Abstract
Background
Extrahepatic metastases have important implications in the clinical management of hepatocellular carcinoma (HCC). The purpose of this study was to validate tumor staging parameters and serum AFP as risk factors of HCC metastasis.
Patients and methods
In this retrospective case–control study, patients with a new diagnosis of HCC (N=236), median age 57 years (range 28–89 years), and male-to-female ratio of 183/53 were divided into a “no-met” group (N=101) without extrahepatic metastasis or a “met” group with extrahepatic metastases (N=135). Metastasis risk factors based on tumor staging parameters (size, number, infiltration, and vascular invasion) and serum AFP level were calculated as odds ratio (OR). Sensitivities of the risk factors as metastasis screening tests were also calculated.
Results
AFP >400 μg/mL, index tumor size >5 cm, and vascular invasion individually had strong association with metastasis, with OR (95% confidence interval) of 11.5 (5.9–22.1), 17.7 (9.0–34.8), and 18.9 (8.2–43.9), respectively, but with moderate sensitivities as metastasis screening tests, with 71.9% (65.7–77.3), 75.6% (69.6–80.7), and 58.5% (52.1–64.7), respectively. Composite multiparametric criteria, eg, a logical union of 1) tumor size outside of Milan criteria, 2) AFP threshold >35 μg/mL, and 3) vascular invasion, had excellent OR up to 55.6 (13.0–237.1) with screening sensitivity 98.5% (95.8–99.6).
Conclusion
Serum AFP, tumor size, and vascular invasion are strongly associated with extrahepatic metastasis of HCC, especially when combined into a multiparametric metastasis prediction criterion.
Keywords: hepatocellular carcinoma, risk factor, α-fetoprotein, stage, metastasis
Introduction
Hepatocellular carcinoma (HCC) is currently eighth leading cause of cancer-related deaths in the USA1 and the fastest growing cancer in mortality.2 Treatment recommendation depends on the patient’s clinical status (eg, liver function and performance status) and tumor stage.3,4 Patients with advanced-stage HCC, defined by the presence of vascular invasion and/or extrahepatic metastasis, are generally not considered candidates for curative treatment and are usually treated with systemic or palliative therapy. The prognosis of advanced-stage HCC is poor with median survival <1 year, in contrast to the ~70% 5-year survival of early-stage HCC.5–7
Extrahepatic metastasis (“metastasis” hereafter) occurs in one-third of patients with HCC,8,9 with the most common sites being lung, lymph nodes, bone, and adrenal glands.10,11 Metastases have important management implications, as locoregional therapies (eg, ablation, resection, and liver transplantation) are no longer effective controlling extrahepatic disease.4,5,12,13 Metastasis is also an independent predictor of poor survival.14–17 Therefore, it is crucial to determine the presence of metastasis at the time of initial HCC diagnosis, as initiation of appropriate therapy will determine survival.11 However, exhaustive metastasis workup, which may include chest CT and bone scintigraphy, may be costly, time consuming, and unnecessary for those with low risk of metastasis.
Several noninvasive prognostic parameters of HCC have been proposed, including tumor size and serum AFP levels. Tumor size is an independent predictor of HCC progression, metastasis development, and overall survival.10,18–20 High levels of AFP are independently associated with metastasis risk and poorer prognosis.21–23 Other parameters, such as vascular invasion and the number of tumors, also have survival implications.23,24 Accordingly, these parameters are integral part of various HCC staging systems.12,25–30 To our knowledge, however, no specific criteria have been proposed for HCC metastasis risk stratification.
We hypothesize that the tumor staging parameters (eg, tumor size, number, infiltration, vascular invasion, and AFP) are associated with synchronous or metachronous metastasis in patients with HCC. The purpose of this study was to validate the tumor staging parameters, either as single-parametric criteria or as multi-parametric criteria, as risk factors of HCC metastasis. If validated, such criteria may allow rapid metastasis risk stratification at the time of diagnostic imaging and facilitate timely management decisions including the need for comprehensive metastasis workup.
Patients and methods
Study design and patient population
This retrospective case–control study at a tertiary-care public hospital was approved by the University of Texas Southwestern Medical Center’s Investigational Review Board. Data were de-identified in compliance with Health Insurance Portability and Accountability Act. The need to obtain informed consent was waived. A review of an HCC clinic database was conducted to identify 457 consecutive patients with a new diagnosis of HCC between January 2005 and December 2011. HCC diagnosis was made either by direct tissue sampling or dynamic contrast-enhanced cross-sectional imaging (CT or MRI) per routine clinical care, interpreted by staff pathologists and radiologists, respectively. Two authors (AGS and ACY) adjudicated each case to confirm that it met diagnostic criteria by histology or the American Association for the Study of Liver Disease.31 Routine evaluation of metastatic disease included chest X-ray and abdominal-pelvic CT (if not already performed). Additional imaging, such as chest CT and bone scan, was performed at the discretion of the treating physician.
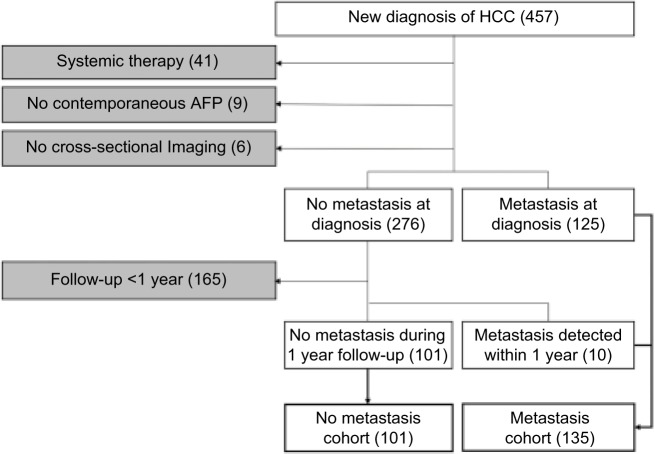
By chart review, patients were divided into the following two cohorts: 1) “no-met” cohort comprised patients without documented metastasis at the time of HCC diagnosis and during the first 12-month follow-up period from the initial diagnosis and 2) “met” cohort with documented metastasis at the time of initial diagnosis or detected during the first 12-month follow-up. A single index imaging study (CT or MRI) was selected for each patient. For those in the no-met group, the index study was the initial imaging examination leading to the HCC diagnosis. For those in the met group, the index imaging study was the pretreatment imaging examination closest to the time of metastasis diagnosis. Patients were excluded from the study if the image/clinical data were incomplete, or determination of the HCC metastasis status was compromised: 1) no documented metastasis but follow-up period <12 months; 2) no available pretreatment CT or MRI; 3) systemic HCC treatment during the follow-up period; 4) index CT/MRI images not available; 5) no contemporaneous pretreatment serum AFP, defined as <3 months of the index CT/MRI; and 6) history of any other cancers. The inclusion–exclusion criteria and the number of patients are graphically summarized in Figure 1.
Figure 1.
Flowchart of inclusion and exclusion criteria (white and gray, respectively) with the number of subjects meeting each criterion in parentheses.
Abbreviation: HCC, hepatocellular carcinoma.
Data collection
For each patient, the index study’s images and its radiology report were reviewed. An axial image series that best depicted the tumor boundary was selected. The size of the dominant (index) tumor, either meeting the AASLD imaging criteria or biopsy proven, was measured as maximum axial diameter. Other nonindex tumors were measured, if they met the imaging criteria or had similar imaging features as the biopsy-proven index tumor. For diffuse infiltrative HCC, its size was coded as 99 cm due to difficulty in delineating the tumor boundary. Presence of vascular invasion (ie, involving either portal vein or hepatic vein) and infiltrative morphology were determined based on the official radiology report, in order to maintain consistency with the patient’s clinical management decisions.
The AFP level (μg/mL) and other demographic and clinical data including the age, sex, and Child-Turcotte-Pugh stage were recorded. Etiology of liver disease, including hepatitis C (positive serum antibody or RNA), hepatitis B (positive surface antigen), alcohol-related liver disease (alcohol intake >40 g/day for ≥10 years), nonalcoholic steatohepatitis (negative work-up for other etiologies in the presence of the metabolic syndrome), and others/unknown, was noted.
A database was constructed using Microsoft Excel (Micro-soft Corporation, Redmond, WA, USA), which recorded tumor size, number, AFP, infiltration, and vascular invasion status, as well as the metastasis status (met vs no-met).
Metastasis risk criteria
Various staging parameters were considered as candidate metastasis risk factors and included AFP, tumor size, number, vascular invasion, and infiltrative morphology. These parameters were primarily derived from the following HCC staging systems: American Joint Commission on Cancer TNM system,25 Barcelona Clinic Liver Cancer system,26 Cancer of the Liver Italian Program score,27 and Chinese University Prognostic Index.28 Although not strictly a staging system, the Milan criteria for liver transplant eligibility12 was also considered. Okuda et al29 and Japan integrated staging score30 were not considered separately, as the former did not include absolute size threshold and the latter used the TNM system for local staging. Although infiltrative morphology is not a part of any existing staging system, its metastasis risk was evaluated as it is associated with poor prognosis.32,33 The continuous variables (ie, AFP, index tumor size, and number of tumors) were stratified into discrete intervals (ie, interval data) according to the threshold values used in different staging systems as follows: AFP intervals 0–35, 35–400, and >400 μg/mL; index tumor size 0–3, 3–5, and >5 cm; and number of tumors 1, 2–3, and >3. Bivariate variables (vascular invasion and infiltrative morphology) were treated as categorical data.
Statistical analysis
Statistical analyses were performed using R Version 3.3.1.34 Summary statistics was calculated for demographic and clinical data, and the differences between the no-met and met cohorts were assessed using Mann–Whitney U test for continuous data and chi-square test for categorical data. Multivariate logistic regression was performed to identify statistically significant independent risk factors of metastasis. Rather than constructing a computationally demanding regression model, however, we considered a series of simple single- or multi-parametric criteria based on previously proposed threshold values. This approach is conceptually analogous to the Milan criteria,12 which incorporates several parameters (tumor size, number, vascular invasion, and extrahepatic metastasis) into a single decision-rule using logical (union or intersection) operations. For each single- and multiparametric risk criteria, the association with metastasis was assessed by constructing the standard 2×2 contingency table and calculating the odds ratio (OR) with 95% confidence intervals (CIs). Treating the risk criteria as a diagnostic test, the sensitivity, specificity, accuracy, and positive and negative predictive values were also calculated with their respective 95% CIs. The metastasis sensitivity between the single- and multiparametric criteria was compared using exact binomial test using R’s Diagnostic Test Comparison for Paired Study Design package (DTCom-Pair). P-values <0.05 were considered statistically significant, after Benjamini–Hotchberg adjustment for multiple testing when appropriate.35
Results
Patient population
The summary statistics of the study population (n=236) is summarized in Table 1, including the demographics, clinical data, and the tumor staging parameters. There was no significant difference in the patient age, sex, race, or liver disease etiology between the met and no-met cohorts. Patients with metastases tended to have more advanced cirrhosis (CPT stage B or C). All tumor staging parameters were significantly worse in the met cohort than in the no-met cohort, with greater AFP values and index tumor size, as well as multifocal disease, vascular invasion, and infiltrative tumor being more frequent. As expected, liver-directed HCC treatment was more common in the no-met cohort.
Table 1.
Demographic and clinical data
| Patient characteristics | Total | No met | Met | P |
|---|---|---|---|---|
| Demographics | ||||
| Number of patients | 236 (100%) | 101 (42.8%) | 135 (57.2%) | |
| Median age (range) (years) | 57 (28–89) | 55 (37–79) | 58 (28–89) | 0.084 |
| Sex (male/female) | 183/53 | 76/25 | 107/28 | 0.500 |
| Race | ||||
| Caucasian | 61 | 27 | 34 | 0.645 |
| Black | 89 | 34 | 55 | |
| Hispanic | 69 | 31 | 38 | |
| Others | 17 | 9 | 8 | |
| Etiology of liver disease | ||||
| HCV | 163 | 71 | 92 | 0.678 |
| HBV | 25 | 8 | 17 | |
| Alcohol | 28 | 12 | 16 | |
| NASH | 13 | 7 | 6 | |
| Others/cryptogenic | 13 | 4 | 9 | |
| Child-Turcotte-Pugh stage | ||||
| A | 99 | 56 | 43 | 0.001 |
| B | 93 | 32 | 61 | |
| C | 43 | 12 | 31 | |
| Unknown | 1 | 1 | 0 | |
| Tumor staging parameters | ||||
| AFP median (IQR) | 148 (12–4116) | 14 (5–81) | 1,725 (113–18,104) | <0.001 |
| Index tumor size (IQR) (cm) | 5.0 (2.9–10.0) | 2.9 (2.0–4.2) | 8.7 (5.4–14.0) | <0.001 |
| Single/multiple | 140/96 | 74/27 | 66/69 | <0.001 |
| Vascular invasion (−/+) | 150/86 | 94/7 | 56/79 | <0.001 |
| Infiltrative tumor (−/+) | 208/28 | 99/2 | 109/26 | <0.001 |
| Loco-regional therapy | ||||
| Treatment (yes/no) | 104/132 | 78/23 | 26/109 | <0.001 |
| Liver transplantation | 4 | 4 | 0 | |
| Chemoembolization | 66 | 56 | 10 | |
| Thermal ablation | 13 | 12 | 1 | |
| Surgical resection | 22 | 20 | 2 | |
| Radioembolization | 6 | 1 | 5 |
Notes: No met, patients with no documented metastasis within 12 months of follow-up; Met, patients with synchronous or metachronous metastasis within 12 months of follow-up. Etiology – others, cryptogenic cirrhosis (N=8) and Wilson’s disease (N=1). (−/+) indicates feature absent/present.
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; NASH, nonalcoholic steatohepatitis
Single-parametric criteria
The metastasis risk of each staging parameter is summarized in Table 2. All staging parameters were associated with metastasis with variable strength, with ORs ranging 2.9–18.9. Multifocality had the weakest and vascular invasion had the strongest association with metastasis. The criteria with greatest sensitivity for extrahepatic metastasis were index tumor size >3 cm (91.9%), Milan size criteria (85.9%), and AFP >35 μg/mL (82.2%). Criteria with greatest specificities were infiltrative tumor (98.0%), number of tumors >3 (95.0%), and vascular invasion (93.1%).
Table 2.
Single-parametric criteria for metastasis
| Criteria | Threshold | Odds ratio | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|
| AFP (μg/mL) | >35 | 8.0 (4.4–14.5) | 82.2 (76.8–86.6) | 63.4 (57.0–69.3) | 74.2 (68.2–79.4) | 75.0 (69.0–80.2) | 72.7 (66.7–78.1) |
| >400 | 11.5 (5.9–22.1) | 66.7 (60.4–72.4) | 85.1 (78.0–89.2) | 74.6 (68.6–79.8) | 85.7 (80.6–89.7) | 65.6 (59.3–71.5) | |
| Index tumor size (cm) | >3 | 15.8 (7.6–32.9) | 91.9 (87.6–94.8) | 58.4 (52.0–64.6) | 77.5 (71.7–82.5) | 74.7 (68.7–79.9) | 84.3 (79.0–88.4) |
| >5 | 17.7 (9.0–34.8) | 75.6 (69.6–80.7) | 85.1 (78.0–89.2) | 79.7 (74.0–84.4) | 87.2 (82.2–90.9) | 72.3 (66.2–77.6) | |
| Number of tumors | >1 | 2.9 (1.6–5.0) | 51.1 (44.7–57.5) | 73.3 (67.2–78.6) | 60.6 (54.2–66.7) | 71.9 (65.8–77.3) | 52.9 (46.4–59.2) |
| >3 | 12.0 (4.6–31.5) | 38.5 (32.5–44.9) | 95.0 (91.4–97.3) | 62.7 (56.3–68.7) | 91.2 (86.8–94.3) | 53.6 (47.2–59.9) | |
| Milan size criteria | a | 16.7 (8.7–32.2) | 85.9 (80.8–89.9) | 73.3 (67.2–78.6) | 80.5 (74.9–85.1) | 81.1 (75.6–85.7) | 79.6 (73.9–84.3) |
| Vascular invasion | – | 18.9 (8.2–43.9) | 58.5 (52.1–64.7) | 93.1 (89.0–95.8) | 73.3 (67.3–78.6) | 91.9 (87.6–94.8) | 62.7 (56.3–68.7) |
| Infiltrative tumor | – | 11.8 (2.7–51.0) | 19.3 (14.7–24.8) | 98.0 (95.2–99.3) | 53.0 (46.6–59.3) | 92.9 (88.7–95.6) | 47.6 (41.3–54.0) |
Notes: Data in parentheses are Wilson’s 95% confidence interval.
Milan size criteria: one tumor <5 cm or up to three tumors <3 cm with no extrahepatic disease or vascular invasion.
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
Logistic regression
Multivariate logistic regression results are summarized in Table 3. Milan size criteria were excluded from this analysis for its obvious correlation (by design) with tumor size and number. After correction for covariates, the following risk factors were independently associated with metastasis: AFP level, index tumor size, and presence of vascular invasion, all with adjusted P-values <0.05. No significance was detected for the number of tumor nodules and infiltrative morphology, after correcting for effects of AFP, tumor size, and vascular invasion.
Table 3.
Tumor staging parameters and multivariate logistic regression
| Risk factor | Categories | # patients | Odds ratio (95% CI) | Unadj P-value | Adj P-value |
|---|---|---|---|---|---|
| α-Fetoprotein (μg/mL) | <35 | 88 (37.3%) | 2.41 (1.52–3.80) | 0.0002 | 0.0008 |
| 35–400 | 43 (18.2%) | ||||
| >400 | 105 (44.5%) | ||||
| Index tumor size (cm) | <3 | 70 (29.7%) | 4.11 (2.54–6.65) | <0.0001 | <0.0001 |
| 3–5 | 49 (20.8%) | ||||
| >5 | 117 (49.6%) | ||||
| Number of tumors | 1 | 140 (59.3%) | 1.82 (1.03–2.98) | 0.0378 | 0.1111 |
| 2–3 | 39 (16.5%) | ||||
| >3 | 57 (24.1%) | ||||
| Vascular invasion | Absent | 150 (63.6%) | 5.90 (2.04–17.04) | 0.0011 | 0.0039 |
| Present | 86 (36.4%) | ||||
| Infiltrative morphologya | Absent | 208 (88.1%) | 3.56 (0.36–17.31) | 0.3529 | 0.8646 |
| Present | 28 (11.9%) |
Notes:
Although infiltrative morphology is not a part of existing staging system, its metastasis risk was evaluated as it is a marker of poor prognosis. The P-values were adjusted using the Benjamini–Hochberg method for multiple comparisons.
Abbreviations: CI, confidence interval; Unadj, unadjusted; Adj, adjusted.
Multiparametric criteria
Of all possible logical combination of the independent risk factors (AFP, tumor size, and vascular invasion), only those that would improve sensitivity by “union” operations (to be used as metastasis screening test) were analyzed and results are shown in Table 4. Other logical combinations of risk factors, or other choices of threshold values and ranges, were not exhaustively considered to control the total number of simultaneous tests and associated penalty on the adjusted P-values. All combination criteria of AFP, size, and vascular invasion performed well with high sensitivities ranging from 86.7 to 98.5% for the detection of extrahepatic metastasis, with variable specificities and ORs. Multiparametric criteria including “Milan size or vascular invasion or AFP >35 μg/mL” had the highest sensitivity and OR of 98.5% and 55.6, respectively. The sensitivity of this multiparametric criteria was significantly better (P<0.001) than those of Milan size criteria, vascular invasion, or AFP >35 μg/mL alone as single-parametric criteria. Therefore, for patients determined to be within the Milan criteria based on imaging, the presence of extrahepatic metastatic disease is virtually excluded if AFP is also <35 μg/mL.
Table 4.
Multiparametric criteria for metastasis
| Criteria | Threshold | Odds ratio | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Index tumor >3 cm or VI | – | 23.7 (10.1–55.8) | 94.8 (91.1–97.1) | 56.4 (50.0–62.7) | 78.4 (72.6–83.2) | 74.4 (68.4–79.6) | 89.1 (84.4–92.5) |
| Index tumor >5 cm or VI | – | 26.3 (13.1–52.9) | 86.7 (81.7–90.5) | 80.2 (74.6–84.8) | 83.9 (78.6–88.1) | 85.4 (80.3–89.4) | 81.8 (76.3–86.3) |
| Milan size criteriaa or VI | – | 25.5 (12.1–53.8) | 91.9 (87.6–94.8) | 69.3 (63.1–74.9) | 82.2 (76.8–86.6) | 80.0 (74.4–84.7)) | 86.4 (81.4–90.3) |
| Index tumor >3 cm or AFP | >35 | 19.8 (6.8–57.8) | 97.0 (93.9–98.7) | 37.6 (31.6–44.0) | 71.6 (65.5–77.0) | 67.5 (61.3–73.2) | 90.5 (86.0–93.7) |
| >400 | 23.7 (9.5–58.8) | 95.6 (92.0–97.7) | 52.5 (46.1–58.8) | 77.1 (71.3–82.1) | 72.9 (66.8–78.2) | 89.8 (85.2–93.2) | |
| Index tumor >5 cm or AFP | >35 | 19.8 (8.7–44.6) | 94.1 (90.2–96.5) | 55.4 (49.2–61.7) | 77.5 (71.7–82.5) | 73.8 (67.8–79.1) | 87.5 (82.6–91.2) |
| >400 | 26.3 (12.9–53.7) | 89.6 (85.0–93.0) | 75.3 (69.3–80.4) | 83.5 (78.1–87.7) | 82.9 (77.5–87.2) | 84.4 (79.2–88.6) | |
| Milan size criteriaa or AFP | >35 | 41.5 (12.4–138.9) | 97.8 (94.8–99.2) | 48.5 (42.3–54.9) | 76.7 (70.8–81.7) | 71.7 (65.6–77.2) | 94.2 (90.4–96.7) |
| >400 | 25.3 (11.5–55.7) | 93.3 (89.3–96.0) | 64.4 (58.0–70.2) | 80.9 (75.4–85.5) | 77.8 (72.0–82.7) | 87.8 (88.0–91.5) | |
| Index tumor >3 cm or VI or AFP | >35 | 24.4 (7.2–82.1) | 97.8 (94.8–99.2) | 35.6 (29.8–42.0) | 71.2 (65.0–76.6) | 67.0 (60.7–72.7) | 92.3 (88.1–95.2) |
| >400 | 33.4 (11.5–97.2) | 97.0 (93.9–98.7) | 50.5 (44.1–56.9) | 77.1 (71.3–82.1) | 72.4 (66.3–77.7) | 92.7 (88.6–95.5) | |
| Index tumor >5 cm or VI or AFP | >35 | 27.6 (10.4–73.1) | 96.3 (92.9–98.2) | 51.5 (45.1–57.8) | 77.1 (71.3–82.1) | 72.6 (66.6–78.0) | 91.2 (86.8–94.3) |
| >400 | 34.8 (15.6–77.5) | 93.3 (89.3–96.0) | 71.3 (65.2–76.7) | 83.9 (78.6–88.1) | 81.3 (75.8–85.8) | 88.9 (84.2–92.4) | |
| Milan size criteriaa or VI or AFP | >35 | 55.6 (13.0–237.1) | 98.5 (95.8–99.6) | 45.5 (39.3–52.0) | 75.8 (69.9–80.9) | 70.7 (64.6–76.2) | 95.8 (92.3–97.9) |
| >400 | 34.2 (13.7–85.0) | 95.6 (92.0–97.7) | 61.4 (55.0–67.4) | 80.9 (75.4–85.5) | 76.8 (70.9–81.8) | 91.2 (86.8–94.3) |
Notes:
Milan size criteria: one tumor <5 cm or up to three tumors <3 cm with no extrahepatic disease or vascular invasion. Data in parentheses are 95% CIs.
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; VI, vascular invasion.
Comparison of criteria
The exact binomial test P-values for the differences in diagnostic sensitivity between the single-, two-, and three-parametric criteria are shown in Table 5. Depending on the tumor size parameter (>3 cm, >5 cm, or Milan size criteria), two-parametric criteria with combination of size parameter and either AFP >35 μg/mL or vascular invasion performed significantly better, and three-parametric criteria with combination of all size parameter, AFP >35 μg/mL, and vascular invasion performed significantly better than two-parametric criteria.
Table 5.
Pairwise sensitivity comparison P-values between single-, two-, and three-parametric criteria
| Metastasis prediction criteria | Index tumor >3 cm or VI | Index tumor >3 cm or AFP >35 mg/mL | Index tumor >3 cm or VI or AFP >35 mg/mL |
|---|---|---|---|
| Index tumor >3 cm | 0.1250 (0.1500) | 0.0156 (0.0312) | 0.0078 (0.0234) |
| Index tumor >3 cm or VI | – | 0.3750 (0.3750) | 0.1250 (0.1500) |
| Index tumor >3 cm or AFP >35 mg/mL | – | – | 0.0074 (0.0234) |
|
| |||
| Index tumor >5 cm or VI | Index tumor >5 cm or AFP >35 mg/mL | Index tumor >5 cm or VI or AFP >35 mg/mL | |
|
| |||
| Index tumor >5 cm | <0.0001 (<0.0001) | <0.0001 (<0.0001) | <0.0001 (<0.0001) |
| Index tumor >5 cm or VI | – | 0.0210 (0.0252) | 0.0002 (0.0003) |
| Index tumor >5 cm or AFP >35 mg/mL | – | – | 0.2500 (0.2500) |
|
| |||
| Milan size criteria or VI | Milan size criteria or AFP >35 mg/mL | Milan size criteria or VI or AFP >35 mg/mL | |
|
| |||
| Milan size criteria | 0.0078 (0.0094) | <0.0001 (<0.0001) | <0.0001 (<0.0001) |
| Milan size criteria or VI | – | 0.0215 (0.0215) | 0.0039 (0.0078) |
| Milan size criteria or AFP >35 mg/mL | – | – | 0.0074 (0.0095) |
Note: Values within parentheses indicate Benjamini–Hochberg adjusted P-values for multiple comparisons.
Abbreviation: VI, vascular invasion.
Discussion
In patients with new diagnosis of HCC, extrahepatic metastasis can profoundly impact treatment options and prognosis. Determination of metastasis risk using readily available imaging and serum AFP data may facilitate timely management decision-making, including the need for exhaustive metastasis workup. Furthermore, correct risk stratification of these patients may help avoid unnecessary morbidity and cost associated to loco-regional therapy. The purpose of this retrospective study in patients with new diagnosis of HCC was to validate the association between tumor staging parameters and synchronous/metachronous metastases, ultimately to enable rapid metastasis risk stratification based on imaging findings in conjunction with AFP.
Among the staging parameters in existing HCC staging systems, we found that index tumor size, vascular invasion, and AFP are independently associated with metastasis. Logical combinations of these parameters tended to be more strongly associated with metastases, often with significantly higher sensitivity for metastasis detection. In particular, the combination of Milan size criteria, vascular invasion, and AFP >35 μg/ml had the highest OR, sensitivity, and negative predictive values; in those patients in whom these criteria were simultaneously negative, metastasis was exceedingly rare.
The current National Comprehensive Cancer Network (NCCN, Version 2.2016) recommendation for confirmed HCC cases includes a chest CT and optional whole-body bone scintigraphy for metastatic workup.36 Utility of whole-body PET imaging has been investigated,37,38 but its use as routine metastasis workup tool remains controversial. Up to 26/109 (24%) of our patients in the met cohort received loco-regional therapy, illustrating the difficulty of predicting extrahepatic metastasis at initial diagnosis with current staging approaches. Our study suggests that only a subset of patients with HCC is higher risk for metastasis who may require formal metastasis workup. Metastasis risk may be easily assessed based multiphasic liver CT or MRI findings in conjunction with serum AFP. In low-risk patients, it may be reasonable to refer immediately to liver-directed therapy, thereby shortening time to therapy and saving cost of additional imaging studies. In our study population, for example, the total cost saving would have been $21,000 in MEDICARE US dollars (or ~$90/patient), assuming the standard metastasis workup consisting of chest CT without contrast and whole body bone scan. Patients otherwise not meeting the low-risk criteria may benefit from comprehensive metastasis workup, and any extrahepatic abnormality found on imaging should be scrutinized with high suspicion of metastatic disease, especially those with high AFP >400 μg/mL, vascular invasion, multifocal, or infiltrative tumor(s), due to their moderate to high specificity for metastases.
Due to retrospective design, this study has several limitations. First, patient assignment into the no-met cohort was based on 12-month metastasis-free survival. This requirement was necessary because patients did not undergo uniform metastasis workup; the decision to obtain chest CT, bone scintigraphy, or PET in addition to routine chest radiograph was made at the discretion of the treatment provider, as per NCCN guideline at the time of their clinical care.39 As this may have led to under-detection of subclinical metastases at initial diagnosis (ie, verification bias), we extended 12 months of clinical and/or imaging observation to allow initially undetected metastasis to declare itself over time. However, this requirement resulted in exclusion of ~1/3 of the potentially eligible patients who were lost during follow-up. Therefore, patients with very aggressive tumors or decompensated cirrhosis may have been underrepresented, as they were not likely to have survived long enough to meet the inclusion/exclusion criteria. Second, the potential therapy effect on metastasis development and detection could not be completely addressed. Patients already receiving systemic therapy at the time of diagnosis were excluded to prevent the confounding effect of systemic therapy on metastases. Majority of the no-met cohort and minority of the met cohort underwent liver-directed therapy. While complete response after such loco-regional therapy likely would not affect the evolution of extrahepatic metastases, partial, stable, or progressive disease could potentially pose additional risk of subsequent metastasis development. However, we believe that this was only a minor concern, since the majority of the met cohort had metastatic disease before initiation of therapy (125 out of 135 in met cohorts), and the possible confounding effect by treatment outcome is expected to be small. This study did not investigate cirrhosis as a risk factor of metastasis. The data on cirrhosis were incomplete, because determination of the cirrhosis status using a reference standard method (random liver biopsy; no serum markers or elastography techniques were available at the time of this study) was often not needed for clinical care. The HCC patient population at this public safety-net hospital has been overwhelmingly (>95%) those with known or suspected cirrhosis, and therefore, this population would not have allowed meaningful sub-analysis, even if the cirrhosis data were available. Finally, this study was conducted in a public safety-net hospital in a large metropolitan area, where patients could present with more advanced HCC in theory compared to insured population undergoing routine HCC surveillance.40,41 The rate of metastatic disease in this population, however, was similar to previous reports.8,9 Also, estimates of the OR, sensitivity, and specificity are independent of disease prevalence and hence our results may be generalizable to other patient populations. Our population was composed entirely of cirrhotic patients, most due to chronic hepatitis C infection; this may influence the frequency of metastasis and AFP threshold level.42–45 Due to these limitations, the metastasis screening criteria need to be further validated prospectively in an independently sampled population.
Conclusion
This retrospective study validated that tumor staging parameters are associated with metastasis risk in patients with new diagnosis of HCC. Patients with low metastasis risk may be identified based on AFP, tumor size, and absence of vascular invasion. In these patients, comprehensive metastasis workup may not be needed, thereby facilitating timely delivery of treatment and eliminating cost for further diagnostic imaging studies.
Acknowledgments
This study was conducted with support from the Center for Translational Medicine, NIH/NCATS grant number UL1TR001105, and NIH/NCATS grant number KL2TR001103. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Center for Translational Medicine, UT Southwestern Medical Center, and its affiliated academic and health care centers, the National Center for Advancing Translational Sciences, or the National Institutes of Health.
Footnotes
Disclosure
AGS is on the Speakers’ Bureau and a consultant for Bayer. ACY is on the Speakers’ Bureau for Bayer and received research funding from Novartis, Merck, and Peregrine. The authors report no other conflicts of interest in this work.
References
- 1.U.S. Cancer Statistics Working Group [webpage on the Internet] United States Cancer Statistics: 1999–2012 Incidence and Mortality Web-based Report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2015. [Accessed September 25, 2017]. Available from: http://www.cdc.gov/uscs. [Google Scholar]
- 2.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134(6):1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 6.Padhya KT, Marrero JA, Singal AG. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2013;29(3):285–292. doi: 10.1097/MOG.0b013e32835ff1cf. [DOI] [PubMed] [Google Scholar]
- 7.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20(11):1781–1787. doi: 10.1111/j.1440-1746.2005.03919.x. [DOI] [PubMed] [Google Scholar]
- 9.Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117(19):4475–4483. doi: 10.1002/cncr.25960. [DOI] [PubMed] [Google Scholar]
- 10.Yoo DJ, Kim KM, Jin YJ, et al. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol. 2011;26(1):145–154. doi: 10.1111/j.1440-1746.2010.06341.x. [DOI] [PubMed] [Google Scholar]
- 11.Katyal S, Oliver JH, 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216(3):698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 12.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz M. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S268–S276. doi: 10.1053/j.gastro.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 14.Sala M, Forner A, Varela M, Bruix J. Prognostic prediction in patients with hepatocellular carcinoma. Semin Liver Dis. 2005;25(2):171–180. doi: 10.1055/s-2005-871197. [DOI] [PubMed] [Google Scholar]
- 15.Chlebowski RT, Tong M, Weissman J, et al. Hepatocellular carcinoma. Diagnostic and prognostic features in North American patients. Cancer. 1984;53(12):2701–2706. doi: 10.1002/1097-0142(19840615)53:12<2701::aid-cncr2820531224>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Calvet X, Bruix J, Gines P, et al. Prognostic factors of hepatocellular carcinoma in the west: a multivariate analysis in 206 patients. Hepatology. 1990;12(4 pt 1):753–760. doi: 10.1002/hep.1840120422. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29(1):62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Yan ZL, Gong RY, et al. Independent factors and predictive score for extrahepatic metastasis of hepatocellular carcinoma following curative hepatectomy. Oncologist. 2012;17(7):963–969. doi: 10.1634/theoncologist.2011-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol. 2002;8(2):193–199. doi: 10.3748/wjg.v8.i2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Xiao GQ, Yan LN, et al. Value of alpha-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J Gastroenterol. 2013;19(11):1811–1819. doi: 10.3748/wjg.v19.i11.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101(3):524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 22.Tangkijvanich P, Anukulkarnkusol N, Suwangool P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31(4):302–308. doi: 10.1097/00004836-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64(8):1700–1707. doi: 10.1002/1097-0142(19891015)64:8<1700::aid-cncr2820640824>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 24.Jun L, Zhenlin Y, Renyan G, et al. Independent factors and predictive score for extrahepatic metastasis of hepatocellular carcinoma following curative hepatectomy. Oncologist. 2012;17(7):963–969. doi: 10.1634/theoncologist.2011-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edge SB, American Joint Committee on Cancer. American Cancer Society . AJCC Cancer Staging Handbook: from the AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. p. xix.p. 718. [DOI] [PubMed] [Google Scholar]
- 26.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 27.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28(3):751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 28.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94(6):1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 29.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56(4):918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 30.Kudo M, Chung H, Haji S, et al. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40(6):1396–1405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 31.Bruix J, Sherman M, Practice Guidelines Committee. American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 32.Yopp AC, Mokdad A, Zhu H, et al. Infiltrative hepatocellular carcinoma: natural history and comparison with multifocal, nodular hepatocellular carcinoma. Ann Surg Oncol. 2015;22(Suppl 3):S1075–S1082. doi: 10.1245/s10434-015-4786-7. [DOI] [PubMed] [Google Scholar]
- 33.Kneuertz PJ, Demirjian A, Firoozmand A, et al. Diffuse infiltrative hepatocellular carcinoma: assessment of presentation, treatment, and outcomes. Ann Surg Oncol. 2012;19(9):2897–2907. doi: 10.1245/s10434-012-2336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 36.NCCN [webpage on the Internet] NCCN Clinical Practice Guidelines in Oncology. 2016. [Accessed September 25, 2017]. [cited August 22, 2016]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 37.Yoon KT, Kim JK, Kim DY, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in detecting extrahepatic metastasis in pretreatment staging of hepatocellular carcinoma. Oncology. 2007;72(Suppl 1):104–110. doi: 10.1159/000111715. [DOI] [PubMed] [Google Scholar]
- 38.Cho Y, Lee DH, Lee YB, et al. Does 18F-FDG positron emission tomography-computed tomography have a role in initial staging of hepatocellular carcinoma? PLoS One. 2014;9(8):e105679. doi: 10.1371/journal.pone.0105679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benson AB, 3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7(4):350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singal AG, Yopp A, S Skinner C, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861–867. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singal AG, Yopp AC, Gupta S, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila) 2012;5(9):1124–1130. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology. 1998;27(1):273–278. doi: 10.1002/hep.510270140. [DOI] [PubMed] [Google Scholar]
- 43.Di Bisceglie AM, Sterling RK, Chung RT, et al. HALT-C Trial Group Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C trial. J Hepatol. 2005;43(3):434–441. doi: 10.1016/j.jhep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Sterling RK, Wright EC, Morgan TR, et al. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am J Gastroenterol. 2012;107(1):64–74. doi: 10.1038/ajg.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gopal P, Yopp AC, Waljee AK, et al. Factors that affect accuracy of alpha-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12(5):870–877. doi: 10.1016/j.cgh.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]