Abstract
Although autologous bone grafts are considered a gold standard for the treatment of bone defects, they are limited by donor site morbidities and geometric requirements. We propose that tissue engineering technology can overcome such limitations by recreating fully viable and biological bone grafts. Specifically, we will discuss the use of bone scaffolds and autologous cells with bioreactor culture systems as a tissue engineering paradigm to grow bone in vitro. We will also discuss emergent vascularization strategies to promote graft survival in vivo, as well as the role of inflammation during bone repair. Finally, we will highlight some recent advances and discuss new solutions to bone repair inspired by endochondral ossification.
Keywords: : bone, tissue engineering, biomimetics
Introduction
In the United States alone, there are 6 million bone fractures per year, and 5–10% of these fractures suffer incomplete healing due to bone loss, failed fixation, infection, and inadequate vascularization.1 Current treatment that involves autografts harvested from other locations in the body has drawbacks, including limited tissue supply, donor site morbidity, infections, and poor integration. Allografts are also widely used, but have been associated with disease transmission and host rejection.2
Tissue engineering provides an important treatment alternative using the patient's own cells in combination with a scaffold for bone repair. Common scaffolding materials include natural and synthetic polymers such as poly (lactic-co-glycolic acid) (PLGA), poly (caprolactone) (PCL), and silk,3–7 and bioceramics such as tricalcium phosphate (TCP) and hydroxyapatite (HAp) resembling the mineral phase in bone.8,9 Decellularized bone (DCB), used in the clinic for bone repair, has also gained interest as bone scaffold due to its native matrix and unique osteoinductive properties.10,11 Biomimicry can guide the development of bone grafts in vitro to promote safe and effective repair. Biomimetic approaches to engineer bone have taken cues from the native bone structure and milieu and involve the use of specialized scaffolds.
In this review, we examine the status of biomimetic approaches for bone tissue engineering (Fig. 1). We first discuss the fabrication of synthetic scaffolds mimicking the native bone, the use of decellularized tissue scaffolds, the cell sources, and bioreactor systems for cultivation of bone grafts in vitro. Next, we discuss the importance of angiogenesis in bone repair and current strategies to promote scaffold vascularization that is critical for establishing blood supply to the bone graft upon implantation. Finally, we discuss how endochondral ossification of engineered cartilaginous template is inspiring a new generation of bone grafts.
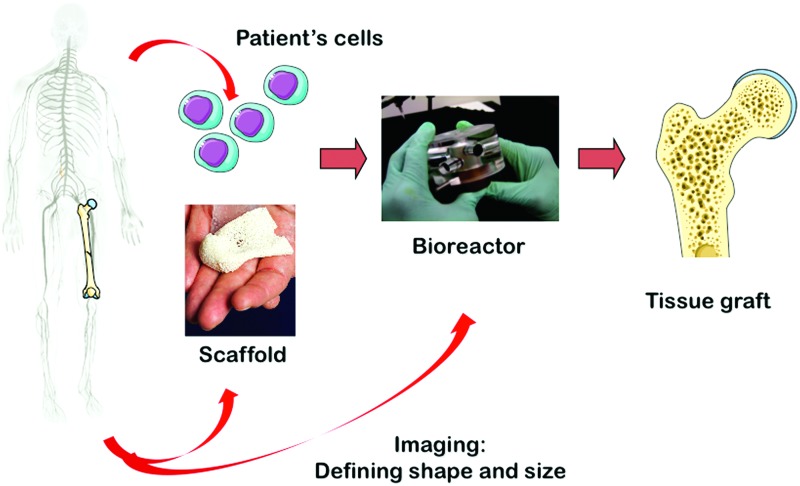
FIG. 1.
The bone tissue engineering paradigm. Bone scaffold, autologous stem cells, and bioreactor culture systems enable the growth of autologous bone grafts in vitro for bone repair applications in vivo. Color images available online at www.liebertpub.com/teb
The Bone Tissue Engineering Paradigm: Scaffold, Cells, and Bioreactor
Developments in fabrication of synthetic bone scaffolds
Scaffold design to support bone formation largely determines the success of bone regeneration. Scaffold material and design in tissue engineering play key roles by providing a suitable structure, mechanical function, and delivery of bioactive factors. In an excellent review of scaffold design for tissue engineering, Hollister said, “The art of scaffolding is where to put the holes and the biofactors,” paraphrasing architect Robert le Ricolais who said, “The art of structure is where to put the holes.”12 Thus, scaffold design must entail the creation of hierarchical porous structures resembling the native tissue to provide mechanical support, mass transport, and incorporation of biomolecules for recruiting cells and directing bone formation.
Synthetic and natural polymers such as PCL and type I collagen, as well as bioceramics such as calcium phosphates (CaPs) and bioactive glasses, have been used to fabricate scaffolds for bone tissue engineering.7,13–15 These materials recapitulate the organic and inorganic phases of the native bone that confer toughness, strength, and osteoconductivity. Although synthetic polymers can form scaffolds with interconnected pores, they lack the mechanical property and osteoconductivity of native bone.
In contrast, scaffolds fabricated from ceramics such as Hap and TCP are osteoconductive and have compressive strengths that are comparable to that of the native bone.14,16 However, ceramic scaffolds lack interconnected pores and are prone to fracture. For these reasons, some investigators have attempted to reinforce ceramic scaffolds with polymers such as PCL and collagen,17,18 whereas others have used natural composite material such as the coral exoskeleton with an inorganic CaP phase growing onto an organic template.19 Ceramic scaffolds are slow to be resorbed in vivo and lack biological factors present in the native bone to promote robust osteogenic differentiation.
Conventional methods to create pores in scaffolds include salt leaching, gas forming, phase separation, and freeze-drying.20 However, these methods offer limited control over the scaffold microarchitecture. Recent advances in additive manufacturing have enabled the creation of scaffolds with precise features and pore structures by 3D printing.18,21,22 Bioceramics have also been successfully incorporated into ink mixtures that are compatible with 3D printing by photopolymerization or extrusion. However, many 3D printed ceramic composite scaffolds require high temperature processing, which then precludes incorporation of biological factors. Still, some noteworthy progress has been made recently in bridging the gap between additive manufacturing and clinical translation.
In one study, a bone scaffold mixture comprising PCL/TCP and a cell-laden hydrogel were alternately printed by extrusion to form a calvarial bone construct that supported bone regeneration in a rat model.23 In another study, HA combined with PCL and PLGA binders and other solvents to form a mixture that printed into a hyperelastic bone scaffold by extrusion. The hyperelastic bone scaffold resisted fracture and permanent deformation under high-impact loads, supported osteogenesis in vitro, and promoted spinal fusion in a rat model, as well as calvarial regeneration in a macaque model.22
Unique benefits of DCB as a scaffold
Despite the many advances in fabrication of synthetic scaffold, DCB remains an attractive and popular choice of scaffold for bone regeneration. The many qualities of DCB include its hierarchical porous structure, mechanical competence, osteoinductivity, and osteoconductivity. Interestingly, most modern research into the use of DCB for bone repair dates back to the seminal work by Urist in the 1960s, who first demonstrated the osteoinductive nature of bone matrix. The inductive agents were subsequently identified as potent trophic factors called bone morphogenetic proteins (BMP), which enhance and regulate cartilage and bone formation.24,25 BMP-2 has since been isolated and become widely used in research and clinic for bone regeneration.26 Scaffold mineralization of DCB was later shown to significantly affect the osteogenesis in vitro.27 Importantly, bone matrix derived from DCB has also been shown to impart osteoconductivity to collagen matrix.28 Together, the endogenous trophic factors and mineral and matrix components within the DCB support bone formation.
Currently, multiple companies are using proprietary methods to test, sterilize, and decellularize bone allografts for clinical use. Notable FDA-approved DCB products include GrafTech®, GraftCage®, BTB Select®, BioCAP Select™, MatriGRAFT®, and ReadiGRAFT®. Proprietary mixes comprising matrices derived from demineralized DCB have also been approved and such products include Grafton®, Osteofil®, ALLOMATRIX®, AlleGro®, Optium DBM®, and OsteoBiologics I/C Graft Chamber®. The large number of DCB products suggests that DCB can be derived safely from animal or cadaveric sources.29
Still, cellular grafts were shown to result in better bone regeneration than acellular controls in several autologous implantation models.30 It is likely that the constituent cells and biological factors are necessary to enhance osteogenesis at the site of bone defect. Thus, personalized bone grafts generated from DCB and autologous cells could provide an effective alternative to autografts for bone reconstruction.
Sources of autologous cells for bone formation
Osteoprogenitor cells have been isolated from adult bone tissue and periosteum, by preparation of explant cultures from dissected tissues, or enzymatic release of progenitor cells from endosteal and periosteal layers.31,32 Such primary human bone and periosteal cells formed bone-like tissues when cultured on porous scaffolds, confirming their osteogenicity.33
Due to their multipotency and proliferative ability, adult mesenchymal stem cells present a more attractive cell source and have been widely used for tissue engineering. Found in a variety of tissues, including the bone marrow, adipose tissue, synovium, and dental pulp, they can reach up to 50 population doublings and are capable of differentiating into bone, cartilage, tendon, ligament, muscle, and adipose cells.
Among the different adult stem cell sources, bone marrow mesenchymal stem cells (BMSCs) isolated from bone marrow stroma are the most studied source for bone regeneration. These cells are commonly isolated based on adherence and growth on tissue culture plastics or immunoselected by characteristic markers. The number of BMSCs within each bone marrow aspirate varies among patients (0.001–0.01% of the nucleated marrow cells), and there is a need for expansion in culture to reach clinically relevant numbers for therapeutic purposes.34,35 Many groups have demonstrated the osteogenic potential of BMSCs and their usefulness toward bone tissue engineering.6,10,36 When differentiated in porous scaffolds under osteoinductive conditions, these cells can form bone-like tissues.
Adipose-derived stem cells (ASCs) were discovered more recently and quickly became an attractive cell source for bone tissue engineering due to their abundance in readily accessible lipoaspirates and their ability to differentiate into multiple lineages.37,38 Depending on patients, several liters of lipoaspirate containing a relatively high frequency of ASCs (1–5% of isolated nucleated cells) can be obtained. Isolation protocols involve density gradient centrifugation of collagenase-digested tissue (lipoaspirate or minced adipose) followed by selection and culture of adherent cell populations. The formation of bone-like tissue from ASCs cultured on scaffolds has already been reported separately.39–42
Bioreactor systems for bone tissue engineering
A key limitation in the development of bone tissues of clinically relevant sizes in vitro is the insufficient transport of nutrients and oxygen. To overcome such transport limitations, bioreactor systems have been developed to support the culture of large engineered bone tissues in vitro.6,10,36,39,41–51 These systems are summarized in Table 1. Rotating wall vessels, spinner flasks, perfusion bioreactors, and compression bioreactors have been used to culture engineered bone in vitro to different effects. In general, rotating wall vessels and spinner flasks support the formation of smaller bone constructs and are limited by suboptimal transport inside the construct core.52 It is also unclear whether these systems promote robust osteogenic differentiation or improvement in bone formation over static culture.
Table 1.
Summary of Bioreactor Systems for Bone Tissue Engineering
| System | Cells | Scaffold | Outcomes (vs. static) | Source |
|---|---|---|---|---|
| Rotating wall vessel | BMSCs | Gelatin-HA | ↑Proliferation | (43) |
| ASCs | ZrO2 based ceramic/HAp | ↑Proliferation, Differentiation | (41) | |
| BMSCs | Gelatin-Coral/Hap | N.A. | (44) | |
| Spinner flasks | ASCs | PET | ↑Proliferation | (42) |
| BMSCs | Collagen | ↑Differentiation | (45) | |
| BMSCs | Silk | ↑Proliferation, Differentiation | (46) | |
| BMSCs | Silk | N.A. | (6) | |
| BMSCs | Gelatin-HA | ↑Proliferation, Differentiation | (43) | |
| BMSCs | Chitosan | ↑Proliferation, Differentiation | (47) | |
| Perfusion | Osteoblasts | Titanium | ↑Proliferation, Differentiation | (48) |
| BMSCs | Titanium/MSC-ECM | N.A. | (36) | |
| BMSCs | Si-TCP/HAp-TCP | ↑Proliferation, Differentiation | (49) | |
| BMSCs | DCB | ↑Proliferation, Differentiation | (10) | |
| BMSCs | DCB | ↑Proliferation, Differentiation | (50) | |
| ASCs | DCB | ↑Proliferation, Differentiation | (39) | |
| ASCs | DCB | N.A. | (51) |
ASC, Adipose-derived stem cell; BMSC, bone marrow mesenchymal stem cell; DCB, decellularized bone; ECM, extracellular matrix; HA, hyaluronic acid; HAp, hydroxyapatite; PET, polyethylene terephthalate; Si-TCP, silicate substituted TCP; TCP, tricalcium phosphate; ZrO2, zirconium dioxide; N.A., not applicable; ↑, increase.
Instead, perfusion bioreactors enable optimal transport throughout the constructs and provide biophysical stimulation to bone-forming cells.49,53 In native bone, mechanosensitive osteocytes enable adaptation to mechanical loading. Through the lacuna-canalicular network, osteocytes communicate with osteoblasts, osteoclasts, and osteoprogenitor cells using paracrine signaling to induce bone formation or resorption.53 Fluid shear stress also modulates the release of nitric oxide (NO) and upregulation of prostaglandin (PG) by osteoblasts and mediates bone maintenance.54,55 More recently, stem cells have also been shown to be mechanosensitive and capable of undergoing mechanically induced osteogenic differentiation. BMSCs and ASCs cultured under flow stimulation upregulated the expression of osteogenic markers and increased deposition of bone matrix proteins.56,57 Mechanotransduction during osteogenic differentiation involve multiple pathways that include calcium signaling and components of the mitogen-activated protein kinase (MAPK)/ERK, Wnt, Hippo, and RhoA/ROCK pathways.58 Mechanosensors, in particular, the primary cilia, have been shown to directly mediate flow-induced osteogenic differentiation of BMSCs.56
In one study, a perfusion system enabled the cultivation of up to six tissue constructs simultaneously, with equal flow rate in all scaffolds, for a wide range of flow rates and scaffold densities (Fig. 2A–E).10 When cultured under perfusion, hMSCs and hASCs proliferated and formed spatially uniform bone on DCB scaffolds.10,39 Shear forces associated with perfusion resulted in the upregulated expression of osteogenic genes and improved bone formation over static culture. Flow velocities within the cultured tissues ranging from 400 to 800 μm/s resulted in the most dense and homogenous matrix deposition.59 Interestingly, flow perfusion synergized with native bone-like matrix to enhance osteogenic differentiation of MSCs on titanium fiber mesh.36 However, the enhancement was abolished upon denaturation of the matrix. Thus, the flow dependence of bone formation within a perfusion bioreactor is complex and could involve the geometry, internal architecture, and cellular maturity of the construct.
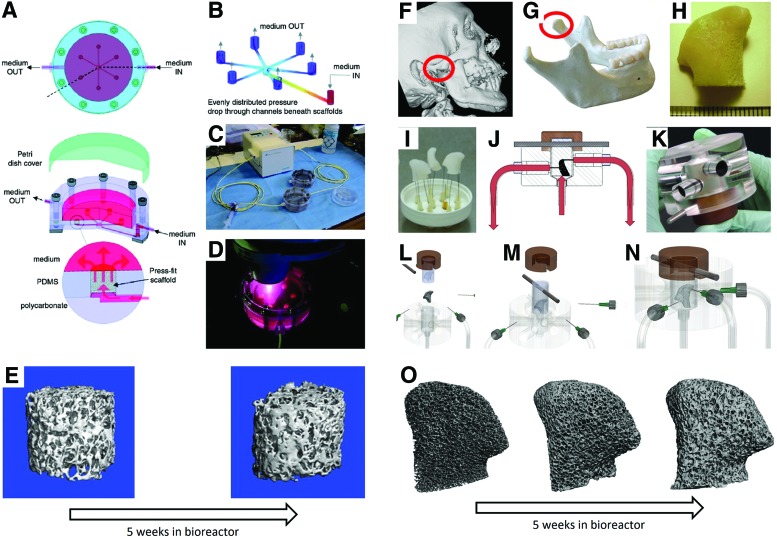
FIG. 2.
The multichamber perfusion bioreactor and an anatomically shaped bioreactor for engineering anatomically shaped osteochondral grafts. Schematic of the bioreactor (A–D) and representative μCT images of a recellularized scaffold cultured over a period of 5 weeks (E). μCT images of the jaw-bone defect used for 3D reconstruction (F, G) and milling of a decellularized bone scaffold into a desired shape (H). Syringes function as channels for perfusion flow of media (E) through the scaffold in the bioreactor (I–N). Representative μCT images of a recellularized scaffold cultured over a period of 5 weeks (O). Reproduced with permission from Grayson et al. (10) (A–E) and (50) (F–O). Color images available online at www.liebertpub.com/teb
Bioreactor for personalized bone reconstruction
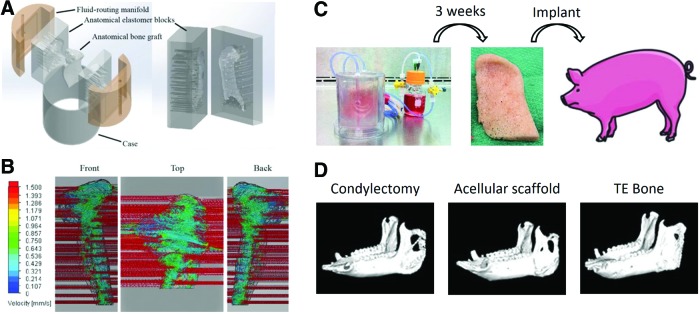
To achieve clinical relevance, the grafts also need to match the defect in terms of shape, architecture, and biomechanical properties. The maintenance of cellularity in large, anatomically shaped bone grafts in vitro necessitates a biomimetic scaffold-bioreactor system. With such a system, the formation of a clinically sized, anatomically shaped, and viable human temporomandibular joint (TMJ)50,51 has been reported. Using computer aided design (CAD) guided by digitized micro computed tomography (μCT) images, TMJ shaped DCB scaffolds and elastomer blocks with corresponding chambers were generated. The scaffolds were seeded with BMSCs and press fitted in the elastomer blocks fitted with channels for controllable perfusion throughout the constructs (Fig. 2F–O). After 5 weeks of culture, fully viable and anatomically shaped bone grafts with physiologic cell density and uniform bone matrix were formed.50 Using a similar system, autologous bone grafts grown from ASCs enhanced regeneration of the ramus–condyle unit (RCU) following condylectomy in a porcine model (Fig. 3).51 The engineered RCU promoted near-complete bone regeneration after 6 months of implantation, whereas acellular scaffolds and untreated controls resulted in extensive fibrous tissue formation at the site of defect.
FIG. 3.
Advanced bioreactor design for culturing anatomically shaped bone grafts. Channels were designed into a customized manifold to optimize perfusion flow throughout the scaffold (A, B). Seeded TMJ grafts were cultured in a bioreactor for 3 weeks and implanted in pigs for 6 months following condylectomy (C). Tissue engineered (TE) bone improved TMJ repair versus empty and acellular controls (D). Reproduced with permission from Bhumiratana and Vunjak-Novakovic (30). TMJ, temporomandibular joint. Color images available online at www.liebertpub.com/teb
Together, the osteoinductive capacity of the DCB, the osteogenic capacity of various adult stem cells (hMSCs, hASCs), and the physiologic benefits of perfusion flow contribute to a comprehensive paradigm of bone tissue engineering (Fig. 1). This paradigm heralds the next generation of autologous bone grafts for bone reconstruction. Major challenges in reconstructing large bone defects remain, such as the ability to prevascularize the graft and establish blood perfusion immediately following implantation to maintain graft viability. In the next section, we discuss the importance of vascularization and current strategies to vascularize bone grafts.
Strategies to Promote Vascularization
Importance of angiogenesis in bone development and repair
Bone development in the limb bud is concomitant with angiogenesis. Flat bones develop by a process called intramembranous ossification, in which MSCs differentiate directly into osteoblasts as capillaries invade from the surrounding tissue.60 The osteoblasts secrete bone matrix nodules and eventually fuse to form woven bone. Long bones develop through endochondral ossification, in which MSCs differentiate into chondrocytes to form a cartilaginous anlage to guide vascularization. Infiltrating blood vessels bring osteoprogenitor cells and recruit osteoclasts to degrade the cartilaginous template, which is then replaced by bone.60–63
Cross talk between endothelial cells (ECs) and osteoblasts is essential for bone formation, acting through the secretion of paracrine factors, especially vascular endothelial growth factor (VEGF), platelet-derived growth factor-BB (PDGF-BB), and BMP.60,61,64 VEGF stimulates the migration of ECs and the formation of an immature vascular network.65 PDGF-BB stabilizes the blood vessels by recruiting pericytes that wrap around the blood vessels and prevent them from regressing.66 The rate of ossification depends on vascularization, and only the cells that are near capillaries contribute to bone formation.61,67
Bidirectional signaling between bone cells and cells of the vasculature plays a major role in bone homeostasis and function.60,61 Bone is a highly vascularized tissue, with most cells positioned within 100 μm of the nearest capillary.65 Blood vessels transport oxygen, nutrients, and cells to bone tissue and are essential for bone viability and health.61 Obstruction of the blood supply often results in skeletal pathologies such as osteonecrosis and osteoporosis.60
Bone repair is also dependent on the blood vessel network.68 Upon disruption to the circulation, bone fracture triggers inflammation.69 Pro-inflammatory signals, tumor necrosis factor-α (TNFα) and interleukin-1β (IL1β), recruit cells for bone formation.70 Osteoclasts degrade necrotic tissue, while osteoprogenitor cells form new bone.71 Clinically, vascular comorbidity is a major risk factor for delayed or nonunion fracture healing.72 Experimentally, stimulation of neovascularization was shown to recover bone healing in hind limb ischemia.73
Prevascularization of bone grafts
Given the importance of angiogenesis in bone development and repair, a successful bone tissue engineering strategy should also address the challenge of vascularization. The coupling of osteogenesis and angiogenesis during skeletal development is well documented.74,75 Cross talk between ECs and osteoprogenitor cells through paracrine signaling and cell–cell contact also enhanced bone formation in vitro.76–79 Importantly, prevascularization of grafts could help graft survival by anastomosis with host vasculature and rapid establishment of blood supply within the graft upon implantation. Thus, investigators have been pursuing different strategies to vascularize bone tissues grown in vitro.
To prevascularize the bone grafts, some investigators chose to include native vessels and vascular bundles such as the femoral artery and vein, the carotid artery, jugular vein, or saphenous bundle in osteoconductive scaffolds.80–82 Following implantation, de novo bone deposition and neovascularization within the grafts were observed.
Other investigators instead chose to vascularize grafts by seeding ECs with perivascular cells and allowing the ECs to form microcapillary-like networks within the grafts.83,84 However, anastomosis between the preformed microvascular structures and host vasculature was limited in some cases. Interestingly, vascular network formation was observed in bone tissue comprising DCB seeded with osteogenically differentiated MSCs and undifferentiated MSCs and ECs in hydrogels.85 The undifferentiated MSCs in the hydrogel assumed roles of perivascular cells and formed more mature vascular networks with the ECs. Upon implantation, the ECs seeded with MSCs resulting in dense and persistent neovasculature that anastomosed with the host vasculature over time. In another 3D coculture study, Hedgehog signaling modulated the morphology of vasculature in vitro and extent of ectopic bone formation in vivo.86 Activation of Sonic Hedgehog (Shh) in the ECs in aggregate culture with MSCs resulted in more perfused lumens and increased formation of mature bone tissue in vivo.
Taken together, modulating the behavior ECs and MSCs in vitro could lead to potent graft vascularization strategies. While it is evident that prevascularization of bone grafts could enhance graft survival and de novo bone formation in vivo, we still need a better understanding of the bioactive factors and cellular responses to control vascularization in vitro more effectively.
Delivery of angiogenic factors
The delivery of pro-angiogenic factors for bone tissue engineering has been extensively investigated. Various combinations of VEGF, PDGF-BB, fibroblast growth factor (FGF), and BMP have been shown to enhance both vascularization and bone formation in vivo.87–92 Notably, growth factors engineered to bind extracellular matrix (ECM) proteins strongly improved tissue repair and reduced unwanted side effects.93 Sequential application recapitulating the temporally defined sequence of VEGF and PDGF-BB activity during angiogenesis could also enhance blood vessel formation.87,94 The delivery of PDGF-BB enhanced vascularization and bone formation in an ovariectomized mouse model of postmenopausal osteoporosis.95 This finding is important because most early tissue engineering studies are conducted in relatively healthy animals, and the patients are likely to have significant comorbidities that will affect bone regeneration.
Moreover, changes in the concentrations, timing, or spatial distribution of angiogenic growth factors during development can result in vascular abnormalities,65 highlighting the importance of careful control over the release of angiogenic factors. Excessive VEGF can lead to leaky vessels that are prone to regression.96,97 Instead, VEGF engineered to bind strongly to ECM proteins reduced vascular permeability.93 Still, it is not possible to engineer a system that fully recapitulates the myriad of growth factors that are highly regulated in terms of dose, timing, and localization, during normal angiogenesis. For this reason, strategies that stimulate the angiogenic properties of the host inflammatory response, which would be expected to more faithfully recapitulate the appropriate processes in angiogenesis, are being investigated.
Harnessing the Inflammatory Response
Role of inflammation in bone repair
Bone is unique in that small fractures heal perfectly, without scarring,98 while large bone defects remain a challenge.99 Therefore, strategies aimed at recapitulation of bone repair represent an attractive approach in tissue engineering.
The process of inflammation is critical for the initiation of bone healing (Fig. 4). Experimentally, removal of the inflammatory milieu contained within the fracture hematoma impairs fracture healing in animal models.100,101 Administration of the pro-inflammatory cytokine TNFα to mouse bone fractures within 24 h after injury significantly enhanced fracture healing.102 Macrophages, the primary cells of the inflammatory response, and their bone-resident cousins, osteoclasts and osteal macrophages, are essential for bone formation during repair.103 Mechanical manipulation of bone fractures in mice significantly altered the behavior of macrophages and modulated bone healing through endochondral or intramembranous ossification.104 Signals from macrophages have also been shown to directly affect skeletal cell differentiation in vitro.105–107
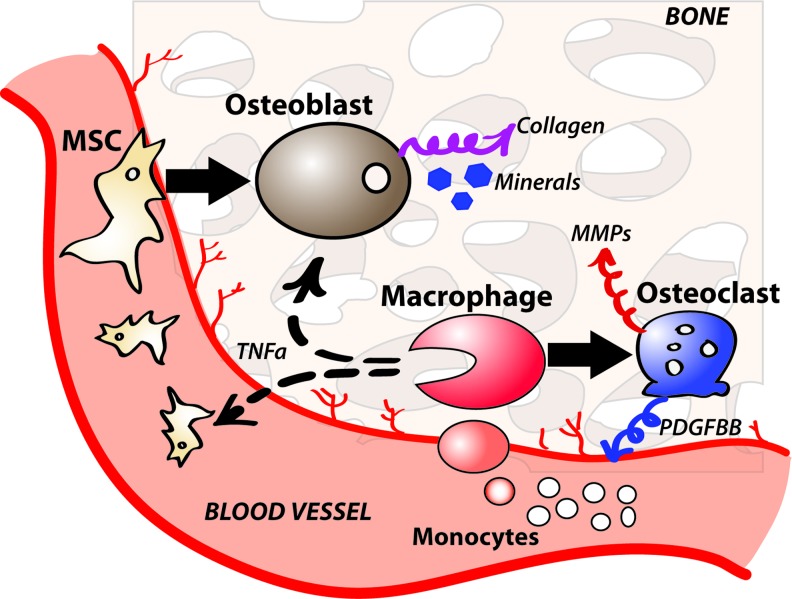
FIG. 4.
Schematics of inflammatory response during bone repair. Circulating monocytes differentiate into macrophages at the site of repair. Macrophages mediate bone formation by secreting trophic factors to promote differentiation of MSCs into osteoblasts and by differentiating into osteoclasts to remodel nascent bone. MSC, mesenchymal stem cell. Color images available online at www.liebertpub.com/teb
Moreover, prolonged inflammation beyond the initial phase (∼4 days) leads to impaired healing in bone108,109 and other tissues.110–112 Thus, inflammation must be tightly controlled, so that it initiates bone repair at early stages of injury yet subsides in a timely manner.
Role of macrophage phenotype in bone formation and angiogenesis
The importance of macrophages in angiogenesis has been demonstrated by its inhibition in animal models depleted of macrophages113–118 or increased angiogenesis when macrophages are transplanted into ischemic tissue.119,120 Preosteoclasts in developing bone in mice have been shown to release PDGF-BB, which is essential for bone vascularization and bone formation.95 Recently, the ability of macrophages to rapidly and dramatically alter their phenotype in response to changing environmental stimuli has provided further insight into the mechanisms of macrophage regulation of bone repair and angiogenesis. Classically activated M1 macrophages secrete pro-inflammatory cytokines, while alternatively activated M2 macrophages regulate the balance of ECM synthesis and remodeling.121
In the normal response to injury, M1 macrophages dominate at early stages (1–3 days) and M2 macrophages control later stages (4–10 days). M1-to-M2 transition was observed during the remodeling phase of long bone repair in a mouse osteotomy model.122 A coculture study recapitulating the transition also showed a significant improvement in osteogenesis by preosteoblastic cells in vitro.123 Instead, a study on persistent inflammation found that healing in numerous tissues was drastically impaired when the transition was disrupted.124 Similarly, elevated levels of pro-inflammatory (i.e., M1 associated) cytokines delayed and impaired healing.108,125,126 A persistent presence of M1 macrophages surrounding an implanted biomaterial at later time points (i.e., later than ∼3 days following implantation) has been associated with chronic inflammation.127–129 M1 polarization of macrophages in response to wear debris from orthopedic implants has also been linked directly to implant loosening.130 M1 polarization has also been shown to reduce the osteogenic effects of macrophages on MSC.105
The roles of different macrophage phenotypes in angiogenesis have also been widely investigated. M2 macrophages are typically described as the angiogenic phenotype,131 although most studies are focused on the M2-like tumor-associated macrophages.132 Macrophages polarized ex vivo to the M2 phenotype promoted angiogenesis subcutaneously in mice133 and in a chick chorioallantoic membrane model.134 Several studies found that M2 macrophages are associated with greater levels of blood vessel infiltration into porous biomaterial scaffolds.128,135 Interestingly, M1 macrophages have also been shown to promote angiogenesis in vitro and in vivo136,137 and affect blood vessel infiltration into scaffolds.138,139
Interestingly, human macrophages polarized to the M1 or M2 phenotypes in vitro contributed to angiogenesis in different ways (Fig. 5a).137 M1 macrophages expressed and secreted factors that promote the initiation of angiogenesis, especially VEGF. M2 macrophages secreted factors involved in later stages of angiogenesis, especially PDGF-BB, which recruits stabilizing pericytes. This study sheds light on the apparent controversy that surrounds the roles of macrophage phenotypes on angiogenesis (Fig. 5b).
FIG. 5.
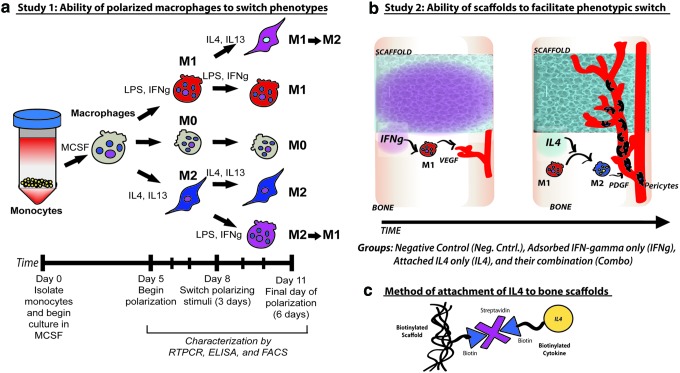
Harnessing the inflammatory response for graft vascularization by modulating macrophage phenotype. Our first study provides in vitro evidence of the phenotypic plasticity of macrophages differentiated from monocytes in peripheral blood (a). Macrophages can be differentiated into M0, M1, and M2 phenotypes and also be induced to switch between the M1 and M2 phenotypes. Schematics of how macrophages can be induced to promote scaffold vascularization. IFN-γ stimulates M1 differentiation to result in the secretion of VEGF that promotes angiogenesis. IL4 stimulates the transition of M1 to M2, which results in the secretion of PDGF and recruitment of pericytes for vasculature stabilization (b). For sequential release of IFN-γ followed by IL4, IFN-γ is adsorbed to the scaffold, whereas IL4 is attached to the scaffold by biotin–streptavidin interaction (c). Reproduced with permission from reference [156]. PDGF, platelet-derived growth factor; IFN-γ, interferon-gamma; VEGF, vascular endothelial growth factor. Color images available online at www.liebertpub.com/teb
Furthermore, M2 macrophages also expressed high levels of tissue inhibitor of metalloproteinase-3 (TIMP3), which inhibits angiogenesis by blocking the actions of MMP9 and VEGF140 and preventing the release of the inflammatory cytokine TNFα.141,142 TIMP3 also stabilized vasculature formation from ECs in vitro.143 In line with these results, media conditioned by M2 macrophages inhibited in vitro sprouting of human umbilical cord-derived endothelial cells on Matrigel. In addition, human macrophages polarized to the M2c phenotype, a distinct subset of M2, promoted EC sprouting in vitro.137 M2c macrophages also secrete high levels of MMP9, which promotes angiogenesis through remodeling of the basement membrane of blood vessels and other independent signaling mechanisms.144,145 Macrophages that are positive for the M2c marker CD163 have been associated with angiogenesis in humans.146,147 Thus, it appears likely that M1 macrophages initiate angiogenesis through the release of VEGF, M2a macrophages stabilize the growing vasculature by recruiting pericytes and regulating the actions of M1 macrophages, and M2c macrophages promote vascularization through remodeling.
Controlling macrophage behavior to promote bone regeneration
Macrophages can also be actively targeted to enhance bone repair. For example, an increased ratio of M1-to-M2 macrophages was found at sites of bisphosphonate-induced osteonecrosis of the jaw in both humans and mice.148 When ex vivo programmed M2 macrophages were infused into the mice, the incidence of osteonecrosis was significantly suppressed. Blocking the pro-inflammatory cytokine IL17 also shifted the macrophage populations toward the M2 phenotype and ameliorated osteonecrosis. In another study, twice weekly administration of IL33 caused M2 polarization of osteal macrophages and inhibited bone loss in a transgenic mouse model of spontaneous joint inflammation.149 These studies suggest that strategies aimed at controlling macrophage polarization have the potential to be applied clinically for the treatment of bone disorders.
Transplantation of MSCs into bone defects predominantly enhances bone repair through immunomodulatory effects, as opposed to their direct differentiation into bone-forming cells. Interestingly, the transplantation of MSCs into rat femoral defects induced an early increase in M1 macrophage recruitment and ultimately enhanced bone healing relative to implants without MSCs, although later time points were not evaluated.150
FTY720 is an agonist of sphingosine 1-P (S1P), which stimulates M2 polarization of macrophages.151 Controlled release of FTY720 from PLGA scaffolds promoted the recruitment of macrophages, scaffold vascularization, and new bone formation in rodents.152–154 Interestingly, the cytokine profile following implantation of gelatin hydrogels that released another S1P agonist, SEW2871, was shown to be primarily M1 at 3 days following implantation and primarily M2 at 10 days, recapitulating the natural sequence observed in normal repair.155
Similarly, decellularized scaffolds can be bone to sequentially release the M1-promoting cytokine interferon-gamma (IFNγ) followed by the M2-promoting IL4 (Fig. 5b, c).156 In a subcutaneous implantation model in mice, IFNγ-releasing scaffolds were more vascularized than control scaffolds, but no effects were observed from the release of IL4 alone or in combination with IFNγ. The lack of vascularization was attributed to the overlapping and conflicting M1 and M2 signals at early time points, as confirmed in vitro by studying the responses of human macrophages to the scaffolds. Future aimed at further separating the M1 and M2 phases could improve bone regeneration outcomes in vivo. Considering the major roles of macrophages in angiogenesis and bone formation, they represent an attractive target for strategies to engineer vascularized bone.
Recapitulation of Endochondral Ossification as a Paradigm for Long Bone Repair
Another exciting direction in bone tissue engineering is inspired by the process of endochondral ossification that drives natural bone development and long bone fracture repair. Before bone formation, mesenchymal progenitors form a cartilaginous callus that serves as a bone anlage. Crucial to this process are the hypertrophic chondrocytes that trigger the transition from the soft callus to the nascent bone by provoking vascular invasion and initial bone template deposition. The use of hypertrophic chondrocytes is an attractive alternative for engineering bone grafts as they can survive in the hypoxic environments of cartilage and, thereby, withstand the time delay necessary for the vascular development they help orchestrate.
In line with this general theme, an induction regimen has been developed for differentiating MSCs into hypertrophic chondrocytes, and cartilaginous templates comprising hypertrophic chondrocytes induced from hMSCs underwent robust endochondral ossification following ectopic implantation.157,158 The usefulness of such a paradigm was confirmed in an orthotopic model, as autografts of cartilaginous callus at bone injury sites resulted in the healing of a separate nonunion bone fracture.159
Notably, endochondral ossification of engineered cartilaginous templates was accompanied by vascular invasion. The newly formed blood vessels consisted of CD31+ ECs and NG2+ pericytes, suggesting maturation of the vascular structure.160 Intriguingly, one study found that hypertrophic chondrocytes reversed differentiation upon their release from the lacunae and assumed the role of pericytes during endochondral ossification.161 Instead, other studies found that hypertrophic chondrocytes underwent transdifferentiation into osteoblasts directly or through a transient pluripotent state.159,162 Although our understanding of the underlying mechanisms is still incomplete, these studies showed that engineered cartilaginous templates comprising hypertrophic chondrocytes promote bone regeneration in vivo. This challenges previous concepts of bone tissue engineering that are focused on osteogenic induction and graft vascularization.
Putting this proposition to the test, cartilaginous templates formed from MSCs enabled the healing of critical-sized and massive femur defects in a rat model.163 Remarkably, the biomechanical strengths of the implant group reached that of a normal femur after 8 weeks, indicative of near complete healing. Although it remains to be seen if such encouraging results can be reproduced in larger animal models, this elegant study shows that a biomimetic strategy recapitulating endochondral ossification is of great interest to bone tissue engineering.
To decouple the roles of the cells and ECM within the cartilaginous template, the in vivo outcomes of devitalized cartilaginous templates and cartilage-derived matrix with or without MSCs were investigated. Whereas apoptosis-driven devitalization maintained the capacity of the cartilaginous template to undergo endochondral ossification in vivo, devitalization by freeze and thaw cycles abolished bone formation.164 This revealed the importance of ECM integrity and endogenous bioactive factors for triggering endochondral ossification in vivo. Cartilage-derived matrix underwent endochondral ossification in vivo only when seeded with MSCs, confirming the role of cells in creating a cartilaginous template suitable for endochondral ossification.165
However, it remains to be seen if MSCs from other sources can form grafts that undergo endochondral ossification. A recent study found epigenetic differences that underpin an endochondral signature in BMSCs, but a lack thereof in MSCs from other sources. When implanted without in vitro conditioning, BMSCs formed bone filled with marrow and cartilaginous remnants, whereas ASCs formed bone without marrow.166
Evidently, we are only beginning to understand how different tissue engineering components enable grafts to undergo endochondral ossification upon implantation. Still, these studies reveal the tremendous promise of this exciting new direction as they enrich the engineers' toolbox and the clinicians' options for next-generation bone reconstruction.
Conclusions and Future Directions
In summary, the bone tissue engineering paradigm comprises an osteoinductive scaffold, an osteogenic cell source, and a bioreactor to improve transport and provide biophysical stimuli. To enhance graft survival and bone regeneration in vivo, the formation of functional blood vessels perfused with blood and a positive interaction with the inflammatory response must be promoted. A particularly promising strategy is to harness the inflammatory response by encouraging the natural sequence of inflammatory and anti-inflammatory signals. Another new direction we discussed is long bone repair by mimicking endochondral ossification.
Most in vivo studies still rely on evidence gleaned from small animals that are young and healthy. Whether the implantation successes of tissue engineered bone grafts can be recapitulated in diseased and larger animals remains to be seen. Much like how the osteoinductive capacity of bone matrix led to the discovery of BMP-2, our continuous pursuit of engineering bone grafts could take lessons from the native bone. We need to refine our understanding of bone development, regeneration, and remodeling to better guide the in vitro cellular responses and the in vivo outcomes. The coupling between ECs and MSCs leading to bone formation, regulation of macrophage response during inflammation, and the processes by which a cartilaginous template becomes a mature bone are just a few important phenomena we are only beginning to understand.
Tissue engineering has the potential of providing fully biological bone grafts for bone repair, and we are currently on the verge of clinical translation. Although autografts remain the gold standard for bone repair, rapid advances in bone tissue engineering seem to be fostering translation, and the tissue-engineered bone technology could soon become clinical practice.
Acknowledgments
The authors gratefully acknowledge the funding support of A*STAR Singapore (NSS Scholarship) and NIH (grants EB002520, EB015888, DE016525, and AR061988) for this work.
Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Cheung C. The future of bone healing. Clin Podiatr Med Surg 22, 631, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betz R.R. Limitations of autograft and allograft: new synthetic solutions. Orthopedics 25, s561, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Fuchs S., Ghanaati S., Orth C., Barbeck M., Kolbe M., Hofmann A., Eblenkamp M., Gomes M., Reis R.L., and Kirkpatrick C.J. Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials 30, 526, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Santos M.I., Tuzlakoglu K., Fuchs S., Gomes M.E., Peters K., Unger R.E., Piskin E., Reis R.L., and Kirkpatrick C.J. Endothelial cell colonization and angiogenic potential of combined nano- and micro-fibrous scaffolds for bone tissue engineering. Biomaterials 29, 4306, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Meinel L., Karageorgiou V., Hofmann S., Fajardo R., Snyder B., Li C., Zichner L., Langer R., Vunjak-Novakovic G., and Kaplan D.L. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J Biomed Mater Res A 71, 25, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Hofmann S., Hagenmuller H., Koch A.M., Muller R., Vunjak-Novakovic G., Kaplan D.L., Merkle H.P., and Meinel L. Control of in vitro tissue-engineered bone-like structures using human mesenchymal stem cells and porous silk scaffolds. Biomaterials 28, 1152, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Burg K.J.L., Porter S., and Kellam J.F. Biomaterial developments for bone tissue engineering. Biomaterials 21, 2347, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Rezwan K., Chen Q.Z., Blaker J.J., and Boccaccini A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27, 3413, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Zhou J., Lin H., Fang T., Li X., Dai W., Uemura T., and Dong J. The repair of large segmental bone defects in the rabbit with vascularized tissue engineered bone. Biomaterials 31, 1171, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Grayson W.L., Bhumiratana S., Cannizzaro C., Chao P.H., Lennon D.P., Caplan A.I., and Vunjak-Novakovic G. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A 14, 1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urist M.R. Bone: formation by autoinduction. Science 150, 893, 1965 [DOI] [PubMed] [Google Scholar]
- 12.Hollister S.J. Porous scaffold design for tissue engineering. Nat Mater 4, 518, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Woodruff M.A, and Hutmacher D.W. The return of a forgotten polymer-Polycaprolactone in the 21st century. Prog Polym Sci 35, 1217, 2010 [Google Scholar]
- 14.Johnson A.J.W, and Herschler B.A. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater 7, 16, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Roohani-Esfahani S.I., Nouri-Khorasani S., Lu Z.F., Appleyard R.C., and Zreiqat H. Effects of bioactive glass nanoparticles on the mechanical and biological behavior of composite coated scaffolds. Acta Biomater 7, 1307, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Roohani-Esfahani S.I., Nouri-Khorasani S., Lu Z., Appleyard R., and Zreiqat H. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite-PCL composites. Biomaterials 31, 5498, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Kane R.J., Weiss-Bilka H.E., Meagher M.J., Liu Y.X., Gargac J.A., Niebur G.L., Wagner D.R., and Roeder R.K. Hydroxyapatite reinforced collagen scaffolds with improved architecture and mechanical properties. Acta Biomater 17, 16, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Seol Y.J., Park D.Y., Park J.Y., Kim S.W., Park S.J., and Cho D.W. A new method of fabricating robust freeform 3D ceramic scaffolds for bone tissue regeneration. Biotechnol Bioeng 110, 1444, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Petite H., Viateau V., Bensaid W., Meunier A., de Pollak C., Bourguignon M., Oudina K., Sedel L., and Guillemin G. Tissue-engineered bone regeneration. Nat Biotechnol 18, 959, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Loh Q.L, and Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev 19, 485, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felzmann R., Gruber S., Mitteramskogler G., Tesavibul P., Boccaccini A.R., Liska R., and Stampfl J. Lithography-based additive manufacturing of cellular ceramic structures. Adv Eng Mater 14, 1052, 2012 [Google Scholar]
- 22.Jakus A.E., Rutz A.L., Jordan S.W., Kannan A., Mitchell S.M., Yun C., Koube K.D., Yoo S.C., Whiteley H.E., Richter C.P., Galiano R.D., Hsu W.K., Stock S.R., Hsu E.L., and Shah R.N. Hyperelastic “bone”: a highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci Transl Med 8, 358ra127, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Kang H.W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., and Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34, 312, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Urist M.R., Delange R.J., and Finerman G.A.M. Bone cell differentiation and growth factors. Science 220, 680, 1983 [DOI] [PubMed] [Google Scholar]
- 25.Wozney J.M., Rosen V., Celeste A.J., Mitsock L.M., Whitters M.J., Kriz R.W., Hewick R.M., and Wang E.A. Novel regulators of bone formation: molecular clones and activities. Science 242, 1528, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Sampath T.K., Muthukumaran N., and Reddi A.H. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc Natl Acad Sci USA 84, 7109, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauney J.R., Jaquiery C., Volloch V., Herberer M., Martin I., and Kaplan D.L. In vitro and in vivo evaluation of differentially demineralized cancellous bone scaffolds combined with human bone marrow stromal cells for tissue engineering. Biomaterials 26, 3173, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Davy D.T. Biomechanical issues in bone transplantation. Orthop Clin North Am 30, 553, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Song J.J, and Ott H.C. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med 17, 424, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Bhumiratana S, and Vunjak-Novakovic G. Concise review: personalized human bone grafts for reconstructing head and face. Stem Cells Transl Med 1, 64, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mailhot J.M, and Borke J.L. An isolation and in vitro culturing method for human intraoral bone cells derived from dental implant preparation sites. Clin Oral Implants Res 9, 43, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Voegele T.J., Voegele-Kadletz M., Esposito V., Macfelda K., Oberndorfer U., Vecsei V., and Schabus R. The effect of different isolation techniques on human osteoblast-like cell growth. Anticancer Res 20, 3575, 2000 [PubMed] [Google Scholar]
- 33.Hutmacher D.W, and Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng 9, S45, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Friedenstein A.J., Chailakhyan R.K., and Gerasimov U.V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinetics 20, 263, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Datta N., Pham Q.P., Sharma U., Sikavitsas V.I., Jansen J.A., and Mikos A.G. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A 103, 2488, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., and Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7, 211, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Cao Y., Sun Z., Liao L., Meng Y., Han Q., and Zhao R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 332, 370, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Frohlich M., Grayson W.L., Marolt D., Gimble J.M., Kregar-Velikonja N., and Vunjak-Novakovic G. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Eng Part A 16, 179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Correia C., Bhumiratana S., Yan L.P., Oliveira A.L., Gimble J.M., Rockwood D., Kaplan D.L., Sousa R.A., Reis R.L., and Vunjak-Novakovic G. Development of silk-based scaffolds for tissue engineering of bone from human adipose-derived stem cells. Acta Biomater 8, 2483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diederichs S., Roker S., Marten D., Peterbauer A., Scheper T., van Griensven M., and Kasper C. Dynamic cultivation of human mesenchymal stem cells in a rotating bed bioreactor system based on the Z (R) RP platform. Biotechnol Progr 25, 1762, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Yasuda K., Inoue S., and Tabata Y. Influence of culture method on the proliferation and osteogenic differentiation of human adipo-stromal cells in nonwoven fabrics. Tissue Eng 10, 1587, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Wang T.W., Wu H.C., Wang H.Y., Lin F.H., and Sun J.S. Regulation of adult human mesenchymal stem cells into osteogenic and chondrogenic lineages by different bioreactor systems. J Biomed Mater Res A 88a, 935, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Ben-David D., Kizhner T., Livne E., and Srouji S. A tissue-like construct of human bone marrow MSCs composite scaffold support in vivo ectopic bone formation. J Tissue Eng Regen M 4, 30, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Meinel L., Karageorgiou V., Fajardo R., Snyder B., Shinde-Patil V., Zichner L., Kaplan D., Langer R., and Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng 32, 112, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Kim H.J., Kim U.J., Leisk G.G., Bayan C., Georgakoudi I., and Kaplan D.L. Bone regeneration on macroporous aqueous-derived silk 3-D scaffolds. Macromol Biosci 7, 643, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Teixeira G.Q., Barrias C.C., Lourenco A.H., and Goncalves R.M. A multicompartment holder for spinner flasks improves expansion and osteogenic differentiation of mesenchymal stem cells in three-dimensional scaffolds. Tissue Eng Part C Methods 20, 984, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bancroft G.N., Sikavitsas V.I., van den Dolder J., Sheffield T.L., Ambrose C.G., Jansen J.A., and Mikos A.G. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A 99, 12600, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjerre L., Bunger C.E., Kassem M., and Mygind T. Flow perfusion culture of human mesenchymal stem cells on silicate-substituted tricalcium phosphate scaffolds. Biomaterials 29, 2616, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Grayson W.L., Frohlich M., Yeager K., Bhumiratana S., Chan M.E., Cannizzaro C., Wan L.Q., Liu X.S., Guo X.E., and Vunjak-Novakovic G. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A 107, 3299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhumiratana S., Bernhard J.C., Alfi D.M., Yeager K., Eton R.E., Bova J., Shah F., Gimble J.M., Lopez M.J., Eisig S.B., and Vunjak-Novakovic G. Tissue-engineered autologous grafts for facial bone reconstruction. Sci Transl Med 8, 343ra83, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sladkova M, and de Peppo G. Bioreactor systems for human bone tissue engineering. Processes 2, 494, 2014 [Google Scholar]
- 53.Burger E.H, and Klein-Nulend J. Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J 13, S101, 1999 [PubMed] [Google Scholar]
- 54.Johnson D.L., McAllister T.N., and Frangos J.A. Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. Am J Physiol 271, E205, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Kavlock K.D, and Goldstein A.S. Effect of pulse frequency on the osteogenic differentiation of mesenchymal stem cells in a pulsatile perfusion bioreactor. J Biomech Eng 133, 091005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoey D.A., Tormey S., Ramcharan S., O'Brien F.J., and Jacobs C.R. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells 30, 2561, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knippenberg M., Helder M.N., Doulabi B.Z., Semeins C.M., Wuisman P.I., and Klein-Nulend J. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng 11, 1780, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Chen J.C, and Jacobs C.R. Mechanically induced osteogenic lineage commitment of stem cells. Stem Cell Res Ther 4, 107, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grayson W.L., Marolt D., Bhumiratana S., Froehlich M., Guo X.E., and Vunjak-Novakovic G. Optimizing the Medium Perfusion Rate in Bone Tissue Engineering Bioreactors. Biotechnol Bioeng 108, 1159, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanczler J.M, and Oreffo R.O. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater 15, 100, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Brandi M.L, and Collin-Osdoby P. Vascular biology and the skeleton. J Bone Miner Res 21, 183, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Gerber H.P., Vu T.H., Ryan A.M., Kowalski J., Werb Z., and Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 5, 623, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Kronenberg H.M. Developmental regulation of the growth plate. Nature 423, 332, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Deckers M.M., van Bezooijen R.L., van der Horst G., Hoogendam J., van Der Bent C., Papapoulos S.E., and Lowik C.W. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 143, 1545, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Cao L, and Mooney D.J. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv Drug Deliv Rev 59, 1340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ball S.G., Shuttleworth C.A., and Kielty C.M. Platelet-derived growth factor receptors regulate mesenchymal stem cell fate: implications for neovascularization. Expert Opin Biol Ther 10, 57, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Gerber H.P, and Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med 10, 223, 2000 [DOI] [PubMed] [Google Scholar]
- 68.Khosla S., Westendorf J.J., and Modder U.I. Concise review: insights from normal bone remodeling and stem cell-based therapies for bone repair. Stem Cells 28, 2124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schindeler A., McDonald M.M., Bokko P., and Little D.G. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol 19, 459, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Mountziaris P.M, and Mikos A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev 14, 179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andersen T.L., Sondergaard T.E., Skorzynska K.E., Dagnaes-Hansen F., Plesner T.L., Hauge E.M., Plesner T., and Delaisse J.M. A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol 174, 239, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lange R.H., Bach A.W., Hansen S.T., Jr., and Johansen K.H. Open tibial fractures with associated vascular injuries: prognosis for limb salvage. J Trauma 25, 203, 1985 [DOI] [PubMed] [Google Scholar]
- 73.Uhrig B.A., Boerckel J.D., Willett N.J., Li M.T., Huebsch N., and Guldberg R.E. Recovery from hind limb ischemia enhances rhBMP-2-mediated segmental bone defect repair in a rat composite injury model. Bone 55, 410, 2013 [DOI] [PubMed] [Google Scholar]
- 74.Kusumbe A.P., Ramasamy S.K., and Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramasamy S.K., Kusumbe A.P., Wang L., and Adams R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santos M.I., Unger R.E., Sousa R.A., Reis R.L., and Kirkpatrick C.J. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials 30, 4407, 2009 [DOI] [PubMed] [Google Scholar]
- 77.Rouwkema J., Westerweel P.E., de Boer J., Verhaar M.C., and van Blitterswijk C.A. The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Eng Part A 15, 2015, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Rouwkema J., de Boer J., and Van Blitterswijk C.A. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng 12, 2685, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Yu H., VandeVord P.J., Gong W., Wu B., Song Z., Matthew H.W., Wooley P.H., and Yang S. Promotion of osteogenesis in tissue-engineered bone by pre-seeding endothelial progenitor cells-derived endothelial cells. J Orthop Res 26, 1147, 2008 [DOI] [PubMed] [Google Scholar]
- 80.Kneser U., Schaefer D.J., Polykandriotis E., and Horch R.E. Tissue engineering of bone: the reconstructive surgeon's point of view. J Cell Mol Med 10, 7, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawamura K., Yajima H., Ohgushi H., Tomita Y., Kobata Y., Shigematsu K., and Takakura Y. Experimental study of vascularized tissue-engineered bone grafts. Plast Reconstr Surg 117, 1471, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Wang L., Fan H., Zhang Z.-Y., Lou A.-J., Pei G.-X., Jiang S., Mu T.-W., Qin J.-J., Chen S.-Y., and Jin D. Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized beta-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials 31, 9452, 2010 [DOI] [PubMed] [Google Scholar]
- 83.Tremblay P.L., Hudon V., Berthod F., Germain L., and Auger F.A. Inosculation of tissue-engineered capillaries with the host's vasculature in a reconstructed skin transplanted on mice. Am J Transplant 5, 1002, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Levenberg S., Rouwkema J., Macdonald M., Garfein E.S., Kohane D.S., Darland D.C., Marini R., van Blitterswijk C.A., Mulligan R.C., D'Amore P.A., and Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol 23, 879, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Tsigkou O., Pomerantseva I., Spencer J.A., Redondo P.A., Hart A.R., O'Doherty E., Lin Y., Friedrich C.C., Daheron L., Lin C.P., Sundback C.A., Vacanti J.P., and Neville C. Engineered vascularized bone grafts. Proc Natl Acad Sci USA 107, 3311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rivron N.C., Raiss C.C., Liu J., Nandakumar A., Sticht C., Gretz N., Truckenmuller R., Rouwkema J., and van Blitterswijk C.A. Sonic Hedgehog-activated engineered blood vessels enhance bone tissue formation. Proc Natl Acad Sci USA 109, 4413, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spiller K.L, and Vunjak-Novakovic G. Clinical translation of controlled protein delivery systems for tissue engineering. Drug Deliv Transl Res 5, 101, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patel Z.S., Young S., Tabata Y., Jansen J.A., Wong M.E., and Mikos A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43, 931, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young S., Patel Z.S., Kretlow J.D., Murphy M.B., Mountziaris P.M., Baggett L.S., Ueda H., Tabata Y., Jansen J.A., Wong M., and Mikos A.G. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A 15, 2347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De la Riva B., Sanchez E., Hernandez A., Reyes R., Tamimi F., Lopez-Cabarcos E., Delgado A., and Evora C. Local controlled release of VEGF and PDGF from a combined brushite-chitosan system enhances bone regeneration. J Control Release 143, 45, 2010 [DOI] [PubMed] [Google Scholar]
- 91.Kaigler D., Silva E.A., and Mooney D.J. Guided bone regeneration using injectable vascular endothelial growth factor delivery gel. J Periodontol 84, 230, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shah N.J., Hyder M.N., Quadir M.A., Dorval Courchesne N.M., Seeherman H.J., Nevins M., Spector M., and Hammond P.T. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc Natl Acad Sci U S A 111, 12847, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martino M.M., Briquez P.S., Guc E., Tortelli F., Kilarski W.W., Metzger S., Rice J.J., Kuhn G.A., Muller R., Swartz M.A., and Hubbell J.A. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 343, 885, 2014 [DOI] [PubMed] [Google Scholar]
- 94.Richardson T.P., Peters M.C., Ennett A.B., and Mooney D.J. Polymeric system for dual growth factor delivery. Nat Biotechnol 19, 1029, 2001 [DOI] [PubMed] [Google Scholar]
- 95.Xie H., Cui Z., Wang L., Xia Z., Hu Y., Xian L., Li C., Xie L., Crane J., Wan M., Zhen G., Bian Q., Yu B., Chang W., Qiu T., Pickarski M., Duong le T., Windle J.J., Luo X., Liao E., and Cao X. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med 20, 1270, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hellberg C., Ostman A., and Heldin C.H. PDGF and vessel maturation. Recent Results Cancer Res 180, 103, 2010 [DOI] [PubMed] [Google Scholar]
- 97.Yancopoulos G.D., Davis S., Gale N.W., Rudge J.S., Wiegand S.J., and Holash J. Vascular-specific growth factors and blood vessel formation. Nature 407, 242, 2000 [DOI] [PubMed] [Google Scholar]
- 98.Carano R.A, and Filvaroff E.H. Angiogenesis and bone repair. Drug Discov Today 8, 980, 2003 [DOI] [PubMed] [Google Scholar]
- 99.Hausman M.R, and Rinker B.D. Intractable wounds and infections: the role of impaired vascularity and advanced surgical methods for treatment. Am J Surg 187, 44S, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Grundnes O, and Reikeras O. The importance of the hematoma for fracture healing in rats. Acta Orthop Scand 64, 340, 1993 [DOI] [PubMed] [Google Scholar]
- 101.Park S.H., Silva M., Bahk W.J., McKellop H., and Lieberman J.R. Effect of repeated irrigation and debridement on fracture healing in an animal model. J Orthop Res 20, 1197, 2002 [DOI] [PubMed] [Google Scholar]
- 102.Glass G.E., Chan J.K., Freidin A., Feldmann M., Horwood N.J., and Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A 108, 1585, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alexander K.A., Chang M.K., Maylin E.R., Kohler T., Muller R., Wu A.C., Van Rooijen N., Sweet M.J., Hume D.A., Raggatt L.J., and Pettit A.R. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 26, 1517, 2011 [DOI] [PubMed] [Google Scholar]
- 104.Wang X., Yu Y.Y., Lieu S., Yang F., Lang J., Lu C., Werb Z., Hu D., Miclau T., Marcucio R., and Colnot C. MMP9 regulates the cellular response to inflammation after skeletal injury. Bone 52, 111, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Champagne C.M., Takebe J., Offenbacher S., and Cooper L.F. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 30, 26, 2002 [DOI] [PubMed] [Google Scholar]
- 106.Freytes D.O., Kang J.W., Marcos-Campos I., and Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem 114, 220, 2013 [DOI] [PubMed] [Google Scholar]
- 107.Pirraco R.P., Reis R.L., and Marques A.P. Effect of monocytes/macrophages on the early osteogenic differentiation of hBMSCs. J Tissue Eng Regen Med 7, 392, 2013 [DOI] [PubMed] [Google Scholar]
- 108.Schmidt-Bleek K., Schell H., Schulz N., Hoff P., Perka C., Buttgereit F., Volk H.D., Lienau J., and Duda G.N. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res 347, 567, 2012 [DOI] [PubMed] [Google Scholar]
- 109.Reikeras O., Shegarfi H., Wang J.E., and Utvag S.E. Lipopolysaccharide impairs fracture healing: an experimental study in rats. Acta Orthop 76, 749, 2005 [DOI] [PubMed] [Google Scholar]
- 110.Krishnamoorthy L. The role of macrophages in human wound healing and their response to a tissue engineered dermal replacement in human chronic wounds [MD thesis]. Wound Healing Research Unit, University of Glasgow, Glasgow, Scotland: 2006 [Google Scholar]
- 111.Khallou-Laschet J., Varthaman A., Fornasa G., Compain C., Gaston A.T., Clement M., Dussiot M., Levillain O., Graff-Dubois S., Nicoletti A., and Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS One 5, e8852, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., and Popovich P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29, 13435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sakurai E., Anand A., Ambati B.K., van Rooijen N., and Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 44, 3578, 2003 [DOI] [PubMed] [Google Scholar]
- 114.Hibino N., Yi T., Duncan D.R., Rathore A., Dean E., Naito Y., Dardik A., Kyriakides T., Madri J., Pober J.S., Shinoka T., and Breuer C.K. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J 25, 4253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Low-Marchelli J.M., Ardi V.C., Vizcarra E.A., van Rooijen N., Quigley J.P., and Yang J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res 73, 662, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fantin A., Vieira J.M., Gestri G., Denti L., Schwarz Q., Prykhozhij S., Peri F., Wilson S.W., and Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kubota Y., Takubo K., Shimizu T., Ohno H., Kishi K., Shibuya M., Saya H., and Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med 206, 1089, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arendt L.M., McCready J., Keller P.J., Baker D.D., Naber S.P., Seewaldt V., and Kuperwasser C. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res 73, 6080, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hisatome T., Yasunaga Y., Yanada S., Tabata Y., Ikada Y., and Ochi M. Neovascularization and bone regeneration by implantation of autologous bone marrow mononuclear cells. Biomaterials 26, 4550, 2005 [DOI] [PubMed] [Google Scholar]
- 120.Hirose N., Maeda H., Yamamoto M., Hayashi Y., Lee G.H., Chen L., Radhakrishnan G., Rao P., and Sasaguri S. The local injection of peritoneal macrophages induces neovascularization in rat ischemic hind limb muscles. Cell Transplant 17, 211, 2008 [DOI] [PubMed] [Google Scholar]
- 121.Mosser D.M, and Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8, 958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schlundt C., El Khassawna T., Serra A., Dienelt A., Wendler S., Schell H., van Rooijen N., Radbruch A., Lucius R., Hartmann S., Duda G.N., and Schmidt-Bleek K. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2015. DOI: 10.1016/j.bone.2015.10.019 [DOI] [PubMed] [Google Scholar]
- 123.Loi F., Córdova L.A., Zhang R., Pajarinen J., Lin, T.-h., Goodman S.B., and Yao Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther 7, 15, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mirza R.E., Fang M.M., Weinheimer-Haus E.M., Ennis W.J., and Koh T.J. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes 63, 1103, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu A.C., Raggatt L.J., Alexander K.A., and Pettit A.R. Unraveling macrophage contributions to bone repair. Bonekey Rep 2, 373, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nassiri S., Zakeri I., Weingarten M.S., and Spiller K.L. Relative expression of pro-inflammatory and anti-inflammatory genes reveals differences between healing and nonhealing human chronic diabetic foot ulcers. J Invest Dermatol 135, 1700, 2015 [DOI] [PubMed] [Google Scholar]
- 127.Brown B.N., Valentin J.E., Stewart-Akers A.M., McCabe G.P., and Badylak S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 30, 1482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Madden L.R., Mortisen D.J., Sussman E.M., Dupras S.K., Fugate J.A., Cuy J.L., Hauch K.D., Laflamme M.A., Murry C.E., and Ratner B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A 107, 15211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hamlet S, and Ivanovski S. Inflammatory cytokine response to titanium chemical composition and nanoscale calcium phosphate surface modification. Acta Biomater 7, 2345, 2011 [DOI] [PubMed] [Google Scholar]
- 130.Rao A.J., Gibon E., Ma T., Yao Z., Smith R.L., and Goodman S.B. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater 8, 2815, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., and Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25, 677, 2004 [DOI] [PubMed] [Google Scholar]
- 132.Mantovani A., Sozzani S., Locati M., Allavena P., and Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23, 549, 2002 [DOI] [PubMed] [Google Scholar]
- 133.Jetten N., Verbruggen S., Gijbels M.J., Post M.J., De Winther M.P., and Donners M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17, 109, 2014 [DOI] [PubMed] [Google Scholar]
- 134.Zajac E., Schweighofer B., Kupriyanova T.A., Juncker-Jensen A., Minder P., Quigley J.P., and Deryugina E.I. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood 122, 4054, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fishman J.M., Lowdell M.W., Urbani L., Ansari T., Burns A.J., Turmaine M., North J., Sibbons P., Seifalian A.M., Wood K.J., Birchall M.A., and De Coppi P. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc Natl Acad Sci U S A 110, 14360, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Willenborg S., Lucas T., van Loo G., Knipper J.A., Krieg T., Haase I., Brachvogel B., Hammerschmidt M., Nagy A., Ferrara N., Pasparakis M., and Eming S.A. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 120, 613, 2012 [DOI] [PubMed] [Google Scholar]
- 137.Spiller K.L., Anfang R.R., Spiller K.J., Ng J., Nakazawa K.R., Daulton J.W., and Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35, 4477, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sussman E.M., Halpin M.C., Muster J., Moon R.T., and Ratner B.D. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng 42, 1508, 2014 [DOI] [PubMed] [Google Scholar]
- 139.Tous E., Weber H.M., Lee M.H., Koomalsingh K.J., Shuto T., Kondo N., Gorman J.H., 3rd, Lee D., Gorman R.C., and Burdick J.A. Tunable hydrogel-microsphere composites that modulate local inflammation and collagen bulking. Acta Biomater 8, 3218, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qi J.H., Ebrahem Q., Moore N., Murphy G., Claesson-Welsh L., Bond M., Baker A., and Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med 9, 407, 2003 [DOI] [PubMed] [Google Scholar]
- 141.Rosenberg G.A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8, 205, 2009 [DOI] [PubMed] [Google Scholar]
- 142.Mohammed F.F., Smookler D.S., Taylor S.E., Fingleton B., Kassiri Z., Sanchez O.H., English J.L., Matrisian L.M., Au B., Yeh W.C., and Khokha R. Abnormal TNF activity in Timp3-/- mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet 36, 969, 2004 [DOI] [PubMed] [Google Scholar]
- 143.Saunders W.B., Bohnsack B.L., Faske J.B., Anthis N.J., Bayless K.J., Hirschi K.K., and Davis G.E. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 175, 179, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jadhav U., Chigurupati S., Lakka S.S., and Mohanam S. Inhibition of matrix metalloproteinase-9 reduces in vitro invasion and angiogenesis in human microvascular endothelial cells. Int J Oncol 25, 1407, 2004 [PubMed] [Google Scholar]
- 145.Ardi V.C., Kupriyanova T.A., Deryugina E.I., and Quigley J.P. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A 104, 20262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koh Y.W., Park C.S., Yoon D.H., Suh C., and Huh J. CD163 expression was associated with angiogenesis and shortened survival in patients with uniformly treated classical Hodgkin lymphoma. PLoS One 9, e87066, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang F.Q., Chen G., Zhu J.Y., Zhang W., Ren J.G., Liu H., Sun Z.J., Jia J., and Zhao Y.F. M2-polarised macrophages in infantile haemangiomas: correlation with promoted angiogenesis. J Clin Pathol 66, 1058, 2013 [DOI] [PubMed] [Google Scholar]
- 148.Zhang Q., Atsuta I., Liu S., Chen C., Shi S., Shi S., and Le A.D. IL-17-mediated M1/M2 macrophage alteration contributes to pathogenesis of bisphosphonate-related osteonecrosis of the jaws. Clin Cancer Res 19, 3176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zaiss M.M., Kurowska-Stolarska M., Bohm C., Gary R., Scholtysek C., Stolarski B., Reilly J., Kerr S., Millar N.L., Kamradt T., McInnes I.B., Fallon P.G., David J.P., Liew F.Y., and Schett G. IL-33 shifts the balance from osteoclast to alternatively activated macrophage differentiation and protects from TNF-alpha-mediated bone loss. J Immunol 186, 6097, 2011 [DOI] [PubMed] [Google Scholar]
- 150.Seebach E., Freischmidt H., Holschbach J., Fellenberg J., and Richter W. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater 10, 4730, 2014 [DOI] [PubMed] [Google Scholar]
- 151.Hughes J.E., Srinivasan S., Lynch K.R., Proia R.L., Ferdek P., and Hedrick C.C. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res 102, 950, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Petrie Aronin C.E., Shin S.J., Naden K.B., Rios P.D., Jr, Sefcik L.S., Zawodny S.R., Bagayoko N.D., Cui Q., Khan Y., and Botchwey E.A. The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1-phosphate receptor targeted drug FTY720. Biomaterials 31, 6417, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Das A., Segar C.E., Hughley B.B., Bowers D.T., and Botchwey E.A. The promotion of mandibular defect healing by the targeting of S1P receptors and the recruitment of alternatively activated macrophages. Biomaterials 34, 9853, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Awojoodu A.O., Ogle M.E., Sefcik L.S., Bowers D.T., Martin K., Brayman K.L., Lynch K.R., Peirce-Cottler S.M., and Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A 110, 13785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim Y.H., Furuya H., and Tabata Y. Enhancement of bone regeneration by dual release of a macrophage recruitment agent and platelet-rich plasma from gelatin hydrogels. Biomaterials 35, 214, 2014 [DOI] [PubMed] [Google Scholar]
- 156.Spiller K.L., Nassiri S., Witherel C.E., Anfang R., Ng J., Nakazawa K., Yu T., and Vunjak Novakovic G. Sequential delivery of cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 37, 194, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mueller M.B, and Tuan R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58, 1377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Scotti C., Tonnarelli B., Papadimitropoulos A., Scherberich A., Schaeren S., Schauerte A., Lopez-Rios J., Zeller R., Barbero A., and Martin I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A 107, 7251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bahney C.S., Hu D.P., Taylor A.J., Ferro F., Britz H.M., Hallgrimsson B., Johnstone B., Miclau T., and Marcucio R.S. Stem cell- derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res 29, 1269, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Scotti C., Piccinini E., Takizawa H., Todorov A., Bourgine P., Papadimitropoulos A., Barbero A., Manz M.G., and Martin I. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci U S A 110, 3997, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Serafini M., Sacchetti B., Pievani A., Redaelli D., Remoli C., Biondi A., Riminucci M., and Bianco P. Establishment of bone marrow and hematopoietic niches in vivo by reversion of chondrocyte differentiation of human bone marrow stromal cells. Stem Cell Res 12, 659, 2014 [DOI] [PubMed] [Google Scholar]
- 162.Zhou X., von der Mark K., Henry S., Norton W., Adams H., and de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. Plos Genet 10, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Harada N., Watanabe Y., Sato K., Abe S., Yamanaka K., Sakai Y., Kaneko T., and Matsushita T. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials 35, 7800, 2014 [DOI] [PubMed] [Google Scholar]
- 164.Bourgine P.E., Scotti C., Pigeot S., Tchang L.A., Todorov A., and Martin I. Osteoinductivity of engineered cartilaginous templates devitalized by inducible apoptosis. Proc Natl Acad Sci U S A 111, 17426, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gawlitta D., Benders K.E.M., Visser J., van der Sar A.S., Kempen D.H.R., Theyse L.F.H., Malda J., and Dhert W.J.A. Decellularized cartilage-derived matrix as substrate for endochondral bone regeneration. Tissue Eng Part A 21, 694, 2015 [DOI] [PubMed] [Google Scholar]
- 166.Reinisch A., Etchart N., Thomas D., Hofmann N.A., Fruehwirth M., Sinha S., Chan C.K., Senarath-Yapa K., Seo E.-Y., Wearda T., Hartwig U.F., Beham-Schmid C., Trajanoski S., Lin Q., Wagner W., Dullin C., Alves F., Andreeff M., Weissman I.L., Longaker M.T., Schallmoser K., Majeti R., and Strunk D. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood 125, 249, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]