Abstract
Recent findings suggest that the melastatin transient receptor potential channel 7 (TRPM7) is overexpressed in many types of cancers and is involved in tumorigenesis. However, its expression pattern and the potential role in bladder cancer remain unclear. The aim of the present study was to investigate the expression status of TRPM7 and its relationship with the development of bladder cancer. In the present study, we observed that the expression of TRPM7 was significantly elevated in bladder cancer tissues compared with that noted in the adjacent non-tumor tissues. Furthermore, increased TRPM7 expression was significantly associated with recurrence, metastasis and prognosis. In addition, after knockdown of the expression of TRPM7 by siRNA, the proliferation and the motility of T24 and 5637 cells were obviously inhibited, and downregulation of TRPM7 was found to play an important role in bladder cancer cell apoptosis. In conclusion, our findings suggest that TRPM7 plays an important role in bladder cancer, and TRPM7 may serve as a potentially unfavorable factor and novel target for human bladder cancer.
Keywords: TRPM7, bladder cancer, prognosis, metastasis, proliferation, motility, apoptosis
Introduction
Bladder cancer is one of the most common genitourinary malignancies arising from the epithelial lining of the urinary bladder. In China, the incidence and mortality rate have been rapidly increasing in the last few decades (1,2). Tobacco use, Schistosoma infection, chemical exposure, diet and lifestyle trends and genetic susceptibility have been reported to be risk factors for the tumorigenesis and progression of bladder cancer (3–5). Standard treatment for bladder cancer patients includes surgical resection or adjuvant chemotherapy and radiation. However, despite advances in diagnosis and therapy, there has been no improvement in the overall survival rate for bladder cancer patients in recent decades. Therefore, identification of novel molecular markers is critical to refining our understanding of the pathogenesis of bladder cancer and for developing more efficient treatment and surveillance strategies.
The transient receptor potential (TRP) channel family includes biological transmembrane proteins that play an important role in various physiologic and pathologic processes by modulating cytoplasmic signaling and cellular responses (6). The melastatin transient receptor potential channel 7 (TRPM7), a member of the TRP channel family, functions as a non-selective cation channel and a protein kinase (7,8). Recent studies have reported that TRPM7 is highly expressed in various types of cancers such as breast, ovarian and prostate cancer, glioblastoma and pancreatic cancer (9–13). Accumulating evidence has also shown that TRPM7 plays an important role in malignant progression including the regulation of cell proliferation, adhesion, apoptosis, gene expression, cell migration and metastasis (14–19). It has been found that TRPM7 regulates cancer cell proliferation and migration mainly by the channel activity mediating influxes of both Ca2+ and Mg2+ (20,21). Moreover, regulation of cell adhesion by TRPM7 includes the combined effect of Ca2+ and kinase-dependent pathways on actomyosin contractility (22). However, the involvement of TRPM7 in the pathogenesis and progression of bladder cancer remains to be detected.
The aim of the present study was to characterize the expression and biological role of TRPM7 in bladder cancer. We found that TRPM7 was overexpressed in bladder cancer. In addition, TRPM7 was strongly correlated with clinicopathological characteristics and poor survival rates in bladder cancer. Furthermore, the effect of TRPM7 on the biological behaviors of bladder cancer was investigated by anti-TRPM7 small interfering RNA (siRNA) assays. The data revealed that TRPM7 promoted bladder cancer proliferation, migration and invasion.
Materials and methods
Tissue specimens
Bladder cancer and paired adjacent normal bladder tissues used for immunohistochemistry were collected from 74 bladder cancer patients who underwent surgical resection in The First Affiliated Hospital of China Medical University from 2008 to 2015. None of patients underwent chemotherapy, radiotherapy or adjuvant treatment before surgery. The present study was approved by the Ethics Committee of the China Medical University. Informed consent was obtained from all patients. Twenty pairs of fresh bladder cancer and adjacent non-tumor bladder tissues used for quantitative PCR were collected from The First Affiliated Hospital of China Medical University and snap frozen in liquid nitrogen until use. The patients had not received any therapy before admission.
Immunohistochemical staining
Immunohistochemistry for TRPM7 expression in bladder cancer tissues was performed using standard methods. Sections were deparaffinized in xylene and hydrated in a graded ethanol series. The sections were then processed in 10 mmol/l citrate buffer (pH 6.0) and heated at 120°C for 5 min to retrieve the antigen. After that, sections were soaking in 3% hydrogen peroxide for 20 min, which served as a blocking agent for endogenous peroxidase activity. After being rinsed in phosphate-buffered saline (PBS; pH 7.2), 10% goat serum was applied for 1 h at room temperature to block non-specific reactions. Sections were incubated with anti-TRPM7 goat polyclonal antibody (diluted 1:50; ab729; Abcam, Cambridge, MA, USA) overnight at 4°C. Horseradish peroxidase-conjugated anti-goat IgG was used as a secondary antibody. After washing, the peroxidase reaction was developed with 3,3′-diaminobenzidine tetrahydrochloride (DAB) chromogen solution. Finally, the sections were counterstained with hematoxylin, dehydrated and coverslipped. All the slides were evaluated independently by two pathologists using a conventional semi-quantitative scoring system according to a previously defined scoring system (16). Briefly, the intensity of the staining in each section was assessed as very strongly positive (3), moderately positive (2), weakly positive (1), or negative (0). The percentage of positive tumor cells were scored as <5% (0), 5–20% (1), 21–50% (2) and >50% (3) of cells. The final score was calculated by multiplying the percentage and the intensity score. A score of ≥4 was defined as high TRPM7 expression and scores of <4 were defined as low TRPM7 expression.
Cell culture and siRNA transfection
Human bladder cancer cell lines (BIU87, 5637 and T24) were cultured in RPMI-1640 medium with 10% fetal bovine serum (FBS) (both from HyClone, Logan, UT, USA) at 37°C in 5% CO2 incubator. We obtained TRPM7 siRNA and the negative control siRNA (NC_siR) from GenePharma Co. Ltd. (Shanghai, China). Before transfection, cells were plated overnight and grew to 30–50% confluency. According to the manufacturer's instructions, the siRNAs were transfected into 5637 and T24 cells using Lipofectamine™ 3000 (Invitrogen, Carlsbad, CA, USA). The sequences of siRNAs are shown in Table I. The efficiency of siRNA was determined by the protein and mRNA levels of TRPM7 in the 48-h post transfected cells.
Table I.
Sequences of the primer pairs for real-time RT-PCR and siRNAs targeting TRPM7.
| RNA names | Sequences (5–3′) |
|---|---|
| TRPM7 | F 5′-TAGCCTTTAGCCACTGGAC-3′ |
| R 5′-GCATCTTCTCCTAGATTTGC-3′ | |
| GAPDH | F 5′-GAAGGTGAAGGTCGGAGTC-3′ |
| R 5′-GAAGATGGTGATGGGATTTC-3′ | |
| TRPM7_siR 1 | S GCGCUUUCCUUAUCCACUUTT |
| A AAUGGAUAAGGAAAGCGCTT | |
| TRPM7_siR 2 | S CCAUAUCCCACAAUCUCAATT |
| A UUGAGAUUGUGGGAUAUGGTT | |
| TRPM7_siR 3 | S GGUGUUCCCAGAAAGGCAATT |
| A UUGCCUUUCUGGGAACACCTT | |
| NC_siR | S UUCUCCGAACGUGUCACGUTT |
| A ACGUGACACGUUCGGAGAATT |
TRPM7, transient receptor potential channel 7; F, forward; R, reverse; S, sense; A, antisense.
Immunofluorescence staining
BIU87, 5637 and T24 cells (5×104 cells/ml) were seeded on 12-mm coverslips for 24 h, fixed with 4% paraformaldehyde for 30 min, and then permeabilized for 20 min with 0.1% Triton X-100 solution and blocked in normal bovine serum for 30 min at room temperature. Cells were incubated overnight at 4°C with anti-TRPM7 (1:50; Abcam) antibody in 2% bovine serum albumin (BSA; BioShop, Burlington, ON, Canada), 2% FBS and 0.2% fish gelatin (Sigma-Aldrich, St. Louis, MO, USA). The cells were then incubated with FITC-labeled secondary antibody for 2 h at room temperature. Nuclei were labeled with 4,6-diamidino-2-phenylindole (DAPI) (2 µg/ml). Fluorescence was visualized with a confocal microscope (Carl Zeiss Inc., Gottingen, Germany).
Quantitative real-time PCR
Total RNA was isolated from bladder cancer and adjacent non-tumor bladder tissues with TRIzol reagent (Invitrogen). cDNA was synthesized using a Transcriptor First Strand cDNA Synthesis kit (Roche, Mannheim, Germany). Then, SYBR-Green Real-Time PCR Master Mix (Applied Biosystems, Foster City, CA, USA) was used. Real-time PCR was performed using Thermal Cycler Dice™ Real-Time System TP800 (Takara, Tokyo, Japan). Amplification conditions consisted of 2 min at 50°C for reverse transcription, 5 min at 95°C for Taq activation followed by 45 cycles of 94°C for 40 sec, one cycle of 58°C for 20 sec and elongation at 72°C for 30 sec. The sequence of primers designed for TRPM7 and GAPDH are listed in Table I. GAPDH served as the internal control for mRNA determination of TRPM7. Results were normalized to GAPDH. The relative gene expression was calculated using 2−ΔΔCt.
Western blot analysis
Cells were lysed by in ice-cold cell RIPA buffer. Protein concentration was measured using the BCA protein assay kit (Pierce, Rockford, IL, USA). Total cellular protein (30 µg) was separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) filter membranes (Millipore, Bedford, MA, USA). The membrane was blocked with 5% non-fat milk in Tris-buffered saline with Tween-20 (TBST) buffer for 2 h at room temperature and incubated overnight at 4°C with a specific primary antibody against TRPM7 (ab85016; 1:500 dilution; Abcam) and mouse-anti-human actin (1:2,000 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) as an internal control. Next, the membranes were incubated at room temperature for 1 h with horseradish peroxidase-conjugated goat anti-mouse IgG (1:2,500 dilution; Santa Cruz Biotechnology, Inc.), and signals were developed using Western Blotting Luminol Reagent (Gene Company Ltd., Hong Kong).
Cell proliferation assays
Cell proliferation was determined using the Cell Counting Kit-8 (CCK-8) assay (Dojindo, Tokyo, Japan). The cells (3×103 cells/well) were seeded into 96-well plates. At time points of 0, 6, 12, 24, 36 and 48 h, 10 µl of CCK-8 was added/well and was incubated at 37°C for 1 h. The absorbance was measured at 450 nm using a plate reader (Model 680; Bio-Rad Laboratories, Hercules, CA, USA) to determine the number of viable cells. All of the experiments were performed three times.
Wound healing assay
Cells (5×105 cells/well) were seeded into 6-well plates. After the cells were grown to 80% confluency, wounds were created by scraping the cells with a 100-µl pipette tip. The 6-well plates were incubated at 37°C and a microscope was used to observe the migrated distance every 12 h.
Transwell assay
The invasion and migration of cells were measured using 8-µm pore-size Transwell chambers with or without Matrigel (BD Biosciences, San Diego, CA, USA). Cells (1×105 cells/well) were seeded into the top chamber with the serum-free medium while the bottom chambers were filled with 500 µl complete RPMI-1640 medium. After 24 h of incubation, the cells remaining on the upper side were carefully removed with cotton swabs, and those cells that had migrated to the lower side were fixed and stained with 1.0% crystal violet. The cells were quantified by counting all of the cells that had migrated through the membrane in five random fields under a light microscope (magnification, ×200). The mean value was calculated from data obtained from three separate chambers.
Flow cytometric analysis for alteration in apoptosis
For apoptosis analysis, after transfection for 48 h, cells were collected, washed with PBS, and stained with FITC/Annexin V Apoptosis Detection Kit I (BD Biosciences), and analyzed by flow cytometric analysis.
Cell cycle analysis
Cells were trypsinized and washed in ice-cold PBS, and then fixed in ice-cold 75% ethanol in PBS overnight. PI/RNase staining buffer (BD Biosciences) was added, and the cells were incubated at 4°C for 30 min. Cell cycle profiles were analyzed using a FACSCalibur flow cytometer (BD Biosciences).
Calculation and statistical analysis
All data are presented as means ± standard deviation (SD) and analyzed using SPSS 21.0 statistical software (SPSS, Inc., Chicago, IL, USA). t-test was used to determine the significance of differences in multiple comparisons. Survival curves were estimated by Kaplan-Meier analysis and compared by the log-rank test. All tests performed were two-sided. P<0.05 was considered statistically significant.
Results
TRPM7 is overexpressed in bladder cancer tissues
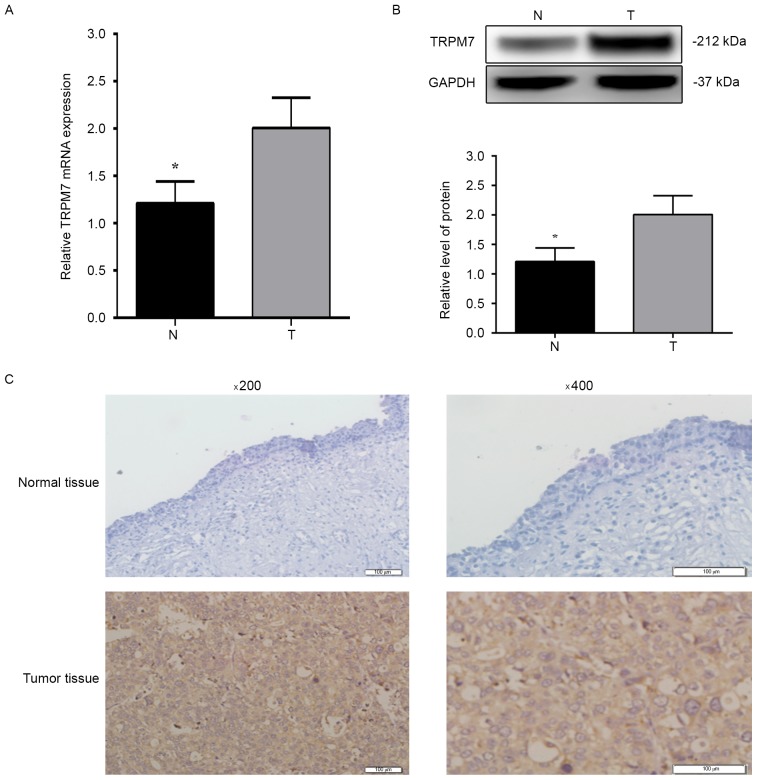
To characterize the expression of TRPM7 in human bladder cancer tissues, we firstly examined the mRNA and protein levels of TRPM7 in 20 pairs of human bladder cancer, and the adjacent non-tumor bladder tissues by qRT-PCR and western blotting. As shown in Fig. 1A and B, the mRNA and protein levels of TRPM7 were significantly increased in the bladder cancer tissues compared with levels in the paired adjacent non-tumor bladder tissues (P<0.05). Next, we performed immunohistochemical analysis to assess the protein expression and subcellular localization of TRPM7 in 74 paraffin-embedded bladder cancer tissues (Fig. 1C). High TRPM7 expression was detected in 47 (63.5%) of the 74 bladder cancer tissues and in 16 (25.80%) of the 62 adjacent non-tumor tissues (P<0.05). These data suggest that TRPM7 is overexpressed in bladder cancer tissues and may be a potential biomarker for bladder cancer.
Figure 1.
Expression of TRPM7 in human bladder cancer tissues. (A) The relative mRNA level of TRPM7 in 20 paired bladder cancer tissues and adjacent non-tumor tissues by qRT-PCR. (B) The relative protein level of TRPM7/GAPDH in bladder cancer tissues and adjacent non-tumor tissues. N, adjacent non-tumor tissues; T, bladder cancer tissues. (C) Immunohistochemistry findings of TRPM7 protein expression in bladder cancer and adjacent non-tumor tissues (original magnification, ×200 and ×400). Low expression of TRPM7 was noted in adjacent non-tumor tissues. High TRPM7 immunohistochemical staining was noted in bladder cancer tissues. The same experiment was repeated at least three times. Values shown are mean ± SD of triplicate measurements and were repeated three times with similar results; *P<0.05.
Relationship between TRPM7 expression and clinicopathological variables in bladder cancer
To investigate the potential role of TRPM7 in bladder cancer, the relationship between TRPM7 expression level and clinicopathological factors was analyzed and is summarized in Table II. As shown, TRPM7 overexpression was associated with recurrence (P<0.01), and metastasis (P=0.021) of patients with bladder cancer. However, TRPM7 exhibited no significant association with other clinicopathological characteristics, such as age, sex, histologic grade, tumor stage and multiplicity (all P>0.05).
Table II.
Relationship between the expression of TRPM7 and clinicopathological factors in 74 bladder cancer patients.
| TRPM7 expression, n | ||||
|---|---|---|---|---|
| Parameters | No. case | Low | High | P-value |
| Sex | 1.0 | |||
| Male | 46 | 17 | 29 | |
| Female | 28 | 10 | 18 | |
| Age, years | 0.432 | |||
| <60 | 21 | 6 | 15 | |
| ≥60 | 53 | 21 | 32 | |
| Histological grade | 0.226 | |||
| Low | 45 | 19 | 26 | |
| High | 29 | 8 | 21 | |
| Tumor stage | 0.333 | |||
| Ta-T1 | 40 | 17 | 23 | |
| T2-T4 | 34 | 10 | 24 | |
| Multiplicity | 0.054a | |||
| Unifocal | 35 | 17 | 18 | |
| Multifocal | 39 | 10 | 29 | |
| Recurrence | <0.01a | |||
| Yes | 47 | 3 | 44 | |
| No | 27 | 24 | 3 | |
| Metastasis | 0.021a | |||
| Yes | 17 | 2 | 15 | |
| No | 57 | 25 | 32 | |
Statistical analyses were performed using the Pearsons χ2 test. P<0.05 was considered significant. TRPM7, transient receptor potential channel 7.
High TRPM7 expression predicts poor prognosis of bladder cancer patients
The prognostic value of TRPM7 was evaluated by Kaplan-Meier analysis. The survival curves indicated that high TRPM7 expression was significantly associated with poor survival of bladder cancer patients. Patients with TRPM7 high expression had worse prognoses than those with low expression of TRPM7 (Fig. 2) (P<0.05).
Figure 2.
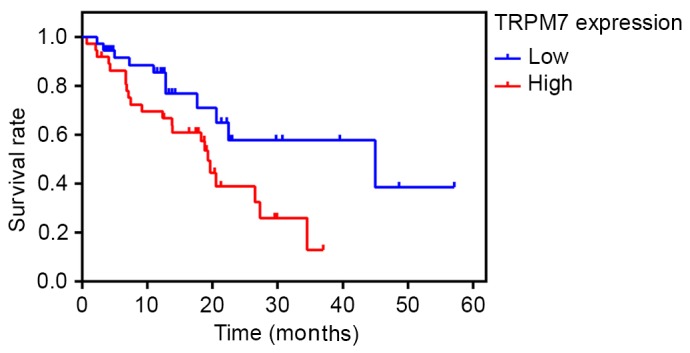
Kaplan-Meier survival curves of 74 patients with bladder cancer based on TRPM7 expression status. The ‘time (months)’ on the x-axis indicates the cancer-specific survival time. Patients in the high expression group had significantly poorer prognosis than those in the low expression group (P<0.05, log-rank test).
Furthermore, univariate and multivariate Cox regression analyses were applied to the clinicopathological characteristics in regards to TRPM7 expression levels. As shown in Table III, multiplicity (P=0.017), recurrence (P<0.01), metastasis (P=0.049) and TRPM7 expression (P<0.01) were all significantly related to bladder cancer patient poor survival. Multivariate analysis showed that high TRPM7 expression was independently associated with the poor prognosis of bladder cancer patients (P=0.035) (Table IV).
Table III.
Survival status and clinicopathological parameters in 74 human bladder cancer tissues.
| Survival status, n | ||||
|---|---|---|---|---|
| Parameters | Total | Alive | Dead | P-value |
| Sex | 1.000 | |||
| Male | 46 | 18 | 28 | |
| Female | 28 | 11 | 17 | |
| Age, years | 0.603 | |||
| <60 | 21 | 7 | 14 | |
| ≥60 | 53 | 22 | 31 | |
| Histological grade | 1.000 | |||
| Low | 45 | 18 | 27 | |
| High | 29 | 11 | 18 | |
| Tumor stage | 0.634 | |||
| Ta-T1 | 40 | 17 | 23 | |
| T2-T4 | 34 | 12 | 22 | |
| Multiplicity | 0.017a | |||
| Unifocal | 35 | 19 | 16 | |
| Multifocal | 39 | 10 | 29 | |
| Recurrence | <0.01a | |||
| Yes | 47 | 4 | 43 | |
| No | 27 | 25 | 2 | |
| Metastasis | 0.049a | |||
| Yes | 17 | 3 | 14 | |
| No | 57 | 26 | 31 | |
| TRPM7 | <0.01a | |||
| Low expression | 27 | 26 | 1 | |
| High expression | 47 | 3 | 44 | |
Statistical analyses were performed using the Pearsons χ2 test. P<0.05 was considered significant. TRPM7, transient receptor potential channel 7.
Table IV.
Contribution of various potential prognostic factors to survival by Cox regression analysis on 74 human bladder cancer tissues.
| Hazard ratio | P-value | 95% CI | |
|---|---|---|---|
| Multiplicity | |||
| Unifocal vs. multifocal | 0.930 | 0.838 | 0.466–1.857 |
| Recurrence | |||
| Yes vs. no | 5.542 | 0.043a | 1.052–29.194 |
| Metastasis | |||
| Yes vs. no | 1.384 | 0.379 | 0.671–2.853 |
| TRPM7 expression | |||
| Low vs. high | 0.089 | 0.035a | 0.009–0.846 |
Statistical analyses were performed using the Cox regression analysis
P<0.05 was considered significant. CI, confidence interval; RPM7, transient receptor potential channel 7.
TRPM7 mRNA and protein levels are higher in 5637 and T24 cells than BIU87 cells
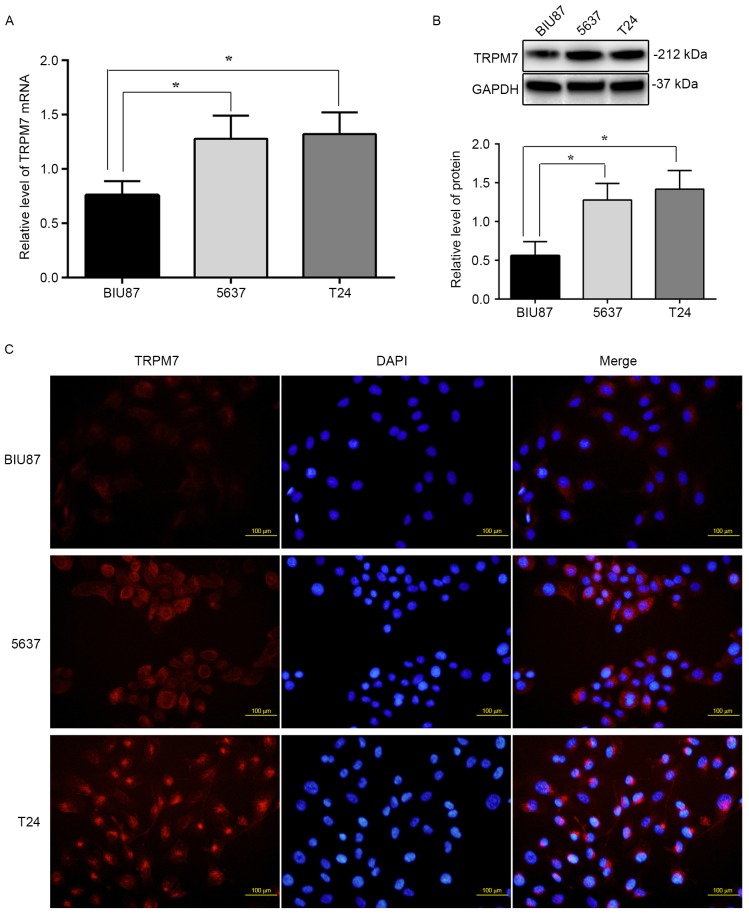
To examine the mRNA and protein levels of TRPM7 in BIU87, 5637 and T24 bladder cancer cell lines, RT-PCR and western blotting were carried out, respectively. Fig. 3A shows that the levels of TRPM7 mRNA in 5637 and T24 cells (normalized to GAPDH, 1.38±0.7, 1.45±0.6; P<0.05) were significantly higher than that noted in the BIU87 cells (0.75±0.4; P<0.05). Western blotting demonstrated that TRPM7 protein levels (normalized to GAPDH) were also higher in the 5637 and T24 cells (1.25±0.41, 1.36±0.49; P<0.05) compared to that noted in the BIU87 cells (0.58±0.32) (Fig. 3B). TRPM7 protein levels were further determined in bladder cancer cell lines using immunofluorescent staining. As shown in Fig. 3C, higher levels of TRPM7 protein were observed in the 5637 and T24 cells compared to that noted in the BIU87 cells in cell culture, and the TRPM7 protein trend was to localize around the nuclear membrane in the 5637 and T24 cells. The fluorescence intensity in the 5637 and T24 cells was 25 or 30% higher than that in the BIU87 cells (P<0.05). Our results provide evidence that TRPM7 channels are highly expressed in bladder cancer cells with a high degree of malignancy, and enrichment of TRPM7 is around the nuclear membrane.
Figure 3.
The difference in mRNA and protein levels of TRPM7 in bladder cancer cell lines. (A) The amount of TRPM7 mRNA in 5637 and T24 cells was significantly higher than that in BIU87 cells (*P<0.05). (B) Western blotting demonstrated that TRPM7 protein level (normalized to GAPDH) was also higher in the 5637 and T24 cells compared to that in the BIU87 cells (*P<0.05). (C) Higher level of TRPM7 protein was observed in 5637 and T24 cells compared to that in the BIU87 cells by immunofluorescent staining (P<0.05), and the TRPM7 protein trend was to localize around the nuclear membrane in the 5637 and T24 cells.
TRPM7 siRNA effectively suppresses TRPM7 expression
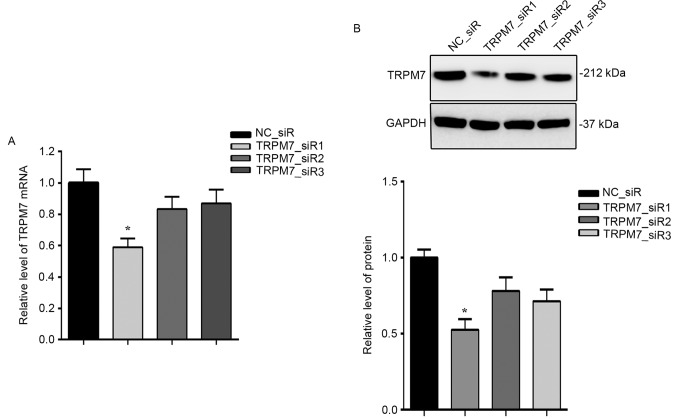
To investigate the role of TRPM7 in bladder cancer, TRPM7 was knocked down using RNAi method. T24 cells were transfected with three siRNAs targeting TRPM7. As illustrated in Fig. 4, the mRNA (Fig. 4A) and protein (Fig. 4B) expression levels of TRPM7 were both significantly inhibited by TRPM7_siR1.
Figure 4.
Expression of TRPM7 in T24 cells is effectively downregulated by specific TRPM7 siRNAs. (A) Interference efficiency at the mRNA level was detected by RT-qPCR. (B) Interference efficiency at the protein level was detected by western blotting compared with NC_siR; *P<0.05.
TRPM7 knockdown inhibits cellular proliferation and induces cell apoptosis
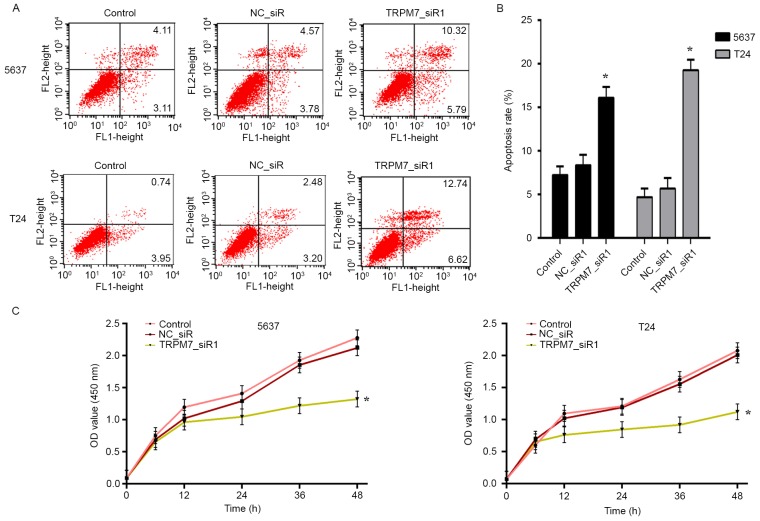
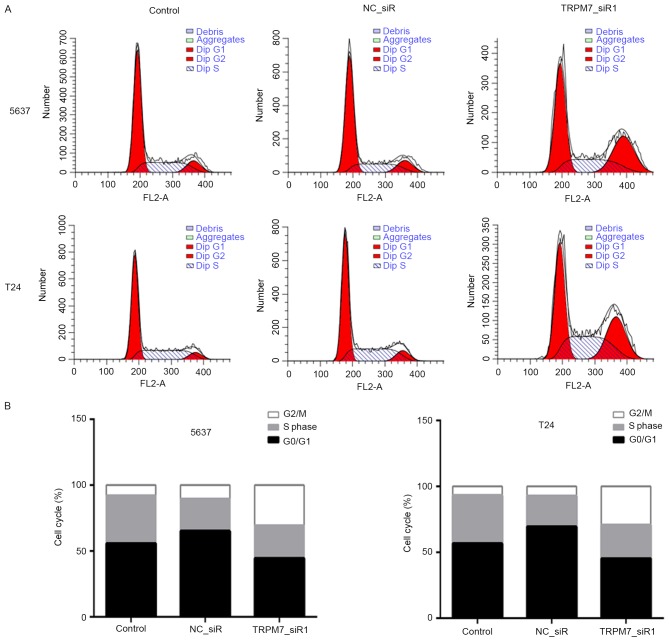
We transfected the 5637 and T24 cells with TRPM7_siR1 and NC_siR. Compared with NC_siR and control groups, flow cytometric analysis revealed that downregulation of TRPM7 significantly induced apoptosis (Fig. 5A). Statistical analysis of apoptosis is shown in Fig. 5B. CCK-8 assay was carried out to assess the effects of TRPM7 knockdown on T24 and 5637 cell proliferation. The CCK-8 assay showed that after downregulation of TRPM7 with TRPM7_siR1, T24 and 5637 cells exhibited a significant decrease in cell proliferation compared with that noted in the control siRNA group (Fig. 5C). Flow cytometric cell cycle analysis revealed that 5637 and T24 cells were blocked in the G2/M phase after transfection with TRPM7_siR1 (Fig. 6A and B).
Figure 5.
Effect of TRPM7 on cell proliferation and apoptosis. 5637 and T24 cells were transfected with TRPM7_siR1 for 48 h and compared with NC_siR and parental cells. (A and B) Apoptotic cells stained with Annexin V and PI were revealed by flow cytometric analysis and the apoptotic rates were statistically analyzed; *P<0.05. (C) Cell proliferation of T24 and 5637 cells was measured by CCK-8 assay. The inhibition rate of T24 and 5637 cells with different treatments; *P<0.05 compared with NC_siR and control group.
Figure 6.
Flow cytometric cell cycle analysis of bladder cancer cells. (A and B) 5637 and T24 cells in the G2/M phase were markedly increased in the TRPM7_siR1 group compared with the control and NC_siR groups.
TRPM7 knockdown inhibits cell motility
We next investigated the effect of TRPM7 on cell motility with wound-healing and Transwell assays. After being incubated with physical-wound and cultured in serum-free medium to exclude the interference of proliferation, the percentage of wound closure at 48 h was significantly lower in the TRPM7_siR1-treated cells than that noted in the control cells (Fig. 7A and B). Transwell assays showed that the silencing of TRPM7 measurably inhibited cell migration and invasion in the Transwell assays (Fig. 7C and D). All the data support that TRPM7 stimulates bladder cancer cell migration and invasion.
Figure 7.
Effect of TRPM7 on cell migration and invasion. (A and B) T24 and 5637 cells transfected with TRPM7 siRNAs showed a slower wound closure rate than cells transfected with non-specific siRNA or control. Scale bar, 50 µm. (C and D) Knockdown of TRPM7 inhibited cell migration and invasion as detected by Transwell assays. Number of cells that invaded through the membrane was counted in 10 fields under magnification, ×200; *P<0.05 compared with NC_siR and control group.
Discussion
It is believed that bladder tumorigenesis and development are multistep pathologic processes involving numerous genetic alterations, of which the inactivation of tumor repressors and activation of oncogenes are critical events in the initiation of bladder tumors. As one of the most fatal carcinomas worldwide, the molecular mechanisms of bladder cancer remain unknown. It is urgent to identify novel molecules which may serve as prognostic factors and therapeutic targets for bladder cancer. In the present study, we found that TRPM7 is highly expressed in bladder cancer and is closely correlated with clinical stages and poor prognosis of bladder cancer patients. However, TRPM7 accelerated bladder cancer cell growth and migration. Taken together, these results suggest that TRPM7 may be a potential regulator in bladder cancer progression.
Calcium (Ca2+) and magnesium (Mg2+) are two important metal elements that contribute to a variety of tumor cell processes such as proliferation, migration and apoptosis. Studies have reported that TRP channels are involved in a variety of basic cellular processes and are crucial for carcinogenesis and cancer development (22,23). TRPM7, belonging to the TRP channel family, is a non-selective cation channel mediating both Ca2+ and Mg2+ flow (7,8). TRPM7 is ubiquitously expressed and essential for diverse physiological and pathophysiological processes such as excitability, gene expression, muscle contraction, cell volume regulation and hormone secretion (20,24). Accumulating studies have confirmed that TRPM7 is aberrantly overexpressed and plays a vital role in various types of cancers (25–28). For example, TRPM7 is high expressed in pancreatic cancer and correlates with tumor size and stage. Moreover, TRPM7 is required for pancreatic cancer invasion (13). However, the contribution of TRPM7 to bladder cancer carcinogenesis remains largely unidentified and needs to be determined.
In the present study, we firstly detected TRPM7 expression in human bladder cancer tissues by RT-PCR, western blot and immunohistochemical analyses. The expression levels of TRPM7 mRNA and protein were significantly higher in bladder cancer than levels in the non-tumor tissues. In addition, a similar consequence was detected in bladder cancer cell lines (BIU87, 5637 and T24). Next, the correlation analysis demonstrated that higher TRPM7 expression level was closely associated with recurrence (P<0.01) and metastasis (P=0.021). All these data suggest that TRPM7 functions as a potential oncogene and plays an important role in the progression of bladder cancer.
Previous research has demonstrated that TRPM7 is correlated with the poor prognosis of various malignancies such as neuroblastoma (19,29). Thus, we performed Kaplan-Meier analysis to investigate the prognostic role of TRPM7 in bladder cancer patients. We found that overexpression of TRPM7 was correlated with the patient overall survival time. In addition, univariate and multivariate analyses revealed that TRPM7 is a significant independent prognostic predictor for bladder cancer patients.
Thus, to further explore the functions of TRPM7 in bladder cancer, we determined the effect of TRPM7 on the behaviors of bladder cancer cells (T24 and 5637). TRPM7 was downregulated via RNAi strategy, the siRNAs targeting TRPM7 were designed and screened in bladder cancer cells, and the most efficient silencing siRNA (TRPM7_siR1) was used for following observations. After TRPM7 was silenced, the CCK-8 assay showed that the proliferation of T24 and 5637 cells was significant inhibited and flow cytometric cell cycle analysis exhibited that cells were blocked in the G2/M phase, and flow cytometric analysis revealed that downregulation of TRPM7 induced apoptosis.
Bladder cancer has a tendency to metastasize, and metastasis is an important characteristic which influences bladder cancer patient prognosis. We used wound-healing and Transwell assays to study the role of TRPM7 in bladder cancer migration and invasion. The loss-of-function experiments showed that the silencing of TRPM7 expression inhibited cell migratory and invasive abilities. All the results suggest that TRPM7 may be a potential tumor promoter in bladder cancer.
In summary, our data confirmed that TRPM7 is overexpressed in bladder cancer tissue. Furthermore, high TRPM7 expression may be involved in the clinical development and poor prognosis of bladder cancer patients. Moreover, we showed that TRPM7 may be involved in cell proliferation, apoptosis, migration and invasion abilities of bladder cancer cells. The present study indicated that TRPM7 has potent oncogenic activity in bladder cancer and TRPM7 channel functions may uncover new strategies in the future to prevent the progression of bladder cancer diseases.
Acknowledgements
The present study was supported by the The National Natural Science Foundation of China (81372723), and The Key Urology Laboratory Foundation of Shenyang City of China (F13-293-1-00).
References
- 1.Pang C, Guan Y, Li H, Chen W, Zhu G. Urologic cancer in China. Jpn J Clin Oncol. 2016;46:497–501. doi: 10.1093/jjco/hyw034. [DOI] [PubMed] [Google Scholar]
- 2.Ye F, Wang L, Castillo-Martin M, McBride R, Galsky MD, Zhu J, Boffetta P, Zhang DY, Cordon-Cardo C. Biomarkers for bladder cancer management: Present and future. Am J Clin Exp Urol. 2014;2:1–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Zalabani AH, Stewart KF, Wesselius A, Schols AM, Zeegers MP. Modifiable risk factors for the prevention of bladder cancer: A systematic review of meta-analyses. Eur J Epidemiol. 2016;31:811–851. doi: 10.1007/s10654-016-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Guillaume L, Guy L. Epidemiology of and risk factors for bladder cancer and for urothelial tumors. Rev Prat. 2014;64(1372–1374):1378–1380. (In French) [PubMed] [Google Scholar]
- 6.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 7.Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 8.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 9.Guilbert A, Gautier M, Dhennin-Duthille I, Rybarczyk P, Sahni J, Sevestre H, Scharenberg AM, Ouadid-Ahidouch H. Transient receptor potential melastatin 7 is involved in oestrogen receptor-negative metastatic breast cancer cells migration through its kinase domain. Eur J Cancer. 2013;49:3694–3707. doi: 10.1016/j.ejca.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Liao QJ, Zhang Y, Zhou H, Luo CH, Tang J, Wang Y, Tang Y, Zhao M, Zhao XH, et al. TRPM7 is required for ovarian cancer cell growth, migration and invasion. Biochem Biophys Res Commun. 2014;454:547–553. doi: 10.1016/j.bbrc.2014.10.118. [DOI] [PubMed] [Google Scholar]
- 11.Lin CM, Ma JM, Zhang L, Hao ZY, Zhou J, Zhou ZY, Shi HQ, Zhang YF, Shao EM, Liang CZ. Inhibition of transient receptor potential melastain 7 enhances apoptosis induced by TRAIL in PC-3 cells. Asian Pac J Cancer Prev. 2015;16:4469–4475. doi: 10.7314/APJCP.2015.16.10.4469. [DOI] [PubMed] [Google Scholar]
- 12.Chen WL, Barszczyk A, Turlova E, Deurloo M, Liu B, Yang BB, Rutka JT, Feng ZP, Sun HS. Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion. Oncotarget. 2015;6:16321–16340. doi: 10.18632/oncotarget.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yee NS, Kazi AA, Li Q, Yang Z, Berg A, Yee RK. Aberrant over-expression of TRPM7 ion channels in pancreatic cancer: Required for cancer cell invasion and implicated in tumor growth and metastasis. Biol Open. 2015;4:507–514. doi: 10.1242/bio.20137088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizuno H, Suzuki Y, Watanabe M, Sokabe T, Yamamoto T, Hattori R, Gotoh M, Tominaga M. Potential role of transient receptor potential (TRP) channels in bladder cancer cells. J Physiol Sci. 2014;64:305–314. doi: 10.1007/s12576-014-0319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng X, Cai C, Wu J, Cai S, Ye C, Chen H, Yang Z, Zeng H, Shen Q, Zou F. TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett. 2013;333:96–102. doi: 10.1016/j.canlet.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 16.Middelbeek J, Kuipers AJ, Henneman L, Visser D, Eidhof I, van Horssen R, Wieringa B, Canisius SV, Zwart W, Wessels LF, et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res. 2012;72:4250–4261. doi: 10.1158/0008-5472.CAN-11-3863. [DOI] [PubMed] [Google Scholar]
- 17.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/S0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 18.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehen'kyi V, Shapovalov G, Skryma R, Prevarskaya N. Ion channnels and transporters in cancer. 5. Ion channels in control of cancer and cell apoptosis. Am J Physiol Cell Physiol. 2011;301:C1281–C1289. doi: 10.1152/ajpcell.00249.2011. [DOI] [PubMed] [Google Scholar]
- 20.Romani A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch Biochem Biophys. 2007;458:90–102. doi: 10.1016/j.abb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 21.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: Implications for health and disease. Physiol Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 22.Guilbert A, Gautier M, Dhennin-Duthille I, Haren N, Sevestre H, Ouadid-Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am J Physiol Cell Physiol. 2009;297:C493–C502. doi: 10.1152/ajpcell.00624.2008. [DOI] [PubMed] [Google Scholar]
- 23.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Xiao L, Luo CH, Zhou H, Hu J, Tang YX, Fang KN, Zhang Y. Overexpression of TRPM7 is associated with poor prognosis in human ovarian carcinoma. Asian Pac J Cancer Prev. 2014;15:3955–3958. doi: 10.7314/APJCP.2014.15.9.3955. [DOI] [PubMed] [Google Scholar]
- 25.Kim BJ, Nah SY, Jeon JH, So I, Kim SJ. Transient receptor potential melastatin 7 channels are involved in ginsenoside Rg3-induced apoptosis in gastric cancer cells. Basic Clin Pharmacol Toxicol. 2011;109:233–239. doi: 10.1111/j.1742-7843.2011.00706.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen JP, Luan Y, You CX, Chen XH, Luo RC, Li R. TRPM7 regulates the migration of human nasopharyngeal carcinoma cell by mediating Ca2+ influx. Cell Calcium. 2010;47:425–432. doi: 10.1016/j.ceca.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, Sumimoto H, Ito Y, Mori Y, Inoue R. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci. 2004;95:403–419. doi: 10.1254/jphs.FP0040273. [DOI] [PubMed] [Google Scholar]
- 28.Yee NS, Kazi AA, Yee RK. Cellular and developmental biology of TRPM7 channel-kinase: Implicated roles in cancer. Cells. 2014;3:751–777. doi: 10.3390/cells3030751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lange I, Koomoa DL. MycN promotes TRPM7 expression and cell migration in neuroblastoma through a process that involves polyamines. FEBS Open Bio. 2014;4:966–975. doi: 10.1016/j.fob.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]