Abstract
Background: Available glucagon formulations are approved for immediate use after reconstitution for severe hypoglycemia emergency treatment. However, they are used in dual-hormone artificial pancreas (insulin and glucagon) studies through subcutaneous infusion pumps over 24 h. Chemical and physical stability of such glucagon use have not been reported in a comprehensive manner.
Materials and Methods: Recombinant Glucagon DNA (Eli Lilly) was used. Compatibility and sterility of glucagon delivery through subcutaneous pump systems were verified. Glucagon degradation through liquid chromatography with tandem mass spectrometry (LC-MS/MS), fibrillation using intrinsic tryptophan fluorescence shift, and bioactivity through a cell-protein kinase A-based fluorescent bioassay were assessed over a range of different physical conditions (temperature, movement, and air bubbles).
Results: Subcutaneous infusion pump systems administered glucagon in sterile conditions and with comparable accuracy to insulin delivery; mean absolute relative difference of actual versus expected weights were 1.2% ± 1.1% for glucagon and 1.1% ± 0.5% for insulin (P = 0.9). In comparison to freshly reconstituted samples, glucagon analyzed through LC-MS/MS was intact at 93.0% ± 7.0% after 24 h (P = 0.42) and 83.04% ± 6.0% after 48 h (P = 0.02) of incubation in pumps at 32°C. Peak wavelengths for Trp fluorescence did not differ from samples exposed to air bubbles or movement whether incubated (in infusion sets for 24 h at 32°) immediately or 24- and 48-h poststorage at 4°C (P = 0.10, 0.70 and 0.80, respectively) and no significant differences in bioactivity (shifts in EC50) were found for the same conditions (P = 0.13, 0.83, and 0.63).
Conclusion: Available glucagon formulations are chemically and physically stable, as well as compatible with delivery through subcutaneous infusion pumps over 24 h and can be used in long-term clinical trials.
Keywords: : Glucagon formulation, Artificial pancreas, Diabetes
Introduction
Glucagon is recently emerging with new clinical uses, reviving the interest in this long ago discovered hormone. In response to hypoglycemia, glucagon is known to counteract the effect of insulin mainly in the liver by increasing hepatic glucose production.1 Up to now, its use has been limited to the treatment of severe hypoglycemia. However, with the development of the dual-hormone artificial pancreas (AP), a new use of glucagon is proposed; it is administered as mini-boluses to prevent and/or treat any type of imminent hypoglycemia.2 Dual-hormone AP (insulin and glucagon) has proved to be beneficial in decreasing time in hypoglycemia and improving overall glucose control with a possible added benefit in comparison to single-hormone AP (insulin only).2–6
Commercially available glucagon formulations have been reserved for emergency treatment of severe hypoglycemia with immediate use after reconstituting the lyophilized glucagon powder in an acidic diluent, discarding any leftovers.7,8 The recommendation for instantaneous use is driven by the chemical and physical instability of glucagon during extended use.9 Glucagon is not soluble in neutral solutions and unstable physically (formation of fibrils and gels that could be cytotoxic) and chemically (degradation through deamidation and oxidation) in aqueous solutions.10–12 The pH of the medium can accelerate either fibrillation when in acidic ranges or degradation under alkaline conditions.13,14 In addition to the nature of the solution and its pH, other physical conditions such as temperature and movement could also affect glucagon stability.14,15
Dual-hormone AP studies have so far only used the approved emergency glucagon kits. However, in contrast to the approved one time shot, the 1 mg/mL glucagon solution is delivered as mini-boluses over 24 h postreconstitution through insulin infusion pump sets.9 While the clinical efficacy of this glucagon use has been shown by the conducted clinical trials, its chemical and physical stability over this time period have not been reported. Such information is needed to endorse the security and efficacy of this glucagon use and consequently facilitate the task of different research groups in advancing this technology. The search for new stable glucagon formulations and analogs is under way, but not fast enough to escort the ongoing shift toward long-term dual-hormone AP trials.13,16–19 The safety and efficacy of current and new formulations, as well as glucagon analogs will also need to be tested in human trials before larger long-term applications.20
We therefore report experiments undertaken to examine different security and efficacy aspects of current glucagon formulations in dual-hormone AP studies. Compatibility and sterility of glucagon delivery through subcutaneous insulin infusion pump systems were verified. Glucagon degradation through liquid chromatography with tandem mass spectrometry (LC-MS/MS), its fibrillation using intrinsic tryptophan fluorescence shift, and its bioactivity through in vitro cell-based bioassay were also assessed in an acidic medium over a range of different physical conditions, including temperature, movement, and presence of air bubbles.
Materials and Methods
Materials
Glucagon kits (Eli Lilly, Canada Toronto, ON, Canada) were used. Each kit consists of a vial containing 1 mg of lyophilized glucagon powder (rDNA origin) and 49.0 mg of lactose, and a syringe with 1 mL diluent (Hyporet® HY7530; 1.2% glycerin, pH 2.8). For all experiments, glucagon reconstitution was carried out according to manufacturer's recommendation. Insulin aspart (Novorapid, Novo Nordisk Mississauga, ON, Canada) was used for the infusion system compatibility experiment. Two types of subcutaneous infusion pump systems were used: from Medtronic (Northridge, CA, including Minimed Paradigm Veo pump, 3 mL cartridge, and Silhouette Paradigm 60 cm et 17 mm infusion set) and from Roche (Indianapolis, IN, including Accu-Chek Spirit Combo Insulin pump, 3.15 mL cartridge and Tender 80 cm et 13 mm infusion set).
Compatibility and sterility of subcutaneous infusion pump systems
Actual and expected weights of glucagon delivered into vials through two different types of subcutaneous infusion pump systems were compared. The same experiments were carried out using insulin as a control. Pumps were programmed to deliver hormones as boluses (12 boluses of 0.5 units each, 10 boluses of 1.0 units, and 5 boluses of 3.0 units) or as continuous infusion (6.0 units infusion over 4 h). Experiments were carried out on Medtronic and Roche subcutaneous pump infusion systems separately.
Sterility testing according to United States Pharmacopeia USP <71> guidelines21: a growth promotion test was first conducted to validate the inability of glucagon to inhibit the growth of microorganisms. Briefly, reconstituted glucagon vials (1 mL) were filtered under sterile conditions and incubated separately with <100 colony-forming units of six different microorganisms in their corresponding culture medium as detailed in Supplementary Table S2 (Supplementary Data are available online at www.liebertpub.com/dia). Test tubes without glucagon were prepared in parallel and served as positive controls. The incubation could be stopped once a comparable growth between the test and control tubes was observed. After validating the growth promotion test, a sterility test was conducted; eight glucagon vials were reconstituted, transferred into Medtronic reservoirs, and infusion sets then retrieved to be incubated with culture media (tryptic soy broth and fluid thioglycollate media) at times 0, 12, 24, and 48 h for 14 days.
Chemical degradation testing through LC-MS/MS
LC-MS/MS was used to determine the percentage of remaining glucagon, which was incubated at 32°C in Medtronic infusion sets for 24 and 48 h with shaking in reference to glucagon samples directly collected at time 0 after preparation. Shaking was in orbital motion at 60 rpm. Presence and quantification of 16 different glucagon degradation fragments that were identified under acidic solutions by Joshi et al. were also assessed.11 ([13C6] Leu14)-glucagon (Bachem, Torrance, CA) was used as an internal standard (IS). Details about IS, glucagon, and its fragments (sequence, parent and MRM MS/MS [Multiple Reaction Monitoring Mass Spectrometry] ions, etc.) are provided in Supplementary Table S1. Acquisition was performed with an ABSciex TripleTOF 5600 system (ABSciex, Foster City, CA) that is equipped with an electrospray interface with a 50 μm iD capillary and coupled to an Eksigent μUHPLC (Eksigent, Redwood City, CA). Analyst TF 1.7 software was used for instrument control and data processing and acquisition. Optimized MRM parameters were used to monitor glucagon, its degradation products, and the IS. Samples were injected by loop overfilling into a 5 μL loop. Samples were infused into MS/MS with an isocratic flow (50 μL/min) of 80% solvent A (H2O, 0.1% formic acid) and 20% solvent B (acetonitrile 100%, 0.1% formic acid) for 0.5 min. Data integration and quantification were performed with MultiQuant software (ABSciex, Foster City, CA) using the area under the curve value.
Fibrillation and bioactivity testing
Testing for fibrillation and bioactivity of glucagon was done under different physical conditions, including temperature, movement, and presence of air bubbles. In dual-hormone AP studies, less than half the glucagon vial is used per 24 h and thus a large amount of reconstituted glucagon ends up being wasted. We therefore tested the feasibility of storing part of the reconstituted glucagon at 4°C for a subsequent refill of the cartridge 24 and 48 h later. Movement was simulated by an orbital shaker at 60 rpm and bubbles were introduced through the catheter into the cartridges. A temperature of 32°C was chosen to simulate that of the skin where the pump is usually worn. A glucagon stock solution: a synthetic glucagon (100 μM Glucagon Stock, Sigma), provided as a powder was diluted in acetic acid to a pH of 2.8 and served as a positive control. Buffer reagents of each experiment with no added glucagon served as negative controls. In total, 13 conditions were tested (Supplementary Fig. S1), including a baseline glucagon sample (S0) retrieved at reconstitution, which served as a comparator. Four samples (S1–S4) were then placed in pump cartridges in an incubator at 32°C to represent the four possible combinations of presence or absence of movement and air bubbles. Glucagon vials were also reconstituted and kept at 4°C for 24 h for samples (S5–S8) and at 4°C for 48 h for samples (S9–S12) before these were eventually placed at 32°C for 24 h in (±) movement and (±) air bubble combinations (Supplementary Fig. S1).
Intrinsic tryptophan fluorescence shift assay for fibrillation: glucagon has a single tryptophan residue, Trp-25, with intrinsic fluorescence characteristics that can be used to study the formation of fibrils. The fluorescence spectrum of the Trp-25 residue typically peaks at a maximum of around 350 nm and is blue shifted to ∼320 nm when incorporated into a fibril structure.21 Samples of 100 μL were loaded in triplicates in 96-well plates and read by Perkin Elmer Envision 2104 multilabel reader spectrophotometer system (Perkinelmer, Inc., Waltham, MA) with excitation at 280 nm and emission at a spectrum ranging from 300 to 450 nm (4 nm increments), and peaks of maximum fluorescence were recorded for each sample.
In vitro cell-based assay for glucagon bioactivity: recombinant CHO-K1 cells that stably express the glucagon receptor (GenBank Acc. NM_000024) and the catalytic domain of human protein kinase A (PKA; GenBank Acc. NM_002730) fused to the N-terminus of enhanced green fluorescent protein were obtained from Thermo Scientific (Pittsburgh, PA). Activation of the glucagon receptor generates cAMP, which redistributes fluorescent PKA from concentrated granules to diffused patterns through the cytoplasm, reducing the overall fluorescent cell signal. Cells were maintained in Ham's F-12 culture medium supplemented with 1% penicillin–streptomycin, 1 mg/mL zeocin, 0.5 mg/mL G418, and 10% fetal bovine serum (FBS) at 37°C, 5% CO2, and 95% humidity. They were incubated overnight in black 96-well plates at a density of 10,000 cells/well and were exposed the next morning to serially diluted glucagon for 30 min at 37°C, 5% CO2 (diluting assay buffer consisted of Ham's F12, 10% FBS, 1% penicillin/streptomycin, 10 mM HEPES, and 0.25% DMSO [dimethyl sulfoxide]). Cells were then fixed in 10% formalin for 20 min, washed with PBS, and labeled with 1 μM Hoechst staining solution. ImageXpress Micro confocal high content imaging system (Molecular Devices, Sunnyvale, CA) was used to capture the images and Multiwavelength Cell Scoring module through MetaXpress 3.1. Software (Molecular Devices, Sunnyvale, CA) was used for fluorescence quantification. A decrease in fluorescence is a measure of the cellular response to glucagon stimulation.
Statistical analysis
Data are presented as mean ± standard deviation where applicable. GraphPad Prism 7.01 software (GraphPad Software, La Jolla, CA) was used for statistical analysis and plotting. Means were compared by t-test and ANOVA. For bioactivity testing, dose–response curves were generated using nonlinear regression (log [dose] vs. response-variable slope curves) with calculation of half maximal effective concentration EC50. Slopes and log EC50 were also compared by extra sum-of-squares F test. A p-value <0.05 was considered a statistically significant result. All experiments were repeated 2–4 times.
Results
Glucagon delivery is sterile and comparable to insulin infusion
Glucagon met the validity tests of growth promotion and showed comparable growth patterns of the different tested microorganisms to control test tubes lacking glucagon (Supplementary Table S2). At a subsequent step, glucagon vials that were reconstituted, transferred into cartridges, and retrieved through infusion sets at times 0, 12, 24, and 48 h were all found to be sterile after 14 days of incubation in appropriate media (Supplementary Table S2). Subcutaneous infusion pump systems delivered glucagon with acceptable accuracy that is comparable to insulin delivery; mean absolute relative difference of actual versus expected weights were 1.2% ± 1.1% for glucagon and 1.1% ± 0.5% for insulin (P = 0.9). Details of weight measurements through different infusion systems are provided in Supplementary Table S3.
Glucagon degradation is minimal after 24 h
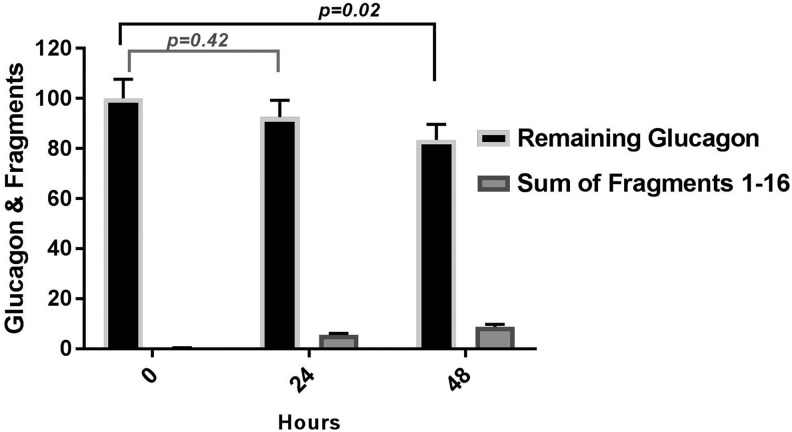
In comparison to reconstituted samples at time 0, glucagon was intact at 93.0% ± 7.0% after 24 h (P = 0.42) and at 83.04% ± 6.0% 48 h later (P = 0.02). We measured the accumulation of each glucagon fragment, # 1–16 (described in Supplementary Table S1). The most abundant fragments (# 1, 3, 14, and 15) were the by-products of aspartyl peptide cleavage. Four of the reported fragments could not be detected in the degradation process (# 6, 7, 11, and 12). The rest were by-products of either glutaminyl deamidation or aspartyl peptide cleavage, or both processes concurrently. We present the remaining glucagon and the sum of all its observed fragments in Figure 1. To note that the fragment intensity is a relative value with the possibility of certain fragments ionizing better than glucagon itself, which may overrepresent the reality. Also, the observed intensity of each degradation fragment was very low (Supplementary Fig. S2); therefore, the documented fragments may not be the sole source of glucagon diminution in time, while adsorption and precipitation could be other likely explanations.
FIG. 1.
Degradation of glucagon kept in infusion pump sets at 32°C with movement for 24 and 48 h using LC-MS/MS, n = 3. LC-MS/MS, liquid chromatography with tandem mass spectrometry.
Fibrillation is minimal and bioactivity is preserved for glucagon with its current use in the AP system
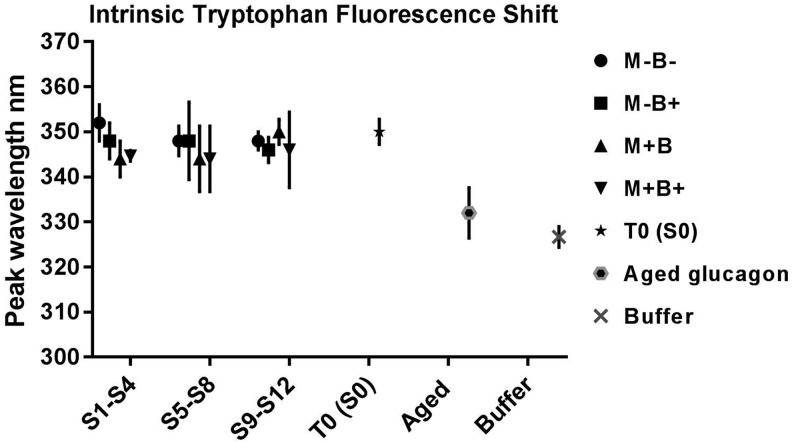
Peaks for Trp intrinsic fluorescence ranged from 344 to 352 nm for all tested conditions (S0–S12 detailed in Supplementary Fig. S1). While blue shifts to peaks of 334 ± 2.8 nm were observed with aged glucagon samples that were kept for 30 days at 4°C. Reagent buffer solutions without added glucagon (negative controls) also showed blue shifts to peaks of 326.7 ± 2.3 nm (Fig. 2). Peak wavelengths did not differ significantly for S1–S4, S5–S8, and S9–S12, when each set of samples was compared to reconstituted glucagon at T0 (S0), P = 0.10, 0.70, and 0.80, respectively, but differed significantly in comparison to aged glucagon (30 days at 4°C) and negative control, P = 0.001, 0.003, and 0.002, respectively (Fig. 2).
FIG. 2.
Intrinsic tryptophan fluorescence shift assay. Peak wavelengths for glucagon samples tested at different physical conditions. M is for movement and B is for air bubbles. Samples are incubated in infusion pump sets for 24 h at 32°C immediately postreconstitution for S1–S4, 24 h postincubation at 4°C for S5–S8, and for 48 h postincubation at 4°C for S9–S12. Aged glucagon is kept for 30 days at 4°C, n = 3.
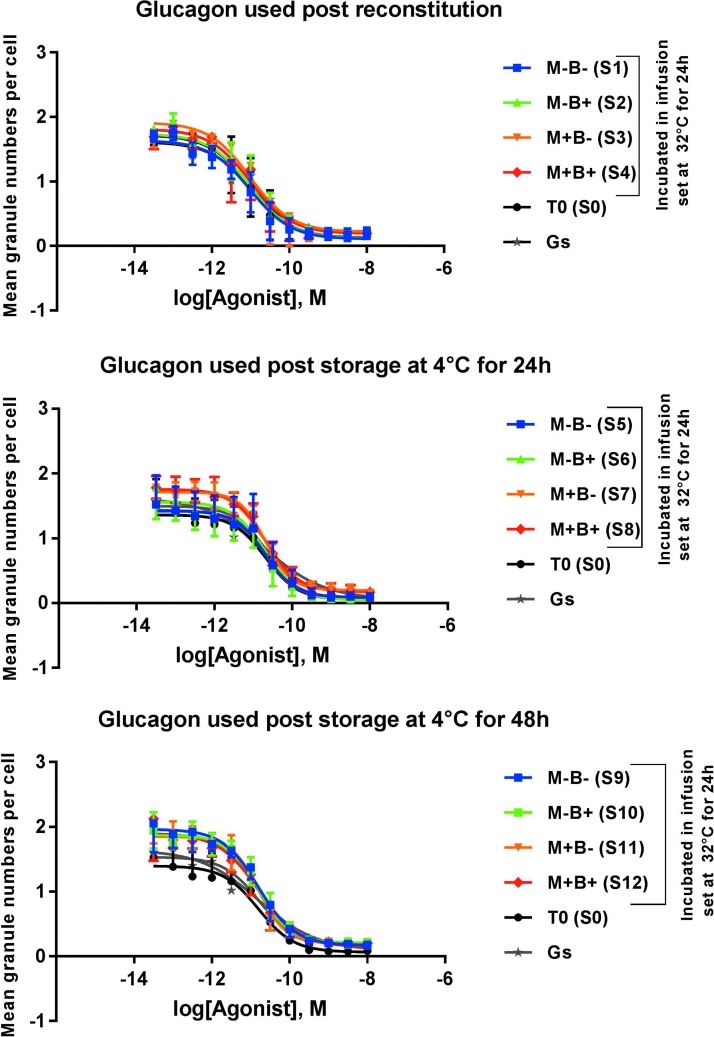
In the cellular based assay for glucagon receptor activation, exposure of glucagon samples to air bubbles or movement in infusion pump cartridges kept for 24 h at 32°C did not adversely affect its bioactivity. No significant shifts in EC50 or slopes of the corresponding dose–response curves of these conditions were found in comparison to glucagon stock or freshly reconstituted lyophilized glucagon, P = 0.13 (Fig. 3). No differences in bioactivity were either observed when the different conditions were tested using glucagon samples kept for 24 h (P = 0.83) or 48 h (P = 0.63) at 4°C after reconstitution (Fig. 3). Individual EC50 comparison is also provided in Supplementary Figure S3. Note that negative controls (buffer solutions without glucagon) showed responses that are similar to those observed at the plateau of maximal dilutions of the glucagon samples.
FIG. 3.
In vitro cellular based bioactivity assay. Dose–response curves for glucagon samples tested at different physical conditions. M is for movement and B is for air bubbles, n = 4.
Discussion
Dual-hormone AP systems that incorporate glucagon to further prevent and treat hypoglycemia are shifting toward long-term clinical testing (weeks to months). New glucagon formulations or analogs that would be stable for extended use are either under development or awaiting the results of adequate clinical studies.13,16,18,19 Meanwhile, this report confirms the ability of insulin infusion pump sets to deliver accurate glucagon micro-boluses, the physical and the chemical stability (sterility, fibrillation, and degradation), and the preserved bioactivity of the commercially available glucagon formulation for the intended dual-hormone AP clinical use.
Two different commonly used infusion pump sets, in our study, delivered glucagon adequately with comparable precision to insulin infusion. Following standard USP modalities, the sterility of glucagon delivery was also confirmed for up to 48 h in the infusion pump. USP specifications for glucagon use (in emergency kits) require a content that is between 65% and 110% of the labeled amount.22 In our study, we have showed a minimal degradation at the end of 24 h with over 90% remaining glucagon. Although 83% of the glucagon was intact after 48 h in infusion pumps, which would still be considered acceptable by USP specifications, this difference was statistically significant. However, more importantly, the USP specifications apply to the 1 mg dose of emergency kit glucagon, which leads to supraphysiologic levels. In contrast, mini-boluses of glucagon are administered (microgram range) in the context of the AP.9 Therefore, we recommend against keeping glucagon for 48 h in the infusion pumps since a narrower range for degradation should be accepted with mini-dosing. The majority of the degradation fragments described by Joshi et al. at acidic pH were detected except for four fragments.11 This can be due to differences in temperature settings with a higher heat stress (60°C) resulting in more degradation by-products. Note that degradation may not be the sole reason for glucagon loss. Adsorption and precipitation are potential players that would be more pronounced at 48 h as well (Fig. 2).
Glucagon is known to fibrillate under acidic conditions, possibly interfering with pump delivery, but most importantly increasing the potential for cytotoxicity.12,13,23 For up to 24 h in infusion pumps, glucagon did not show any gel formation to the naked eye or any signs of significant fibrillation through Trp fluorescence shift assays under any of the tested physical conditions, including storage at 4°C for 24 or 48 hrs before use. Glucagon forms random coils of α-helices in aqueous solutions that would transition to β-sheet structures with time.21 Several methods in addition to Trp shift assay have been suggested for peptide fibrillation assessment such as transmission electronic microscopy (TEM), thioflavin T binding assay, and circular dichroism analysis, among others.24 Often a combination of different methods are used in extensive studies dedicated solely to studying peptide fibrillation processes.21,24 Advanced visual assays like TEM may be more precise at detecting first signs of fibrillation than fluorescence shift assays.16 While we have relied on Trp shift assay, which may have missed some degree of fibrillation, our results are congruent with a study by Onoue et al. who used different methods, including TEM.12 Authors have shown that in acidic solutions, glucagon would fibrillate quickly at concentrations exceeding 2.5 mg/dL, while it was free of fibrils for up to 24 h at 1 mg/dL. With extended aging, glucagon would fibrillate regardless of lower concentrations.12
Both fibrillation and degradation are expected to affect the bioactivity of glucagon. In our study, despite showing no significant fibrillation or degradation after 24 h of incubation in an infusion set, the preservation of glucagon potency in these settings needs to be confirmed. Previous studies failed to show a decrease in bioactivity when fibrillated glucagon was used in animals (commercial glucagon formulation aged up to 7 days).25,26 A shift in bioactivity was, however, observed in cellular bioassays of glucagon degraded at alkaline pH.10 This was mostly marked for fragments deamidated on Gln 3 and/or isomerized on Asp 9, while it was unpredictable for other degradation fragments.10 Our spectrometry analysis detected fragments with Gln 3 deamidation (fragment 10), but at very low levels (Supplementary Fig. S2). The PKA cell-based assay used in our study cannot differentiate the effect of fibrillation or degradation separately, but is a proven useful tool to assess overall glucagon potency.13,27 We have tested physical conditions that could possibly affect glucagon bioactivity: temperature of 32°C to simulate a skin temperature close to which the pump is worn, movement to imitate a person's activity, and air bubbles in the setting of an infusion set kept for 24 h. All of these did not affect glucagon bioactivity (Fig. 3). We have further demonstrated that storing part of the reconstituted glucagon at 4°C for 24 and 48 h before use in the pump did not affect its bioactivity. Over half of the 1 mg glucagon reconstituted from an emergency kit is often wasted since only intermittent mini-boluses are administered in the AP.9 Our results therefore suggest the possibility of minimizing glucagon wasting and cutting down the high cost associated with glucagon use in long-term research trials.
Conclusion
Awaiting stable and clinically safe new glucagon formulations, this report confirms the stability of commercially available glucagon when used for 24 h under different physical conditions associated with use in the AP setting. The possibility of storing reconstituted glucagon at 4°C for 24 and 48 h before pump use has also been demonstrated, which could potentially downsize the costs associated with glucagon use in the long-term clinical trials.
Supplementary Material
Acknowledgment
We would like to acknowledge the guidance of Dr. W. Kenneth Ward on the choice of the cell-based bioassay and the assistance of our laboratory members: Mrs. Diane Mignault, Mrs. Annie Tardif, and Mrs. Melika Forde Lewis. We equally acknowledge PhenoSwitch Bioscience Inc., QC, Canada, and Sterigen Inc., QC, Canada, for their assistance in spectrometry and sterility analyses, respectively. This study was supported by funds from Canadian Diabetes Association and NIH Grant No. 1DP3DK106930-01, Fondation J-A De Sève Chair held by R.R.-L., and Canadian Institutes of Health Research, and Fonds de Recherche Santé Québec scholarships held by N.T. Glucagon and insulin were in-kind contributions from Eli Lilly, Canada, and Novo Nordisk, respectively.
Author Disclosure Statement
R.R.-L. has received consultant's or speaker's honorariums or grants from Abbott, Amgen, AstraZeneca, Becton Dickinson, Boehringer Ingelheim, Carlina Technology, Eli Lilly, Janssen, Lifescan, Medtronic, Merck, Novartis, Neomed, Novo Nordisk, Roche, Sanofi-Aventis, Takeda, and Valeant; R.R.L. has also a patent for type 2 diabetes biomarkers, extending life of catheters, and intellectual property for AP. The other authors declare no competing interests.
References
- 1.Schade DS, Woodside W, Eaton RP: The role of glucagon in the regulation of plasma lipids. Metabolism 1979;28:874–886 [DOI] [PubMed] [Google Scholar]
- 2.Thabit H, Hovorka R: Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia 2016;59:1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haidar A, Legault L, Matteau-Pelletier L, et al. : Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:595–604 [DOI] [PubMed] [Google Scholar]
- 4.Haidar A, Legault L, Messier V, et al. : Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol 2015;3:17–26 [DOI] [PubMed] [Google Scholar]
- 5.Haidar A, Rabasa-Lhoret R, Legault L, et al. : Single- and dual-hormone artificial pancreas for overnight glucose control in type 1 diabetes. J Clin Endocrinol Metab 2016;101:214–223 [DOI] [PubMed] [Google Scholar]
- 6.Taleb N, Emami A, Suppere C, et al. : Comparison of two continuous glucose monitoring systems, Dexcom G4 Platinum and Medtronic Paradigm Veo Enlite System, at rest and during exercise. Diabetes Technol Ther 2016;18:561–567 [DOI] [PubMed] [Google Scholar]
- 7.GLUCACON (rDNA Origin) Product Monograph: Glucagon for Injection, rDNA origin, 1 mg glucagon per vial. ©Eli Lilly Canada Inc., Toronto, Ontario; submission control no. 139332; approved July 9, 2012; 32 pp [Google Scholar]
- 8.GLUCAGEN® HYPOKIT Product Monograph: GLUCAGEN® and GLUCAGEN® HYPOKIT, 1 mg. Novo Nordisk Canada Inc., Mississauga, Ontario; submission control no. 192245; approved June 1, 2016; pp. 1–36 [Google Scholar]
- 9.Haidar A, Smaoui MR, Legault L, Rabasa-Lhoret R: The role of glucagon in the artificial pancreas. Lancet Diabetes Endocrinol 2016;4:476–479 [DOI] [PubMed] [Google Scholar]
- 10.Caputo N, Castle JR, Bergstrom CP, et al. : Mechanisms of glucagon degradation at alkaline pH. Peptides 2013;45:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi AB, Rus E, Kirsch LE: The degradation pathways of glucagon in acidic solutions. Int J Pharm 2000;203:115–125 [DOI] [PubMed] [Google Scholar]
- 12.Onoue S, Ohshima K, Debari K, et al. : Mishandling of the therapeutic peptide glucagon generates cytotoxic amyloidogenic fibrils. Pharm Res 2004;21:1274–1283 [DOI] [PubMed] [Google Scholar]
- 13.Jackson MA, Caputo N, Castle JR, et al. : Stable liquid glucagon formulations for rescue treatment and bi-hormonal closed-loop pancreas. Curr Diab Rep 2012;12:705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen JS, Flink JM, Dikov D, Otzen DE: Sulfates dramatically stabilize a salt-dependent type of glucagon fibrils. Biophys J 2006;90:4181–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steiner SS, Li M, Hauser R, Pohl R: Stabilized glucagon formulation for bihormonal pump use. J Diabetes Sci Technol 2010;4:1332–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakhtiani PA, Caputo N, Castle JR, et al. : A novel, stable, aqueous glucagon formulation using ferulic acid as an excipient. J Diabetes Sci Technol 2015;9:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caputo N, Jackson MA, Castle JR, El Youssef , et al. Biochemical stabilization of glucagon at alkaline pH. Diabetes Technol Ther 2014;16:747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pohl R, Li M, Krasner A, De Souza E: Development of stable liquid glucagon formulations for use in artificial pancreas. J Diabetes Sci Technol 2015;9:8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newswanger B, Ammons S, Phadnis N, et al. : Development of a highly stable, nonaqueous glucagon formulation for delivery via infusion pump systems. J Diabetes Sci Technol 2015;9:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taleb N, Haidar A, Messier V, et al. : Glucagon in artificial pancreas systems: potential benefits and safety profile of future chronic use. Diabetes Obes Metab 2017;19:13–23 [DOI] [PubMed] [Google Scholar]
- 21.Moorthy BS, Ghomi HT, Lill MA, Topp EM: Structural transitions and interactions in the early stages of human glucagon amyloid fibrillation. Biophys J 2015;108:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.USP 37-NF 32. Official monographs: glucagon for injection. 2014:3158–3159
- 23.Pedersen JS: The nature of amyloid-like glucagon fibrils. J Diabetes Sci Technol 2010;4:1357–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghodke S, Nielsen SB, Christiansen G, et al. : Mapping out the multistage fibrillation of glucagon. FEBS J 2012;279:752–765 [DOI] [PubMed] [Google Scholar]
- 25.El-Khatib FH, Jiang J, Gerrity RG, Damiano ER: Pharmacodynamics and stability of subcutaneously infused glucagon in a type 1 diabetic Swine model in vivo. Diabetes Technol Ther 2007;9:135–144 [DOI] [PubMed] [Google Scholar]
- 26.Ward WK, Massoud RG, Szybala CJ, et al. : In vitro and in vivo evaluation of native glucagon and glucagon analog (MAR-D28) during aging: lack of cytotoxicity and preservation of hyperglycemic effect. J Diabetes Sci Technol 2010;4:1311–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almholt K, Tullin S, Skyggebjerg O, et al. : Changes in intracellular cAMP reported by a Redistribution assay using a cAMP-dependent protein kinase-green fluorescent protein chimera. Cell Signal 2004;16:907–920 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.