ABSTRACT
Stereotactic body radiation therapy (SBRT) of local tumor would induce an abscopal effect that has been observed in several kinds of human cancers; one important mechanism may involve the improved activation of the host immune system. The immune checkpoint inhibitor can overcome immune tolerance and enhance the activation of antitumor T cells. The combined treatment of SBRT and checkpoint inhibitor may represent a new promising therapeutic approach. Herein, we reported a patient with metastatic renal cell carcinoma (RCC) treated with concurrent SBRT and anti-PD-1 antibody, pembrolizumab, by which the patient achieved an amazingly systemic complete response in only 2.2 months after starting treatment. This case report indicates that the advanced RCC may benefit from the combining treatment of local SBRT and PD-1 inhibitor and provide a useful paradigm worthy of establishing a clinical trial for patients with advanced renal cell carcinoma.
KEYWORDS: Programmed death-1, radiation therapy, renal cell carcinoma
Abbreviations
- RT
radiotherapy
- SBRT
stereotactic body radiation therapy
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- PD-1
programmed death-1
- RCC
renal cell carcinoma
Introduction
The abcsopal effect refers to a rare phenomenon of tumor regression in lesions distant from the targeted site of radiotherapy (RT), and has been known as a rare unexplained phenomenon in patients receiving local radiotherapy.1 The biologic mechanism underlying this effect has not been completely understood, but it may be mediated by RT-induced activation of host immune system, including exposing cryptic tumor antigens, enhancing tumor-antigen presentation, and also altering the tumor microenvironment.2 Preliminary data suggest that stereotactic body radiotherapy (SBRT), whereby tumor cells were exposed to higher doses of radiation delivered in a limited number of fractions, cannot only effectively destroy tumor cells directly but might also more efficiently induce the activation of anti-tumor immune response than radiotherapy with conventional fractions.3,4 However, although RT alone may be efficient at releasing cryptic or neo- tumor antigens, tumor cell would cunningly induced immune-suppression and immune-tolerance to prevent therapeutically effective antitumor immune response.2,5
Immunotherapeutic strategies developed to overcome immune tolerance and enhance the activation of antitumor T cells represent a new promising therapeutic approach.6 An immune checkpoint inhibitor, ipilimumab, a monoclonal against the human cytotoxic T-lymphocyte antigen-4 (CTLA-4), has demonstrated activity in metastatic melanoma treatment. Nivolumab and pembrolizumab, against another immune checkpoint molecular programmed death-1 (PD-1), also have presented anti-tumor activity in metastatic melanoma (approved by FDA in 2014) and non-small cell lung cancer (approved by FDA in 2015) treatment. Therefore, the combination between RT and immunotherapy might be a promising approach to improve anti-tumor effect.2 Till now, there are several case reports showing that local radiation in combination with anti-CTLA-4 antibody ipilimumab resulted in an abscopal effect in patients with metastatic melanoma7,8,9,10 or NSCLC .11
Renal cell carcinoma (RCC) is a rapidly progressing malignant tumor of urinary system. Approximately one-third of patients with renal cell carcinoma have metastatic disease at initial presentation.12 Metastatic RCC (mRCC) has been notoriously resistant to conventional radiotherapy and chemotherapy and is almost incurable condition. Current therapeutic approaches of metastatic RCC involve surgery, targeted therapy and various types of immunotherapy. Although these treatment options have demonstrated progression-free survival benefit, most patients with mRCC eventually experience progression.13,14 Therefore, patients with mRCC continue to present a therapeutic challenge. Nevertheless, the FDA approved anti-PD-1 antibody, nivolumab, in November 2015 for the treatment of advanced metastatic RCC based on the results of a phase III trial with the objective response rate of 25% and the media overall survival of 25 months, despite only 1% complete response.15 Herein, we report a patient with metastatic RCC who achieved a systemic complete response in only 2.2 months after starting treatment with concurrent SBRT and anti-PD-1 antibody, pembrolizumab.
Case report
In February 2016, a 54-year-old male presented with a left-sided abdominal and lumbar pain. A computed tomography (CT) scan of the chest and abdomen revealed a left-sided renal tumor with 10.1 cm maximum diameter, and multiple mediastinal and retroperitoneal nodules (Fig. 1A), and he underwent nephrectomy in February 23, 2016. The pathological exam showed a moderately to poorly differentiated clear cell renal carcinoma (Fig. 1A) and verified metastatic lesions in retroperitoneal nodules (31/31). He was staged T2aN1M1 (stage IV), according to the American Joint Commission on Cancer 7th edition. After radical nephrectomy, the patient was initiated on sunitinib 50 mg daily for 4 weeks with 2-week interval, and 6 weeks was a cycle. After the 1th cycle, the patient presented with dysphagia and bilateral cervical lymph node enlargement in May 2016, and a CT scan in May 18, 2016 demonstrated an increase in number and size of mediastinal, retroperitoneal and pelvis nodules, and multiple new enlarged cervical lymph nodes (the largest one, 2.10 cm × 1.45 cm × 1.30 cm), and the esophagus was compressed by a mediastinal enlarged lymph node (Fig. 2A, the third column).
Figure 1.
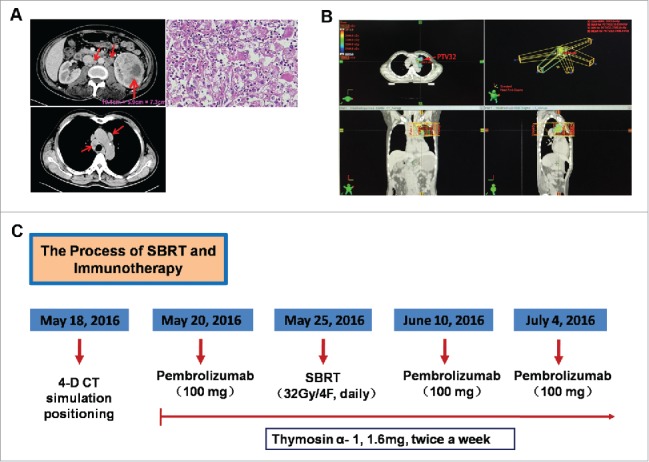
The diagnosis imaging of renal cell carcinoma and the whole process of treatment. A. CT scan before nephrectomy treatment (February 23, 2016); H&E staining of tumor tissue showed a clear cell renal carcinoma. B. The para-aortic enlarged mass was selected as RT target and an additional 0.5 cm margin was added to create a planning target volume (PTV); a total of 32 Gy was administered in 4 fractions to PTV with 6-MV photons by means of a coplanar 5-field intensity-modulated, image-guided technique. C. The whole process of treatment with the concurrent RT and pembrolizumab.
Figure 2.
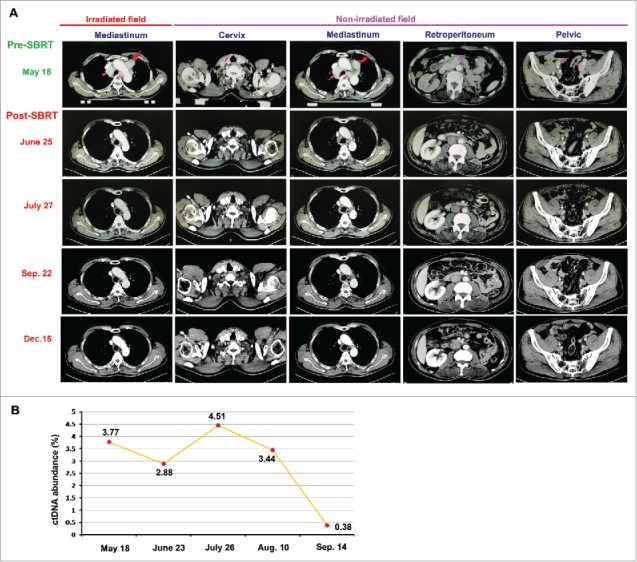
Tumor regressed after the combined treatment with RT and pembrolizumab. A. On June 25, 2016 (a month after starting treatment with RT), an enhanced CT scan showed the dramatically decreased volumes of tumors, compared with the images before combined treatment (May 18, 2016), not only in irradiated field but also in non-irradiated fields; more importantly, the CT scan images on July 27, 2016 demonstrated that all metastases, including the mass in the RT field and other metastatic lesions out of the RT fields, had completely resolved, consistent with a systemic complete response by RECIST criteria. B. The first detection of ctDNA abundance in May 18, 2016 showed 3.77% (ctDNA abundance = ctDNA in peripheral blood / total free DNA in peripheral blood); there was a remarkable decrease of ctDNA abundance (2.28%) measured in June 23, 2016; and only 0.38% ctDNA abundance could be detected in Sep. 14, 2016.
The patient was thereafter initiated on pembrolizumab and local RT to metastatic lymph nodes in left mediastinum with the intent to generate an abscopal response. The experimental nature of this approach was extensively discussed with the patient, who was informed of the only 7 available reports in melanoma and one in NSCLC,2 but the lack of available evidence for the patient with renal cell carcinoma.
The patient was initiated on pembrolizumab 2 mg/kg body weight IV over 30 minutes in May 20, 2016, given every-3-weeks for 4 cycles, following initial 4 doses, pembrolizumab was administered every 12 weeks until tumor progression. The patient was simulated in the supine position and the para-aortic enlarged mass was selected as radiotherapy target and contoured as the gross tumor volume (GTV). An additional 0.5 cm margin was added to create a planning target volume (PTV) (Fig. 1B). In May 25, 2016, a total of 32 Gy was administered in 4 fractions over a period of 4 consecutive days to PTV in mediastinum with 6-MV photons by means of a coplanar 5-field intensity-modulated, image-guided radiation therapy (Fig. 1B). The planning evaluation of PTV and normal tissues was shown in Table 1 and 2. Additionally, thymosinα-1 was administered at twice weekly dose of 1.6 mg by subcutaneous injection during the process of SBRT and pembrolizumab therapy. The whole process of treatment with RT and pembrolizumab was shown in Fig. 1C. The patient can tolerate RT and pembrolizumab without any treatment-related adverse events.
Table 1.
Dose design in irradiated target field.
| PTV |
PTV volume |
Prescribed dose (Gy) |
V100%/VPTV (R100%) |
V50%/ VPTV (R50%) |
Dose at 2cm distance from PTV (Gy) |
| PTV-32 | 85.72 cm3 | 32Gy (8Gy × 4F) | Objective: <1.2–1.5 | Objective: <3.5–4.8 | Objective: <21.12–27.52 |
| Actual: 0.91 | Actual: 4.49 | Actual: 24 |
Table 2.
OAR maximum dose constraints for SBRT.
| OAR*\regimen | 4 Fractions |
|---|---|
| Spinal cord | Objective: 30 Gy |
| Actual: 10.95 Gy | |
| Esophagus | Objective: 32.5 Gy |
| Actual: 27.35 Gy | |
| Heart | Objective: 35 Gy |
| Actual: 7 Gy | |
| Great vessels | Objective: 55 Gy |
| Actual: 32Gy | |
| Trachea and ipsilateral bronchus | Objective: 32.5 Gy |
| Actual: 17.35 Gy | |
| Stomach | Objective: 35 Gy |
| Actual: 0.14 Gy | |
| Lung-Left | Objective: V20<10–15% |
| Actual: V20 = 5.2% | |
| Lung-Right | Objective: V20<10–15% |
| Actual: Dmax = 13Gy |
OAR, organ at risk.
Results
The patient's symptoms of dysphagia disappeared and the enlarged cervical lymph nodes markedly reduced in number and size just 8 d after starting treatment with RT. A neck-chest and abdomen CT scan (June 25, 2016) was performed a month after starting treatment with RT. The scan images demonstrated a dramatic treatment response of the patient's known diseases. Not only was an objective response detected in the para-aortic mass in the RT field, but striking responses were also observed at other metastatic sites out of the RT fields (Fig. 2A). A month later, another CT scan of neck-chest and abdomen (July 27, 2016, only 2.2 months after starting treatment with pembrolizumab) was performed to re-assess the response to the combined treatment. The CT scan images demonstrated that all metastases, including the mass in the RT field and other metastatic lesions out of the RT fields, had completely resolved, consistent with a systemic complete response by RECIST criteria (Fig. 2A). The CT scan in September 22, 2016 (in 4 months after starting treatment with pembrolizumab) showed a sustained complete response of all lesions (Fig. 2A). After that, the patient was followed up and reassessed by CT scan every 3 months. The latest CT scan in December 15, 2016 (in about 7 months after starting treatment with pembrolizumab) consistently showed a complete response of all lesions (Fig. 2A).
Measurement of circulating tumor DNA (ctDNA) has been used as a powerful biomarker for noninvasive assessment of cancer burden and enables detection of microscopic disease before it can be seen on CT scans.16-19 We therefore measured the abundance of ctDNA in blood to predict cancer burden before and during treatment with pembrolizumab and RT. Before the combined treatment, the first detection of ctDNA abundance in May 18, 2016 showed 3.77% (ctDNA abundance = ctDNA in peripheral blood / total free DNA in peripheral blood) (Fig. 2B). A month later, there was a remarkable decrease of ctDNA abundance (2.28%) measured in June 23, 2016 (Fig. 2B). However, unexpectedly, there was a sharp rise of ctRNA abundance observed a month later (measured in July 26). After that, the value of ctDNA abundance began to decrease when measured in Aug. 10. Importantly, only 0.38% ctDNA abundance could be detected in September 14, 2016 (Fig. 2B), which was consistent with a systemic complete response shown in CT images just in 2.2 months after starting treatment with pembrolizumab.
Discussion
A PD-1 checkpoint inhibitor, nivolumab, has shown an overall survival benefit in a randomized, phase II trial and a randomized, phase III trial involving patients with advanced metastatic renal-cell carcinoma, yet objective response rates remain modest, between 20% and 25%, with only 0–2% complete response rates;15,20 and the time to achieving an objective response ranged from 1.4 to 24.8 months and median time to response was 3.5 to 3.7 months.15 Increasing the response rate to anti-PD-1 treatment becomes an area of active investigation. SBRT whereby exposure of tumor cells to higher doses of radiation in limited fractions has been demonstrated a function as an antitumor vaccine in situ,3,21 may produce an abscopal effect, although it is a rare phenomenon in patients receiving local radiotherapy. Several cases have been reported that local SBRT improved systemic immunotherapy effect of anti-CTLA-4 antibody, ipilimumab, in patients with metastatic melanoma7-9,22 or NSCLC.11 Herein, we reported the first patient with advanced renal cell carcinoma who received concurrent treatment of a PD-1 checkpoint inhibitor, pembrolizumab, and SBRT. A rapid objective response was seen just 8 d after the start of treatment with SBRT and 13 d after the start of pembrolizumab. Importantly, a systemic complete response was seen only 2.1 months after starting treatment with SBRT and 2.2 months after pembrolizumab. This marvelous effect observed herein provides further evidence supporting that SBRT induces the activation of host anti-tumor immune response and the combined treatment with SBRT and PD-1 checkpoint inhibitor will be a well-promising therapeutic approach whereby SBRT would strikingly shorten the time to objective response and improve a systemic complete response rate of anti-PD-1 immunotherapy in patients with advanced metastatic renal cell carcinomas.
The sequencing of SBRT and immunotherapy may be an important aspect impacting on the therapeutic effect of this combined strategy. The immunotherapy agent of choice most probably decides the optimal sequencing of SBRT. Varies studies in preclinical models have shown that SBRT given after starting checkpoint blockade immunotherapy or concurrent SBRT and checkpoint blockade immunotherapy has much better effect than SBRT given before checkpoint blockade immunotherapy. One possible mechanism is that stimulating antigen-presenting cells and effector T cells by checkpoint blockade immunotherapy will allow these cells to be readily available to respond to the efflux of tumor antigens generated by SBRT and additionally, the altered tumor microenvironment by checkpoint blockade immunotherapy could maximize therapeutic effects of SBRT.2 However, another converse theory hypothesizes that SBRT given before immunotherapy will generate de novo tumor antigens and broke any pre-existing peripheral immune tolerance of tumor, and thus reinforce the anti-tumor response of immunotherapy. Therefore, the sequencing of SBRT and immunotherapy is a controversial issue confronting oncologist to design a clinical trait today. In this case, the patient received SBRT 5 d after starting PD-1 blockade and demonstrated miraculous systemic complete response in only 2.2 months. Therefore, this case report may provide a useful paradigm worthy of establishing a clinical trial for patients with advanced renal cell carcinoma.
Thymosin α-1 is a naturally occurring polypeptide that regulates immune cell development and function.23-25 Researches have shown that thymosin α-1acted as an endogenous regulator of both the innate and adaptive immunity and thus increased immune effector cell activity which induced antitumor responses and led to more efficient antigen presentation, and thymosin α-1 also enhanced tumor cell immunogenicity through increasing the expression of MHC I molecules in tumor cells.25-27 Herein, thymosin α-1 was used during the process of SBRT and pembrolizumab so as to get a better stimulation of the host anti-tumor immune response.
Additionally, dynamic measurement of ctDNA has been used as a potential predictor for noninvasive assessment of cancer burden.16-19 However, we here observed a rebounding rise of ctDNA abundance before its sharp decrease. The most possible explanation is that mass death of tumor cells in a relatively short time probably releases a great number of ctDNA into circulating blood. Therefore, the ctDNA abundance as a predictor of tumor progression or burden may be more significant when it could be dynamically detected for a longer time.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank the stereotactic body radiation therapy group of Nanfang Hospital. We also appreciated GenomiCare Biotechnology (Shanghai) Co., Ltd. for the measure of ctDNA abundance.
References
- 1.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953; 26:234–41; PMID:13042090; https://doi.org/ 10.1259/0007-1285-26-305-234 [DOI] [PubMed] [Google Scholar]
- 2.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): A curative approach? Nat Rev Clin Oncol 2016; 13:516–24; PMID:26951040; https://doi.org/ 10.1038/nrclinonc.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys 2012; 84:879–80; PMID:23078897; https://doi.org/ 10.1016/j.ijrobp.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012; 83:1306–10; PMID:22208977; https://doi.org/ 10.1016/j.ijrobp.2011.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J Natl Cancer Inst 2013; 105:256–65; PMID:23291374; https://doi.org/ 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252–64; PMID:22437870; https://doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al.. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366:925–31; PMID:22397654; https://doi.org/ 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, Swetter SM, Knox SJ. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol 2012; 5:404–7; PMID:23323154; https://doi.org/ 10.1593/tlo.12280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013; 2:899–906; PMID:24403263; https://doi.org/ 10.1002/cam4.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 2013; 85:293–5; PMID:22560555; https://doi.org/ 10.1016/j.ijrobp.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013; 1:365–72; PMID:24563870; https://doi.org/ 10.1158/2326-6066.CIR-13-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedke J, Gauler T, Grunwald V, Hegele A, Herrmann E, Hinz S, Janssen J, Schmitz S, Schostak M, Tesch H, Zastrow S, Miller K. Systemic therapy in metastatic renal cell carcinoma. World J Urol 2017; 35:179-88; PMID:27277600; https://doi.org/ 10.1007/s00345-016-1868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finley DS, Pantuck AJ, Belldegrun AS. Tumor biology and prognostic factors in renal cell carcinoma. Oncologist 2011; 16(Suppl 2):4–13; PMID:21346035; https://doi.org/ 10.1634/theoncologist.2011-S2-04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escudier B, Porta C, Schmidinger M, Algaba F, Patard JJ, Khoo V, Eisen T, Horwich A. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25(Suppl 3):i49–56; PMID:25210086; https://doi.org/ 10.1093/annonc/mdu259 [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al.. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373:1803–13; PMID:26406148; https://doi.org/ 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al.. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008; 14:985–90; PMID:18670422; https://doi.org/ 10.1038/nm.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B, et al.. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013; 368:1199–209; PMID:23484797; https://doi.org/ 10.1056/NEJMoa1213261 [DOI] [PubMed] [Google Scholar]
- 18.Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, Parkinson C, Chin SF, Kingsbury Z, Wong AS, et al.. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 2013; 497:108–12; PMID:23563269; https://doi.org/ 10.1038/nature12065 [DOI] [PubMed] [Google Scholar]
- 19.Yong E. Cancer biomarkers: Written in blood. Nature 2014; 511:524–6; PMID:25079538; https://doi.org/ 10.1038/511524a [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, et al.. Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol 2015; 33:1430–7; PMID:25452452; https://doi.org/ 10.1200/JCO.2014.59.0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Liu L, Yu D, Kandimalla ER, Sun HB, Agrawal S, Guha C. An in situ autologous tumor vaccination with combined radiation therapy and TLR9 agonist therapy. PLoS One 2012; 7:e38111; PMID:22666458; https://doi.org/ 10.1371/journal.pone.0038111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber NK, Young RJ, Barker CA, Wolchok JD, Chan TA, Yamada Y, Friguglietti L, Beal K. Ipilimumab and whole brain radiation therapy for melanoma brain metastases. J Neurooncol 2015; 121:159–65; PMID:25273687; https://doi.org/ 10.1007/s11060-014-1617-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Low TL, Thurman GB, McAdoo M, McClure J, Rossio JL, Naylor PH, Goldstein AL. The chemistry and biology of thymosin. I. Isolation, characterization, and biological activities of thymosin alpha1 and polypeptide beta1 from calf thymus. J Biol Chem 1979; 254:981–6; PMID:216684 [PubMed] [Google Scholar]
- 24.Goldstein AL. History of the discovery of the thymosins. Ann N Y Acad Sci 2007; 1112:1–13; PMID:17600284; https://doi.org/ 10.1196/annals.1415.045 [DOI] [PubMed] [Google Scholar]
- 25.Pierluigi B, D'Angelo C, Fallarino F, Moretti S, Zelante T, Bozza S, De Luca A, Bistoni F, Garaci E, Romani L. Thymosin alpha1: The regulator of regulators? Ann N Y Acad Sci 2010; 1194:1–5; PMID:20536444; https://doi.org/ 10.1111/j.1749-6632.2010.05465.x [DOI] [PubMed] [Google Scholar]
- 26.Garaci E, Pica F, Matteucci C, Gaziano R, D'Agostini C, Miele MT, Camerini R, Palamara AT, Favalli C, Mastino A, et al.. Historical review on thymosin alpha1 in oncology: Preclinical and clinical experiences. Expert Opin Biol Ther 2015; 15(Suppl 1):S31–9; PMID:26096345; https://doi.org/ 10.1517/14712598.2015.1017466 [DOI] [PubMed] [Google Scholar]
- 27.Maio M, Mackiewicz A, Testori A, Trefzer U, Ferraresi V, Jassem J, Garbe C, Lesimple T, Guillot B, Gascon P, et al.. Large randomized study of thymosin alpha 1, interferon alfa, or both in combination with dacarbazine in patients with metastatic melanoma. J Clin Oncol 2010; 28:1780–7; PMID:20194853; https://doi.org/ 10.1200/JCO.2009.25.5208 [DOI] [PubMed] [Google Scholar]


