Abstract
Recent studies have demonstrated that orchestrated gene activity and expression support synchronous activity of brain networks. However, there is a paucity of information on the consequences of single gene function on overall brain functional organization and connectivity and how this translates at the behavioral level. In this study, we combined mouse mutagenesis with functional and structural magnetic resonance imaging (MRI) to determine whether targeted inactivation of a single gene would modify whole-brain connectivity in live animals. The targeted gene encodes GPR88 (G protein-coupled receptor 88), an orphan G protein-coupled receptor enriched in the striatum and previously linked to behavioral traits relevant to neuropsychiatric disorders. Connectivity analysis of Gpr88-deficient mice revealed extensive remodeling of intracortical and cortico-subcortical networks. Most prominent modifications were observed at the level of retrosplenial cortex connectivity, central to the default mode network (DMN) whose alteration is considered a hallmark of many psychiatric conditions. Next, somatosensory and motor cortical networks were most affected. These modifications directly relate to sensorimotor gating deficiency reported in mutant animals and also likely underlie their hyperactivity phenotype. Finally, we identified alterations within hippocampal and dorsal striatum functional connectivity, most relevant to a specific learning deficit that we previously reported in Gpr88−/− animals. In addition, amygdala connectivity with cortex and striatum was weakened, perhaps underlying the risk-taking behavior of these animals. This is the first evidence demonstrating that GPR88 activity shapes the mouse brain functional and structural connectome. The concordance between connectivity alterations and behavior deficits observed in Gpr88-deficient mice suggests a role for GPR88 in brain communication.
Keywords: : default mode network, Gpr88, mouse brain functional connectivity
Introduction
Neurons form structural and functional networks that drive brain function and behavior (Van Essen, 2013). Connectome genetics, or the analysis of brain connectivity in relation to gene expression and function, addresses how disease genes influence brain connectivity in humans (Richiardi et al., 2015; Thompson et al., 2013), and also links gene transcriptional patterns with neural network activities in both humans and mice (Richiardi et al., 2015). These studies, however, remain correlative in nature. Deep understanding of cognitive and behavioral development, adaptation, or dysfunction also requires adapted approaches to identify molecular and network determinants of healthy and pathological brains. Our recent work, allying targeted mouse mutagenesis and fine-grained magnetic resonance (MR)-based neuroimaging of live animals, revealed a gene-to-network signature for the mu opioid receptor with predominant alteration of pain/aversion networks (Mechling et al., 2016). This proof-of-principle study, based on open-ended whole-brain connectivity analysis, demonstrates the power of combined gene knockout/MRI to decipher consequences of a single gene inactivation on brain networks and potentially predict behavioral outcomes of genetic dysfunction. In this study, we developed a similar approach to tackle the function of the orphan Gpr88 (G protein-coupled receptor 88) receptor gene, encoding another G protein-coupled receptor whose ligand remains unknown, and discovered brain network mechanisms underlying major GPR88-controlled behaviors.
GPR88 is a striatal-enriched G protein-coupled receptor, expressed in rodents, monkeys, and humans during development and in adulthood (Massart et al., 2016). In humans, the Gpr88 gene was associated with bipolar disorders and schizophrenia (Del Zompo et al., 2014), and the potential of GPR88 as a target to treat psychiatric disorders has attracted increasing interest. In mice, deletion of the Gpr88 gene has been studied with a primary focus on striatal-mediated behaviors, and null mutant mice show motor coordination deficits (Logue et al., 2009; Meirsman et al., 2016a; Quintana et al., 2012), reduced prepulse inhibition (Logue et al., 2009), stereotypies (Meirsman et al., 2016a), and modified cue-based learning (Meirsman et al., 2016a; Quintana et al., 2012). Recently, we found that Gpr88 knockout mice also show improved hippocampal-dependent learning and reduced anxiety levels (Meirsman et al., 2016b). This receptor therefore controls a much larger behavior repertoire than anticipated and, beyond motor activity, also engages spatial learning, emotional processing, and the evaluation of environmental stimulus value. In this study, we examined Gpr88 knockout mice using combined resting-state functional MR imaging (rsfMRI)/diffusion tensor imaging (DTI) in live animals to identify neural networks depending on Gpr88 gene activity and used open-ended whole-brain connectivity analysis to determine predominant alterations that would predict major functions of Gpr88 related to neuropsychiatric conditions.
Materials and Methods
Additional detailed methods are provided in the Supplementary Data (Supplementary Data are available online at www.liebertpub.com/brain).
MRI experiments
Animals
MRI was performed on two groups (n = 14/group) of 7–8-week-old live adult male mice (74.9% C57B/6J, 25% 129/SvPas, 0.05% FVB/N, 0.05% SJL/J): wild-type control mice (CTRL: n = 14) and the Gpr88 knockout group (Gpr88−/−: n = 14), respectively. All animal experiments were performed in accordance with the guidelines and ethics on animal experimentation established by the German and French laws: ethical allowance 35_9185.81/G-13/15 from Regierungspräsidium Freiburg, Germany, and CREMEAS, 2003-10-08-[1]-58, Strasbourg-France, respectively.
Animal preparation
The animals were briefly anesthetized with isoflurane during imaging preparation (stereotaxic fixation of the mouse head, attachment of physiological monitoring devices). To avoid the inhibitory effects of isoflurane on the blood oxygen level-dependent (BOLD) signal, anesthesia was further switched to medetomidine (MD—Domitor; Pfizer, Karlsruhe, Germany). Moderate MD sedation was initially induced by a subcutaneous (s.c.) bolus injection (0.3 mg MD/kg body weight in 100 μL 0.9% NaCl solution); 15 min later, the animals received a continuous s.c. infusion of MD through an MRI-compatible catheter (0.6 mg/kg body weight in 200 μL/h) subcutaneously inserted at the mouse shoulder level. After rsfMRI acquisition, MD infusion was stopped and replaced by anesthesia through isoflurane (∼1.5 vol%) for further scanning performed on respiration triggering. Isoflurane induces a deeper anesthesia, important for avoiding the movement artifacts for diffusion imaging. Mouse physiological conditions (including temperature, respiration, and blood oxygen saturation) were monitored continually during the imaging session.
MRI data acquisition
Mouse brain MRI data were acquired with a 7 T small-bore animal scanner (Biospec 70/20, Bruker, Germany) and a mouse head adapted CryoCoil (MRI CryoProbe, Bruker, Germany).
• rsfMRI: Data were acquired with T2*-weighted, single-shot, gradient-echo echo-planar imaging (GE-EPI) sequence (echo time [TE]/repetition time [TR] = 10 ms/1700 ms). The mouse brain (excluding the cerebellum) was covered using 12 axial slices of 0.7 mm thickness, with a field of view (FOV) of 19.2 × 12 mm2 and an acquisition matrix of 128 × 80, which resulted in a planar resolution of 150 × 150 μm2. Two hundred volumes were recorded in an interlaced manner for each run.
• T2: High-resolution morphological images were acquired using Turbo rapid acquisition with relaxation enhancement (RARE) T2 sequence (TE/TR = 50 ms/6514 ms, two averages at RARE factor of 4). The whole brain, including cerebellum, was covered using 48 slices (0.3 mm slice thickness) at planar spatial resolution of 51 × 51 μm2 with an FOV of 1.3 × 1.0 cm2 and an acquisition matrix of 256 × 196.
• High-angular resolution diffusion imaging (HARDI) was performed using four-shot DTI-EPI sequence. Twenty-five axial slices of 0.5 mm thickness were acquired at a resolution of 94 × 94 μm2 with an FOV of 1.5 × 1.2 cm2 and an acquisition matrix of 160 × 128, covering the equivalent partition of the brain as for the rsfMRI scan (TE/TR = 27 ms/3750 ms); Δ = 10 ms, diffusion gradient duration (δ) = 4 ms, b factor of 1000 s/mm2, 30 noncollinear diffusion gradient directions.
MRI data preprocessing
Imaging data were preprocessed (Mechling et al., 2016) using MATLAB (The MathWorks, Natick, MA) along with the fMRI tool of statistical parametric mapping SPM8* and its SPM Mouse† toolbox (Sawiak et al., 2013), which includes functions for realignment, coregistration, and segmentation of mouse brain data (see detailed procedure in Supplementary Data: “Data preprocessing”). Briefly, the preprocession pipeline included an initial realignment of the 200 volumes of rsfMRI data to the first one using a least square approach and a six-parameter rigid body transformation in space to correct for motion in each single scan. The coregistration function also was used to align the rsfMRI (first time point) volumes of each mouse to its respective A0 images obtained from HARDI (HARDI acquisition with a diffusion weighting factor bfactor = 0 mm2/s—no diffusion gradient applied) and T2 image volume. SPM Mouse brain template was further refined by including additional high-resolution mouse brain images to create a tissue probability map (TPM) template. We used this template for spatial normalization and alignment of rsfMRI mouse brain images, morphological T2-weighted images, the A0 images, and the parametric maps derived from diffusion tractography (fiber density [FD] and fractional anisotropy maps). We applied a Gaussian smoothing with a kernel of full width at half maximum of 0.4 × 0.4 × 1 mm3 to all TPM-aligned rsfMRI image volumes (Mechling et al., 2014). Furthermore, the whole brain was parceled using an in-house-developed MATLAB and Allen Mouse Brain Atlas (AMBA) (Lein et al., 2007). The AMBA was aligned and resliced to our template using the SPM8 toolbox, changing its initial resolution to our template's resolution of 165 × 230 × 135 voxels with a voxel size of 0.07 × 0.07 × 0.07 mm3.
MRI data postprocessing
Resting-state functional magnetic resonance imaging
Independent component analysis
High-dimensional (100 components) spatial group independent component analysis (ICA) (Calhoun et al., 2001) using the MATLAB-based toolbox GIFT (group ICA of fMRI toolbox—v1.3i, www.nitrc.org/projects/gift) was carried out on 28 combined control (CTRL) and Gpr88−/− datasets. Infomax algorithm was used to decompose the entire BOLD data set into spatially independent components (ICs) without any hypothesis paradigms (Hyvärinen and Oja, 2000). We further investigated the robustness of the identified components using ICASSO algorithm (Himberg et al., 2004).
Estimation of the number of components is an important step while decomposing the entire BOLD signal into spatially ICs or sources. Underestimation of the components may result in mixing various components (Margulies et al., 2010; van de Ven et al., 2004), whereas overestimation can result in splitting reliable networks (Esposito et al., 2003; Moritz et al., 2005), decreasing the stability of IC estimates (Li et al., 2007). Therefore, we used ICASSO algorithm (Himberg et al., 2004) to assess the stability pattern by bootstrapping and randomizing initial conditions for different numbers of ICs. The quality index Iq (values ranging from 0 to 1) was used as a quantitative measure of robustness of the identified components evaluating compactness and isolation of each cluster (Hübner et al., 2017; Mechling et al., 2014). We verified the consistency of the results when progressively achieving a high spatial definition (in accordance to fine anatomical details) of the functional clustering patterns with 10, 15, 20, 40, 60, 80, 100, and 120-ICASSO, respectively. Using the percentage of components revealing quality index (Iq) > 0.75 as a stability criterion, we observed a clear degradation of the IC estimates for 120-ICASSO, justifying our choice of 100-ICASSO analysis.
The mean resulting patterns were displayed as spatial color-coded z-maps onto T2-weighted morphological images (threshold |z| > 3, corresponding to p < 0.00135) and on coregistered AMBA (Lein et al., 2007). The color coding represents the dependence of the time course in each voxel compared with the mean time course of the respective component in arbitrary units. Coregistration with AMBA allowed for automatic identification of anatomic brain areas covered by IC patterns. From the 100-ICASSO results, 12 artifactual components related to cerebrospinal fluid, vascular, or movement-related pseudo activations were excluded from analysis after visual inspection and overlay on AMBA. From these aggregate components and the original data, we computed spatial, back-reconstructed, individual subject components using a spatial-temporal regression approach (see also Supplementary Data). We further used the back-reconstructed, individual spatial maps to create incidence maps for each IC. Relevant examples are the incidence maps illustrating the patterns of SS, CP, ACB, and AMY functional clusters (Supplementary Fig. S1c; Supplementary Data are available online at www.liebertpub.com/brain). The incidence maps illustrate the spatial distribution and the reproducibility of the IC pattern over each animal group (CTRL and Gpr88−/−). The color-coded incidence of a voxel reflects in how many of the animals it was found to belong. These examples show low intragroup variability of ICA patterns and extremely high similarity between group patterns.
The results substantiate our further approach of using the meaningful 88 group ICASSO functional clusters (Supplementary Fig. S2) as nodes in the generation of mouse brain functional connectivity matrices (MBFC) of CTRL and Gpr88−/− groups of animals through partial correlation (PC). Conducting the 100-ICASSO separately on each animal group would have eventually resulted in slightly different nodes of connectivity and difficulties to directly compare the group results.
Pearson PC analysis
The PC coefficients (Pearson) between each pair of ICs derived with ICASSO were calculated and used to create an 88 × 88 adjacency PC matrix for each animal as well as two average matrices, representative for each experimental group (group-specific PC matrices: Fig. 1a). Each element of the matrix represented the strength of direct connectivity between two components (nodes). The PC matrices were then normalized using Fisher's z-transformation. The significance of positive and negative correlations between pairs of components was further assessed by using a two-sided one-sample t-test and thresholding at p < 0.05.
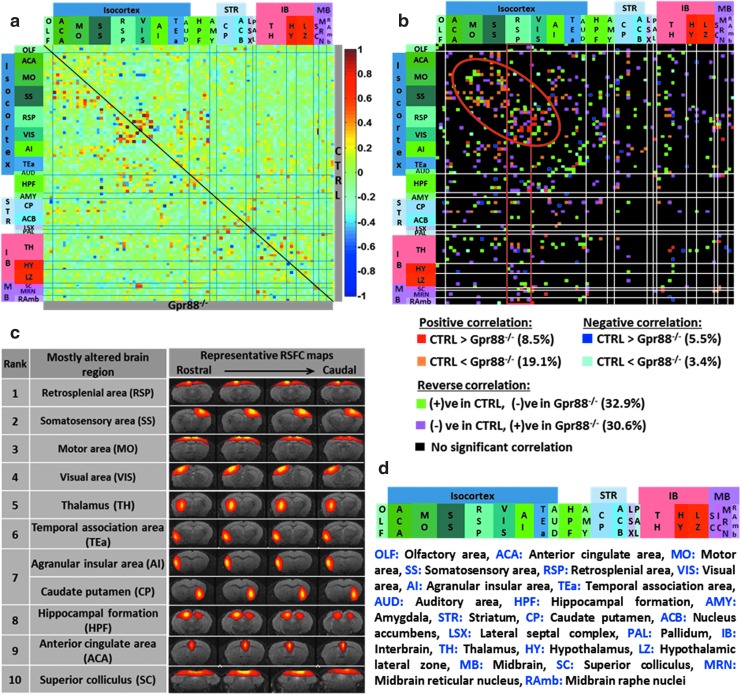
FIG. 1.
Quantitative mapping of functional network alterations in Gpr88−/− mice reveals a strong Gpr88-dependent activity signature in live animals: (a) Group-specific resting-state FC matrices (CTRL above and Gpr88−/− group below the diagonal), including both positive and negative internodal correlations (derived via partial correlation methods). The nodes were defined through functional segregation using 100-ICASSO. Functional nodes were grouped and assigned to corresponding anatomical regions through coregistration on the Allen Mouse Brain Atlas (d). (b) Direct intergroup (CTRL vs. Gpr88−/−) statistical comparison of connectivity matrices (two-sample t-test, p < 0.01, FDR corrected) is shown as a 2D matrix. The Gpr88 genetic inactivation induced widespread modifications of internode connectivity. The red circle points to the cluster of strong intracortical FC remodeling in the absence of Gpr88, whereas the red square points to extensive significant FC alterations of the RSP area connectivity. (c) Nodes of brain area with highest number of statistically significant connectivity changes are ranked (two-sample t-test, p < 0.01, FDR corrected). Their functional pattern is overlaid on four T2-weighted anatomical images. From top to bottom: retrosplenial area (RSP), somatosensory area (SS), motor area (MO), visual area (VIS), thalamus (TH), temporal association area (TEa), superior colliculus (SC), caudate-putamen (CP), agranular insular area (AI), hippocampal formation (HPF), and anterior cingulate area (ACA). (d) Assignment of brain regions from rostral to caudal direction and their corresponding localization on the sagittal mouse brain template adapted from the Allen Mouse Brain Atlas. The anatomical overlap of components was identified using a MATLAB-based and in-house-developed postprocessing tool, which is based on coregistration of the data on the Allen Mouse Brain Atlas (AMBA) (Lein et al., 2007) (http://mouse.brain-map.org/static/atlas). We assigned the component to the region or anatomical subdivision (in case of large areas) with maximum overlay. Nevertheless, components are partly touching other regions (subdivisions). CTRL, control; FC, functional connectivity; FDR, false discovery rate; GPR88, G protein-coupled receptor 88; RSP, retrosplenial.
Direct intergroup (CTRL vs. Gpr88−/−) statistical comparison of the group matrices was further performed. The functional connectivity (FC) alterations between groups were considered significant after assessment using a two-sample t-test (p < 0.01; false discovery rate [FDR] corrected). A group comparison matrix (GCM) was generated (Fig. 1b) that color coded the statistically significant intergroup differences of connectivity. GCM was further used to count the significantly changed connections for each node (IC) and we further ranked nodes on the basis of highest number of such statistically significant differences in connectivity across the two genotypes (Fig. 1c).
Seed-based correlation analysis
To evaluate the alterations of functional networks, several brain areas that showed high number of correlation alterations between groups were selected as the regions of interest (ROIs). As all the data were normalized onto AMBA, each ROI was extracted using this atlas and used as the seed region to perform FC analysis of preprocessed rsfMRI data. Correlation coefficients were then computed (two-tailed t-test, p < 0.001) between the seed region and the averaged time series of the remaining whole brain for each group and were converted to z values using Fisher's r-to-z transformation. We further performed voxel-level general linear model (p < 0.001) corrected for multiple comparisons using a random field theory approach (Worsley et al., 1996) to statistically evaluate FC remodeling on the group level for each specific seed.
Diffusion-based tractography
Modifications in the brain structural connectivity were assessed using HARDI and further tractography using a global fiber tracking approach (Harsan et al., 2013; Reisert et al., 2011). FD maps were used to measure brain microstructural modifications. Statistically significant differences were evaluated using a two-sample t-test (p < 0.05, familywise error [FWE] corrected) (detailed procedure is described in Supplementary Data).
Results
GPR88 expression in the mouse brain
We verified the expression pattern of GPR88 in the control (CTRL) mice by in situ hybridization (Supplementary Fig. S1a). We observed the receptor expressed in the layers 4 and 5 of the somatosensory cortex (SS), caudate-putamen (CP), amygdala, nucleus accumbens (ACB), and olfactory tubercle (OT) in support of several previously reported literatures (Becker et al., 2008; Ghate et al., 2007; Massart et al., 2016; Mizushima et al., 2000; Van Waes et al., 2011).
Elementary functional clusters identified through group 100 ICA
We applied high-dimensional, data-driven spatial ICA (using 100 components) combined with ICASSO on rsfMRI datasets from CTRL and Gpr88−/− animal groups. Eighty-eight reliable functional clusters were identified whose spatial pattern covered neuroanatomical regions defined by the Allen Mouse Brain Atlas (AMBA) (Supplementary Fig. S2). We further validated the reproducibility of the group ICASSO patterns in each animal and in each experimental group through back reconstruction (see the Materials and Methods section and Supplementary Fig. S1b, c). The results demonstrated low intragroup variability of the component pattern and extremely high similarity between groups. We could associate the spatial pattern of some of these components with areas strongly expressing Gpr88 receptors in normal conditions (Supplementary Fig. S1b). Both left and right hemispheric patterns were obtained and presented in Supplementary Figure S1 for cortical (SS) and subcortical areas (CP, ACB, and AMY). We further used the 88 group ICASSO functional clusters (Supplementary Fig. S2) as nodes in the generation of resting-state brain FC (rsFC) matrices of CTRL (Fig. 1a—above the diagonal) and Gpr88−/− (Fig. 1a—below the diagonal) groups of animals (two-sided one-sample t-test, p < 0.05) (see the Materials and Methods section). All these nodes correspond to anatomically well-defined brain regions and were rearranged according to their association with brain areas in the rostrocaudal axis (Fig. 1d).
Deletion of GPR88 receptor induces extensive FC remodeling of the mouse brain
We quantitatively evaluated the impact of GPR88 receptor deletion on the mouse brain FC using direct statistical intergroup comparison of CTRL and Gpr88−/− MBFC matrices (see the Materials and Methods section and Fig. 1b). We detected significant and widespread alterations of internode connectivity (Fig. 1b; two-sample t-test, p < 0.01, FDR corrected). The 2D matrix representation (Fig. 1b) captured the causal effect of targeted Gpr88 gene disruption at the level of whole brain networks. The extent of Gpr88-dependent connectional activity appeared surprisingly broad within cortical areas, particularly retrosplenial (RSP), sensory areas (somatosensory [SS], and motor cortex [MO]), as well as the visual cortex (VIS) (Fig. 1b, red circle). Furthermore, to define the Gpr88 signature on the mouse brain connectome, we ranked the brain areas on the basis of the number of statistically significant differences (p < 0.01, FDR corrected) in connectivity across the two genotypes (Fig. 1c). There was a clear dominance of connectivity changes for cortical-related nodes, with seven nodes from the top 10 being associated with isocortex. RSP showed the strongest remodeling (rank 1, Fig. 1c) of connectivity. Along with this area—classically included in the default mode network (DMN) across species (Raichle, 2015), VIS (rank 4, Fig. 1c), thalamus (TH, rank 5, Fig. 1c), temporal association area (TEa, rank 6, Fig. 1c), hippocampal formation (HPF, rank 8, Fig. 1c), and anterior cingulate area (ACA, rank 9, Fig. 1c) are brain regions present in the top 10 of our hierarchy. They were previously described as part of a DM-like network in the C57Bl/6 mouse strain (Liska et al., 2015; Hübner et al., 2017). This result suggests a strong modification of resting-state brain activity in Gpr88−/− mice involving DMN.
Additional to this network, the intergroup comparison of FC matrices revealed significant changes (two-sample t-test, p < 0.01, FDR corrected) of the MO (rank 3, Fig. 1c) and the sensory (SS, rank 2; and VIS, rank 4, Fig. 1c) cortical connectivity, as well as the subcortical striatal (particularly involving the caudate-putamen [CP], rank 7, Fig. 1c) and HPF (rank 8, Fig. 1c) circuitries. Moreover, superior colliculus (SC) of the midbrain area—a major node for mediating sensorimotor transformations (Simon, 2008), was ranked tenth among the most altered brain regions (SC, rank 10, Fig. 1c). All together, these findings suggest several salient features of the Gpr88−/− mouse brain architecture. These are massive intracortical and cortico-subcortical rsFC modifications, particularly involving DMN core areas along with the sensorimotor and cortico-striatal pathway. To further strengthen these findings, we performed seed analysis using the brain areas highlighted in the ICASSO-based ranking and anatomically defining the seeds on the basis of coregistration with the AMBA (see the Methods section).
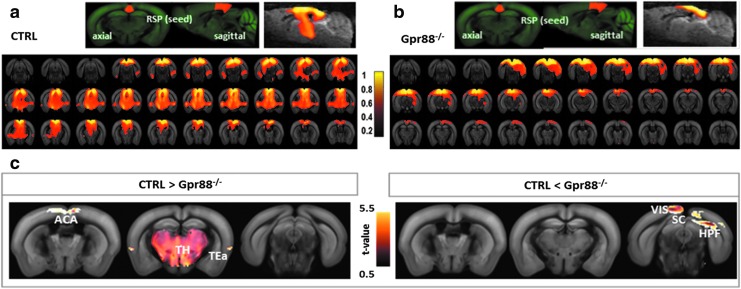
Altered DMN patterns in Gpr88−/− mice
The RSP was ranked on top of substantial FC differences across CTRL and Gpr88−/− mice. We selected bilateral RSP as a seed region for mapping its connectivity patterns across the whole brain (two-tailed t-test, p < 0.001) and identify the DMN. Similar to previous work using the seed-based correlation approach (Hübner et al., 2017; Sforazzini et al., 2014), our results support the idea of a mouse DMN network with RSP as a core area. Its positive connections with the medial and caudal ACA, HPF, TEa, and VIS system (Fig. 2a) portray, in the CTRL group, similarities with the posterior DMN obtained for humans (Di and Biswal, 2014). Relative to this CTRL pattern, RSP in the Gpr88−/− group exhibited clearly reduced FC with ACA, TEa, and massive decreased FC with TH (Fig. 2a vs. b, c). This result demonstrates modification of the DMN in mice lacking the GPR88 receptor. This major modification, which we did not observe in mu opioid receptor knockout mice in our previous work (Mechling et al., 2016), is consistent with the large extent of behavioral alterations reported in Gpr88−/− mice.
FIG. 2.
Gpr88 deletion strongly reshapes the DMN pattern, defined as positive RSP cortex FC: (a) DMN pattern in the CTRL animal group. RSP cortex demonstrated strong coherent fluctuations of the BOLD signal with rostromedial ACA, TEa, and VIS areas, HPF, and thalamus. (b) Altered DMN pattern in the Gpr88−/− mouse brains is characterized by abolished FC of RSP with rostral ACA, TEa, and subcortical brain regions (HPF and TH), but strong connections mostly with the VIS area, including superior colliculus. (c) Statistically significant differences in the DMN patterns when comparing CTRL and Gpr88−/− groups (GLM, p < 0.001, corrected). The left panel shows the brain regions positively correlated with RSP for which correlations are significantly stronger in the CTRL than in the Gpr88−/− group. The right panel shows areas with FC stronger in the mutant group compared with the CTRL. The color scale at the middle indicates the corresponding T-value. BOLD, blood oxygen level-dependent; DMN, default mode network; GLM, general linear modeling.
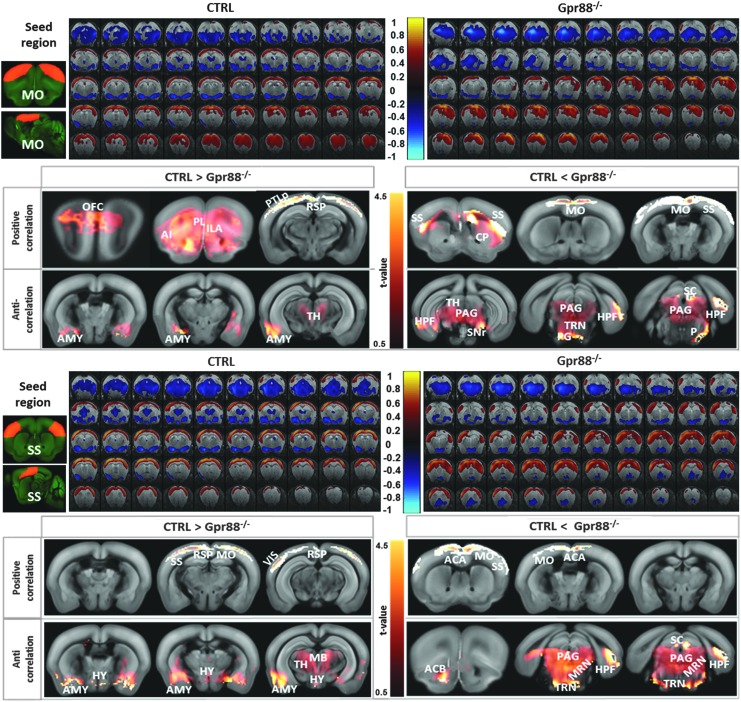
Remodeling of motor and sensory cortical rsFC in Gpr88−/− mice
Sensory (SS) and motor (MO) areas were ranked second and third in the quantification of FC alterations of Gpr88−/− mice. Seed-based analysis using MO as ROI revealed extensive cortical and subcortical rsFC modifications in the Gpr88−/− group (Fig. 3a). We quantified the group statistical significance of the alterations using voxel-level general linear modeling corrected for multiple comparisons using the random field theory approach (p < 0.001, see the Materials and Methods section). When compared with the CTRL group, Gpr88−/− MO showed salient features of reduced (Fig. 3b, CTRL > Gpr88−/−) or stronger (Fig. 3b, CTRL < Gpr88−/−) rsFC with specific brain areas.
FIG. 3.
Remodeled sensory–motor FC underscores the hyperactive or distracted behavior in mutant mice: BOLD rsfMRI correlation maps for (a) MO and (c) SS. Corresponding correlation maps of the CTRL and Gpr88−/− groups were overlaid on atlas brain slices (two-tailed t-test, p < 0.001). The color scale indicates the correlation value (positive correlations from 0 to +1: dark red to yellow and negative correlations from 0 to −1: dark blue to turquoise). (b, d) Comparative analysis of the statistically significant FC (GLM, p < 0.001, corrected) resulting from the seed regions: MO and SS, respectively. The left panel shows positive and anticorrelated brain regions with the respective seed, stronger in the CTRL than Gpr88−/− group. The right panel shows more strongly correlated regions in the mutant group compared with the CTRL. The color scale at the middle indicates the corresponding T-value. rsfMRI, resting-state functional magnetic resonance imaging.
From positive correlation analysis (Fig. 3a, correlations from 0 to 1, and Fig. 3b—positive correlation), the Gpr88−/− group showed decreased (CTRL > Gpr88−/−) FC between MO and orbitofrontal cortex, agranular insular area (AI), and limbic areas (prelimbic cortex—PL, infralimbic cortex—ILA, nucleus accumbens—ACB), as well as caudal RSP and parietal cortex (PTLp). However, stronger rsFC (Fig. 3b, positive correlation CTRL < Gpr88−/−) was quantified between MO and CP and MO and SS, as well as within the MO. These strong modifications of striato-motor connectivity are particularly relevant to highest Gpr88 expression in the striatum and the hyperactive phenotype observed in this model (Logue et al., 2009; Meirsman et al., 2016a; Quintana et al., 2012). Negative correlation analysis (Fig. 3a, correlations from −1 to 0, and Fig. 3b—anticorrelations) revealed reduced MO connectivity with amygdala (AMY) and posterior thalamic nuclei (TH) (Fig. 3b, anticorrelation CTRL > Gpr88−/−), while stronger anticorrelations in the Gpr88−/− group compared with CTRL were obtained with HPF and midbrain (MB) areas, including superior colliculus (SC) or periaqueductal gray (PAG).
Along with remodeled MO connectivity, the Gpr88−/− SS cortex showed significant rsFC alterations (Fig. 3c, d). From positive correlation analysis (Fig. 3c, correlations from 0 to 1, and Fig. 3d—positive correlation), the Gpr88−/− group revealed decreased rsFC (CTRL > Gpr88−/−) between SS and the mid-caudal isocortex, including MO, RSP, and VIS areas. Stronger rsFC (Fig. 3d, positive correlation CTRL <Gpr88−/−) was quantified between SS and rostral isocortex, including MO and ACA. These intracortical modifications of rsFC in the Gpr88−/− group correlate with modifications of the brain connectivity matrix derived from ICASSO analysis (Fig. 1b). Negative correlation analysis (Fig. 3c, correlations from −1 to 0, and Fig. 3d—anticorrelations) revealed reduced SS connectivity with AMY, TH, hypothalamus (HY), and MB areas (Fig. 3d, anticorrelation CTRL > Gpr88−/−), while stronger anticorrelations in the Gpr88−/− group were obtained between SS and ACB, HPF, and MB areas, including SC or PAG (Fig. 3d, anticorrelation CTRL < Gpr88−/−).
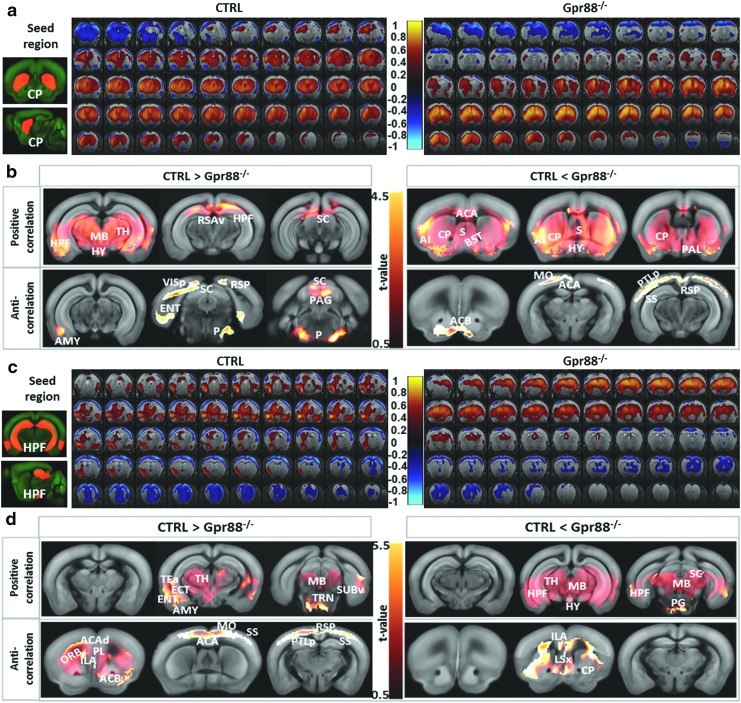
Modified caudate-putamen and HPF connectivity in Gpr88−/−mice
Other brain areas were ranked high in the quantification of FC alterations in mutant mice. Notable are caudate-putamen (CP) and HPF, where Gpr88 is highly enriched for the former and virtually absent for the latter. Our previous behavioral study investigated whether altered striatal function would translate into a modification of hippocampal/striatal balance in learning (Meirsman et al., 2016a). A dedicated, dual-solution cross-maze task revealed that Gpr88−/− mice perform better in the allocentric versus egocentric component of the task for both acquisition and reversal learning, demonstrating facilitation of hippocampus-dependent behavior at the expense of striatal-dependent responses (Meirsman et al., 2016a). We therefore compared FC across these two brain structures using seed-based correlation analysis.
From positive correlation analysis (Fig. 4a, correlations from 0 to 1, and Fig. 4b—positive correlation), the Gpr88−/− CP showed strongly decreased rsFC toward HPF, TH, and MB. CP rsFC was, however, increased in the Gpr88−/− mice (Fig. 4b, positive correlation CTRL < Gpr88−/−) toward ACA and rostral subcortical area, including septal complex (S), pallidum (PAL), bed nuclei of the stria terminalis (BST), and AI. Negative correlation analysis (Fig. 4a, correlations from −1 to 0, and Fig. 4b—anticorrelations) revealed reduced CP connectivity with AMY, entorhinal (ENT), and VIS cortices, as well as SC and pontine olivary nuclei (P), in the Gpr88−/− group. However, stronger anticorrelations were obtained in the Gpr88−/− group between CP and ACB, as well as MO, SS, ACA, and PTLp cortical areas.
FIG. 4.
FC patterns of dorsal striatum (CP) (a, b) and hippocampal formation (HPF) (c, d) are altered in Gpr88−/− mouse brains: BOLD rsfMRI correlation maps for (a) CP and (c) HPF. Corresponding correlation maps of the CTRL and Gpr88−/− groups were overlaid on T2-weighted anatomical brain slices (two-tailed t-test, p < 0.001). The color scale indicates the correlation value (positive correlations from 0 to +1: dark red to yellow and negative correlations from 0 to −1: dark blue to turquoise). (b, d) Detailed analysis of the statistically significant FC modifications (GLM, p < 0.001, corrected) of CP and HPF in the Gpr88−/− group. The left panel shows positive and anticorrelated brain regions with the respective seed stronger in the CTRL than Gpr88−/− group, while the right panel shows more strongly correlated regions in the mutant group compared with the CTRL. The color scale at the middle indicates the corresponding T-value.
Seed-based correlation analysis quantified strong hippocampal connectivity alterations in the Gpr88−/− group. Positive correlation analysis (Fig. 4c, correlations from 0 to 1, and Fig. 4d—positive correlation) showed decreased positive correlations between HPF and AMY, ENT, and TEa, as well as rostral TH and MB in the Gpr88−/− group. Meanwhile, HPF increased its positive rsFC toward caudal TH nuclei, SC, rostral MB, and pontine gray (PG) (Fig. 4d—positive correlation CTRL < Gpr88−/−). Negative correlation analysis (Fig. 4b, correlations from −1 to 0, and Fig. 4d—anticorrelations) of HPF network indicated decreased connectivity between HPF and frontal limbic system, including orbital (ORB), PL, and ACB areas, as well as ACA, MO, SS, RSP, and PTLp cortical regions, in the Gpr88−/− mice. Increased anticorrelated rsFC was, however, quantified between HPF and CP, lateral septal nuclei (LSX), and ILA (Fig. 4d—anticorrelation CTRL <Gpr88−/−). Altogether, the extensive striato-hippocampal rsFC modifications corroborate the modified striato-hippocampal learning phenotype that we previously described for Gpr88−/− mice (Meirsman et al., 2016a).
Structural connectivity assessment
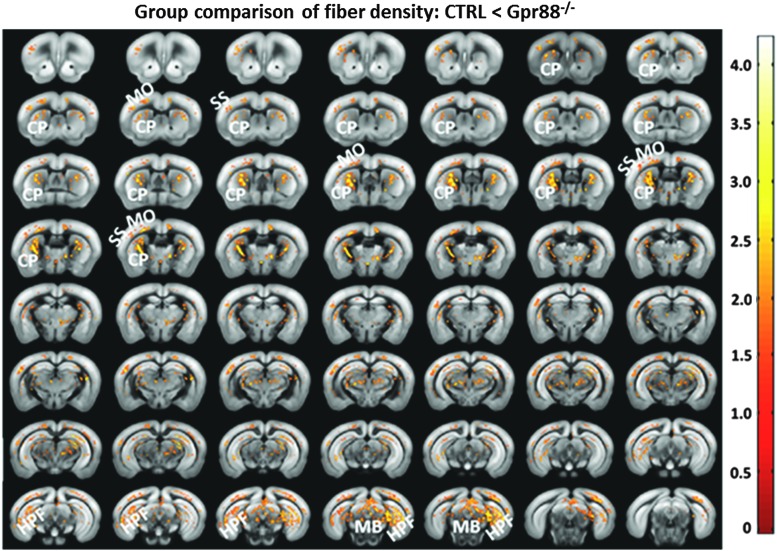
To noninvasively verify if Gpr88 gene deletion impacted the underlying microstructure of brain functional networks, we used DTI and fiber tractography-derived parameters (FD) for measuring structural connectivity modifications (Fig. 6). Significant increase of FD values was detected in Gpr88−/− animals compared with the CTRL group (voxel-wise statistical group comparison, p < 0.05, FWE corrected, contrast CTRL < Gpr88−/−) in brain areas with altered rsFC. These areas included CP, MO, SS, HPF, parts of TH, and MB. No significant changes could be detected when examining the CTRL > Gpr88−/− for FD contrast. For generation of FD maps, we used a global fiber-tracking algorithm that was previously validated for mouse and human DTI data (see the Materials and Methods section). A similar pattern of significant modifications was also quantified when performing group statistics on the fractional anisotropy maps (data not shown) derived after calculation of the diffusion tensor.
FIG. 6.
Significant alterations of the fiber density in Gpr88−/− mice: Statistical significance was evaluated using a two-sample t-test (p < 0.05, FWE corrected). The panel shows the regions with higher fiber density in the mutant mice compared with the CTRL. Corresponding T-value scale is shown. FWE, familywise error.
Discussion
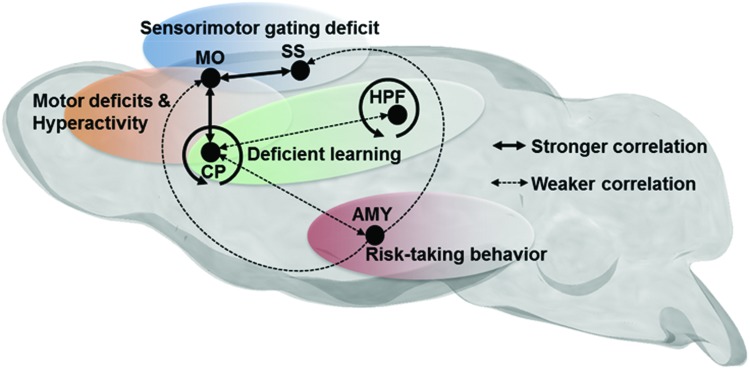
During rest, endogenous fluctuations in low-frequency BOLD signals are synchronized between different regions—widely distributed throughout the brain, forming dynamic FC networks (Cabral et al., 2014). The genetic and molecular factors that regulate the development and behavior of these networks remain undefined. In this study, we focused on the Gpr88 gene and discovered significant modifications of brain connectional patterns in live Gpr88−/− mice. This gene encodes an orphan G protein-coupled receptor whose ligand remains unknown and is expressed as early as embryonic day 16 in the rat (Massart et al., 2016) and P0 in the mouse (our unpublished data). Altered FC in mutant mice may therefore result either from the lack of GPR88 receptor activity during development, and thus reflect compensatory modifications, or from the absence of tonic GPR88 activity in the adult, or both. Inducible gene knockout approaches in the future should clarify respective contributions of early and adult GPR88 expression. The strong modifications of connectional patterns observed in adult Gpr88−/− mice may potentially underpin behavioral alterations of these mutant mice. Table 1 summarizes behavioral phenotypes reported for Gpr88−/− mice, which relate to altered rsFC observed in this study, and the relevant connectivity alterations are summarized in Figure 5.
Table 1.
Summary Behavioral Deficits Reported in Gpr88−/− Mice
| System | Behavioral test | Behavior results |
|---|---|---|
| Sensorimotor gating deficit | Prepulse inhibition of the acoustic startle response assay | Sensorimotor gating deficit (Logue et al., 2009) |
| Motor deficits and hyperactivity | Stereotypy | Increased stereotypy (Meirsman et al., 2016a) |
| Rotarod | Impaired motor coordination or strength (Quintana et al., 2012) | |
| Grip test | No difference in muscle strength (Meirsman et al., 2016a) | |
| Basal locomotor activity | Increased basal locomotor activity in novel and familiar environments (Quintana et al., 2012) | |
| Basal locomotor activity | Increased locomotor activity and lack of habituation to a novel environment. (Meirsman et al., 2016a) | |
| Basal locomotor activity | Increased locomotor activity (Meirsman et al., 2016b) | |
| Learning deficiency | Operant behavior, two-way active avoidance procedure | Impaired avoidance learning, acquisition, and integration of visual or auditory cues (Quintana et al., 2012) |
| Morris water maze | Visuospatial memory and learning were intact (Quintana et al., 2012) | |
| A water-based U maze | Impairments in cue-based learning (Quintana et al., 2012) | |
| Rotarod | Motor coordination and learning impairment (Meirsman et al., 2016a) | |
| Y-maze | Increased exploration in new environments (Meirsman et al., 2016a) | |
| Novel object recognition test | Improved learning and recognition memory (Meirsman et al., 2016a) | |
| Dual-solution cross-maze task | Improved ability to distinguish between goal-directed responses and habitual behavior (Meirsman et al., 2016a) | |
| Fear conditioning | Impaired contextual fear and cue-related fear expression (Meirsman et al., 2016b) | |
| Risk-taking behavior | Elevated-plus maze test | Reduced anxiety levels (Meirsman et al., 2016a) |
| Elevated-plus maze test | Decreased anxiety behaviors (Meirsman et al., 2016b) | |
| Light–dark test | Exhibit increased risk-taking behaviors (Meirsman et al., 2016b) | |
| Marble-burying test | Lower anxiety behaviors (Meirsman et al., 2016a) | |
| Marble-burying test | Decreased threat avoidance and more risk-taking behaviors (Meirsman et al., 2016b) | |
| Nest building | Decreased anxiety (Meirsman et al., 2016a) | |
| Novelty preference | Increased novelty approach/low anxiety behaviors (Meirsman et al., 2016b) | |
| Novelty-suppressed feeding test | Decreased conflict anxiety (Meirsman et al., 2016a) | |
| Novelty-suppressed feeding test | Increased novelty approach/low anxiety behaviors (Meirsman et al., 2016b) | |
| Social interaction test | Increased social behaviors (Meirsman et al., 2016b) |
Mutant mouse phenotypes are displayed in relation to brain connectivity domains as represented in Figure 5.
FIG. 5.
Schematic representation of dominant resting-state FC alterations in Gpr88−/− mice, associated with specific behavioral deficits previously reported for these mutant mice, and summarized in Table 1. Bold lines represent stronger correlations between regions, whereas dashed lines represent weaker correlations.
The repertoire of Gpr88−/− mice behavioral phenotypes was often discussed with respect to dysfunctions of cell types and brain areas expressing Gpr88 in normal conditions. Particularly, the aberrant activation of striatal GABAergic medium spiny neurons (Quintana et al., 2012)—in the absence of a functional GPR88 receptor—was suggested to be a major contributor to behavioral deficits observed in Gpr88−/− mice. However, one has to consider that such perturbations of brain activity are not confined to a single locus, instead they spread along axonal pathways to influence other regions' activity and alter the way these areas communicate with each other. This rsfMRI study allowed a whole brain and hypothesis-free analysis of brain connectivity, unraveling the intrinsic mouse brain functional communication and highlighting the complex highly organized topology of functional networks as previously observed in rodents (Biswal et al., 1995; Liang et al., 2011; Ma et al., 2016; Smith et al., 2013; Zerbi et al., 2015; Zhang et al., 2010), with its central player, the DMN (Raichle et al., 2001). Defined as a set of brain regions that show high neuronal activity during rest (Fox and Raichle, 2007; Raichle, 2015), DMN raised a lot of interest as it was demonstrated that its coherent activity is perturbed in pathological conditions, including psychiatric disorders (Raichle, 2015; Zhou et al., 2014). We and other groups found the DM-like network in the healthy mouse brain (Grandjean et al., 2016; Hübner et al., 2017; Sforazzini et al., 2014) to be modified in mouse models of brain pathologies (Grandjean et al., 2016; Hübner et al., 2017). In this study, we show that Gpr88 deletion in mice strongly perturbs the coherent activity of DMN, with major impact on the connectivity of RSP, the core player of this network. RSP connectivity with mid-rostral part of DMN, including the medial and anterior cingulate cortex as well as cortico-subcortical DMN subcomponents and temporal association area, is strongly suppressed in the Gpr88−/− brains.
These large alterations of DMN upon deletion of the Gpr88 gene are consistent with broad behavioral alterations mentioned in this mouse model and relate to patterns observed in human studies of psychiatric and neurologic disorders. Indeed FC modifications in the DMN have been reported, for instance, in bipolar disorder (Brady et al., 2016; Öngür et al., 2010; Wang et al., 2016), schizophrenia (Garrity et al., 2007; Kühn and Gallinat, 2012; Whitfield-Gabrieli and Ford, 2012), or attention-deficit/hyperactivity disorder (ADHD) (Castellanos and Proal, 2012; Castellanos et al., 2008; Fair et al., 2010; Fassbender et al., 2009; Hoekzema et al., 2014; Sonuga-Barke and Castellanos, 2007; Sun et al., 2012; Uddin et al., 2008). Emerging studies propose ADHD as a DMN disorder (Castellanos and Proal, 2012; Castellanos et al., 2008; Fair et al., 2010; Fassbender et al., 2009; Sonuga-Barke and Castellanos, 2007), and decreased DMN rsFC for adults (Castellanos et al., 2008; Hoekzema et al., 2014; Uddin et al., 2008) as well as adolescents suffering from ADHD (Sun et al., 2012) was reported. Gpr88 receptor, as well as the behavioral traits of the Gpr88−/− mice, was associated over time with the pathophysiology of such disorders (Del Zompo et al., 2014; Logue et al., 2009). This broad impact of Gpr88 deletion on the topology of resting-state connectivity has to be considered in a developmental context as well, as in normal brain, Gpr88 shows differential expression over time in various brain areas. It is therefore likely that the global remodeling observed here results from the lack of GPR88 during development, at least for a large part.
Beside a strongly modified DMN pattern, we show extensive alterations of whole brain Gpr88−/− FC matrix (Fig. 1) and, most interestingly, within intracortical connectivity. Indeed, recent work demonstrates intracellular redistribution of GPR88 during cortical lamination in the normal brains (Massart et al., 2016). In the cortical plate of the developing cortex, GPR88 presents a classical G protein-coupled receptor (GPCR) plasma membrane/cytoplasmic localization that shifts, on the day of birth, to nuclei of neurons progressively settling during postnatal development in layers V to II. It is likely that Gpr88 influences, to some extent, the development of intracortical functional communication and that deletion of the receptor in Gpr88−/− mice leads to remodeling of cortical functional pathways, as seen here. Particularly clear is the alteration of somatomotor connectivity (SS-MO) and SS-MO-ACA functional connections linked with the observed sensorimotor gating deficiency (Logue et al., 2009; Meirsman et al., 2016a) and risk-taking behavior (Meirsman et al., 2016b) of Gpr88−/− mice. Sensorimotor gating is the process of screening or gating of the sensory and motor/cognitive information to enable uninterrupted processing of the most salient aspects of the external and internal environment (Butler et al., 1990). Sensorimotor gating deficiency reflects central inhibitory functioning deficiency and underlies symptoms of ADHD (Holstein et al., 2013) in adults and schizophrenia patients (Braff and Geyer, 1990; Geyer et al., 2001). Prepulse inhibition is a well-validated operational measure of sensorimotor gating in humans and animals (Geyer et al., 2001) that is found to be disrupted in Gpr88−/− mice (Logue et al., 2009). Beyond the widespread whole-brain functional disconnections detected in schizophrenic patients (Liang et al., 2006; Welsh et al., 2010), several studies report specific rsFC disruption in DMN and sensorimotor networks (Kaufmann et al., 2015; Tang et al., 2012) similar to our findings in mice lacking the GPR88 receptor. These connectivity features are suggestive of inhibitory control deficits, hyperactivity, and impulsivity symptoms, as seen in human ADHD (Choi et al., 2013; McLeod et al., 2014; Mostert et al., 2016; Tian et al., 2008). Most remarkably, the cortico-striatal connectivity (particularly MO–striatum FC) is strongly perturbed in Gpr88−/− mice, congruent to the cardinal resting-state network perturbations observed in ADHD (Castellanos and Proal, 2012; Castellanos et al., 2006; Oldehinkel et al., 2016).
Striatal Gpr88−/− FC changes are paralleled by structural modification of the striato-cortical pathways (Fig. 6), revealed in our study through DTI and high-resolution fiber mapping (Harsan et al., 2013). This modified striatocortical circuitry structure (especially the CP-MO altered component) is consistent with rsFC data and may underpin the hyperactive behavior described in the Gpr88−/− model (Meirsman et al., 2016a; Quintana et al., 2012). The involvement of Gpr88 in shaping striatal connections and therefore underlying hyperactive features is supported by the most abundant expression of the receptor in this brain area, including CP (Ghate et al., 2007; Mizushima et al., 2000) and ACB.
Our analysis also indicates that amygdala connectivity to somatosensory and motor cortical area as well as caudate-putamen is weakened in Gpr88 knockout mice. This observation is consistent with the notion that amygdala might now drive increased risk-taking (RT) behavior in a potentially dangerous environment, leading to an apparent reduced anxiety in Gpr88−/− mice (Meirsman et al., 2016b). This phenotype was previously associated with altered gene transcription, dopamine levels, and neuronal morphology in amygdala (Alisch et al., 2014; Yokoyama et al., 2005) and consistent with the rsFC patterns reported in young adult RT behavior (Cox et al., 2010; DeWitt et al., 2014; Touroutoglou et al., 2014). In addition, hypersynchrony of the BOLD signal in the striatum of Gpr88−/− mice, as observed in our study (Fig. 4a, b), has been reported earlier as a major contributor to adolescent RT behavior (Galvan, 2010).
The striatum, densely populated by GPR88 receptors in normal animals, showed perturbed functional cross talk not only with the hippocampus but also the prefrontal cortex, limbic area, and MB in Gpr88−/− mice. Additionally, HPF rsFC was altered. These striatal and hippocampal FC perturbations might underlie the modified learning phenotype of Gpr88−/− mice, observed in a behavioral test that specifically addresses the striatum-hippocampus balance in learning (Meirsman et al., 2016a; Quintana et al., 2012). The dorsal striatum (CP), a major hub of the basal ganglia network, is involved in several functional domains, including learning, cognition, and motivation (Mestres-Missé et al., 2012; Miyachi et al., 2002; Yin et al., 2009). In rodents, CP lesions disrupt acquisition of habits and impair goal-directed learning (Yin et al., 2004, 2005). Human neuroimaging studies also report the involvement of CP activity in the development of habits and goal-directed behavior (Liljeholm et al., 2011; Tanaka et al., 2008; Tricomi et al., 2009). Hippocampus, on the other hand, plays crucial roles in working and episodic memory (Aggleton and Brown, 2006; Cabeza and Nyberg, 2000). Our study therefore reveals a particular deficit in the striatal-hippocampal dialog at the neural network level, which likely explains the preferred allocentric (hippocampus) versus egocentric (striatum) behavioral strategy adopted by mutant mice in performing the cross-maze task (Meirsman et al., 2016a).
Thus far, the intrinsic functional architecture revealed spatially distinct brain regions that exhibit altered rsFC in the Gpr88−/− mice, concordant with hyperactive characteristics of mutant mice and evocative of connectivity alterations observed in ADHD patients. In humans, disrupted functional communications between brain regions is often accompanied by microstructural abnormalities in the white matter, which are thought to contribute to behavioral functioning in ADHD (Nagel et al., 2011). Therefore, we also measured the microstructural integrity in the Gpr88−/− mouse brain through DTI and fiber tractography (Harsan et al., 2013) and mapped the FD, one of the primary indices used to quantitatively measure structural integrity (Konrad and Eickhoff, 2010; Nagel et al., 2011), with diffusion-based tractography. FD values were significantly higher in Gpr88−/− mice (two-sample t-test, p < 0.05, FWE corrected) particularly along the striato-cortical pathway (Fig. 6) linking the striatum (CP) and cortical areas, like MO and SS. Former studies report disturbed structural connectivity of the cortico-striatal network in both adults and children with ADHD in comparison with healthy subjects (Konrad and Eickhoff, 2010; Tamm et al., 2012). Taken together, functional and structural connectivity modifications in the sensorimotor and cortico-striatal circuitry observed in Gpr88−/− mice are consistent with the prevailing neurobiological hypothesis of ADHD, which identifies these networks as a probable substrate for cognitive and behavioral impairments seen in ADHD patients (Bush et al., 2005; Castellanos and Proal, 2012; Castellanos et al., 2006; Holstein et al., 2013; Oldehinkel et al., 2016; van Ewijk et al., 2012).
Conclusion
In conclusion, we provide here the first evidence of Gpr88 involvement in reshaping the mouse brain functional and structural circuitry. The remodeled network architecture and topology underlie the large behavior deficits described in mice lacking a functional GPR88 receptor. Our study therefore suggests GPR88 as an influential player in brain connectivity and likely a susceptibility gene for psychiatric conditions in humans.
Supplementary Material
Acknowledgments
This project was funded with support from the NeuroTime Erasmus+: Erasmus Mundus program of the European Commission. This publication/communication reflects the views only of the author, and the Commission cannot be held responsible for any use that may be made of the information contained therein. This study was supported by the ATHOS Consortium (Fonds Unique Interministériel, Région Alsace, Domain Therapeutics Illkirch, France, and Prestwick Chemicals Illkirch, France). The authors also thank the National Institutes of Health (NIH-NIAAA No. 16658 and NIH-NIDA No. 005010) for financial support. The authors acknowledge the funding grants from Brain Links Brain Tools (BLBT) cluster of excellence from Freiburg (MouseNet 31 project).
Author Disclosure Statement
No competing financial interests exist.
References
- Aggleton JP, Brown MW. 2006. Interleaving brain systems for episodic and recognition memory. Trends Cogn Sci 10:455–463 [DOI] [PubMed] [Google Scholar]
- Alisch RS, Chopra P, Fox AS, Chen K, White ATJ, Roseboom PH, et al. 2014. Differentially methylated plasticity genes in the amygdala of young primates are linked to anxious temperament, an at risk phenotype for anxiety and depressive disorders. J Neurosci 34:15548–15556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JaJ, Befort K, Blad C, Filliol D, Ghate A, Dembele D, et al. 2008. Transcriptome analysis identifies genes with enriched expression in the mouse central extended amygdala. Neuroscience 156:950–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Brady RO, Tandon N, Masters GA, Margolis A, Cohen BM, Keshavan M, Öngür D. 2016. Differential brain network activity across mood states in bipolar disorder. J Affect Disord 207:367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. 1990. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry 47:181–188 [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. 2005. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol. Psychiatry 57:1273–1284 [DOI] [PubMed] [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA. 1990. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. Am J Psychiatry 147:1308–1312 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. 2000. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12:1–47 [DOI] [PubMed] [Google Scholar]
- Cabral J, Kringelbach ML, Deco G. 2014. Exploring the network dynamics underlying brain activity during rest. Prog Neurobiol 114:102–131 [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. 2001. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. 2008. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 63:332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. 2012. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci 16:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. 2006. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci 10:117–123 [DOI] [PubMed] [Google Scholar]
- Choi J, Jeong B, Lee SW, Go HJ. 2013. Aberrant development of functional connectivity among resting state-related functional networks in medication-naïve ADHD children. PLoS One 8:e83516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Gotimer K, Roy AK, Castellanos FX, Milham MP, Kelly C. 2010. Your resting brain CAREs about your risky behavior. PLoS One 5:e12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Zompo M, Deleuze JF, Chillotti C, Cousin E, Niehaus D, Ebstein RP, et al. 2014. Association study in three different populations between the GPR88 gene and major psychoses. Mol Genet Genomic Med 2:152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt SJ, Aslan S, Filbey FM. 2014. Adolescent risk-taking and resting state functional connectivity. Psychiatry Res Neuroimaging 222:157–164 [DOI] [PubMed] [Google Scholar]
- Di X, Biswal BB. 2014. Modulatory interactions between the default mode network and task positive networks in resting-state. PeerJ 2:e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F. Seifritz E, Formisano E, Morrone R, Scarabino T, Tedeschi G, Cirillo S, Goebel R, Di Salle F. 2003. Real-time independent component analysis of fMRI time-series. Neuroimage 20:2209–2224 [DOI] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TGC, Mills KL, et al. 2010. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry 68:1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. 2009. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res 1273:114–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711 [DOI] [PubMed] [Google Scholar]
- Galvan A. 2010. Adolescent development of the reward system. Front Hum Neurosci 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. 2007. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry 164:450–457 [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. 2001. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl.) 156:117–154 [DOI] [PubMed] [Google Scholar]
- Ghate A, Befort K, Becker JAJ, Filliol D, Bole-Feysot C, Demebele D, et al. 2007. Identification of novel striatal genes by expression profiling in adult mouse brain. Neuroscience 146:1182–1192 [DOI] [PubMed] [Google Scholar]
- Grandjean J, Azzinnari D, Seuwen A, Sigrist H, Seifritz E, Pryce CR, Rudin M. 2016. Chronic psychosocial stress in mice leads to changes in brain functional connectivity and metabolite levels comparable to human depression. Neuroimage 142:544–552 [DOI] [PubMed] [Google Scholar]
- Harsan LA, Dávid C, Reisert M, Schnell S, Hennig J, von Elverfeldt D, Staiger JF. 2013. Mapping remodeling of thalamocortical projections in the living reeler mouse brain by diffusion tractography. Proc Natl Acad Sci U S A 110:E1797–E1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg J, Hyvärinen A, Esposito F. 2004. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22:1214–1222 [DOI] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos-Quiroga JA, Richarte Fernández V, Bosch R, Soliva JC, et al. 2014. An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Hum Brain Mapp 35:1261–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein DH, Vollenweider FX, Geyer MA, Csomor PA, Belser N, Eich D. 2013. Sensory and sensorimotor gating in adult attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res 205:117–126 [DOI] [PubMed] [Google Scholar]
- Hübner N, Mechling AE, Lee HL, Reisert M, Bienert T, Hennig J, et al. 2017. The connectomics of brain demyelination: functional and structural patterns in the cuprizone mouse model. Neuroimage 146:1–18 [DOI] [PubMed] [Google Scholar]
- Hyvärinen A, Oja E. 2000. Independent component analysis: algorithms and applications. Neural Netw 13:411–430 [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Skåtun KC, Alnæs D, Doan NT, Duff EP, Tønnesen S, et al. 2015. Disintegration of sensorimotor brain networks in schizophrenia. Schizophr Bull 41:1326–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. 2010. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp 31:904–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. 2012. The neural correlates of subjective pleasantness. Neuroimage 61:289–294 [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445:168–176 [DOI] [PubMed] [Google Scholar]
- Li Y-O, Adali T, Calhoun VD. 2007. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp 28:1251–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. 2006. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport 17:209–213 [DOI] [PubMed] [Google Scholar]
- Liang Z, King J, Zhang N. 2011. Uncovering intrinsic connectional architecture of functional networks in awake rat brain. J Neurosci 31:3776–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeholm M, Tricomi E, O'Doherty JP, Balleine BW. 2011. Neural correlates of instrumental contingency learning: differential effects of action-reward conjunction and disjunction. J Neurosci 31:2474–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska A, Galbusera A, Schwarz AJ, Gozzi A. 2015. Functional connectivity hubs of the mouse brain. Neuroimage 115:281–291 [DOI] [PubMed] [Google Scholar]
- Logue SF, Grauer SM, Paulsen J, Graf R, Taylor N, Sung MA, et al. 2009. The orphan GPCR, GPR88, modulates function of the striatal dopamine system: a possible therapeutic target for psychiatric disorders? Mol Cell Neurosci 42:438–447 [DOI] [PubMed] [Google Scholar]
- Ma Z, Perez P, Ma Z, Liu Y, Hamilton C, Liang Z, Zhang N. 2016. Functional atlas of the awake rat brain: a neuroimaging study of rat brain specialization and integration. Neuroimage. pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Böttger J, Long X, Lv Y, Kelly C, Schäfer A, Goldhahn D, Abbushi A, Milham MP, Lohmann G, Villringer A. 2010. Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. MAGMA 23:289–307 [DOI] [PubMed] [Google Scholar]
- Massart R, Mignon V, Stanic J, Munoz-Tello P, Becker JAJ, Kieffer BL, et al. 2016. Developmental and adult expression patterns of the G-protein-coupled receptor GPR88 in the rat: establishment of a dual nuclear-cytoplasmic localization. J Comp Neurol 524:2776–2802 [DOI] [PubMed] [Google Scholar]
- McLeod KR, Langevin LM, Goodyear BG, Dewey D. 2014. Functional connectivity of neural motor networks is disrupted in children with developmental coordination disorder and attention-deficit/hyperactivity disorder. Neuroimage Clin 4:566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechling AE, Arefin T, Lee HL, Bienert T, Reisert M, Ben Hamida S, et al. 2016. Deletion of the mu opioid receptor gene in mice reshapes the reward-aversion connectome. Proc Natl Acad Sci U S A 113:11603–11608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechling AE, Hübner NS, Lee HL, Hennig J, von Elverfeldt D, Harsan LA. 2014. Fine-grained mapping of mouse brain functional connectivity with resting-state fMRI. Neuroimage 96:203–215 [DOI] [PubMed] [Google Scholar]
- Meirsman AC, Le Merrer J, Pellissier LP, Diaz J, Clesse D, Kieffer BL, Becker JAJ. 2016a. Mice lacking GPR88 show motor deficit, improved spatial learning, and low anxiety reversed by delta opioid antagonist. Biol Psychiatry 79:917–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirsman AC, Robé A, de Kerchove d'Exaerde A, Kieffer BL. 2016b. GPR88 in A2AR neurons enhances anxiety-like behaviors. eNeuro 3 DOI: 10.1523/ENEURO.0202-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestres-Missé A, Turner R, Friederici AD. 2012. An anterior-posterior gradient of cognitive control within the dorsomedial striatum. Neuroimage 62:41–47 [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. 2002. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res 146:122–126 [DOI] [PubMed] [Google Scholar]
- Mizushima K, Miyamoto Y, Tsukahara F, Hirai M, Sakaki Y, Ito T. 2000. A novel G-protein-coupled receptor gene expressed in striatum. Genomics 69:314–321 [DOI] [PubMed] [Google Scholar]
- Moritz CH, Carew JD, McMillan AB, Meyerand ME. 2005. Independent component analysis applied to self-paced functional MR imaging paradigms. Neuroimage 25:181–192 [DOI] [PubMed] [Google Scholar]
- Mostert JC, Shumskaya E, Mennes M, Onnink AMH, Hoogman M, Kan CC, et al. 2016. Characterising resting-state functional connectivity in a large sample of adults with ADHD. Prog Neuropsychopharmacol Biol Psychiatry 67:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Bathula D, Herting M, Schmitt C, Kroenke CD, Fair D, Nigg JT. 2011. Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50:283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel M, Beckmann CF, Pruim RHR, van Oort ESB, Franke B, Hartman CA, et al. 2016. Attention-deficit/hyperactivity disorder symptoms coincide with altered striatal connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging 1:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF. 2010. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res 183:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Sanz E, Wang W, Storey GP, Güler AD, Wanat MJ, et al. 2012. Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue-dependent behaviors. Nat Neurosci 15:1547–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. 2015. The brain's default mode network. Annu Rev Neurosci 38:433–447 [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert M, Mader I, Anastasopoulos C, Weigel M, Schnell S, Kiselev V. 2011. Global fiber reconstruction becomes practical. Neuroimage 54:955–962 [DOI] [PubMed] [Google Scholar]
- Richiardi J, Altmann A, Milazzo AC, Chang C, Chakravarty MM, Banaschewski T, et al. 2015. Correlated gene expression supports synchronous activity in brain networks. Science 348:1241–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawiak SJ, Wood NI, Williams GB, Morton AJ, Carpenter TA. 2013. Voxel-based morphometry with templates and validation in a mouse model of Huntington's disease. Magn Reson Imaging 31:1522–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforazzini F, Schwarz AJ, Galbusera A, Bifone A, Gozzi A. 2014. Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. Neuroimage 87:403–415 [DOI] [PubMed] [Google Scholar]
- Simon SS. 2008. Merging of the senses. Front Neurosci 2:13–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. 2013. Resting-state fMRI in the Human Connectome Project. Neuroimage 80:144–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. 2007. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev 31:977–986 [DOI] [PubMed] [Google Scholar]
- Sun L, Cao Q, Long X, Sui M, Cao X, Zhu C, et al. 2012. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naïve boys with attention deficit hyperactivity disorder. Psychiatry Res 201:120–127 [DOI] [PubMed] [Google Scholar]
- Tamm L, Barnea-Goraly N, Reiss AL. 2012. Diffusion tensor imaging reveals white matter abnormalities in Attention-Deficit/Hyperactivity Disorder. Psychiatry Res 202:150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SC, Balleine BW, O'Doherty JP. 2008. Calculating consequences: brain systems that encode the causal effects of actions. J Neurosci 28:6750–6755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wang L, Cao F, Tan L. 2012. Identify schizophrenia using resting-state functional connectivity: an exploratory research and analysis. Biomed Eng Online 11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Ge T, Glahn DC, Jahanshad N, Nichols TE. 2013. Genetics of the connectome. Neuroimage 80:475–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Jiang T, Liang M, Zang Y, He Y, Sui M, Wang Y. 2008. Enhanced resting-state brain activities in ADHD patients: a fMRI study. Brain Dev 30:342–348 [DOI] [PubMed] [Google Scholar]
- Touroutoglou A, Bickart KC, Barrett LF, Dickerson BC. 2014. Amygdala task-evoked activity and task-free connectivity independently contribute to feelings of arousal. Hum Brain Mapp 35:5316–5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, et al. 2008. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods 169:249–254 [DOI] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Prvulovic D, Roeder CH, Linden DEJ. 2004. Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum Brain Mapp 22:165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. 2013. Cartography and connectomes. Neuron 80:775–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. 2012. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev 36:1093–1106 [DOI] [PubMed] [Google Scholar]
- Van Waes V, Tseng KY, Steiner H. 2011. GPR88- a putative signaling molecule predominantly expressed in the striatum: cellular localization and developmental regulation. Basal Ganglia 1:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhong S, Jia Y, Sun Y, Wang B, Liu T, et al. 2016. Disrupted resting-state functional connectivity in nonmedicated bipolar disorder. Radiology 280:529–536 [DOI] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. 2010. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull 36:713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. 2012. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76 [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. 1996. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4:58–73 [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. 2004. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci 19:181–189 [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilário MRF, Clouse E, Holloway T, Davis MI, et al. 2009. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci 12:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. 2005. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 22:513–523 [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Suzuki E, Sato T, Maruta S, Watanabe S, Miyaoka H. 2005. Amygdalic levels of dopamine and serotonin rise upon exposure to conditioned fear stress without elevation of glutamate. Neurosci Lett 379:37–41 [DOI] [PubMed] [Google Scholar]
- Zerbi V, Grandjean J, Rudin M, Wenderoth N. 2015. Mapping the mouse brain with rs-fMRI: an optimized pipeline for functional network identification. Neuroimage 123:11–21 [DOI] [PubMed] [Google Scholar]
- Zhang N, Rane P, Huang W, Liang Z, Kennedy D, Frazier JA, King J. 2010. Mapping resting-state brain networks in conscious animals. J Neurosci Methods 189:186–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Zhuang Y, Gong H, Wang B, Wang X, Chen Q, et al. 2014. Altered inter-subregion connectivity of the default mode network in relapsing remitting multiple sclerosis: a functional and structural connectivity study. PLoS One 9:e101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.