Abstract
The current treatment recommendation for T2-3N0M0 glottic squamous cell carcinoma (SCC) in the Nordic countries comprises of radiotherapy (RT) and chemoradiotherapy (CRT). Tumor radiosensitivity varies and another option is primary surgical treatment, which underlines the need for predictive markers in this patient population. The aim of the present study was to investigate the relation of the proteins WRAP53β, survivin and p16INK4a to RT/CRT response and ultimate outcome of patients with T2-T3N0 glottic SCC. Protein expression was determined using immunohistochemistry on tumors from 149 patients consecutively treated with RT or CRT at Helsinki University Hospital, Karolinska University Hospital, and Linköping University Hospital during 1999–2010. Our results demonstrate a significantly better 5-year relapse-free survival, disease-free survival (DFS), disease-specific survival and overall survival of patients with T3N0 tumors treated with CRT compared with RT alone. Patients with tumors showing a cytoplasmic staining of WRAP53β revealed significantly worse DFS compared with those with nuclear staining. For survivin, we observed a trend towards better 5-year DFS in patients with strong nuclear survivin expression compared with those with weak nuclear survivin expression (P=0.091). Eleven (7%) tumors showed p16 positivity, with predilection to younger patients, and this age group of patients with p16-positive SCC had a significantly better DFS compared with patients with p16-negative SCC. Taken together, our results highlight WRAP53β as a potential biomarker for predicting RT/CRT response in T2-T3N0 glottic SCC. p16 may identify a small but distinct group of glottic SCC with favorable outcome. Furthermore, for T3N0 patients better outcome was observed following CRT compared to RT alone.
Keywords: head and neck cancer, radiotherapy, chemoradiotherapy, survival, recurrence, biomarker, WRAP53β, survivin, p16
Introduction
Annually, 160,000 new laryngeal cancers are diagnosed globally. In the Nordic countries, nearly 700 new cases are reported each year (2009–2013) with ~230 laryngeal cancer-related deaths annually (2006–2010) (1). Laryngeal cancer, of which 90% represents squamous cell carcinoma (SCC), is subclassified according to localization into supraglottic, glottic and subglottic carcinoma. In glottic SCC, lymph node metastases are rare at presentation and thus, the treatment objective is to achieve permanent local control. Currently, the management of T2N0 and T3N0 glottic SCC consists mainly of radiotherapy (RT) which is combined with chemotherapy (chemoradiotherapy, CRT) in locally advanced cases.
Recent studies from Finland and Sweden show suboptimal treatment results for patients with T2-T3 laryngeal SCC (2,3), and poor results regarding T3N0 patients are also a concern in the United States (4). Although the reasons for these poor results are currently unknown, it has been speculated that the use of organ preservation treatment (RT or CRT) as an alternative to radical surgery (often total laryngectomy) may be one of the culprits (4,5). In this setting, there is a great need for predictive markers to identify the patients that will respond favorably to definitive oncological treatment. With proper pre-treatment stratification, oncological treatment could be directed to ‘responders’ whereas known ‘non-responders’ could undergo primary surgical therapy for improved outcome. We have previously explored a number of potential markers, in vitro and in vivo, that could potentially identify head and neck SCC (HNSCC) patients who would benefit from RT. Through these studies we have identified two proteins, survivin and WRAP53β, which were significantly associated with positive response to RT and longer overall survival (OS) in patients with HNSCC (6,7).
Survivin (BIRC5), the smallest member of the inhibitor of apoptosis family, is a multifunctional protein that acts both as an inhibitor of apoptosis and a regulator of mitosis (8). It is also involved in cell proliferation, chromosome movement, and regulation of response to cellular stress (9). Survivin exists both in the nucleus and in the cytoplasm, and its subcellular localization is suggested to correspond with its different functions (10,11). In oral SCC, Lo Muzio et al (12) have reported expression of survivin as a negative prognostic factor, whereas Freier et al (13) have showed a correlation between high survivin expression and improved OS.
The scaffold protein WRAP53β (alias TCAB1, WDR79), encoded by the WRAP53 gene (WD40 encoding RNA antisense to p53) (14), controls the intracellular localization of factors involved in splicing, telomere elongation and DNA repair (15). Lower expression of nuclear WRAP53β correlates with shorter survival not only for HNSCC, but also for ovarian and breast cancer and in addition, correlates with disruption of the DNA damage response in ovarian tumors (7,16,17). Moreover, inherited mutations in WRAP53β cause the cancer-predisposition disorder dyskeratotis congenita where patients mainly develop hematological malignancies and head and neck cancer, altogether indicating that the WRAP53β protein acts as a tumor suppressor. At the same time, overexpression of WRAP53β is observed in different cancer types, head and neck, lung and rectal cancer (18), however, the clinical relevance of such overexpression remains unclear.
HPV infection has been well established as a favorable prognostic marker in oropharyngeal SCC and the expression of p16INK4a is used as surrogate marker for the infection. The favorable prognostic value of p16 expression remains irrespective of the administered treatment (19). In laryngeal SCC the incidence of HPV or p16-positivity is low compared with oropharynx (20) and a link between p16/HPV and treatment outcome has not been found (21,22).
The primary aim of this study was to investigate survivin, WRAP53β and p16 expression as potential predictive tumor biomarkers for oncological treatment outcome in a selective HNSCC patient group: T2N0-T3N0 glottic SCC treated with definitive RT or CRT.
Materials and methods
Patient selection
Registries of the Helsinki University Hospital, Karolinska University Hospital, and Linköping University Hospital were utilized to identify patients treated for primary T2N0 or T3N0 glottic laryngeal SCC during 1999–2010. The study was approved by the Regional Ethics Review Boards at Linköping University, Karolinska Institute, and Helsinki University Hospital.
The inclusion criteria were as follows: tumor of glottic origin, absence of neck metastasis at presentation (i.e. classification T2N0 or T3N0), primary curatively aimed oncological treatment (definitive RT or CRT), completion of treatment, availability of histological biopsy material for analysis, and follow-up of at least six months after the treatment for living patients. Clinical patient data extracted from the hospital registries included tumor characteristics, details on primary treatment (RT doses), possible salvage therapy, and follow-up information including recurrences and causes of death. Regarding treatment, RT was given in 2 Gy fractions 5 days a week until the target dose was reached. The mean RT dose was 68 Gy (median, 68 Gy; range, 46–80 Gy). All except 1 patient received a minimum dose of 62 Gy. For CRT, cisplatinum 40 mg/m2 was administered once a week concomitantly with RT to a total of six doses.
Immunohistochemistry
Standard hematoxylin and eosin stained slides of the clinical biopsies were first evaluated by a pathologist to confirm diagnosis and assure tumor content in paraffin blocks. New sections from the tumor biopsies were thereafter mounted on positively charged slides and deparaffinized in Aqua DePar (Biocare Medical, Pacheco, CA, USA). For WRAP53β, sections were pretreated with 10 mM citrate buffer (DakoCytomation epitope retrieval solution) in a hot water bath (up to 100°C) for 40 min, blocked with Envision peroxidase block (BCPX968) for 5 min, and incubated for 30 min at room temperature with a rabbit polyclonal anti-WRAP53-C2 antibody diluted 1:1,000 (Innovagen AB, Lund, Sweden). WRAP53β was stained with the EnVision System-HRP (DAB) kit (DakoCytomation), followed by counterstaining for 1 min with Tachas hematoxylin.
For survivin, sections were blocked for endogenous peroxidase, and thereafter subjected to heat-induced antigen retrieval. Automated IHC was performed using a LabVision Autostainer 480S (Thermo Fisher Scientific, Runcorn, UK). A rabbit polyclonal anti-survivin antibody 1:400 (Thermo Fisher Scientific) was diluted in UltraAb Diluent (Thermo Fisher Scientific, Fremont, CA, USA) and applied to the slides for 30 min at room temperature. The slides were further incubated with the secondary reagent (anti-rabbit horseradish peroxidase-conjugated UltraVision; Thermo Fisher Scientific, Runcorn, UK) for 30 min at room temperature. Following the washing steps, the slides were developed for 10 min using the avidin-biotin peroxidase staining technique (Vector Elite; Vector Laboratories, Burlingame, CA, USA) using 3,3-diaminobenzidine as the substrate. The slides were counterstained with Mayers hematoxylin for 5 min (Sigma-Aldrich, St. Louis, MO, USA).
For p16 analysis, the CINtec Histology kit with monoclonal mouse antibody (clone E6H4; Mtm Laboratories AG, Heidelberg, Germany) was used according to the manufacturers instructions. Results were evaluated independently by one pathologist (S.G.) and two additional investigators (A.H. and K.T./L.F.) without knowledge of patient treatment or outcome. Upon disagreement a consensus score was agreed upon.
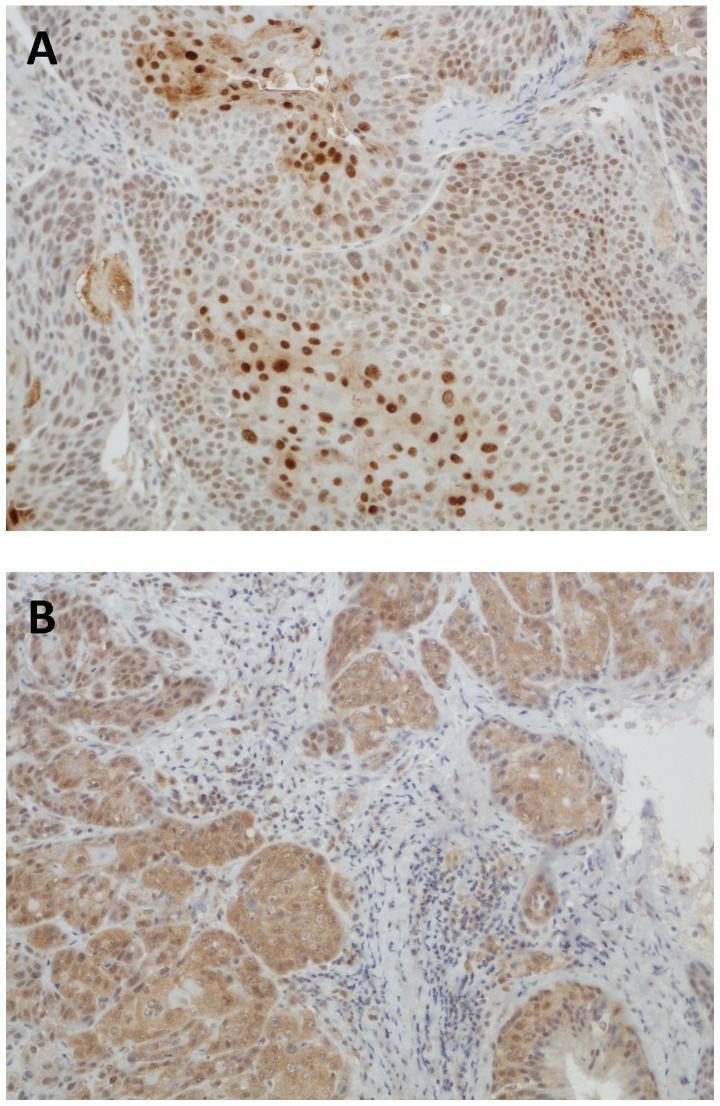
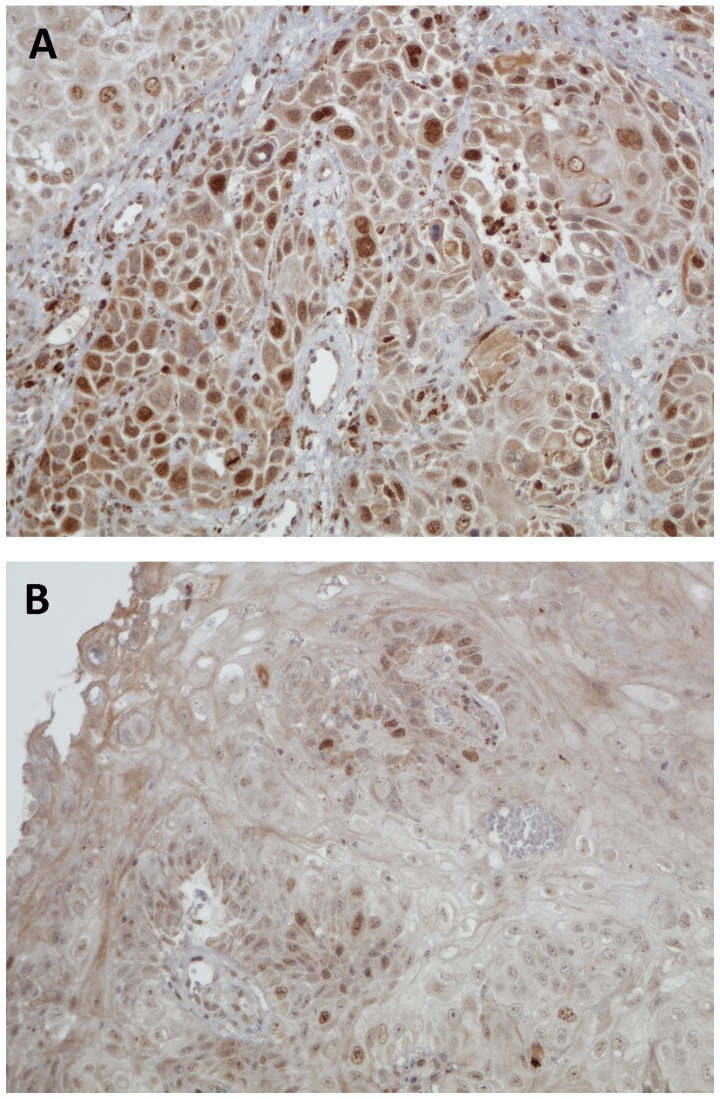
For WRAP53β and survivin the staining intensity was scored as follows: 0 (none), 1 (weak), 2 (moderate), or 3 (strong). Intensity of 0–1 was considered negative and 2–3 was considered positive. Due to slight differences in staining frequency, the percentage of WRAP53β-positive tumor cells was scored 0 (0%), 1 (<10%), 2 (11–50%) or 3 (>50%); for survivin 0 (0%), 1 (<10%), 2 (11–50%), 3 (51%-80%) or 4 (>80%). The predominant subcellular localization was determined by the difference in staining intensity between the nucleus and the cytoplasm. In analyses of WRAP53β, predominantly nuclear or equal staining in the nucleus and the cytoplasm was considered nuclear as previously described (7). For survivin, predominantly nuclear, equal nuclear and cytoplasmic, and predominantly cytoplasmic were analyzed separately. Examples of staining patterns of WRAP53β and survivin are shown in Figs. 1 and 2.
Figure 1.
Immunohistochemical staining of WRAP53β. (A) Nuclear staining. (B) Cytoplasmic staining.
Figure 2.
Immunohistochemical staining of survivin. (A) Strong nuclear staining. (B) Weak nuclear staining.
All p16 slides were scored for intensity of p16 staining in the nucleus and cytoplasm as: 0 (none), 1 (weak), 2 (moderate) or 3 (strong), with 2 or 3 being considered positive if the majority of tumor cells (>70%) showed staining both in the nucleus and cytoplasm (23).
Statistical methods
Comparisons of demographic factors and recurrence with protein expression results were conducted by using the Chi-squared test and P≤0.05 was considered significant. Overall survival (OS, death as endpoint), disease-specific survival (DSS, laryngeal cancer death as endpoint), disease-free survival (DFS, recurrence or death of any cause as endpoint) and relapse-free survival (RFS: recurrence as endpoint) were calculated using Kaplan-Meier curves. OS and DSS times were calculated from the date of diagnosis to the date of event or last follow-up. DFS and RFS times were calculated from the date of treatment completion to the date of event or last follow-up. Significance of differences between patient groups regarding survival was determined using the log-rank (Mantel-Cox) test. P≤0.05 was considered significant. SPSS version 22.0 was used for all statistical analyses.
Results
Clinical data
Altogether 149 patients matched the inclusion criteria. Patient and tumor characteristics and primary treatment are shown in Table I. The median follow-up was 67 months (mean, 77; range, 9–163). Incomplete response to primary treatment was observed in 13 out of the 149 patients (9%). None of the T3N0 patients who received CRT as primary treatment had residual tumor after treatment whereas 5 patients (23%) treated with RT had residual tumor (P=0.018). This difference was not noted in T2N0 patients. Patients with residual tumor after primary treatment had a significantly lower 5-year DSS compared with patients with complete response to primary treatment (51 vs. 87%; P=0.009). The recurrence rates after primary treatment for T2N0 and T3N0 patients were 23 and 45%, respectively (P=0.006). The median time to recurrence was 10 months (mean, 19; range, 3–112). Recurrence expectedly led to lower 5-year OS (no recurrence, 71%, recurrence, 42%; P=0.001). Five-year OS and DSS for T2N0 and T3N0 patients were 69 and 91 and 45 and 69%, respectively.
Table I.
Patient and tumor characteristics as well as primary treatment.
| Characteristics | No. of patients | % of patients |
|---|---|---|
| Age (years) | ||
| <60 | 58 | 39 |
| ≥60 | 91 | 61 |
| Sex | ||
| Male | 143 | 96 |
| Female | 6 | 4 |
| Smoking | ||
| Ever | 128 | 86 |
| Never | 10 | 7 |
| N/A | 11 | 7 |
| Histological grade | ||
| I | 33 | 22 |
| II | 82 | 55 |
| III | 16 | 11 |
| N/A | 18 | 12 |
| T class | ||
| T2N0 | 105 | 71 |
| T3N0 | 44 | 30 |
| Treatment | ||
| T2 | ||
| RT | 94 | 90 |
| CRT | 11 | 10 |
| T3 | ||
| RT | 22 | 50 |
| CRT | 22 | 50 |
| RT dose (Gy) | ||
| <60 | 1 | 1 |
| 60-69 | 99 | 66 |
| ≥70 | 49 | 33 |
N/A, not available; RT, radiotherapy; CRT, chemoradiotherapy.
In the T3N0 group, significant differences in favor of CRT over RT were observed in RFS, DFS, OS and DSS (Table II). Moreover, patients with T3N0 tumors who received CRT showed a significantly lower recurrence rate (1 out of 22; 5%) compared with patients who were treated with RT alone (11 out of 22; P<0.001). For patients with T2N0, a significant difference in 5-year DFS was observed favoring CRT, but no statistical differences were observed in OS, DSS or RFS.
Table II.
OS, DSS, DFS and RFS in T2N0, T3N0 patients treated with RT or CRT.
| T2N0 (n=105) | T3N0 (n=44) | |||||
|---|---|---|---|---|---|---|
| RT (n=94) | CRT (n=11) | P-value | RT (n=22) | CRT (n=22) | P-value | |
| 5-year OS | 66.9 | 90.9 | 0.147 | 18.2 | 72.4 | 0.001 |
| 5-year DSS | 89.8 | 100 | 0.302 | 49.5 | 85.7 | 0.018 |
| 5-year DFS | 54.9 | 90.9 | 0.046 | 13.6 | 54.5 | 0.001 |
| 5-year RFS | 74.8 | 100 | 0.085 | 43.6 | 66.2 | 0.039 |
Results are shown in % and significant p-values are shown in bold. RT, radiotherapy; CRT, chemoradiotherapy; OS, overall survival; DSS, disease-specific survival; DFS, disease-free survival; RFS, relapse-free survival.
IHC staining of WRAP53β
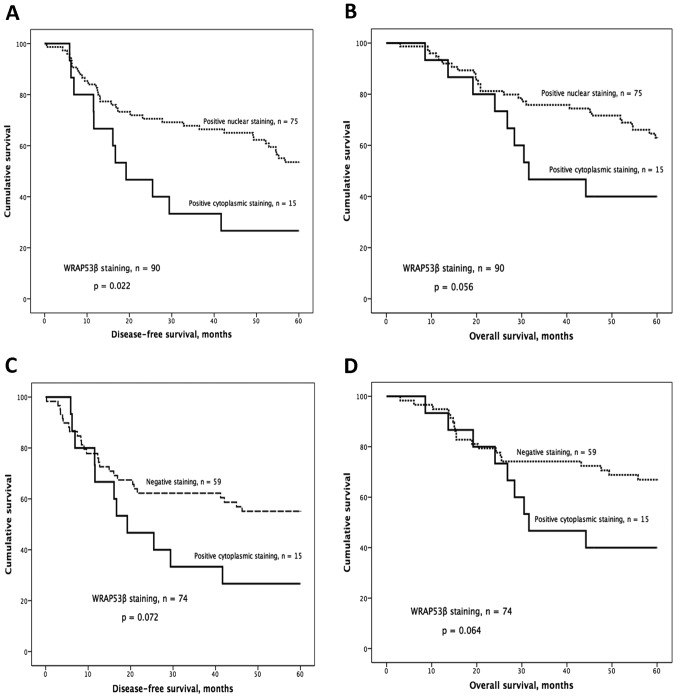
The distribution and subcellular localization of WRAP53β were as follows: 75 positive nuclear, 15 positive cytoplasmic and 59 negative. Kaplan-Meier analysis showed significantly worse DFS and a strong tendency for worse OS for patients with tumors showing cytoplasmic staining compared with patients with nuclear staining (P=0.022 for DFS, P=0.056 for OS; Fig. 3A and B). This trend was also observed when comparing patients with cytoplasmic staining to those classified as negative (P=0.072 for DFS; P=0.064 for OS; Fig. 3C and D).
Figure 3.
Kaplan-Meier curves and WRAP53β IHC staining. (A) Cytoplasmic staining was associated with significantly shorter disease-free survival (DFS) and a trend towards shorter overall survival (OS; B) compared to nuclear staining. This trend was also observed in DFS and OS (C and D) comparing cytoplasmic staining to negative.
IHC staining of survivin
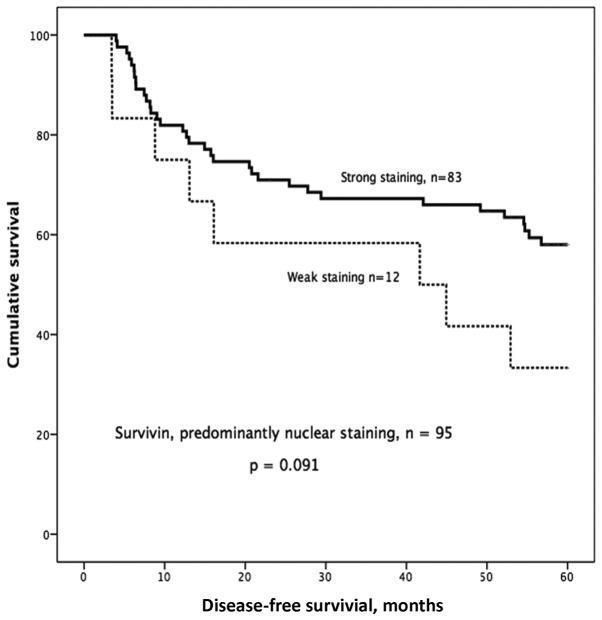
Based on our earlier study (6) we decided to look more specifically at the group with predominantly nuclear staining and of the 148 samples, 95 showed predominantly nuclear staining, 35 showed predominantly cytoplasmic staining, and 18 showed equal staining intensity in the cytoplasm and nucleus. One sample was excluded due to staining failure. In the group with predominantly nuclear staining, we observed a trend towards better 5-year DFS in patients with strong nuclear survivin expression compared with those with weak nuclear survivin expression (P=0.091; Fig. 4).
Figure 4.
Kaplan-Meier curves and survivin IHC staining. Strong nuclear survivin showed a trend towards longer disease-free survival (DFS) compared to weak nuclear survivin.
IHC staining of p16
Moderate or strong p16 staining was only found in 11 (7%) of the tumors. The rest of the 138 tumors had weak or no staining. When the staining of all 149 samples was examined, no significant differences in OS, DSS, DFS or RFS between patients with p16-positive and -negative tumors were found. However, none of the patients with p16-positive tumors had a residual tumor after treatment compared with 9% residual tumor rate in patients with p16-negative tumors. This finding, however, did not reach statistical significance (P=0.287).
Regarding demographics, samples from patients under the age of 60 years (n=58) had higher frequency of p16 positivity compared with older patients (16 vs. 3%; P=0.017). In the cohort of young patients, never-smokers had more commonly p16-positive tumors than current or former smokers (50 vs. 10%; P=0.022). Furthermore, none of the 8 p16-positive tumors in this younger patient group recurred compared with 36% (18/50) recurrence in p16-negative tumors (P=0.041). Regarding survival outcomes in this younger patient group, no significant differences were observed between p16-positive and -negative patients (OS 100 vs. 68%; P=0.083, DSS 100 vs. 86%, P=0.276 or RFS 100 vs. 65%, P=0.073). However, a significant difference was observed in DFS (p16-positive, 100 vs. p16-negative, 50%; P=0.021).
The associations between WRAP53β and DFS remained statistically significant when patients with tumors staining positive for p16 were excluded. Different combinations of p16INK4a, WRAP53β and survivin scores gave no additional prognostic value.
Discussion
Studies regarding prognostic/predictive markers in HNSCC often include tumors from different sites in a single series. However, it is widely acknowledged that etiological factors, metastatic potential, treatment approaches and patient outcomes vary considerably between tumor sites. Previous studies regarding WRAP53β, survivin and p16 have been prone to confounding factors induced by inclusion of tumors from different HNSCC sites, TNM classes, stages and primary treatments. To the best of our knowledge this is the first study examining these potential prognostic biomarkers in a large (n=149), yet, consecutive patient series of a single HNSCC subsite (glottic SCC) with selected TNM class tumors (T2-3N0) with uniform treatment (RT or CRT).
We observed a significantly improved outcome for T3N0 patients treated with CRT compared with those treated with RT alone. This improvement in outcome with CRT has been previously demonstrated in a prospective randomized trial in advanced laryngeal cancer patients (24). In T2N0 patients, a significant difference in 5-year DFS was observed favoring CRT, but no statistical differences were observed in OS, DSS, or RFS. It should be noted, however, that the number of CRT patients in the T2N0 group (n=11; 10%) was small for comparison. Our results are in line with those from Nishimura et al (25) and Akimoto et al (26), who reported improved larynx preservation and DFS but no survival benefit for T2N0 glottic SCC patients treated with CRT.
To the best of our knowledge, this is the first study to investigate the expression of WRAP53β specifically in laryngeal SCC. Our previous study on a heterogeneous HNSCC patient population suggested that the nuclear localization of WRAP53β was associated with improved response to RT and improved OS (7). In the present study, our results suggest that predominant cytoplasmic localization of WRAP53β is a potential predictive marker of poor OS and DFS in glottic SCC. Similarly, in breast cancer, Silwal-Pandit et al (17) have reported negative nuclear/positive cytoplasmic WRAP53β to be associated with reduced survival. There are several possible explanations for these findings, related to presumed decreased nuclear function of the WRAP53β protein if potentially trapped in the cytoplasm. One example could be related to telomerase dysfunction, an event known to occur in WRAP53β-deficient cells and previously linked to radioresistance (27,28). Loss of WRAP53β protein has also been shown to disturb repair of DNA double-strand breaks, resulting in increased genomic instability (16,29).
In the present study, survivin did not appear as strongly associated with outcome in glottic SCC as it did in our previous investigation of a heterogeneous HNSCC study population (6). We have demonstrated in vitro that downregulation of survivin in two HNSCC cell lines led to decreased proliferation and increased radioresistance (6). One difference between our studies is the IHC staining pattern observed, having been homogeneously nuclear in the former study and more heterogeneous nuclear and cytoplasmic in the present study. It is interesting that when isolating the patients whose tumors showed predominantly nuclear expression, a trend for improved DFS was observed for those with strong nuclear staining, but difficulties in standardizing the IHC staining may reduce the utility of this marker.
Although p16 expression is well recognized as a prognostic factor for oropharyngeal SCC, in laryngeal SCC its prognostic value remains unclear according to largest studies available by Morshed et al (22) (n=93) and Young et al (21) (n=307). Unfortunately, their series contain tumors from variable laryngeal subsites (glottic, supraglottic and subglottic), variable stages (T1-4 and N0-N3), and variable treatments (both surgical and non-surgical), thus, complicating the evaluation of outcome differences between groups. However, the lack of general prognostic significance proved true also in our highly homogeneous patient material of T2-3N0 glottic SCC treated with RT/CRT, when observed together as single series. The incidence of p16 expression in this study is in agreement with Young et al (21) and Castellsagué et al (20) who noted incidences of 6.5 and 3%, respectively. Higher prevalence of p16 or HPV-positivity in laryngeal SCC has previously been associated with female sex (21), non-smokers (30) and younger age (31). We also found a significant predominance of p16-positivity in younger (<60 years) patients. Among these patients, non-smokers were more frequently p16-positive than smokers, albeit smoking had no significant relation to p16 positivity in the whole cohort.
Although no significant link between HPV/p16 positivity and laryngeal SCC treatment outcome has been established in general, some authors have observed trends towards less recurrence among HPV/p16-positive patients (30,31). In our material, none of the p16-positive laryngeal SCCs in patients under 60 years recurred (P=0.041), and DFS was also significantly better for this group. Taken together, these previous and current findings suggest that there may be a distinct subgroup of laryngeal SCCs with HPV as a major etiological factor. This group may also have an improved outcome compared with laryngeal SCCs with no HPV association. Unfortunately, HPV- or p16-positive tumors form a small minority of laryngeal SCC. Larger studies or meta-analyses are needed to reach sufficient statistical power to assess its utility as a prognostic factor. Also, the insufficient sensitivity of p16 as a surrogate marker (32) calls for proper assessment of HPV status in these p16-positive tumors, which could not be conducted in the current study.
In conclusion, the present study demonstrates markedly improved outcome for T3N0 glottic SCC patients treated with CRT as compared to those treated with RT alone. Furthermore, our results suggest that cytoplasmic WRAP53β may be a potential predictive marker of poor response to RT/CRT in glottic laryngeal cancer. While HPV or p16 positivity is rare in laryngeal SCC, it may prove significant in identifying a prognostically separate subgroup in the future. Prospective studies are warranted to test the value of predictive markers of RT response in HNSCC such as WRAP53β, to help us to proceed with more individualized treatment decisions and better outcomes for our patients.
Acknowledgements
The present study was supported by the Swedish Cancer Society (2010/545), the County Council of Östergötland, the Research Funds of Linköping University Hospital, the Finnish Cancer Society, the Finnish Medical Foundation, the Finnish-Norwegian Medical Foundation and Helsinki University Hospital Research Funds (TYH2015204).
References
- 1.Engholm G, Ferlay J, Christensen N, Bray F, Gjerstorff ML, Klint A, Køtlum JE, Olafsdóttir E, Pukkala E, Storm HH. NORDCAN - a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49:725–736. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- 2.Haapaniemi A, Koivunen P, Saarilahti K, Kinnunen I, Laranne J, Aaltonen LM, Närkiö M, Lindholm P, Grénman R, Mäkitie A, et al. Finnish Head and Neck Oncology Working Group: Laryngeal cancer in Finland: A 5-year follow-up study of 366 patients. Head Neck. 2016;38:36–43. doi: 10.1002/hed.23834. [DOI] [PubMed] [Google Scholar]
- 3.Wennerberg J. 5th World Congress of IFHNOS and the 2014 Annual Meeting of the AHNS. New York: 2014. A population based perspective on treatment and outcome of glottic laryngeal carcinoma stage T3 and T4 - does organ preservation jeopardize survival? [Google Scholar]
- 4.Hoffman HT, Porter K, Karnell LH, Cooper JS, Weber RS, Langer CJ, Ang KK, Gay G, Stewart A, Robinson RA. Laryngeal cancer in the United States: Changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(Suppl 111):1–13. doi: 10.1097/01.mlg.0000236095.97947.26. [DOI] [PubMed] [Google Scholar]
- 5.Olsen KD. Reexamining the treatment of advanced laryngeal cancer. Head Neck. 2010;32:1–7. doi: 10.1002/hed.21294. [DOI] [PubMed] [Google Scholar]
- 6.Farnebo L, Tiefenbock K, Ansell A, Thunell LK, Garvin S, Roberg K. Strong expression of survivin is associated with positive response to radiotherapy and improved overall survival in head and neck squamous cell carcinoma patients. Int J Cancer. 2013;133:1994–2003. doi: 10.1002/ijc.28200. [DOI] [PubMed] [Google Scholar]
- 7.Garvin S, Tiefenböck K, Farnebo L, Thunell LK, Farnebo M, Roberg K. Nuclear expression of WRAP53β is associated with a positive response to radiotherapy and improved overall survival in patients with head and neck squamous cell carcinoma. Oral Oncol. 2015;51:24–30. doi: 10.1016/j.oraloncology.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Garg H, Suri P, Gupta JC, Talwar GP, Dubey S. Survivin: A unique target for tumor therapy. Cancer Cell Int. 2016;16:49. doi: 10.1186/s12935-016-0326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altieri DC. Targeting survivin in cancer. Cancer Lett. 2013;332:225–228. doi: 10.1016/j.canlet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li F, Yang J, Ramnath N, Javle MM, Tan D. Nuclear or cytoplasmic expression of survivin: what is the significance? Int J Cancer. 2005;114:509–512. doi: 10.1002/ijc.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: Molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67:5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 12.Lo Muzio L, Farina A, Rubini C, Pezzetti F, Stabellini G, Laino G, Santarelli A, Pannone G, Bufo P, de Lillo A, et al. Survivin as prognostic factor in squamous cell carcinoma of the oral cavity. Cancer Lett. 2005;225:27–33. doi: 10.1016/j.canlet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Freier K, Pungs S, Sticht C, Flechtenmacher C, Lichter P, Joos S, Hofele C. High survivin expression is associated with favorable outcome in advanced primary oral squamous cell carcinoma after radiation therapy. Int J Cancer. 2007;120:942–946. doi: 10.1002/ijc.22380. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoudi S, Henriksson S, Corcoran M, Méndez-Vidal C, Wiman KG, Farnebo M. Wrap53, a natural p53 antisense transcript required for p53 induction upon DNA damage. Mol Cell. 2009;33:462–471. doi: 10.1016/j.molcel.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Henriksson S, Farnebo M. On the road with WRAP53β: Guardian of Cajal bodies and genome integrity. Front Genet. 2015;6:91. doi: 10.3389/fgene.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedström E, Pederiva C, Farnebo J, Nodin B, Jirström K, Brennan DJ, Farnebo M. Downregulation of the cancer susceptibility protein WRAP53β in epithelial ovarian cancer leads to defective DNA repair and poor clinical outcome. Cell Death Dis. 2015;6:e1892. doi: 10.1038/cddis.2015.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silwal-Pandit L, Russnes H, Borgen E, Skarpeteig V, Vollan Moen HK, Schlichting E, Kåresen R, Naume B, Børresen-Dale AL, Farnebo M, et al. The sub-cellular Localization of WRAP53 has prognostic impact in breast cancer. PLoS One. 2015;10:e0139965. doi: 10.1371/journal.pone.0139965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rassoolzadeh H, Böhm S, Hedström E, Gad H, Helleday T, Henriksson S, Farnebo M. Overexpression of the scaffold WD40 protein WRAP53β enhances the repair of and cell survival from DNA double-strand breaks. Cell Death Dis. 2016;7:e2267. doi: 10.1038/cddis.2016.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer CA, Zlobec I, Green E, Probst S, Storck C, Lugli A, Tornillo L, Wolfensberger M, Terracciano LM. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126:1256–1262. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 20.Castellsagué X, Alemany L, Quer M, Halec G, Quirós B, Tous S, Clavero O, Alòs L, Biegner T, Szafarowski T, et al. ICO International HPV in Head and Neck Cancer Study Group: HPV involvement in head and neck cancers: Comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108:djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 21.Young RJ, Urban D, Angel C, Corry J, Lyons B, Vallance N, Kleid S, Iseli TA, Solomon B, Rischin D. Frequency and prognostic significance of p16INK4A protein overexpression and transcriptionally active human papillomavirus infection in laryngeal squamous cell carcinoma. Br J Cancer. 2015;112:1098–1104. doi: 10.1038/bjc.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morshed K, Polz-Dacewicz M, Szymański M, Polz D. Short-fragment PCR assay for highly sensitive broad-spectrum detection of human papillomaviruses in laryngeal squamous cell carcinoma and normal mucosa: Clinico-pathological evaluation. Eur Arch Otorhinolaryngol. 2008;265(Suppl 1):S89–S96. doi: 10.1007/s00405-007-0569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, Solomon B, Choi J, OSullivan B, Kenny LM, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura G, Tsukuda M, Mikami Y, Matsuda H, Horiuchi C, Taguchi T, Takahashi M, Kawakami M, Watanabe M, Niho T, et al. Efficacy of concurrent chemoradiotherapy for T1 and T2 laryngeal squamous cell carcinoma regarding organ preservation. Anticancer Res. 2009;29:661–666. [PubMed] [Google Scholar]
- 26.Akimoto T, Nonaka T, Kitamoto Y, Ishikawa H, Ninomiya H, Chikamatsu K, Furuya N, Hayakawa K, Mitsuhashi N, Nakano T. Radiation therapy for T2N0 laryngeal cancer: A retrospective analysis for the impact of concurrent chemotherapy on local control. Int J Radiat Oncol Biol Phys. 2006;64:995–1001. doi: 10.1016/j.ijrobp.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Berardinelli F, Nieri D, Sgura A, Tanzarella C, Antoccia A. Telomere loss, not average telomere length, confers radiosensitivity to TK6-irradiated cells. Mutat Res. 2012;740:13–20. doi: 10.1016/j.mrfmmm.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 28.McCaul JA, Gordon KE, Minty F, Fleming J, Parkinson EK. Telomere dysfunction is related to the intrinsic radio-resistance of human oral cancer cells. Oral Oncol. 2008;44:261–269. doi: 10.1016/j.oraloncology.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Henriksson S, Rassoolzadeh H, Hedström E, Coucoravas C, Julner A, Goldstein M, Imreh G, Zhivotovsky B, Kastan MB, Helleday T, et al. The scaffold protein WRAP53beta orchestrates the ubiquitin response critical for DNA double-strand break repair. Genes Dev. 2014;28:2726–2738. doi: 10.1101/gad.246546.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalfert D, Celakovsky P, Laco J, Ludvikova M. The role of protein p16INK4a in glottic laryngeal squamous cell carcinoma. Pathol Oncol Res. 2014;20:909–915. doi: 10.1007/s12253-014-9773-y. [DOI] [PubMed] [Google Scholar]
- 31.Baumann JL, Cohen S, Evjen AN, Law JH, Vadivelu S, Attia A, Schindler JS, Chung CH, Wirth PS, Meijer CJ, et al. Human papillomavirus in early laryngeal carcinoma. Laryngoscope. 2009;119:1531–1537. doi: 10.1002/lary.20509. [DOI] [PubMed] [Google Scholar]
- 32.Jouhi L, Hagström J, Atula T, Mäkitie A. Is p16 an adequate surrogate for human papillomavirus status determination? Curr Opin Otolaryngol Head Neck Surg. 2017;25:108–112. doi: 10.1097/MOO.0000000000000341. [DOI] [PubMed] [Google Scholar]