Abstract
Development of synthetic biomaterials imbued with inorganic and organic characteristics of natural bone that are capable of promoting effective bone tissue regeneration is an ongoing goal of regenerative medicine. Calcium phosphate (CaP) has been predominantly utilized to mimic the inorganic components of bone, such as calcium hydroxyapatite, due to its intrinsic bioactivity and osteoconductivity. CaP-based materials can be further engineered to promote osteoinductivity through the incorporation of osteogenic biomolecules. In this study, we briefly describe the microstructure and the process of natural bone mineralization and introduce various methods for coating CaP onto biomaterial surfaces. In particular, we summarize the advantages and current progress of biomimetic surface-mineralizing processes using simulated body fluids for coating bone-like carbonated apatite onto various material surfaces such as metals, ceramics, and polymers. The osteoinductive effects of integrating biomolecules such as proteins, growth factors, and genes into the mineral coatings are also discussed.
Keywords: : simulated body fluids, bone, bone tissue engineering, calcium phosphate, polymer scaffolds
Introduction
Musculoskeletal disorders and diseases cost over $180 billion annually in the United States.1 Demand for bone grafts and the financial burden are expected to rapidly increase due to increasing life expectancy.2,3 Autograft bone is currently the clinical gold standard for treating critical size bone defects. Bone autografts, however, have the disadvantages of generating a second surgical site, donor-site morbidity, and diminished patient quality of life due to surgical burden.4–6 Allograft bone and xenograft bone are alternative options to autograft bone, but these run the risk of transmission of infection, immune rejection, toxicity associated with sterilization, and being inefficient at osteoinduction.4,5,7–9 Therefore, the field of tissue engineering and regenerative medicine has been focused on alternative ways of regenerating healthy tissue to replace diseased or damaged bone tissue.
Desirable characteristics of biomaterials for bone tissue engineering are as follows: possessing a bioactive surface10–12; having the capacity to promote new bone formation from the surrounding established bone (osteoconductivity)13,14; and having the ability to induce osteoblastic differentiation (osteoinductivity).13,14 To achieve these properties, researchers have focused on developing scaffolds using a multitude of materials, including natural products, synthetic polymers, and metals that offer ideal properties for tissue engineering. However, these products still fall short of the gold standard: autografts. To improve the bone regeneration properties of scaffolds, researchers have coated them with various forms of apatite, which mimic the natural bone surface thus providing an ideal environment for osteogenesis and increase the structural stability of the scaffold. A promising approach to surface coating materials is using simulated body fluids (SBFs). The high concentration of calcium and phosphate in these fluids promotes the formation of calcium phosphate (CaP) crystalline structures similar to the apatite found in native bone. In this study, the use of SBFs as a biomimetic technique will be discussed through investigation of SBF analytical use, applicable substrates, and biomolecule incorporation.
Bone is a complex tissue that consists of an inorganic phase intimately embedded into an organic extracellular matrix (ECM). The mass of dehydrated bone is ∼70% inorganic and 30% organic.15 The inorganic phase of bone contributes to the structural support of the skeletal system. Bone mineral comprises mainly of carbonated hydroxyapatite (carbonated apatite)16 and differs from hydroxyapatite in that it is a nonstoichiometric apatite with a Ca/P ratio that may range from 1.50 to 1.90 depending on age, gender, bone site, and pathophysiological conditions. This nonstoichiometric chemical composition of bone mineral is mainly due to the presence of ionic substitutions, such as CO32− and HPO42− that may be substituted for PO43−, while Na+, Mg 2+, and K2+ may replace Ca2+ of hydroxyapatite.17,18 It is anticipated that the poorly crystalline (i.e., amorphous) property, in conjunction with nonstoichiometric chemistry, of carbonated apatite contributes to it possessing a higher solubility than hydroxyapatite.19–21 The organic ECM is predominantly composed of collagens, type I collagen in particular. In addition, noncollagenous proteins known as small integrin-binding ligand N-linked glycoproteins (SIBLING proteins) comprise 10–15% of total bone protein content.22
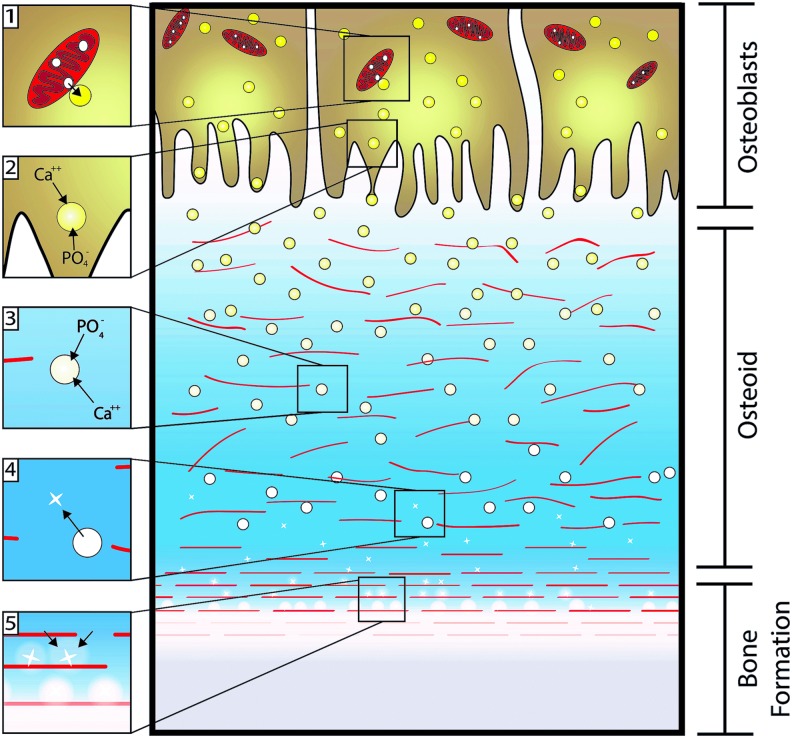
Bone mineralization occurs during development, remodeling of existing bone, and fracture repair.15,23 To form new bone, osteoblasts are recruited to the area through a variety of factors such as transforming growth factor-β and bone morphogenetic proteins (BMPs). Then, the osteoblasts begin to deposit a new collagenous matrix for mineralization to occur.24,25 The osteoblasts then accumulate Ca2+ and PO43− within polarized matrix vesicles promoted by phosphatases, calcium binding proteins, and potential mitochondria vesicles.26 The matrix vesicles are then released from osteoblasts and continue to concentrate Ca2+ and PO43−.27 Once precipitation occurs, the matrix vesicles release hydroxyapatite nanocrystals and amorphous calcium phosphate into the local environment.15 The collagen framework, now coated with highly acidic fibrils such as SIBLlNG proteins, provides an anchoring point for the nanocrystals.16,24 The attached crystals and charged regions of the collagen act as nucleation sites for crystal growth by converting the high levels of Ca2+, PO43−, and amorphous calcium phosphate into ordered carbonated apatite (Fig. 1).26,28 Over time, osteoblasts become embedded within the bone mineral and collagen matrix, and differentiate to osteocytes or undergo apoptosis.15
FIG. 1.
Natural osteoblast-mediated bone mineralization. (1 and 2) Osteoblast matrix vesicles accumulate calcium and phosphate ions (transitioning from yellow to white) from the cytosol and mitochondria, and are released toward the newly formed collagen matrix (red lines). (3) The released vesicles continue to concentrate calcium and phosphate ions until precipitation occurs, drawing from the ion-rich environment. (4) The newly formed apatite crystals (white crosses) are released into the environment, (5) providing nucleation sites for continued apatite growth. Adapted from Mescher 2013, The McGraw-Hill Companies, Inc.28 Color images available online at www.liebertpub.com/tea
Approaches for Mineralizing Biomaterials
Biomaterials coated with CaP have been widely used for bone regeneration due to their excellent intrinsic bioactivity and osteoconductivity. CaP-based coatings can be further engineered to incorporate biomolecules that promote osteoinductivity. CaP coatings were first investigated in the early 1980s to treat the surface of titanium (Ti) metal implants so as to enhance the bonding ability of the implant to the bone. Since then, various methods have been developed to provide bioactivity to nonbioactive materials using various coating techniques such as thermal spraying,29–33 sputter coating,34–36 sol–gel deposition,37–39 hot isostatic pressing,40,41 and dip coating.42 Each of these methods has advantages and disadvantages (Table 1).43
Table 1.
Calcium Phosphate Coating Techniques for Biomaterials
| Method | Advantages | Disadvantages | Process conditions | Coating thickness | References |
|---|---|---|---|---|---|
| Thermal spraying | Widely commercialized with low processing cost; high coating rate | Biomolecules cannot be incorporated due to high processing temperature; large minimum thickness for uniform coating; low adhesive strength; risk of delamination | High processing temperature (>8,000°C) | 30–200 μm | Huang et al.29; De Groot et al.30; Yang et al.31; Li et al.32; Gross et al.33 |
| Sputter coating | Can form thin and uniform coating; high adhesive strength | Long processing time; expensive; hard to control Ca/P ratio when processed by RF magnetic sputtering; | Low processing temperature | <3 μm | Surmenev34; Ding35; Wolke et al.36 |
| Sol–gel deposition | Can uniformly form thin coatings on complex substrates | Expensive raw material; some processes require high sintering process | Low processing temperature | <1 μm | Nguyen et al.37; Liu et al.38; Manso et al.39 |
| Hot isostatic pressing | Can produce dense coatings | Cannot coat complex substrates; expensive; high temperature and high pressure involved | High processing temperature and pressure | 0.2–2.0 mm | Li et al.40; Onoki and Hashida41 |
| Dip coating | Inexpensive; short processing time; can coat complex substrates | Needs sintering process; thermal expansion mismatch | High sintering temperature | 0.05–0.5 mm | Mavis and Taş42 |
| Biomimetic coating with SBF solution | Can form bone-like apatite; mild processing conditions allow incorporation of biomolecules; can coat complex substrates | Long processing time; ionic concentration and pH of SBF need to be maintained by replenishment; uniform coating under static conditions is limited by certain thicknesses | Biocompatible; pH close to or slightly lower than 7.4; atmospheric pressure; incubation at 37°C | <30 μm | Shin et al.45; Kohn et al.46; Liu et al.49; Habibovic et al.50; Kokubo and Yamaguchi52; Choi and Murphy93 |
Adapted from Ong et al. (2009), with permission from Springer Science + Business Media.43
CaP, calcium phosphate; RF, radio frequency; SBF, simulated body fluid.
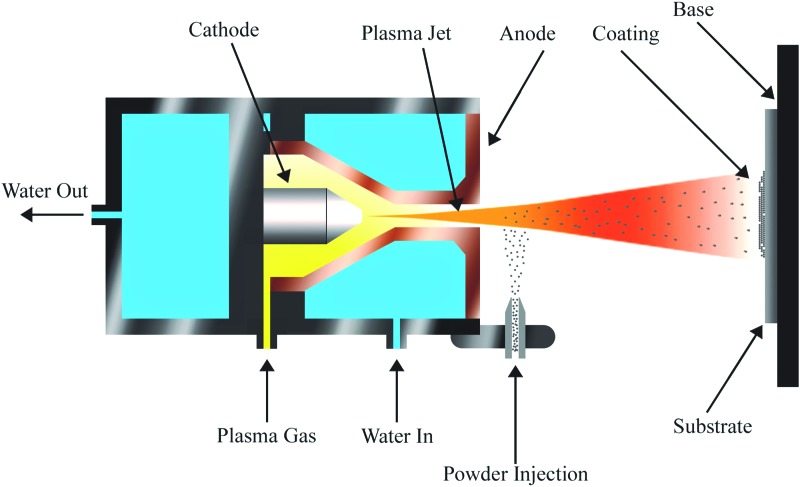
Thermal spraying is one of the most successful and widely commercialized techniques used for CaP coating.29,30 The technique involves feeding the coating material into a plasma jet, where the sample is heated to >8,000°C and then propelled toward the desired surface (Fig. 2).44 The high processing temperature may limit the selection of underlying substrate materials, and be problematic when incorporation of heat labile biological molecules is desired. It also requires a relatively large thickness (30–200 μm) to achieve uniform coating31 and is therefore not ideal for small-sized materials or intricate structures. In addition, this technique has the disadvantage of low adhesive strength and risk of delamination.
FIG. 2.
Plasma spray coating. A plasma jet is created when the plasma gas passes through the electric field generated by the anode and cathode. Then, the coating powder is injected into the plasma jet, which rapidly propels the material onto the substrate. Adapted from Bosco et al. 2012, MDPI.44 Color images available online at www.liebertpub.com/tea
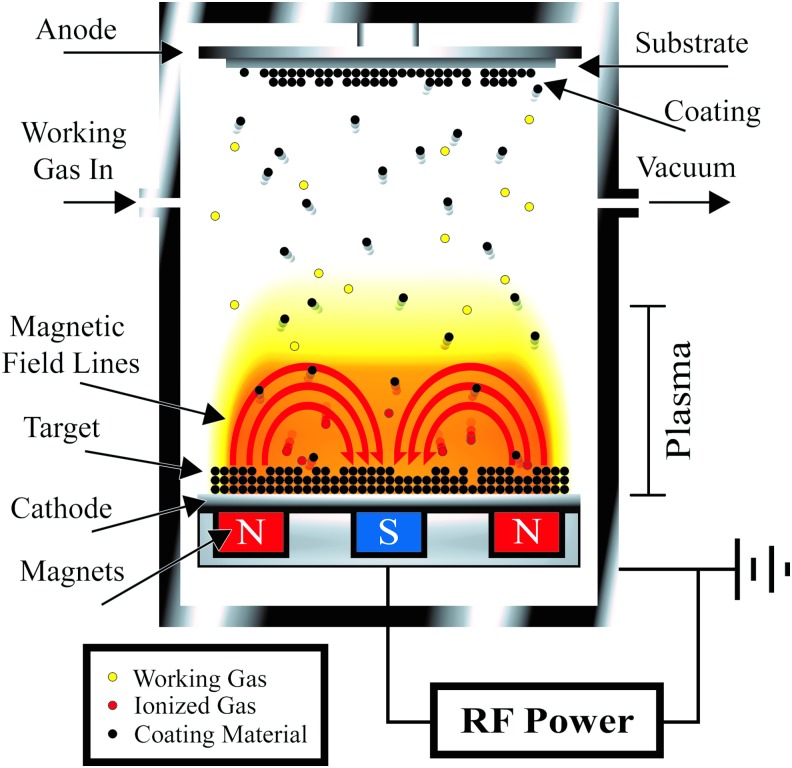
Radio frequency (RF) magnetron sputtering involves an RF generator, a magnetron, and an ionizable gas.34 The generator and magnetron efficiently convert the gas into the plasma, which is directed to bombard the coating material. The coating material is then ejected toward the desired substrate (Fig. 3).44 This method provides a great deal of control over the coating material by fabricating thin (<1 μm), uniform CaP coatings with high adhesive strength. However, RF magnetron sputtering is a time-intensive and high-cost method that only coats the visible surface of the substrate.34
FIG. 3.
RF magnetron sputter coating. The magnetic field forces the plasma close to the cathode, and the alternating current of the RF generator prevents charge build up in concentrated areas. The high-energy ions of the plasma bombard the coating material (target) ejecting the material toward the substrate producing a thin uniform coat. Adapted from Bosco et al. 2012, MDPI.44 RF, radio frequency. Color images available online at www.liebertpub.com/tea
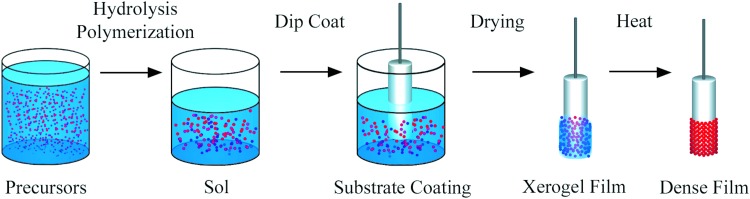
Of all of the aforementioned methods, sol–gel deposition is the only one that can achieve uniform coating throughout a porous matrix.37 The sol–gel method involves forming a solution (“sol”) containing the calcium and phosphate to be coated, followed by dipping the substrate into the solution and allowing it to dry to form a viscous gel-like layer.38 The gel-like coating can be calcinated to form a hardened layer of apatite on the substrate (Fig. 4). Since the sol is highly fluid, the sol–gel deposition technique is able to coat the interior of a porous substrate. However, high processing costs and expensive raw materials for this method are often prohibitive.31
FIG. 4.
Sol–gel coating. The precursor solution undergoes polymerization resulting in a gel-like solution (Sol). Then, the substrate is dipped into the Sol, removed, and allowed to dry to a thick film. Xerogel film is sintered using a drying oven. This leads to polycondensation and enhances the mechanical properties of the final dense film. Color images available online at www.liebertpub.com/tea
Some techniques, such as thermal spraying, dynamic mixing method, and isostatic pressing, require high processing temperatures and are therefore not amenable to polymers with relatively low melting temperatures. Also, many techniques, such as plasma spraying, sputter coating, pulsed laser deposition, and dynamic mixing method, are limited to one-sided (“line of sight”) coating, as their coating processes are unidirectional. This is not desirable for coating materials with three‐dimensional (3D) complex structures.
Biomimetic Mineralization by SBFs
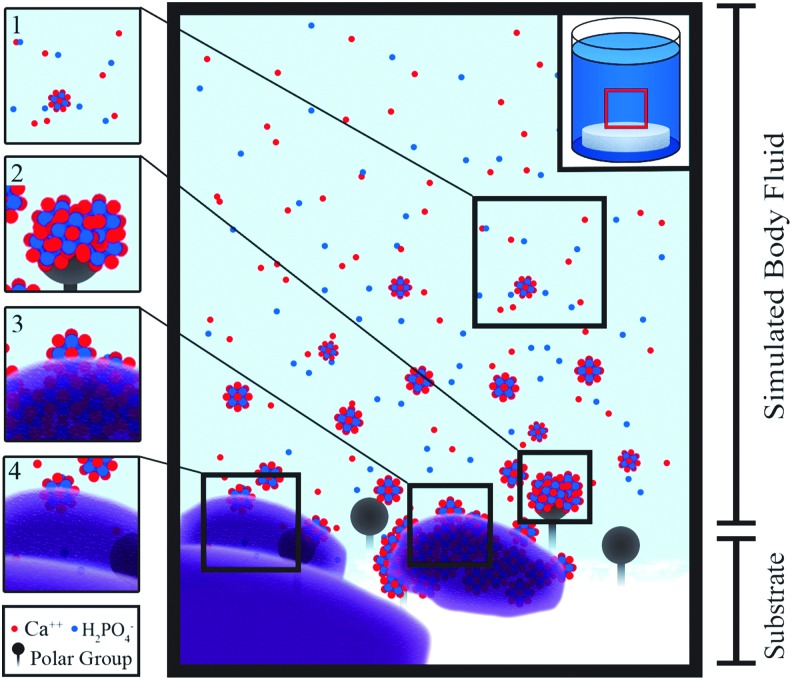
A promising alternative CaP coating method is mineralization of material surfaces using a supersaturated solution known as SBFs. SBFs comprised ions at similar concentrations to those found in blood plasma. This coating technique is performed under biological conditions in terms of temperature, pressure, and pH, forming carbonated apatite on a substrate, which is similar in chemical composition and material properties (crystallinity and dissolution rate) to bone mineral (Fig. 5). Mineral formation using this biomimetic process is governed by both the surface characteristics of the materials and the immersion parameters, such as the composition of the SBF, ionic strength, pH, temperature, and immersion time.45–50 Since the CaP layers are coated using aqueous SBF solutions, surfaces of highly complex structure such as 3D interconnective porous scaffolds can be uniformly coated, unlike other conventional “line-of-sight” CaP coating techniques described above. The coating conditions of this technique, such as pH and temperature, are similar to those of body fluid, which allows for a wide range of candidate materials to be coated with CaP. Furthermore, these biocompatible conditions enable the potential use of biomolecules sensitive to pH and temperature, such as proteins, growth factors, and genes.
FIG. 5.
SBF-mediated mineralization. (1) The high concentrations of calcium and phosphate ions contained in the SBF begin to form prenucleation crystals. (2) The amorphous solids are attracted to the polar surface groups of the substrate. (3 and 4) Apatite crystals are deposited. (5) The formed apatite is a nucleation site allowing for continued crystal growth. SBF, simulated body fluid. Color images available online at www.liebertpub.com/tea
Kokubo et al. introduced the concept of biomimetic mineralization using SBFs in 1990.51 Glass-ceramic A-W (apatite-wollastonite) was soaked in various aqueous solutions possessing similar ionic concentrations and pH levels to human blood plasma. After incubation for 7–30 days, they reported that an apatite phase had formed on the glass-ceramic surface. Since this initial report, the use of SBF to form bone-like apatite has been extended to various types of materials such as metals, ceramics, and biodegradable polymers. Over the past three decades, SBF has been widely used and developed for the following purposes: (1) bioactivity assessment for biomaterials, (2) surface coating of biomaterials to improve osteoconductivity, and (3) incorporation of biomolecules into mineral coatings.
Bioactivity assessment
From the 1970s, several materials have been reported as bioactive through their ability to integrate with host bone tissue via apatite formed on the interface between the material and the bone subsequent to implantation in vivo. From these observations, it has been suggested that the apatite-forming ability in vivo can be pretested in vitro using solutions that simulate body fluid. Numerous biomaterials such as metals,52 natural polymers,53–55 synthetic polymers,56,57 and organic/inorganic composite materials58,59 have been tested for their potential apatite-forming ability in the presence of SBF solutions.10,11,52–61 Various methods for testing the bioactivity in vitro using SBF solutions have been standardized.62 A recently published review article reported on how in vitro apatite-forming ability in the presence of SBF could often successfully predict actual bioactivity of biomaterials in vivo using animal models.60 SBFs also have been used to test other material properties such as polymer biodegradation,63,64 and wear or corrosion behavior of metals.65,66
Surface coating of biomaterials to promote osteoconductivity
The apatite coating technique using immersion in SBFs can be applied to various types of materials, including metals,52 ceramics,51,67,68 polymers,45,69–72 and organic/inorganic composite materials.73 To achieve successful apatite coating, the surfaces of the materials need to be modified to be functionally activated.
Metals
Ti metal and its alloys are among the most commonly used metals for dental implants and bone substitutions. Various kinds of surface treatments have been attempted to confer bioactivity or apatite-forming ability on Ti metal and its alloys. Heat treatment with NaOH solution can form a sodium hydrogen titanate (NaxH2-xTiyO2y+1; 0<x<2) layer with functional groups of Na+ and O2− on the surface. The treated Ti metal formed bone-like apatite on its surface after immersion in SBF, while the nontreated Ti metal did not.74 Surface treatment methods have been modified to enhance osteoconductivity of Ti metal, including NaOH/CaCl2/heat treatments, H2SO4/HCl/heat treatments, and NaOH/acid/heat treatments.
Metal implants coated with bone-like mineral apatite using SBFs also resulted in enhanced osteoconductivity in vivo compared with noncoated metal implants. Significantly greater bonding strength of the interface between the implant and the bone was obtained with bone-like mineral-coated Ti alloy implants in goat femurs, compared to noncoated implant groups.19,75 This enhanced bonding strength can be attributed to the precoated bone-like mineral layer that promotes new bone deposition onto the osteoconductive surfaces.
Ceramics
Ceramics are another class of biomaterials that have been extensively studied because of their superior bioactivity and potential application for dental or skeletal tissue repair.51,68,76–79 Ceramics are well suited for SBF treatment because, once immersed in SBF, ceramics can release ions such as calcium and silica. These released ions contribute to nucleation and subsequent surface mineralization, thus forming bone-like mineral apatite. Many types of ceramics, such as Bioglass 45S5, glass-ceramic A-W, and glasses in the Na2O-CaO-B2O3-Al2O3-SiO2-P2O5 system, were able to become bioactive by forming mineral layers on their surfaces in the presence of SBF. These ceramics were confirmed to bind to the living bone through newly formed CaP layers at the interface between the implant and the bone when they were implanted in vivo.10
Polymers
Although metals and ceramics serve as adequate materials for bone and dental implants due to their potential bioactivity, the nondegradability of these materials limits their application for bone tissue engineering. Adjustable biodegradability of the scaffold is essential for bone tissue engineering. The scaffold needs to persist for sufficient time to allow new bone tissue formation to occur. Then, as the scaffold degrades, it will be substituted with the regenerated bone. Therefore, natural and synthetic biodegradable polymers have been widely used as scaffold materials for tissue engineering due to their advantages such as controllable biodegradation and tunable scaffold properties.
Natural polymer-based scaffolds that are nontoxic and bioactive can provide cells with a biocompatible microenvironment. Natural polymers used as scaffold materials can be divided into proteins (collagen,76,80,81 silk,82 and gelatin80,83,84) or carbohydrates (cellulose85–87 and chitin54,55,88,89). Multiple natural polymers have been incubated in SBF to obtain biomimetic mineral properties and then used in organic/inorganic scaffolds for the purposes of promoting tissue regeneration.
Biomimetic surface mineralization by SBF immersion has also utilized multiple types of synthetic polymers such as poly(lactide-co-glycolide) (PLGA),45,47,49,69,72,90–98 poly-L-lactide,98,99 poly(2-hydroxyethylmethacrylate), poly(ɛ-caprolactone),99,100 and polyhydroxyalkanoate.56 Compared to natural polymers, synthetic polymers have found widespread application as scaffold candidate materials, as their mechanical and chemical properties can be specifically controlled.71,101,102
Incorporation of Biomolecules by Biomimetic Mineralization
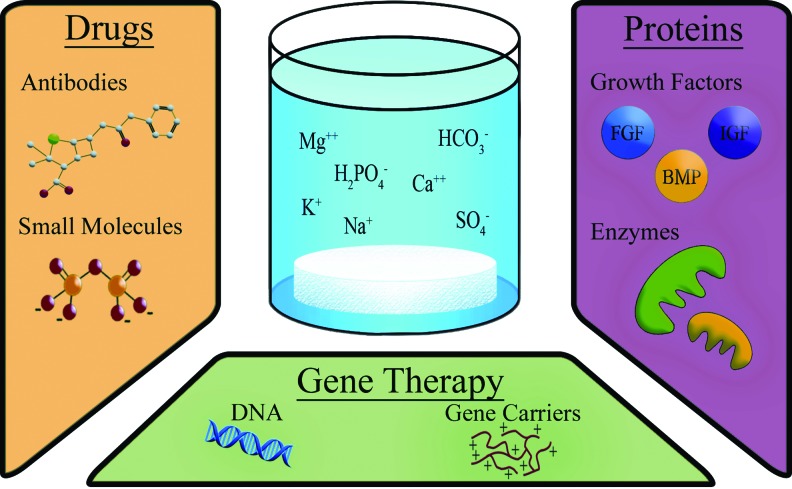
Although biomimetic mineral coatings provide the underlying scaffold with osteoconductivity, they do not directly confer osteoinductivity. To overcome this limitation, current research has been focused on integrating drugs or biological molecules such as proteins and genes into the mineral coatings (Fig. 6). In this study, some examples of drugs, proteins, and genes incorporated into the CaP coatings will be introduced, and their therapeutic benefits will be assessed.
FIG. 6.
Incorporation of biomolecules by biomimetic mineralization. To further functionalize the coating created by SBFs, proteins, drugs, or genes can be incorporated into the apatite coatings. In this study, the ions present in SBF are displayed alongside the different therapeutic molecules that can be incorporated into the apatite coating of the substrate. BMP, bone morphogenetic protein; FGF, fibroblast growth factor; IGF, insulin-like growth factor. Color images available online at www.liebertpub.com/tea
Drugs
Currently, there are a multitude of drug molecules being investigated for their potential incorporation into the CaP coatings to reduce inflammation and enhance osteogenesis. Incorporation of antibiotics into the CaP layer can prevent postoperative infection at the surgical site promoting favorable osteointegration of dental/skeletal implants and bone substituting materials.103 This is highly desirable, because of the prevalence of peri-implantitis. Unfortunately, conventional CaP coating techniques, such as plasma spraying, and isostatic pressing involving nonphysiological processing conditions, such as high temperature and high pressure, do not allow incorporation of drugs into the CaP coatings. Various antibiotics were successfully incorporated into CaP coatings on Ti implants using SBFs.103 The Ti surface was initially coated with a thin layer of amorphous carbonated apatite by immersing the metal in a supersaturated SBF solution, and then antibiotics were coprecipitated. Loading ability, release kinetics, and efficacy of the antibiotics were evaluated. Antibiotics containing carboxyl groups, such as cephalothin, carbenicillin, and cefamandole, possessed higher binding affinities and slower releasing kinetics, suggesting that the chemical structure of the antibiotics determined their binding/chelating affinity to calcium-rich mineral coatings.103
Bisphosphonates (BPs) are primary agents for treating osteoporosis. However, current publications have demonstrated that systemic delivery of BPs results in inefficient dose delivery to the target site and causes toxic side effects, such as gastric ulcers and BP-related osteonecrosis of the jaw. All BPs have the same backbone (P-C-P),104 which provides them with a high calcium-binding affinity and enables the incorporation of BPs into CaP layers through SBF-mediated coating. For example, alendronate sodium (AS), an approved BP, was successfully incorporated into calcium-deficient hydroxyapatite coatings on Ti alloys using a biomimetic coating process.105 The release profile of AS was controllable through modification of the SBF-mediated incorporation. These results suggest that drug incorporation in the mineral coating through SBF mediation can be optimized to provide a localized, long-acting administration to achieve a therapeutic dose.
Proteins and growth factors
Proteins can be adsorbed onto mineral surfaces forming organic/inorganic hybrids. Once bone-like mineral coatings are formed on substrate materials, proteins are then adsorbed on the mineral surface via electrostatic interactions between proteins and the mineral apatite. As this interaction occurs after the surface is established, proteins do not integrate into the mineral structure nor alter mineral formation. Proteins can also be incorporated within the mineral coating by coprecipitation in SBF solution. These different methods (surface adsorption vs. coprecipitation within mineral structure) affect the release kinetics of the incorporated proteins. The surface-adsorbed proteins demonstrate a burst release profile, whereas the coprecipitated proteins show a more controlled and sustained release profile, for the proteins are physically incorporated with the mineral layers.106–110
As a proof of concept, osteogenic growth factors were combined with mineral coatings to further the osteogenic capabilities of the biomaterial. After CaP layers were formed using conventional coating methods, BMP-2 was superficially deposited on the outer surface of these mineral coatings, through either adsorption106 or chemical surface treatment.107 Both of these superficially adsorbed growth factors were released with a pattern of initial burst release (higher release rate), however, this release profile is not the ideal delivery kinetics for biological outcomes.
Sustained release of proteins or growth factors allows for long-term delivery within the therapeutic range and can be achieved by incorporating proteins or growth factors into the mineral coating. This concept of controlling release profile has been applied to the biomimetic mineralization process. Coprecipitation incorporates biomolecules, such as proteins, growth factors, enzymes, and drugs, into the bone-like mineral coatings. BMP-2-incorporated mineral coatings fabricated by a biomimetic coprecipitation technique in SBF have been compared to mineral coatings with superficially adsorbed BMP-2.108,109 A pharmacologically favorable low dose of BMP-2 was gradually released from the groups containing BMP-2 incorporated by coprecipitation, whereas a burst release of BMP-2 was observed from the groups with superficially adsorbed BMP-2. With the same amount of BMP-2 loaded by the two different methods, a more sustained osteogenic response was observed in the groups where BMP-2 was incorporated by coprecipitation.110 In another study, the delivery mode and efficacy of BMP-2 were tested using Ti-alloy (Ti6A14V) discs implanted subcutaneously in the dorsal region of rats for up to 5 weeks. Significantly improved bone volume and density of the regenerated bone were observed in the groups that provided sustained delivery of BMP-2.49,108,109 Furthermore, the release kinetics of insulin-like growth factor-1 (IGF-1) was linear with a sustained profile, when IGF-1 was incorporated within mineral coatings by coprecipitation.111
Gene therapy
Gene therapy has attracted scientific interest due to its advantages over protein-based growth factor delivery. Current problems with protein-based delivery include the following: (1) continuous administration of protein-based growth factors is required for biological outcomes, (2) optimized spatiotemporal delivery is challenging and, if not achieved, cost and efficacy are prohibitive, and (3) multiple doses are required due to the short half-life of protein-based growth factors.112,113
Developing gene delivery carriers has been extensively studied,92,96,113–123 and biomimetic mineralization using SBF has been investigated as a means to synthesize a nonviral gene delivery agent. The physiological conditions (temperature, pH, ionic composition of SBF) used during the biomimetic mineralization process allow incorporation of genetic material with low risk of denaturing the DNA. The negative charge on the DNA provides a nucleation site for the high concentration of calcium ions to precipitate forming CaP/DNA complexes in the SBF. Therefore, precipitation of DNA/calcium-containing composites has been successfully formulated by using SBFs or modified SBFs for nonviral gene delivery.115,121,124 Prefabricated coprecipitates of model DNA (lambda DNA) encapsulated in CaP (DNA/CaP) were adsorbed onto 2D PLGA plates and 3D interconnective porous PLGA scaffolds. Although human bone cell line (SaOS-2) was successfully transfected onto 2D plates and 3D scaffolds, more than 95% of the initially adsorbed DNA/CaP was released within 2 days.124
Naked plasmid DNA (pDsRed pDNA) was superficially adsorbed onto mineral-coated PLGA film.94 The release kinetics of pDNA was modulated by both the intrinsic properties of the minerals formed in different SBFs and the extrinsic conditions such as pH and ionic composition of the testing solutions. The same group also demonstrated pDNA (pMetLuc and pEGFP-N1)-Lipofectamine complexes adsorbed to mineral-coated tissue culture polystyrene for testing optimized surface-mediated transfection by adjusting the carbonate content in the SBF solutions.92
As with proteins, DNA can be associated with mineral coatings of prospective implants either through superficial binding of the DNA to the mineral surface by adsorption or by incorporation within mineral structure by coprecipitation. To compare surface adsorption and coprecipitation of plasmid DNA during biomimetic mineralization in terms of transfection efficiencies, plasmid DNA encoding for the β-gal gene was complexed with Lipofectamine, and integrated with bone-like mineral coatings using the two different methods. DNA-lipoplex stability was retained in both methods, but coprecipitated DNA-lipoplexes induced higher transfection efficiencies compared to adsorbed DNA-lipoplexes.96
Limitations of Biomimetic Mineralization Using SBFs
Although biomimetic mineralization can be widely applied to enhance the tissue regenerative capacity of implanted materials, there are still a number of drawbacks and hurdles to overcome. The formation of a continuous layer of mineral coating substrate materials is a process that takes longer than other conventional CaP coating methods. A few approaches have been proposed to accelerate the biomimetic mineral coating process.125,126 For instance, substrate surfaces can be functionalized before immersion in SBFs. The surfaces of substrate materials, such as PLGA, can be functionalized by treating them with NaOH solution resulting in the exposure of more hydroxyl functional groups on the PLGA surface and therefore increasing the capacity to bind Ca2+ ions in SBFs.45,92,96 Another approach for accelerating the mineralization process is to adjust concentrations of selected ions, typically calcium and phosphorus, in SBF solutions.48,127
Manufacturing challenges arise when using biomimetic mineral coating techniques to coat 3D interconnective porous scaffolds due to the fact that biomimetic coating under static conditions cannot uniformly coat the inner surfaces of 3D scaffolds. The nonuniform coating is particularly problematic when the scaffold is large and the pore size is small. To overcome this issue, a dynamic perfusion technique can be applied to achieve uniform coating throughout the thickness of the scaffolds.128
A disadvantage of using the coprecipitation technique is the low efficiency of incorporation of biomolecules into mineral coatings. Only a small proportion of biomolecules in SBF can be entrapped within the mineral deposited onto the underlying substrate material.
Conclusions and Future Directions
Over the last three decades, the process of regenerating bone using SBF has made significant advances. However, the lack of SBF use in clinical practice indicates the need for further advances to overcome the translational challenges. Valuable studies investigating the role CaP in bone tissue engineering demonstrate the importance of this inorganic phase with regard to bioactivity, osteoconduction, and osteoinduction. In this review, we provided evidence that biomimetic mineralization is a promising approach to generating CaP coatings on supportive structures made from ceramics, metals, or polymers. Coating these materials with bone-like mineral layers not only increases load-bearing mechanical strength but also provides bioactivity, osteoconductivity, and, once incorporated with relevant biomolecules, osteoinductivity. In addition, it was highlighted that coprecipitation of proteins/growth factors and DNA within the mineral coatings can provide sustained delivery of these biomolecules, thereby enhancing bone tissue regeneration.
Potential future directions for biomimetic mineralization using SBF include surface mineralization of complex scaffolds fabricated by 3D bioprinting technology, improvement of SBF coating rate through surface preparation, and optimization of apatite coatings containing relevant therapeutic agents for specific diseases.
Acknowledgments
We acknowledge the financial support from the 2017 Martin “Bud” Schulman Postdoctoral Fellowship Award by the American Association of Orthodontists Foundation and the Lyle and Sharon Bighley Endowed Chair of Pharmaceutical Sciences.
Disclosure Statement
No competing financial interests exist.
References
- 1.The Henley J. Kaiser Family Foundation. http://kff.org/slideshow/how-much-does-the-u-s-spend-to-treat-different-diseases Accessed January25, 2017
- 2.Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997–2004. Arthritis Care Res 59, 481, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Nwachukwu B.U., Bozic K.J., Schairer W.W., Bernstein J.L., Jevsevar D.S., Marx R.G., and Padgett D.E. Current status of cost utility analyses in total joint arthroplasty: a systematic review. Clin Orthop Relat Res 473, 1815, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damien C.J., and Parsons J.R. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater 2, 187, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Kao S.T., and Scott D.D. A review of bone substitutes. Oral Maxillofac Surg Clin North Am 19, 513, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Kessler P., Thorwarth M., Bloch-Birkholz A., Nkenke E., and Neukam F. Harvesting of bone from the iliac crest—comparison of the anterior and posterior sites. Br J Oral Maxillofac Surg 43, 51, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bauer T.W., and Muschler G.F. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res 371, 10, 2000 [PubMed] [Google Scholar]
- 8.Eppley B.L., Pietrzak W.S., and Blanton M.W. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg 16, 981, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Moreau M.F., Gallois Y., Baslé M.-F., and Chappard D. Gamma irradiation of human bone allografts alters medullary lipids and releases toxic compounds for osteoblast-like cells. Biomaterials 21, 369, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Kokubo T., and Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27, 2907, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Bohner M., and Lemaitre J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 30, 2175, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Pina S., Oliveira J.M., and Reis R.L. Biomimetic strategies to engineer mineralized human tissues. In: Antoniac I., ed. Handbook of Bioceramics and Biocomposites. Switzerland: Springer, 2016, pp. 503–519. [Google Scholar]
- 13.Albrektsson T., and Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J 10, S96, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vacanti C., and Pietrzak W.S. Musculoskeletal Tissue Regeneration: Biological Materials and Methods. New Jersey: Springer Science and Business Media, 2008 [Google Scholar]
- 15.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3(Suppl 3), S131, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veis A., and Dorvee J.R. Biomineralization mechanisms: a new paradigm for crystal nucleation in organic matrices. Calcif Tissue Int 93, 307, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeGeros R.Z. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res 395, 81, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Rey C., Combes C., Drouet C., and Glimcher M.J. Bone mineral: update on chemical composition and structure. Osteoporos Int 20, 1013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrere F., Van Der Valk, C., Dalmeijer R., Van Blitterswijk, C., De Groot K., and Layrolle P. In vitro and in vivo degradation of biomimetic octacalcium phosphate and carbonate apatite coatings on titanium implants. J Biomed Mater Res A 64, 378, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Pan H., and Darvell B.W. Effect of carbonate on hydroxyapatite solubility. Cryst Growth Des 10, 845, 2010 [Google Scholar]
- 21.Dorozhkin S.V. Amorphous calcium phosphates. J Biomim Biomater Tissue Eng 7, 27, 2010 [Google Scholar]
- 22.Termine J.D. Non-collagen proteins in bone. In: Evered D., and Harnett S., eds. Cell and Molecular Biology of Vertebrate Hard Tissues. Chichester, UK: John Wiley & Sons Ltd, 1988, p. 178. [Google Scholar]
- 23.Claes L., Recknagel S., and Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 8, 133, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Nudelman F., Pieterse K., George A., Bomans P.H., Friedrich H., Brylka L.J., Hilbers P.A., de With G., and Sommerdijk N.A. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater 9, 1004, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen G., Deng C., and Li Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8, 272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boonrungsiman S., Gentleman E., Carzaniga R., Evans N.D., McComb D.W., Porter A.E., and Stevens M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Natl Acad Sci U S A 109, 14170, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson H.C. Matrix vesicles and calcification. Curr Rheumatol Rep 5, 222, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Mescher A.L. Chapter 8. Bone. In: Mescher A.L., ed. Junqueira's Basic Histology, 13e. New York, NY: The McGraw-Hill Companies, 2013, pp. 138–159. [Google Scholar]
- 29.Huang Y., He J., Gan L., Liu X., Wu Y., Wu F., and Gu Z.-W. Osteoconductivity and osteoinductivity of porous hydroxyapatite coatings deposited by liquid precursor plasma spraying: in vivo biological response study. Biomed Mater 9, 065007, 2014 [DOI] [PubMed] [Google Scholar]
- 30.De Groot, K., Geesink R., Klein C., and Serekian P. Plasma sprayed coatings of hydroxylapatite. J Biomed Mater Res 21, 1375, 1987 [DOI] [PubMed] [Google Scholar]
- 31.Yang Y., Kim K.-H., and Ong J.L. A review on calcium phosphate coatings produced using a sputtering process—an alternative to plasma spraying. Biomaterials 26, 327, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Li H., Khor K., and Cheang P. Titanium dioxide reinforced hydroxyapatite coatings deposited by high velocity oxy-fuel (HVOF) spray. Biomaterials 23, 85, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Gross K., Berndt C., and Herman H. Amorphous phase formation in plasma‐sprayed hydroxyapatite coatings. J Biomed Mater Res A 39, 407, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Surmenev R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf Coat Technol 206, 2035, 2012 [Google Scholar]
- 35.Ding S.-J. Properties and immersion behavior of magnetron-sputtered multi-layered hydroxyapatite/titanium composite coatings. Biomaterials 24, 4233, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Wolke J., Van Dijk K., Schaeken H., De Groot K., and Jansen J. Study of the surface characteristics of magnetron‐sputter calcium phosphate coatings. J Biomed Mater Res A 28, 1477, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Nguyen H.Q., Deporter D.A., Pilliar R.M., Valiquette N., and Yakubovich R. The effect of sol-gel-formed calcium phosphate coatings on bone ingrowth and osteoconductivity of porous-surfaced Ti alloy implants. Biomaterials 25, 865, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Liu D.M., Troczynski T., and Tseng W.J. Water-based sol-gel synthesis of hydroxyapatite: process development. Biomaterials 22, 1721, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Manso M., Langlet M., Jiménez C., and Martınez-Duart J. Microstructural study of aerosol-gel derived hydroxyapatite coatings. Biomol Eng 19, 63, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Li J., Forberg S., and Hermansson L. Evaluation of the mechanical properties of hot isostatically pressed titania and titania-calcium phosphate composites. Biomaterials 12, 438, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Onoki T., and Hashida T. New method for hydroxyapatite coating of titanium by the hydrothermal hot isostatic pressing technique. Surf Coat Technol 200, 6801, 2006 [Google Scholar]
- 42.Mavis B., and Taş A.C. Dip coating of calcium hydroxyapatite on Ti‐6Al‐4V substrates. J Am Ceram Soc 83, 989, 2000 [Google Scholar]
- 43.Ong J.L., Yang Y., Oh S., Appleford M., Chen W., Liu Y., Kim K.-H., Park S., Bumgardner J., and Haggard W. Calcium phosphate coating produced by a sputter deposition process. In: Leon B., and Jansen J.A., eds. Thin Calcium Phosphate Coatings for Medical Implants. New York: Springer, 2009, pp. 178–198. [Google Scholar]
- 44.Bosco R., Van Den Beucken J., Leeuwenburgh S., and Jansen J. Surface engineering for bone implants: a trend from passive to active surfaces. Coatings 2, 95, 2012 [Google Scholar]
- 45.Shin K., Jayasuriya A.C., and Kohn D.H. Effect of ionic activity products on the structure and composition of mineral self assembled on three‐dimensional poly (lactide‐co‐glycolide) scaffolds. J Biomed Mater Res A 83, 1076, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohn D.H., Shin K., Hong S.-I., Jayasuriya A.C., Leonova E.V., Rossello R.A., Krebsbach P.H., Landis W., and Sodek J. Self-assembled mineral scaffolds as model systems for biomineralization and tissue engineering. Presented at the Proceedings of the Eighth International Conference on the Chemistry and Biology of Mineralized Tissues, Toronto, 2005 [Google Scholar]
- 47.Choi S., and Murphy W.L. A screening approach reveals the influence of mineral coating morphology on human mesenchymal stem cell differentiation. Biotechnol J 8, 496, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S.-S., Park M.S., Gwak S.-J., Choi C.Y., and Kim B.-S. Accelerated bonelike apatite growth on porous polymer/ceramic composite scaffolds in vitro. Tissue Eng 12, 2997, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Liu Y., Wu G., and de Groot K. Biomimetic coatings for bone tissue engineering of critical-sized defects. J R Soc Interface 7, S631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Habibovic P., Barrere F., Blitterswijk C.A., Groot K., and Layrolle P. Biomimetic hydroxyapatite coating on metal implants. J Am Ceram Soc 85, 517, 2002 [Google Scholar]
- 51.Kokubo T., Kushitani H., Sakka S., Kitsugi T., and Yamamuro T. Solutions able to reproduce in vivo surface‐structure changes in bioactive glass‐ceramic A‐W3. J Biomed Mater Res 24, 721, 1990 [DOI] [PubMed] [Google Scholar]
- 52.Kokubo T., and Yamaguchi S. Novel bioactive materials developed by simulated body fluid evaluation: surface-modified Ti metal and its alloys. Acta Biomater 44, 16, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Suárez‐González D., Barnhart K., Saito E., Vanderby R., Hollister S.J., and Murphy W.L. Controlled nucleation of hydroxyapatite on alginate scaffolds for stem cell‐based bone tissue engineering. J Biomed Mater Res A 95, 222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prabaharan M., and Sivashankari P. Prospects of bioactive chitosan-based scaffolds in tissue engineering and regenerative medicine. In: Chitin and Chitosan for Regenerative Medicine. Springer, 2016, pp. 41–59. [Google Scholar]
- 55.Muthukumar T., Aravinthan A., Sharmila J., Kim N.S., and Kim J.-H. Collagen/chitosan porous bone tissue engineering composite scaffold incorporated with Ginseng compound K. Carbohydr Polym 152, 566, 2016 [DOI] [PubMed] [Google Scholar]
- 56.Misra S.K., Ansari T.I., Valappil S.P., Mohn D., Philip S.E., Stark W.J., Roy I., Knowles J.C., Salih V., and Boccaccini A.R. Poly (3-hydroxybutyrate) multifunctional composite scaffolds for tissue engineering applications. Biomaterials 31, 2806, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Rasoulianboroujeni M., Yazdimamaghani M., Khoshkenar P., Pothineni V.R., Kim K.M., Murray T.A., Rajadas J., Mills D.K., Vashaee D., and Moharamzadeh K. From solvent-free microspheres to bioactive gradient scaffolds. Nanomedicine 13, 1157, 2016 [DOI] [PubMed] [Google Scholar]
- 58.Sa Y., Yang F., de Wijn J.R., Wang Y., Wolke J.G., and Jansen J.A. Physicochemical properties and mineralization assessment of porous polymethylmethacrylate cement loaded with hydroxyapatite in simulated body fluid. Mater Sci Eng C 61, 190, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Mi H.-Y., Jing X., Salick M.R., Cordie T.M., Peng X.-F., and Turng L.-S. Morphology, mechanical properties, and mineralization of rigid thermoplastic polyurethane/hydroxyapatite scaffolds for bone tissue applications: effects of fabrication approaches and hydroxyapatite size. J Mater Sci 49, 2324, 2014 [Google Scholar]
- 60.Zadpoor A.A. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater Sci Eng C 35, 134, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Kokubo T., and Takadama H. Simulated body fluid (SBF) as a standard tool to test the bioactivity of implants. In: Bauerlein E., ed. Handbook of Biomineralization: Biological Aspects and Structure Formation. Weinheim, Germany: Wiley-VCH Verlag GmbH, 2008, pp. 97–109. [Google Scholar]
- 62.International Organization for Standardization (ISO). Implants for surgery, in vitro evaluation for apatite-forming ability of implant materials (ISO 23317:2012), 2012
- 63.Li Z., Song G.-L., and Song S. Effect of bicarbonate on biodegradation behaviour of pure magnesium in a simulated body fluid. Electrochimica Acta 115, 56, 2014 [Google Scholar]
- 64.Armentano I., Dottori M., Fortunati E., Mattioli S., and Kenny J. Biodegradable polymer matrix nanocomposites for tissue engineering: a review. Polym Degrad Stab 95, 2126, 2010 [Google Scholar]
- 65.Kumar P., Babu P.D., Mohan L., Anandan C., and Grips V.W. Wear and corrosion behavior of Zr-doped DLC on Ti-13Zr-13Nb biomedical alloy. J Mater Eng Perform 22, 283, 2013 [Google Scholar]
- 66.Zhang Y., Ai J., Wang D., Hong Z., Li W., and Yokogawa Y. Dissolution properties of different compositions of biphasic calcium phosphate bimodal porous ceramics following immersion in simulated body fluid solution. Ceram Int 39, 6751, 2013 [Google Scholar]
- 67.Liu X., Ding C., and Chu P.K. Mechanism of apatite formation on wollastonite coatings in simulated body fluids. Biomaterials 25, 1755, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Li P., Nakanishi K., Kokubo T., and de Groot K. Induction and morphology of hydroxyapatite, precipitated from metastable simulated body fluids on sol-gel prepared silica. Biomaterials 14, 963, 1993 [DOI] [PubMed] [Google Scholar]
- 69.Murphy W.L., Kohn D.H., and Mooney D.J. Growth of continuous bonelike mineral within porous poly (lactide-co-glycolide) scaffolds in vitro. J Biomed Mater Res 50, 50, 2000 [DOI] [PubMed] [Google Scholar]
- 70.Gkioni K., Leeuwenburgh S.C., Douglas T.E., Mikos A.G., and Jansen J.A. Mineralization of hydrogels for bone regeneration. Tissue Eng Part B Rev 16, 577, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Kretlow J.D., and Mikos A.G. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng 13, 927, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Wu G., Liu Y., Iizuka T., and Hunziker E.B. Biomimetic coating of organic polymers with a protein-functionalized layer of calcium phosphate: the surface properties of the carrier influence neither the coating characteristics nor the incorporation mechanism or release kinetics of the protein. Tissue Eng Part C Methods 16, 1255, 2010 [DOI] [PubMed] [Google Scholar]
- 73.Zhang C., Cao M., Lan J., Wei P., Cai Q., and Yang X. Regulating proliferation and differentiation of osteoblasts on poly (l‐lactide)/gelatin composite nanofibers via timed biomineralization. J Biomed Mater Res A 104A, 1968, 2016 [DOI] [PubMed] [Google Scholar]
- 74.Kim H.M., Miyaji F., Kokubo T., Nishiguchi S., and Nakamura T. Graded surface structure of bioactive titanium prepared by chemical treatment. J Biomed Mater Res 45, 100, 1999 [DOI] [PubMed] [Google Scholar]
- 75.Habibovic P., Li J., Van Der Valk C.M., Meijer G., Layrolle P., Van Blitterswijk C.A., and De Groot K. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials 26, 23, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Marelli B., Ghezzi C.E., Barralet J.E., Boccaccini A.R., and Nazhat S.N. Three-dimensional mineralization of dense nanofibrillar collagen-bioglass hybrid scaffolds. Biomacromolecules 11, 1470, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Kokubo T., Kim H.-M., and Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials 24, 2161, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Kokubo T. Bioactive glass ceramics: properties and applications. Biomaterials 12, 155, 1991 [DOI] [PubMed] [Google Scholar]
- 79.Abe Y., Kokubo T., and Yamamuro T. Apatite coating on ceramics, metals and polymers utilizing a biological process. J Mater Sci Mater Med 1, 233, 1990 [Google Scholar]
- 80.Kuttappan S., Mathew D., and Nair M.B. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering-A mini review. Int J Biol Macromol 93, 1390, 2016 [DOI] [PubMed] [Google Scholar]
- 81.Yang H.S., La W.-G., Bhang S.H., Lee T.-J., Lee M., and Kim B.-S. Apatite-coated collagen scaffold for bone morphogenetic protein-2 delivery. Tissue Eng Part A 17, 2153, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Lee M.J., Park J.B., Kim H.H., Ki C.S., Park S.Y., Kim H.J., and Park Y.H. Surface coating of hydroxyapatite on silk nanofiber through biomineralization using ten times concentrated simulated body fluid and the evaluation for bone regeneration. Macromol Res 22, 710, 2014 [Google Scholar]
- 83.Liu X., Smith L.A., Hu J., and Ma P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 30, 2252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ren L., Tsuru K., Hayakawa S., and Osaka A. Novel approach to fabricate porous gelatin-siloxane hybrids for bone tissue engineering. Biomaterials 23, 4765, 2002 [DOI] [PubMed] [Google Scholar]
- 85.Singh B., Panda N., Mund R., and Pramanik K. Carboxymethyl cellulose enables silk fibroin nanofibrous scaffold with enhanced biomimetic potential for bone tissue engineering application. Carbohydr Polym 151, 335, 2016 [DOI] [PubMed] [Google Scholar]
- 86.Fang B., Wan Y.-Z., Tang T.-T., Gao C., and Dai K.-R. Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng Part A 15, 1091, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Joshi M.K., Pant H.R., Tiwari A.P., Maharjan B., Liao N., Park C.H., and Kim C.S. Three-dimensional cellulose sponge: fabrication, characterization, biomimetic mineralization, and in vitro cell infiltration. Carbohydr Polym 136, 154, 2016 [DOI] [PubMed] [Google Scholar]
- 88.Saravanan S., Leena R., and Selvamurugan N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int J Biol Macromol 93, 1354, 2016 [DOI] [PubMed] [Google Scholar]
- 89.Rao R.R., Jiao A., Kohn D.H., and Stegemann J.P. Exogenous mineralization of cell-seeded and unseeded collagen-chitosan hydrogels using modified culture medium. Acta Biomater 8, 1560, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy W.L., Peters M.C., Kohn D.H., and Mooney D.J. Sustained release of vascular endothelial growth factor from mineralized poly (lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 21, 2521, 2000 [DOI] [PubMed] [Google Scholar]
- 91.Murphy W.L., Hsiong S., Richardson T.P., Simmons C.A., and Mooney D.J. Effects of a bone-like mineral film on phenotype of adult human mesenchymal stem cells in vitro. Biomaterials 26, 303, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Choi S., Yu X., Jongpaiboonkit L., Hollister S.J., and Murphy W.L. Inorganic coatings for optimized non-viral transfection of stem cells. Sci Rep 3, 1567, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choi S., and Murphy W.L. The effect of mineral coating morphology on mesenchymal stem cell attachment and expansion. J Mater Chem 22, 25288, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi S., and Murphy W.L. Sustained plasmid DNA release from dissolving mineral coatings. Acta Biomater 6, 3426, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luong L.N., Hong S.I., Patel R.J., Outslay M.E., and Kohn D.H. Spatial control of protein within biomimetically nucleated mineral. Biomaterials 27, 1175, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Luong L.N., McFalls K.M., and Kohn D.H. Gene delivery via DNA incorporation within a biomimetic apatite coating. Biomaterials 30, 6996, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luong L.N., Ramaswamy J., and Kohn D.H. Effects of osteogenic growth factors on bone marrow stromal cell differentiation in a mineral-based delivery system. Biomaterials 33, 283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karaman O., Kumar A., Moeinzadeh S., He X., Cui T., and Jabbari E. Effect of surface modification of nanofibres with glutamic acid peptide on calcium phosphate nucleation and osteogenic differentiation of marrow stromal cells. J Tissue Eng Regen Med 10, E132, 2016 [DOI] [PubMed] [Google Scholar]
- 99.Saito E., Suarez-Gonzalez D., Rao R.R., Stegemann J.P., Murphy W.L., and Hollister S.J. Use of micro-computed tomography to nondestructively characterize biomineral coatings on solid freeform fabricated poly(L-lactic acid) and poly(ɛ-caprolactone) scaffolds in vitro and in vivo. Tissue Eng Part C Methods 19, 507, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gaharwar A.K., Mukundan S., Karaca E., Dolatshahi-Pirouz A., Patel A., Rangarajan K., Mihaila S.M., Iviglia G., Zhang H., and Khademhosseini A. Nanoclay-enriched poly (ɛ-caprolactone) electrospun scaffolds for osteogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A 20, 2088, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Place E.S., George J.H., Williams C.K., and Stevens M.M. Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev 38, 1139, 2009 [DOI] [PubMed] [Google Scholar]
- 102.Szpalski C., Wetterau M., Barr J., and Warren S.M. Bone tissue engineering: current strategies and techniques—part I: scaffolds. Tissue Eng Part B Rev 18, 246, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Stigter M., Bezemer J., De Groot K., and Layrolle P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J Control Release 99, 127, 2004 [DOI] [PubMed] [Google Scholar]
- 104.Golomb G., Schlossman A., Saadeh H., Levi M., Van Gelder J.M., and Breuer E. Bisacylphosphonates inhibit hydroxyapatite formation and dissolution in vitro and dystrophic calcification in vivo. Pharm Res 9, 143, 1992 [DOI] [PubMed] [Google Scholar]
- 105.Zhou H., Lawrence J.G., Touny A.H., and Bhaduri S.B. Biomimetic coating of bisphosphonate incorporated CDHA on Ti6Al4 V. J Mater Sci Mater Med 23, 365, 2012 [DOI] [PubMed] [Google Scholar]
- 106.Ono I., Gunji H., Kaneko F., Sait T., and Kuboki Y. Efficacy of hydroxyapatite ceramic as a carrier for recombinant human bone morphogenetic protein. J Craniofac Surg 6, 238, 1995 [DOI] [PubMed] [Google Scholar]
- 107.Kim H.M., Miyaji F., Kokubo T., and Nakamura T. Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J Biomed Mater Res 32, 409, 1996 [DOI] [PubMed] [Google Scholar]
- 108.Liu Y., Huse R., De Groot, K., Buser D., and Hunziker E. Delivery mode and efficacy of BMP-2 in association with implants. J Dent Res 86, 84, 2007 [DOI] [PubMed] [Google Scholar]
- 109.Liu Y., De Groot K., and Hunziker E. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone 36, 745, 2005 [DOI] [PubMed] [Google Scholar]
- 110.Liu Y., De Groot K., and Hunziker E. Osteoinductive implants: the mise-en-scene for drug-bearing biomimetic coatings. Ann Biomed Eng 32, 398, 2004 [DOI] [PubMed] [Google Scholar]
- 111.Jayasuriya A.C., and Shah C. Controlled release of insulin‐like growth factor‐1 and bone marrow stromal cell function of bone‐like mineral layer‐coated poly (lactic‐co‐glycolic acid) scaffolds. J Tissue Eng Regen Med 2, 43, 2008 [DOI] [PubMed] [Google Scholar]
- 112.Partridge K.A., and Oreffo R.O. Gene delivery in bone tissue engineering: progress and prospects using viral and nonviral strategies. Tissue Eng 10, 295, 2004 [DOI] [PubMed] [Google Scholar]
- 113.Park T.G., Jeong J.H., and Kim S.W. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev 58, 467, 2006 [DOI] [PubMed] [Google Scholar]
- 114.Jang J.H., Bengali Z., Houchin T.L., and Shea L.D. Surface adsorption of DNA to tissue engineering scaffolds for efficient gene delivery. J Biomed Mater Res A 77, 50, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shen H., Tan J., and Saltzman W.M. Surface-mediated gene transfer from nanocomposites of controlled texture. Nat Mater 3, 569, 2004 [DOI] [PubMed] [Google Scholar]
- 116.Kong H.J., Liu J., Riddle K., Matsumoto T., Leach K., and Mooney D.J. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat Mater 4, 460, 2005 [DOI] [PubMed] [Google Scholar]
- 117.Salem A.K., Searson P.C., and Leong K.W. Multifunctional nanorods for gene delivery. Nat Mater 2, 668, 2003 [DOI] [PubMed] [Google Scholar]
- 118.Intra J., and Salem A.K. Fabrication, characterization and in vitro evaluation of poly (D, L‐lactide‐co glycolide) microparticles loaded with polyamidoamine-plasmid DNA dendriplexes for applications in nonviral gene delivery. J Pharm Sci 99, 368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang X.-Q., Intra J., and Salem A.K. Conjugation of polyamidoamine dendrimers on biodegradable microparticles for nonviral gene delivery. Bioconjug Chem 18, 2068, 2007 [DOI] [PubMed] [Google Scholar]
- 120.Cao X., Deng W., Wei Y., Su W., Yang Y., Wei Y., Yu J., and Xu X. Encapsulation of plasmid DNA in calcium phosphate nanoparticles: stem cell uptake and gene transfer efficiency. Int J Nanomedicine 6, 3335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nouri A., Castro R., Santos J.L., Fernandes C., Rodrigues J., and Tomás H. Calcium phosphate-mediated gene delivery using simulated body fluid (SBF). Int J Pharm 434, 199, 2012 [DOI] [PubMed] [Google Scholar]
- 122.Chowdhury E., Kunou M., Nagaoka M., Kundu A., Hoshiba T., and Akaike T. High-efficiency gene delivery for expression in mammalian cells by nanoprecipitates of Ca-Mg phosphate. Gene 341, 77, 2004 [DOI] [PubMed] [Google Scholar]
- 123.Sun B., Tran K.K., and Shen H. Enabling customization of non-viral gene delivery systems for individual cell types by surface-induced mineralization. Biomaterials 30, 6386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kofron M.D., and Laurencin C.T. Development of a calcium phosphate co-precipitate/poly (lactide-co-glycolide) DNA delivery system: release kinetics and cellular transfection studies. Biomaterials 25, 2637, 2004 [DOI] [PubMed] [Google Scholar]
- 125.Wen H., Wolke J., De Wijn J., Liu Q., Cui F., and De Groot K. Fast precipitation of calcium phosphate layers on titanium induced by simple chemical treatments. Biomaterials 18, 1471, 1997 [DOI] [PubMed] [Google Scholar]
- 126.Tanahashi M., Yao T., Kokubo T., Minoda M., Miyamoto T., Nakamura T., and Yamamuro T. Apatite coated on organic polymers by biomimetic process: improvement in its adhesion to substrate by NaOH treatment. J Appl Biomater 5, 339, 1994 [DOI] [PubMed] [Google Scholar]
- 127.Barrere F., Van Blitterswijk C., De Groot K., and Layrolle P. Nucleation of biomimetic Ca-P coatings on Ti6Al4V from a SBF× 5 solution: influence of magnesium. Biomaterials 23, 2211, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Oliveira A., Malafaya P., Costa S., Sousa R., and Reis R. Micro-computed tomography (μ-CT) as a potential tool to assess the effect of dynamic coating routes on the formation of biomimetic apatite layers on 3D-plotted biodegradable polymeric scaffolds. J Mater Sci Mater Med 18, 211, 2007 [DOI] [PubMed] [Google Scholar]