proportions of patients on lenalidomide maintenance across the three studies are similar in the treatment duration categories of ≥ 1, ≥ 2, and ≥ 3 years. The cessation of lenalidomide maintenance in the IFM study is the primary reason for the smaller percentage of patients on lenalidomide maintenance at ≥ 4 years compared with the other studies. These duration differences may have influenced the magnitude of improvement in PFS, OS, and PFS2 but did not seem to reduce salvage treatment efficacy after PD. A recent retrospective analysis showed a correlation between length of lenalidomide maintenance therapy after ASCT and length of OS in patients with NDMM.27 None of these studies examined a predetermined maintenance duration. Future study designs may evaluate different lengths of maintenance therapy, particularly in patients who achieve minimal residual disease–negative status.
In this analysis, the incidence rates of hematologic and solid tumor SPMs with lenalidomide maintenance were higher compared with placebo or observation. At a median follow-up time of 79.5 months, the rates of hematologic and solid tumor SPMs with lenalidomide maintenance before PD were 5.3% and 5.8%, respectively. These results are comparable to a previous meta-analysis of lenalidomide therapy in patients with NDMM that showed cumulative 5-year incidence rates of hematologic and solid tumor SPMs of 3.1% and 3.8%, respectively.28 The cumulative incidence rate of developing an SPM before PD is higher for patients who received lenalidomide maintenance, and the cumulative incidence rates of PD and death as a result of MM are higher for patients who received placebo or observation. Overall, the risk of developing PD is greater than that of developing an SPM.
An important treatment goal for patients with NDMM is to achieve and maintain remission or long-term disease control.29,30 Maintenance therapy after ASCT can be considered a valid approach toward achieving long-term disease control, delaying time to relapse and second-line treatment, and prolonging survival. A previously published meta-analysis of RCTs comparing thalidomide- or lenalidomide-based maintenance versus thalidomide- and lenalidomide-free maintenance or no maintenance therapy showed in stratified analyses that both maintenance therapies improve PFS, but not OS, in the transplantation setting.31 The study used published data from 2012 and 2014 of the same three trials in this analysis. The OS data were less mature, and the analysis did not use primary-source, patient-level data.
This study demonstrates a statistically significant and clinically meaningful improvement in OS with lenalidomide maintenance. With new, highly active, triplet induction regimens enhancing depth and duration of response as well as ongoing studies evaluating the optimal timing of ASCT,32-36 the use of lenalidomide maintenance for transplantation-eligible patients can be considered a standard of care. The costs of maintenance therapy should be weighed against the costs of shorter survival, earlier progression, and earlier use of subsequent lines of therapies for patients without maintenance. Understanding the role of minimal residual disease detection and immune reconstitution after ASCT, as well as developing early end points as surrogates for long-term outcomes, should allow us to develop clinical strategies to further improve OS.
ACKNOWLEDGMENT
We thank Kristina Hernandez and Peter Simon for medical writing assistance, which was sponsored by Celgene Corporation.
Footnotes
Supported by Celgene Corporation. The Cancer and Leukemia Group B 100104 trial was funded by the National Cancer Institute and National Heart, Lung, and Blood Institute and conducted by the Alliance for Clinical Trials in Oncology, Eastern Cooperative Oncology Group, and Blood and Marrow Transplant Clinical Trials Network. The Gruppo Italiano Malattie Ematologiche dell'Adulto RV-MM-PI-209 trial was funded by Celgene Corporation. The Intergroupe Francophone du Myélome 2005-02 trial was funded by the Programme Hospitalier de Recherche Clinique, the Swiss Group for Clinical Cancer Research, and a grant from Celgene Corporation.
Presented in part at the 52nd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2016, and the 21st Congress of the European Hematology Association, Copenhagen, Denmark, June 9-12, 2016.
Processed as a Rapid Communication manuscript.
Clinical trial information: NCT00551928, NCT00551928, NCT00430365.
See accompanying Editorial on page 3269
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Philip L. McCarthy
Honoraria: Celgene, Bristol-Myers Squibb, Sanofi, Takeda, The Binding Site, Karyopharm Therapeutics
Consulting or Advisory Role: Celgene, Janssen, Bristol-Myers Squibb, Sanofi, Karyopharm Therapeutics
Research Funding: Celgene (Inst)
Sarah A. Holstein
Consulting or Advisory Role: Celgene, Takeda
Research Funding: Celgene (Inst)
Travel, Accommodations, Expenses: Celgene
Maria Teresa Petrucci
Honoraria: Janssen-Cilag, Celgene, Bristol-Myers Squibb, Takeda, Amgen
Paul G. Richardson
Consulting or Advisory Role: Celgene
Research Funding: Celgene, Millennium
Cyrille Hulin
Honoraria: Celgene, Amgen, Bristol-Myers Squibb, Novartis, Janssen-Cilag, Takeda
Patrizia Tosi
No relationship to disclose
Sara Bringhen
Honoraria: Celgene, Janssen-Cilag, Bristol-Myers Squibb
Consulting or Advisory Role: Amgen, Mundipharma, Karyopharm Therapeutics
Pellegrino Musto
Honoraria: Celgene, Janssen-Cilag, Sanofi, Amgen, Takeda, Bristol-Myers Squibb
Kenneth C. Anderson
Consulting or Advisory Role: Celgene, Millennium, Gilead Sciences, Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: C4 Therapeutics, Oncopep, Acetylon
Denis Caillot
No relationship to disclose
Francesca Gay
Honoraria: Celgene, Janssen, Takeda, Bristol-Myers Squibb, Amgen
Consulting or Advisory Role: Amgen, Roche, Takeda, Celgene, Seattle Genetics
Philippe Moreau
Honoraria: Celgene, Takeda, Novartis, Janssen-Cilag, Amgen
Consulting or Advisory Role: Celgene, Takeda, Janssen, Novartis, Amgen
Gerald Marit
Travel, Accommodations, Expenses: Celgene, Janssen-Cilag, The Binding Site
Sin-Ho Jung
No relationship to disclose
Zhinuan Yu
Employment: Celgene
Stock or Other Ownership: Celgene
Benjamin Winograd
Employment: Celgene
Stock or Other Ownership: Celgene
Robert D. Knight
Employment: Celgene
Stock or Other Ownership: Celgene
Antonio Palumbo
Employment: Takeda
Honoraria: Genmab, Janssen-Cilag, Takeda
Consulting or Advisory Role: Genmab, Janssen-Cilag, Takeda
Research Funding: Genmab (Inst), Janssen-Cilag (Inst), Takeda (Inst)
Michel Attal
No relationship to disclose
REFERENCES
- 1.National Comprehensive Cancer Network : Clinical practice guidelines in oncology: Multiple myeloma (version 1.2017) https://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf
- 2.Palumbo A, Cavallo F, Gay F, et al. : Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 371:895-905, 2014 [DOI] [PubMed] [Google Scholar]
- 3.McCarthy PL, Holstein SA: Role of stem cell transplant and maintenance therapy in plasma cell disorders. Hematology (Am Soc Hematol Educ Program) 2016:504-511, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attal M, Lauwers-Cances V, Hulin C, et al. : Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 376:1311-1320, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrello I: Can we change the disease biology of multiple myeloma? Leuk Res 36:S3-S12, 2012. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attal M, Harousseau JL, Stoppa AM, et al. : A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med 335:91-97, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Child JA, Morgan GJ, Davies FE, et al. : High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 348:1875-1883, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Attal M, Harousseau JL, Facon T, et al. : Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med 349:2495-2502, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Paiva B, Gutiérrez NC, Rosiñol L, et al. : High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood 119:687-691, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Rawstron AC, Child JA, de Tute RM, et al. : Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: Impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol 31:2540-2547, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. : Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood 123:3073-3079, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harousseau JL, Avet-Loiseau H, Attal M, et al. : Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: Long-term analysis of the IFM 99-02 and 99-04 trials. J Clin Oncol 27:5720-5726, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Lahuerta JJ, Mateos MV, Martínez-López J, et al. : Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: Sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol 26:5775-5782, 2008 [DOI] [PubMed] [Google Scholar]
- 14.van de Velde HJ, Liu X, Chen G, et al. : Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica 92:1399-1406, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Lopez J, Blade J, Mateos MV, et al. : Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood 118:529-534, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Barlogie B, Anaissie E, Haessler J, et al. : Complete remission sustained 3 years from treatment initiation is a powerful surrogate for extended survival in multiple myeloma. Cancer 113:355-359, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Hoering A, Crowley J, Shaughnessy JD, Jr, et al. : Complete remission in multiple myeloma examined as time-dependent variable in terms of both onset and duration in Total Therapy protocols. Blood 114:1299-1305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreau P, Attal M, Facon T: Frontline therapy of multiple myeloma. Blood 125:3076-3084, 2015 [DOI] [PubMed] [Google Scholar]
- 19.McCarthy PL, Owzar K, Hofmeister CC, et al. : Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 366:1770-1781, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attal M, Lauwers-Cances V, Marit G, et al. : Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 366:1782-1791, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Palumbo A, Hajek R, Delforge M, et al. : Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med 366:1759-1769, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. : Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med 371:906-917, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Weber DM, Chen C, Niesvizky R, et al. : Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med 357:2133-2142, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Dimopoulos M, Spencer A, Attal M, et al. : Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 357:2123-2132, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Quach H, Ritchie D, Stewart AK, et al. : Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 24:22-32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson GH, Davies FE, Pawlyn C, et al: Lenalidomide is a highly effective maintenance therapy in myeloma patients of all ages: Results of the phase III Myeloma XI study. Blood 128, 2016 (abstr 1143) [Google Scholar]
- 27.Mian I, Milton DR, Shah N, et al. : Prolonged survival with a longer duration of maintenance lenalidomide after autologous hematopoietic stem cell transplantation for multiple myeloma. Cancer 122:3831-3837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palumbo A, Bringhen S, Kumar SK, et al. : Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: A meta-analysis of individual patient data. Lancet Oncol 15:333-342, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Palumbo A, Anderson K: Multiple myeloma. N Engl J Med 364:1046-1060, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Stewart AK, Richardson PG, San-Miguel JF: How I treat multiple myeloma in younger patients. Blood 114:5436-5443, 2009 [DOI] [PubMed] [Google Scholar]
- 31. doi: 10.1093/jnci/djv342. Wang Y, Yang F, Shen Y, et al: Maintenance therapy with immunomodulatory drugs in multiple myeloma: A meta-analysis and systematic review. J Natl Cancer Inst 108:djv342, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajkumar SV: Doublets, triplets, or quadruplets of novel agents in newly diagnosed myeloma? Hematology (Am Soc Hematol Educ Program) 2012:354-361, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Dhakal B, Girnius S, Hari P: Recent advances in understanding multiple myeloma. F1000Research, 2016. doi:10.12688/f1000research.8777.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson PG, Weller E, Lonial S, et al. : Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 116:679-686, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. : A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood 120:1801-1809, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. doi: 10.1093/annonc/mdx096. Moreau P, San Miguel J, Sonneveld P, et al: Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 10.1093/annonc/mdx096 [epub ahead of print on April 27, 2017] [DOI] [PubMed] [Google Scholar]





