Abstract
Purpose
Sorafenib and lenvatinib are oral multikinase inhibitors targeting vascular endothelial growth factor receptor (VEGFR) and approved for radioiodine (RAI)-refractory differentiated thyroid cancer (DTC). However, there are no approved second- or third-line therapies. MET is implicated in resistance to VEGFR inhibitors. Cabozantinib is an oral multikinase inhibitor targeting MET in addition to VEGFR and is approved for medullary thyroid cancer. In a phase I study of cabozantinib, five of eight patients with DTC previously treated with a VEGFR-targeted therapy had an objective response to cabozantinib.
Patients and Methods
Patients with RAI-refractory disease with Response Evaluation Criteria in Solid Tumor (RECIST) measurable disease and evidence of progression on prior VEGFR-targeted therapy were enrolled in this single-arm phase II study. The cabozantinib starting dose was 60 mg/day orally but could be escalated to 80 mg if the patient did not experience a response. Patients underwent tumor assessment according to RECIST v1.1 every 8 weeks. In this study, if at least five of 25 response-evaluable patients had an objective response, cabozantinib would be considered a promising agent in this patient population.
Results
Twenty-five patients were enrolled. The median age was 64 years, and 64% of patients were men. Twenty-one patients had received only one prior VEGFR-targeted therapy (sorafenib, pazopanib, or cediranib), and four patients had received two such therapies. The most common treatment-related adverse events were fatigue, weight loss, diarrhea, palmar-plantar erythrodysesthesia, and hypertension. One drug-related death was noted. Of the 25 patients, 10 (40%) had a partial response, 13 (52%) had stable disease, and two (8%) had nonevaluable disease. The median progression-free survival and overall survival were 12.7 months and 34.7 months, respectively.
Conclusion
Cabozantinib demonstrated clinically significant, durable objective response activity in patients with RAI-refractory DTC who experienced disease progression while taking prior VEGFR-targeted therapy.
INTRODUCTION
Thyroid cancer is the most common endocrine neoplasm and also the most rapidly increasing cancer in the United States, with more than 62,000 new diagnoses in 2015.1 Nearly two thirds of patients are younger than 55 years of age, and most are women. Although the prognosis is good, with more than 90% of patients experiencing long-term survival, patients who develop advanced or radioiodine (RAI)-refractory disease represent a major therapeutic challenge.2
Thyroid cancers are classified on the basis of histology into differentiated thyroid cancer (DTC), medullary thyroid cancer (MTC), and anaplastic thyroid cancer. DTC accounts for 90% of patients, and includes papillary thyroid cancer, follicular thyroid cancer (FTC), Hürthle cell thyroid cancer (HTC), and poorly differentiated thyroid carcinoma (PDTC). Standard approaches for metastatic DTC include surgery, RAI, and thyroid-stimulating hormone (TSH) suppression with levothyroxine. Doxorubicin-based chemotherapy has historically been used as salvage therapy, but has only modest activity and an unfavorable toxicity profile. More recently, pathways including angiogenesis inhibition and inhibition of aberrant intracellular signaling in the MAPK and PI3K/AKT/mTOR pathways have been successfully exploited.3 Several multikinase inhibitors (MKIs), mainly those targeting vascular endothelial growth factor receptor (VEGFR) and other kinases, such as axitinib, sorafenib, sunitinib, pazopanib, and lenvatinib, have been studied in RAI-refractory DTC. Promising results led to phase III trials of sorafenib4 and levantinib,5 with both drugs improving progression-free survival (PFS) compared with placebo. As a result, both agents have been approved by the Food and Drug Administration (FDA). Despite these recent successes, most patients develop resistance to these agents. To date, there has been no study specifically exploring the role of MKIs, in the second- or third-line setting after disease progression, on prior VEGFR-targeted therapy for patients with RAI-refractory DTC.
Cabozantinib is an oral MKI that is a potent inhibitor of c-MET, RET, and VEGFR,6 which are important mediators of tumor growth and angiogenesis and are associated with a poor prognosis.7-10 Furthermore, c-MET has been implicated in resistance to angiogenesis inhibitors.11-13 In the clinic, a phase III trial of cabozantinib versus placebo in patients with MTC showed a 7.2-month increase in median PFS in patients receiving cabozantinib (hazard ratio [HR], 0.28; P < .0001), leading to FDA approval.14 In DTC, early experience with cabozantinib in a phase I trial demonstrated an objective response in five of eight patients with DTC (62%) previously treated with one prior VEGFR-targeted therapy.15 On the basis of the unique targeting profile of cabozantinib, inhibiting c-MET in addition to RET and VEGFR, and the promising activity demonstrated in the phase I study, we hypothesized that cabozantinib would be effective in this patient population.
PATIENTS AND METHODS
Patients
Patients with advanced thyroid cancer (papillary, follicular, Hürthle cell, or poorly differentiated) aged ≥ 18 years were eligible if they met the following criteria: measurable disease by Response Evaluation Criteria in Solid Tumor (RECIST) v1.1; RECIST v1.1 progression on prior VEGFR-targeted therapy (up to two lines of prior VEGFR-targeted therapy were allowed), RAI-refractory disease as defined by one or more of the following criteria: (1) one or more measurable lesions that did not demonstrate RAI uptake; (2) one or more measurable lesions progressive by RECIST v1.1 within 12 months of prior RAI therapy; (3) one or more measurable lesions present after a cumulative RAI dose of > 600 mCi; (4) adequate organ function and Eastern Cooperative Oncology Group performance status of 0 or 1. Major exclusion criteria are provided in the Appendix (online only). The study was approved by the institutional review board at each of the participating centers and the National Cancer Institute (NCI) and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Written, informed consent was obtained from all patients before study entry. The multicenter trial was coordinated by The Academic and Community Cancer Research United (ACCRU) and funded by the NCI (ClinicalTrials.gov identifier: NCT01811212) and the International Thyroid Oncology Group (ITOG).
Study Treatment
Cabozantinib was administered orally at a starting dose of 60 mg daily in 28-day cycles. This dose was chosen because of the poor tolerance of the 140-mg/day dose in a phase I trial,15 where 11 of 15 patients required dose reduction of cabozantinib to 60 mg/day. Patients who tolerated cabozantinib with no ≥ grade 2 treatment-related AEs could have their dose increased to 80 mg daily. Those patients experiencing ≥ grade 2 treatment-related AEs had their dose reduced to 40 mg daily (and again to 20 mg daily, if necessary). Treatment was continued until disease progression, unacceptable toxicity, or withdrawal of consent. Of note, patients who had continued clinical benefit (such as symptomatic or tumor marker improvement or decreased tumor burden compared with baseline or limited progression in a nontarget lesion treated with radiation or surgery) were allowed to receive therapy even if they met criteria for progressive disease per RECIST v1.1.
Assessment
Physical examination, laboratory tests (hematology, chemistry, thyroglobulin [Tg], TSH), and ECGs were performed at baseline and every 4 weeks while the patient was receiving therapy. For the first two cycles, physical examination, AE evaluation, and limited laboratory testing were performed every 2 weeks. Of note, echocardiogram or multiple-gated acquisition scans were not routinely performed. Computed tomography or magnetic resonance imaging scans of the neck, chest, and abdomen, as well as bone scans, were performed at baseline and every 8 weeks to assess response per RECIST v1.1. Brain imaging at baseline and every 8 weeks was required for patients with known brain metastases. AEs were assessed per NCI Common Terminology Criteria for Adverse Events (version 4.0).
Statistical Design
The primary end point was the objective response rate, defined as the proportion of patients who had a partial response (PR) or complete response by RECIST v1.1 criteria out of all evaluable patients who received any protocol treatment. All enrolled eligible patients who had begun treatment were considered evaluable for response. On the basis of the findings in the phase I trial of cabozantinib for the patients with DTC, we determined that a true overall response rate (ORR) of ≥ 30% would be promising in this patient population. To test the hypothesis that the ORR was ≥ 30% (v the null hypothesis that it was, at most, 10%), we used a two-stage Simon minimax design that required a minimum of 16 and a maximum of 25 response-evaluable patients and constrained the type I error to 10% with 90% power. If two or more of the first 16 patients responded, the study would continue to accrue a total of 25 patients. If five or more of 25 evaluable patients responded, cabozantinib would be considered promising and recommended for further study. Assuming a binomial distribution of the ORR, 95% CIs were calculated for the ORR estimate. Kaplan-Meier methods were used to estimate overall survival (OS) and PFS. Each of these variables was measured from the treatment start date to the date of the event (ie, death or disease progression). Patients who were event-free at the date of the last follow-up were censored at that time. Graphical analyses and Spearman rank correlation tests were used to assess relationships between tumor markers and percentage of tumor reduction.
RESULTS
Patients
Between September 2013 and January 2015, 25 patients were enrolled by ITOG investigators at six centers in United States. Data presented here include collected data as of August 15, 2016. Patient characteristics are listed in Table 1. All patients with RAI-refractory DTC had measurable disease and disease progression while receiving at least one line of prior VEGFR-targeted therapy. The majority of patients had aggressive histology (28% PDTC, 20% HTC, 16% FTC), and a high frequency of bone (84%), liver (36%), and brain (20%) metastases was observed. Patients had high tumor burden at study entry, and in addition to RAI, patients were heavily pretreated with systemic cytotoxic chemotherapy and/or targeted therapies: seven patients had received at least two lines, three had received three lines, and one had received four lines of systemic therapy, including VEGR-targeted therapy. Of five patients with brain metastasis at study entry, all had stable brain metastasis and had discontinued corticosteroids for least for 2 weeks before study entry, four patients had undergone radiation (stereotactic, n = 3; whole brain, n = 1) and one patient had undergone surgery for brain metastasis.
Table 1.
Baseline Patient Characteristics

Efficacy
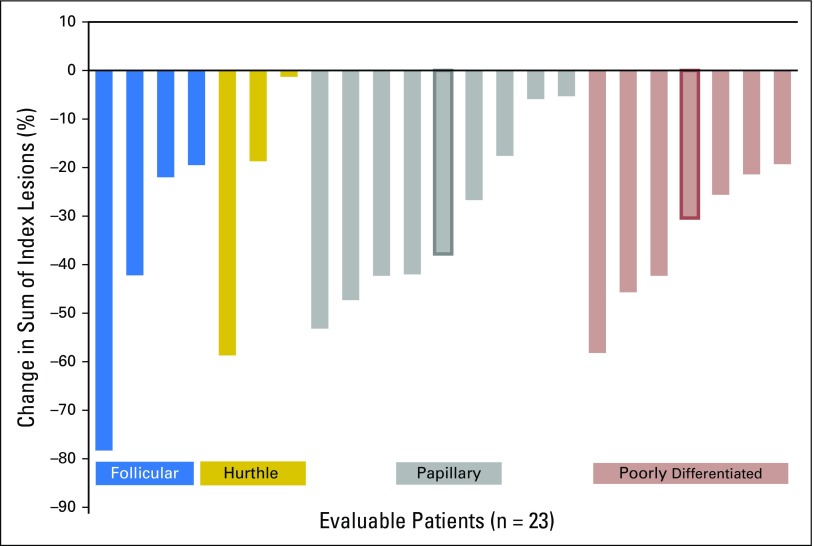
All patients were included in the efficacy analysis. Of the 25 patients, 10 (40%) had a confirmed PR, 13 (52%) had stable disease (SD; including two patients with unconfirmed PR), and two (8%) were not evaluable because of a lack of imaging studies (one patient had clinical progression and the other discontinued treatment because of AEs). Median time to achieve PR was 2 months (range, 2 to 8 months), and median duration of PR was 11.3 (95% CI, 10.3 to not evaluable) months. Figure 1 shows the best objective response assessed by RECIST v1.1. Objective responses were seen across all histologic types, including PDTC and even in patients who had brain metastasis at study entry (two with PR, two with SD, and one with unconfirmed PR).The number of prior VEGFR therapies was associated with response: PR was achieved in 10 of 21 patients (48%) with only one prior VEGFR-targeted therapy, whereas zero of four responses (0%) were seen in patients who received two prior VEGFR-targeted therapies. However, variable degrees of tumor reduction (6%, 19%, and 30%) were seen in three evaluable patients who received two prior VEGFR-targeted therapies. The changes in serum Tg levels during therapy were evaluated for each patient in relation to the percentage of change in target lesions over time and showed significant correlation between Tg response and radiographic objective responses (P = .0178).
Fig 1.
Waterfall plot of the best Response Evaluation Criteria in Solid Tumor response in 23 evaluable patients. Bold border indicates patients with an unconfirmed partial response, considered as stable disease. Ten (40%) patients had a confirmed partial response.
Progression-Free Survival and Overall Survival
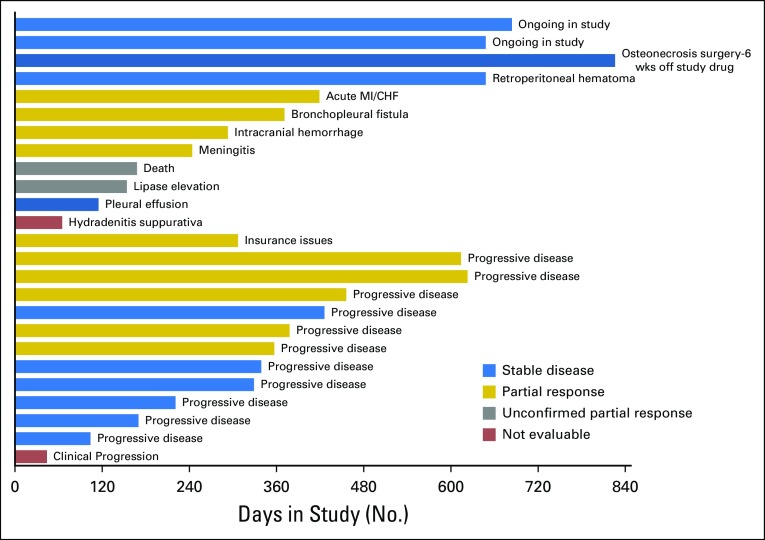
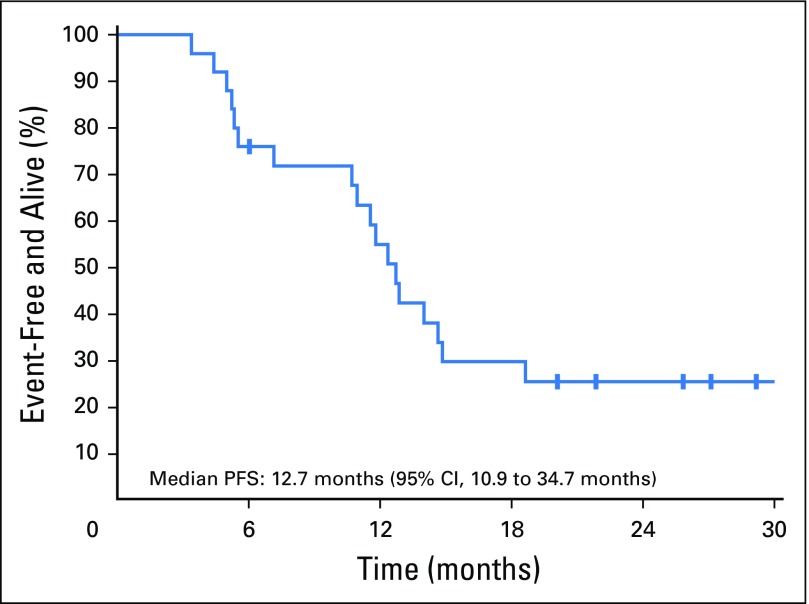
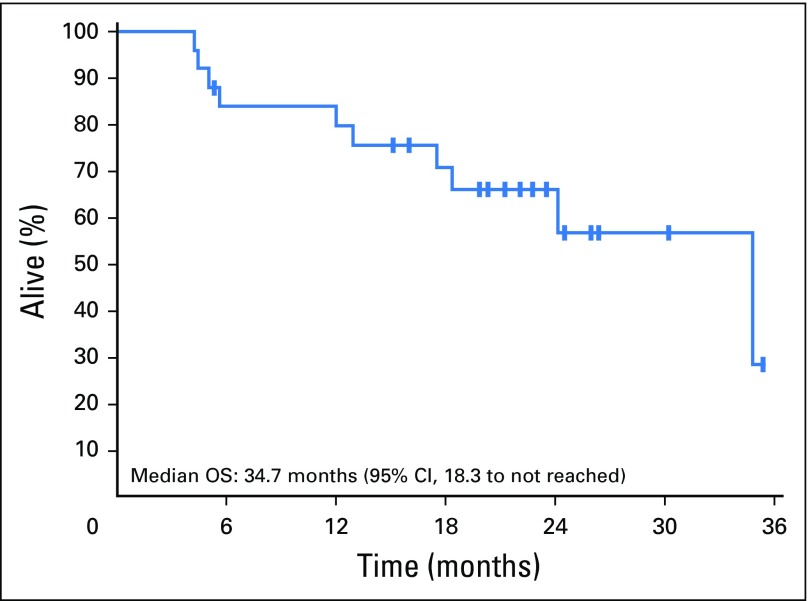
Median duration of follow-up was 22.8 (95% CI, 21.2 to 30.2) months. Two patients remained on cabozantinib at the time of data cutoff, and 23 patients left the study for the following reasons: 11 for progressive disease, nine for AEs, one for clinical progression, one for insurance issues, and one because of death (Fig 2). The pattern of progressive disease after achieving nadir SD or PR was interesting in that only four patients had a 20% increase in the sum of target lesions compared with the nadir response, whereas target lesions in seven patients maintained reduction or stability. In these seven patients, progression was defined based on new lesions (n = 4) or unequivocal progression of nontarget lesions (n = 3). Median PFS was 12.7 (95% CI, 10.9 to 34.7) months (Fig 3), and the estimated PFS at 12 months was 55% (95% CI, 38% to 79%) and 25% (95% CI, 13% to 50%) at 24 months. Median OS was 34.7 (95% CI, 18.3 to not reached) months (Fig 4), and estimated OS at 12 months was 80% (95% CI, 65% to 97%) and at 24 months was 66% (95% CI, 49% to 88%).
Fig 2.
Best response, study duration, and reasons for leaving the study in 25 patients. Each bar represents a patient. Text on right side of each bar represents reason for off study. Colors indicate best response: blue, stable disease; gold, partial response; gray, unconfirmed partial response; red, not evaluable. CHF, congestive heart failure; MI, myocardial infarction, wks, weeks.
Fig 3.
Kaplan-Meier curve of progression-free survival (PFS).
Fig 4.
Kaplan-Meier curve of overall survival (OS).
Dose Modifications
Seven patients (28%) were treated at 60 mg/day of cabozantinib, whereas four (16%) had a dose escalation to 80 mg/day and 14 (56%) had a dose reduction to 40 mg/day (n = 6; 24%) or to 20 mg/day (n = 8; 32%). A total of 275 cycles (a cycle was 28 days) of cabozantinib were administered to 25 patients; dosing was as follows: 21% of cycles at 20 mg/day, 25% of cycles at 40 mg/day, 38% of cycles at 60 mg/day, and 16% of cycles at 80 mg/day. The most common AEs prompting dose reduction were palmar-plantar erythrodysesthesia (n = 5) and fatigue (n = 4), whereas diarrhea, vomiting, weight loss, mucositis, neutropenia, and increase in AST/ALT/lipase were other AEs that resulted in dose reduction. Of four patients treated with cabozantinib at the 80-mg/day dose, three had SD and one had PR, whereas 10 PRs were observed at all doses (five at 20 mg/day, one at 40 mg/day, three at 60 mg/day, and one at 80 mg/day). Median duration of therapy was 20 months in five patients who had a dose reduction to 20 mg/day and achieved PR.
Adverse Events
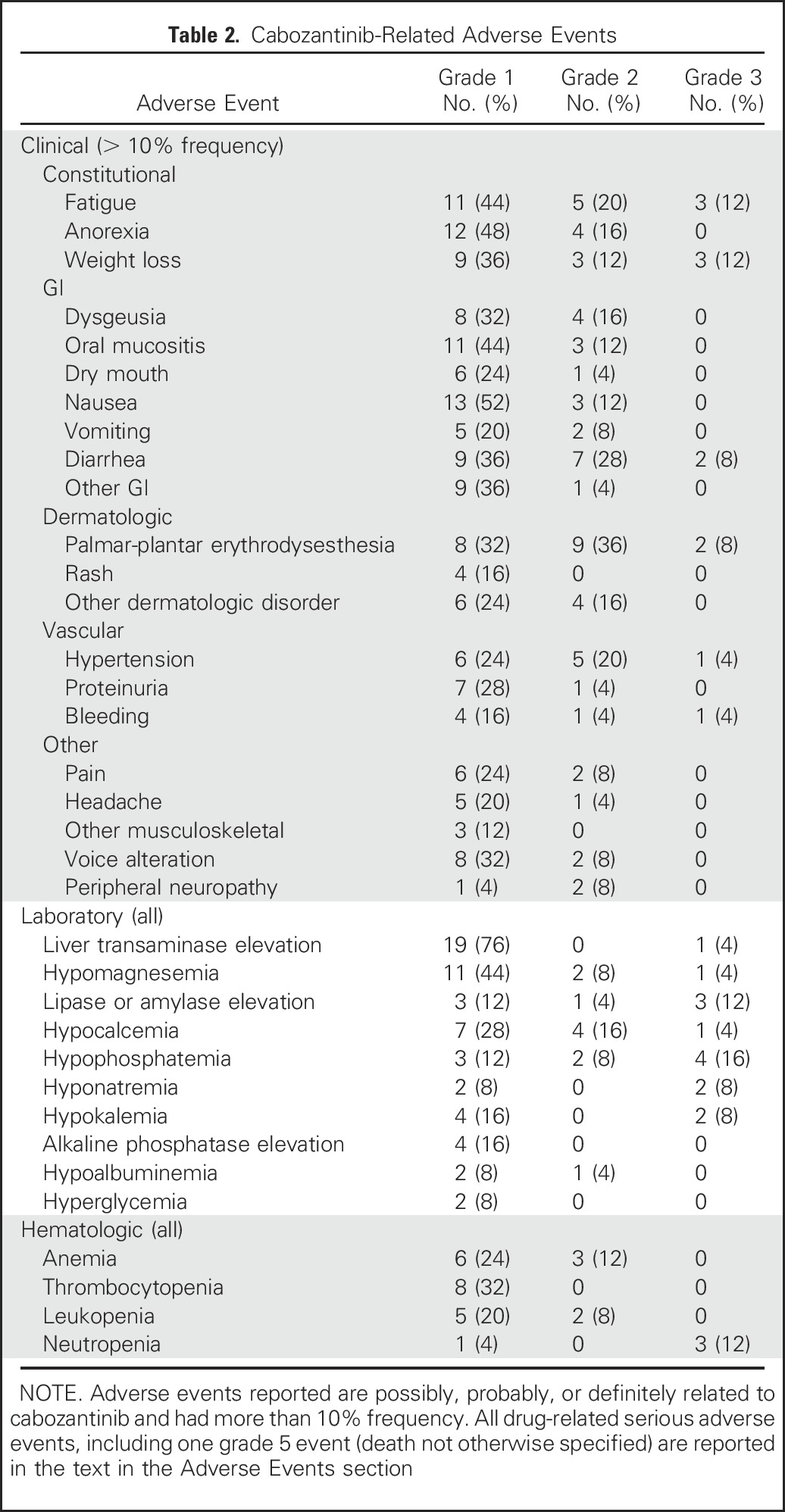
Table 2 lists the most common (≥ 10% frequency) grade 1 to 3 clinical AEs and all laboratory AEs possibly, probably, or definitely attributed to cabozantinib. Notable serious adverse events (AEs) included grade 1 thrombotic thrombocytopenic purpura (n = 1), grade 2 deep venous thrombosis (n = 1), grade 4 perianal hidradenitis suppurativa (n = 1), and grade 3 AEs (n = 6) comprising left ventricular systolic dysfunction, asymptomatic increased lipase, osteonecrosis of the jaw, decubitus ulcer, pneumonia, and meningitis (one of each events). The patient with grade 4 perianal hidradenitis suppurativa had a history of similar AE while receiving pazopanib and lenvatinib previously. The patient with grade 3 osteonecrosis of the jaw was receiving denosumab and had undergone a tooth extraction 6 months earlier. There was one death (grade 5 event) possibly attributable to cabozantinib. This patient was admitted to the hospital (10 days before death) with diarrhea, dehydration, and acute kidney injury that resolved fully within 3 days. At that time, 6-month restaging studies showed PR, stable brain metastasis, and left common iliac vein thrombosis for which the patient was started on a therapeutic dose of enoxaparin. Cabozantinib was withheld since the first day of hospitalization. On day 3 after discharge to the nursing home, the patient died after sudden onset of seizure-like activity and limb paralysis, and the family opted for comfort care. Autopsy was not performed. Of note, there were six bleeding events that consisted of two events of grade 1 epistaxis, one event of grade 1 hematuria, one event of grade 1 rectal bleeding; one event of grade 2 intracranial bleeding (ICH) associated with a new diagnosis of brain metastasis, and one event of grade 3 retroperitoneal hematoma. Although all patients were receiving levothyroxine for postsurgical hypothyroidism and had suppressed TSH (n = 24) at study entry, six patients developed an increase in TSH levels of 1.1 to 8.6 mIU/L during study treatment.
Table 2.
Cabozantinib-Related Adverse Events
DISCUSSION
To our knowledge, this is the first prospective clinical trial evaluating the use of MKIs specifically after first- or second-line failure of prior VEGFR-targeted therapy. All patients had RAI-refractory DTC and had progressive disease as evaluated by RECIST v1.1 while on the previous VEGFR-targeted therapy. This trial demonstrated the feasibility of a unique collaboration between NCI and ITOG to support an investigator-initiated multicenter clinical trial in a rare cancer. Our data demonstrate that in the salvage setting, cabozantinib is effective, with an impressive rate of 40%, a median PFS of 12.7 months, and a median OS of 34.7 months. It is important to note that although at least one previous VEGFR-targeted therapy was required for the eligibility of patients with RAI-refractory DTC, many patients had received other types of systemic therapies. In addition, our study included patients with poor prognostic factors, including aggressive thyroid cancer histology (64% with PDTC, FTC, or HTC), nonlung metastasis (bone, 84%; liver, 36%; and brain, 20%) and a high tumor burden. Thyroglobulin was a fairly good surrogate marker for response to therapy, although it did not correlate in a minority of patients.
Our results are consistent with a recent publication by Dadu et al16 in which 60 patients with DTC who discontinued first-line sorafenib because of progression or AEs were evaluated. In this retrospective study, seven of 17 patients (41%) achieved a PR to salvage therapy such as VEGFR-targeted therapy (including cabozantinib, lenvatinib, pazopanib, or sunitinib) or vemurafenib. Furthermore, the median PFS of 12.7 months in our study was similar to 15.1 months reported in a subset analysis of the phase III SELECT trial in which 66 patients randomly assigned to lenvatinib were previously treated with a VEGFR-targeted MKI.5 But unlike our study, progressive disease while receiving treatment with VEGFR-targeted therapy was not required for entry into SELECT. Thus, patients in SELECT might have had VEGFR-targeted-therapy–sensitive disease but discontinued because of AEs. In a phase II trial of BRAF-mutated DTC, a 27% PR was reported with vemurafenib in 22 patients previously treated with VEGFR-targeted MKI.17 In that group of patients, median PFS was 8.9 months (95% CI, 5.5 months to not reached), and median OS was 14.4 months (95% CI, 8.2 to 29.5 months). In contrast, longer median PFS and OS were observed in our trial of cabozantinib as salvage therapy; this could reflect improved efficacy of cabozantinib or a different patient population and/or patient characteristics.
In our study, cabozantinib was generally well tolerated; the most common AEs were expected and were mild to moderate in severity, including fatigue, weight loss, hand-foot skin reaction, GI effects (anorexia, nausea, mucositis, diarrhea), and hypertension. Dose delays and decreases were used to manage AEs, in addition to aggressive symptomatic management. Like other VEGFR-targeted MKIs, cabozantinib was associated with serious AEs such as bleeding, deep vein thrombosis, left ventricular systolic dysfunction, and osteonecrosis of the jaw. Also, there was a grade 5 event thought to be related to ICH that was possibly related to cabozantinib as well as concomitant enoxaparin. Four of five patients with previously treated brain metastasis at study entry did not develop ICH, and one patient who developed grade 2 ICH while participating in the study for 7.4 months was diagnosed with brain metastasis at that time. Future studies may require brain imaging in all patients at study entry and during the study to diagnose and treat otherwise asymptomatic metastasis before treatment with cabozantinib. It is notable that the doses of cabozantinib used in this clinical trial were well below the FDA-approved dose of 140 mg daily (capsule formulation) in MTC. Our trial was designed using a dose of 60 mg daily (tablet formulation) with the possibility of dose escalation to 80 mg daily. Similar to other cabozantinib trials,15,18-20 the drug is effective at these lower doses, leading to responses in 40% of patients. Whether the drug is better tolerated at these lower doses is not clear, but there is an ongoing clinical trial comparing two different starting doses—60 mg and 140 mg daily (ClinicalTrials.gov identifier: NCT 01896479).
The mechanisms of cabozantinib in inducing PRs in the second-line setting are not fully established, although they may be related to c-MET–driven resistance induced by previous VEGFR-targeted therapy. Neither the thyroid cancer histologic subtype nor dose of cabozantinib was associated with response, whereas one (as opposed to two) prior VEGFR-targeted treatment was associated with a better response rate. Finally, although we did not obtain comprehensive genetic profiling of the tumors at study entry, maximum tumor shrinkage was observed in patients with FTCs harboring NRAS mutations and a patient with papillary thyroid cancer harboring KRAS mutation. Thus, tumor genotype as a predictor of response to cabozantinib warrants further study. In conclusion, cabozantinib is an effective salvage therapy in patients with RAI-refractory DTC who have experienced disease progression on previous VEGFR-targeted therapies.
ACKNOWLEDGMENT
The authors thank the teams at International Thyroid Oncology Group, the Academic and Community Cancer Research United, and Cancer Therapy Evaluation Program for their contributions and support for the trial, as well as the patients for their participation.
Appendix
Major exclusion criteria included radioiodine (RAI) treatment ≤ 6 weeks of study treatment, prior treatment with cabozantinib or other tyrosine kinase inhibitor that targets MET, presence of cavitary lung lesions, need for concomitant therapeutic anticoagulation, active brain metastases or epidural disease, and patients who had not recovered to baseline or ≤ grade 1 adverse event related to all prior therapies (except alopecia and other nonclinically significant adverse events).
Of note, patients with brain metastases previously treated with whole-brain radiation or radiosurgery or patients with epidural disease previously treated with radiation or surgery who were asymptomatic and did not require steroid treatment of at least 2 weeks before starting study treatment were eligible. Baseline brain imaging with contrast-enhanced computed tomography or magnetic resonance imaging scans for patients with known brain metastases was required to confirm eligibility.
Footnotes
Supported in part through the International Thyroid Oncology Group, Ohio State University Comprehensive Cancer Center Grant No. N01-CM-2011-00070; The University of Texas MD Anderson Cancer Center's Cancer Center Support Grant No. CA16672, and University of Chicago Grant No. N01-CM-2011-00071C.
Presented in part at the International Thyroid Congress, Lake Buena Vista, FL, October 18-23, 2015.
Clinical trial information: NCT01811212.
AUTHOR CONTRIBUTIONS
Conception and design: Susan Geyer, Lori J. Wirth, John Wright, Manisha H. Shah
Administrative support: John Wright, Manisha H. Shah
Provision of study materials or patients: Maria E. Cabanillas, Jonas A. de Souza, Lori J. Wirth, Stephen V. Liu, Manisha H. Shah
Collection and assembly of data: Maria E. Cabanillas, Jonas A. de Souza, Lori J. Wirth, Michael E. Menefee, Stephen V. Liu, Komal Shah, Manisha H. Shah
Data analysis and interpretation: Maria E. Cabanillas, Jonas A. de Souza, Susan Geyer, Lori J. Wirth, Michael E. Menefee, Stephen V. Liu, John Wright, Manisha H. Shah
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cabozantinib As Salvage Therapy for Patients With Tyrosine Kinase Inhibitor–Refractory Differentiated Thyroid Cancer: Results of a Multicenter Phase II International Thyroid Oncology Group Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Maria E. Cabanillas
Consulting or Advisory Role: Loxo
Research Funding: Roche/Genentech, Eisai, Kura, Exelixis
Jonas A. de Souza
No relationship to disclose
Susan Geyer
No relationship to disclose
Lori J. Wirth
Consulting or Advisory Role: Amgen, Blueprint Genetics, Eisai, Loxo, Merck
Michael E. Menefee
Consulting or Advisory Role: Eisai
Travel, Accommodations, Expenses: Eisai
Stephen V. Liu
Consulting or Advisory Role: Genentech, Boehringer Ingelheim, Pfizer, ARIAD, Celgene, Eli Lilly, Bristol-Myers Squibb
Research Funding: Genentech/Roche, Pfizer, Threshold Pharmaceuticals, Clovis Oncology, Corvus Pharmaceuticals, Esanex, Bayer, OncoMed, Ignyta, Merck, MedImmune
Komal Shah
No relationship to disclose
John Wright
No relationship to disclose
Manisha H. Shah
Consulting or Advisory Role: Eisai, Loxo
Research Funding: Eisai, Merck, Bristol-Myers Squibb
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2015. CA Cancer J Clin 65:5-29, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Shaha AR, Shah JP, Loree TR: Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg 174:474-476, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Woyach JA, Shah MH: New therapeutic advances in the management of progressive thyroid cancer. Endocr Relat Cancer 16:715-731, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Brose MS, Nutting CM, Jarzab B, et al. : Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 384:319-328, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlumberger M, Tahara M, Wirth LJ, et al. : Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621-630, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Guessous F, Kofman A, et al. : XL-184, a MET, VEGFR-2 and RET kinase inhibitor for the treatment of thyroid cancer, glioblastoma multiforme and NSCLC. IDrugs 13:112-121, 2010 [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen JG, Burrows J, Salgia R: c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett 225:1-26, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Newton RC, Scherle PA: Developing c-MET pathway inhibitors for cancer therapy: Progress and challenges. Trends Mol Med 16:37-45, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Roskoski R, Jr: VEGF receptor protein-tyrosine kinases: Structure and regulation. Biochem Biophys Res Commun 375:287-291, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Birchmeier C, Birchmeier W, Gherardi E, et al. : Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4:915-925, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Chattopadhyay C, El-Naggar AK, Williams MD, et al. : Small molecule c-MET inhibitor PHA665752: Effect on cell growth and motility in papillary thyroid carcinoma. Head Neck 30:991-1000, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Shojaei F, Lee JH, Simmons BH, et al. : HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res 70:10090-10100, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Eder JP, Vande Woude GF, Boerner SA, et al. : Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res 15:2207-2214, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Elisei R, Schlumberger MJ, Müller SP, et al. : Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31:3639-3646, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabanillas ME, Brose MS, Holland J, et al. : A phase I study of cabozantinib (XL184) in patients with differentiated thyroid cancer. Thyroid 24:1508-1514, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dadu R, Devine C, Hernandez M, et al. : Role of salvage targeted therapy in differentiated thyroid cancer patients who failed first-line sorafenib. J Clin Endocrinol Metab 99:2086-2094, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brose MS, Cabanillas ME, Cohen EE, et al. : Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: A non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol 17:1272-1282, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith MR, Sweeney CJ, Corn PG, et al. : Cabozantinib in chemotherapy-pretreated metastatic castration-resistant prostate cancer: Results of a phase II nonrandomized expansion study. J Clin Oncol 32:3391-3399, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee RJ, Saylor PJ, Michaelson MD, et al. : A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. Clin Cancer Res 19:3088-3094, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drilon A, Wang L, Hasanovic A, et al. : Response to cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 3:630-635, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]