Abstract
Purpose
Notch1, a trans-membrane receptor, has been recently shown to aid in the determination of thyroid cell fate associated with tumorigenesis. The present study aimed to investigate the clinical relevance of Notch1 and its role in the regulation of differentiated thyroid cancer (DTC) behavior.
Experimental design
We examined Notch1 expression level and its relationship with clinicopathologic features and outcomes of DTC. Notch1 intracellular domain (NICD) was further characterized both in vitro and in vivo by gain-of-function assays using an inducible system.
Results
Notch1 expression levels were down-regulated in primary DTC tissue samples compared with contralateral non-tumor and benign thyroid tissues. Decreased Notch1 expression in DTC was associated with advanced patient age (p=0.032) and the presence of extrathyroidal invasion (p=0.005). Patients with lower Notch1 expression had a significantly higher recurrence rate (p=0.038). Restoration of NICD in a stably doxycycline-inducible metastatic DTC cell line reduced cell growth and migration profoundly. Using an orthotopic thyroid cancer model, NICD induction significantly reduced the growth of the primary thyroid tumor and inhibited the development of lung metastasis. SERPINE1 was discovered by microarray as the most significant gene down-regulated by NICD. Further validation showed that induction of NICD reduced SERPINE1 expression in a dose-dependent manner while restoration of a relative higher level of SERPINE1was observed with NICD back to minimal level. Additionally, SERPINE1 knock-down inhibited DTC cell migration.
Conclusions
Notch1 regulates the aggressive phenotypes of DTC, which could be mediated by SERPINE1 inhibition. Notch1/SERPINE1 axis warrants further investigation as a novel therapeutic target for advanced DTC.
Keywords: Notch1, thyroid cancer, cell migration, tumor metastases, SERPINE1
INTRODUCTION
Notch1 is a multifunctional trans-membrane receptor and its signaling is activated upon a series of sequential proteolytic cleavages. The last cleavage within the trans-membrane domain of the receptor allows the release and translocation of the intracellular domain of Notch1 (NICD) into the nucleus where it associates with DNA binding proteins to assemble a transcriptional complex that activates downstream target genes (1). Since Notch1 activation implements cellular development, proliferation, and survival in variety of contexts, the aberrant gain or loss of Notch1 signaling has been directly linked to various types of diseases including cancer (2-5).
Notch1 signaling acts either as an oncogene or a tumor suppressor (6). It was first found as a proto-oncogene of T cell acute lymphoblastic leukemia (T-ALL) (1,2). Later on, growing evidence supported by recent studies showed that Notch1 signaling can also have a potent tumor suppressor function in both hematological malignancies and solid tumors including B cell malignancies as well as head and neck squamous cell carcinoma (7-9). The dual function of Notch1 is not only determined by cell context but by the tumor stage as well (10). Notch pathway was reported to be up-regulated in a genetic mouse model for hepatocellular carcinoma, suggesting an oncogenic role of Notch1 in cancer development. Unexpectedly, inhibition of Notch signaling in this model accelerated the hepatocellular carcinoma progression (11).
Differentiated thyroid cancer (DTC), which includes papillary and follicular cancer, comprises over 90% of all thyroid cancers (12). Despite the generally good prognosis, DTC patients with aggressive phenotypes, like extrathyroidal invasion or metastases, reveal a much higher disease recurrence rate, which is still challenging for clinical management (13,14). Recently, the Notch1 pathway has garnered great interest as a potential target of treating aggressive thyroid cancers (15-18). However, unlike the consistent observation on the loss of Notch1 expression in anaplastic thyroid carcinoma (17,19,20), the expression pattern and biological function of Notch1 in DTC are controversial. Notch1mRNA level has been reported to be down-regulated in thyroid tumors and activation of the signaling cascade is involved in the expression of thyrocyte differentiation markers (17). Additionally, it has been found that Notch1 mediates growth suppression of DTC cells, suggesting Notch1 activation as a possible therapeutic strategy for aggressive DTCs (16). To the contrary, DTCs with the BRAF mutation or active MAPK signaling due to the genetic alteration in the RAS gene have shown overexpression of Notch1 and its downstream effector Hes1 (21), which can be blocked by high iodine (22).
The lack of the consensus on the role of Notch1 in DTC may be due to the different molecular subtype of each case (23), which interfere with the activation of Notch1 through the crosstalk of multiple pathways. In the current study, the function of Notch1 signaling was investigated through a doxycycline-inducible NICD construct in DTC cells without the BRAF mutation. Using this gain-of-function assay, we evaluated the dose-dependent effects of NICD on DTC cell growth and migration. Additionally, an orthotopic thyroid cancer model was utilized to assess tumor growth and metastases withNotch1 activation. We also identified and validated serpin peptidase inhibitor, clade E, member 1 (SERPINE1) as a new downstream target of Notch1 signaling through microarray analysis.
MATERIALS AND METHODS
Collection of thyroid specimens
Seventy-six thyroid tissue samples were obtained from patients who underwent thyroid surgery at the University of Wisconsin. The protocol was approved by the institutional review board (IRB) of the University. Tissues were obtained in the operating room after removal, immediately frozen in liquid nitrogen and kept at −80°C until analysis. Among these tissues, 38 were cancer tissues including 29 classical papillary, 7 follicular variant, 1 tall cell variant of PTC, 1 diffuse sclerosing PTC, and 22 were corresponding paired normal thyroid tissues in most of these patients when available. Another 16 samples were obtained from thyroid patients with multi nodular goiter as benign controls.
Maintenance of thyroid cancer cell lines
Four human DTC cell lines used in this study were as following: the papillary cancer cell lines TPC1 and BCPAP were kindly provided by Dr. Daniel Ruan (Brigham and Women’s Hospital, Boston, MA) and Dr. Rebecca Schweppe (University of Colorado Denver, CO), respectively; the follicular cancer cell lines FTC133 and FTC236 were purchased from European Collection of Cell Cultures (ECACC) through Sigma-Aldrich (St Louis, MO). TPC1 and BCPAP were maintained in RPMI-1640 (Invitrogen Life Technologies, Carlsbad, CA) medium supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich). FTC133 and FTC236 were maintained in DMEM/Ham’s F-12 (1:1; Invitrogen) medium supplemented with 10% FBS, 0.01 U/mL of thyroid-stimulating hormone (TSH) and 10 μg/mL of insulin (Sigma-Aldrich). All cell lines were grown at 37 °C in a humidified atmosphere containing 5% CO2. To verify the authenticity of the cell lines, DNA short tandem repeat STR analysis was performed by either DDC (DNA Diagnostics Center, Fairfield, OH) (24) or ECACC. All four cell lines were tested and were found to be authentic.
Isolation of mRNA and analysis of mRNA expression levels by quantitative real-time PCR
Total RNA was isolated from human tissue samples or cultured cells using silica-gel membrane-based spin-column technology (Qiagen Inc., Valencia, CA) according to manufacturer’s instruction. To ensure the purity and integrity of RNA, each sample was examined by electrophoresis on an agarose gel and was quantified by NanoDrop (Thermo Scientific, Waltham, MA). Total RNA (2μg) was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Sequences for each pair of PCR primers were listed in Supplementary Table S1. The quantitative real-time PCR reactions were performed on MyiQ Thermal Cycler (Bio-Rad Laboratories). Target gene expression was normalized to GAPDH levels in respective samples as an internal standard, and the comparative cycle threshold (ΔCt) method was used to calculate relative expression levels of target genes.
Establishment of inducible Notch1 cell line
A 2.3-kb NICD fragment (amino acids 1759 –2556) from pTAN1-cDNA was subcloned into the pRevTRE vector. The cloned construct (pRevTRE-Notch1) was confirmed by DNA sequencing. FTC236-Notch1 inducible cell line was created using a similar method as described previously (4). In brief, the parental FTC236 cells were first transfected with plasmid pRevTet-On (Clontech, Mountain View, CA) containing the Tet-responsive transcriptional activator and selected in medium containing 0.5 mg/mL G418 (Mediatech, Inc, Manassas, VA). The resulting G418-resistant, FTC236-Tet-on clone with the highest inducibility was transfected with either pRevTRE-Notch1or the empty vector (pRevTRE). Transfected cells were selected in 1.0 mg/mL hygromycin (Invitrogen). Resistant FTC236-Notch1 and FTC236-TRE clones were treated with doxycycline and screened for the presence of Notch1 protein by Western blot analysis.
Western blot analysis
FTC236-Notch1 and FTC236-TRE cells were treated with doxycycline at various concentrations up to 1μg/mL. Samples were collected for protein analysis at different time points of either doxycycline treatment or doxycycline withdrawal. The protein lysates were prepared as previously described (25). The samples were then analyzed by Western blot analysis for Notch1, SERPINE1, cyclin B1, cyclin D1, p21Waf1/Cip1, b-actin, and GAPDH. Detailed method is described in the Supplementary Material.
Cell viability assay
Viable cells were determined by a 3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Sigma) after treatment as described previously (19), Please refer to the Supplementary Materials for detailed description of the method.
Clonogenic assay
Cell proliferation of FTC236-Notch1 and -TRE cells treated with different concentrations of doxycycline was measured by clonogenic assay. Detailed method is described in Supplementary Material.
Detection of cell apoptosis by flow cytometry
Cell apoptosis was detected by flowcytometry analysis using PE Annexin V Apoptosis Detection Kit I (BD Pharmingen) according to the manufacturer’s instructions. Detailed method is described in the Supplementary Material.
Cell migration assay
The experiment was carried out using the CytoSelect Cell Migration Assay Kit (Cell Biolabs, Inc.) containing polycarbonate membrane inserts (8-mm pore size) in a 24-well plate. Detailed method is described in the Supplementary Material.
Orthotopic thyroid cancer model
FTC236-Notch1 and FTC236-TRE cells were first labeled with luciferase (see Methods in Supplementary Material). Animal studies were performed in compliance with our animal experiment protocol approved by the University of Wisconsin Madison Animal Care and Use Committee. SCID mice around 6 weeks old (Charles River Laboratories) were used in this orthotopic model. Surgery was performed to expose the right thyroid gland, and 1 _ 106 cells were injected into the lobe as described previously (26). We defined the day of tumor implantation as day 0 of the experiment. After one week to allow tumor development, animals were fed daily with either doxycycline-containing food (0.625 g/kg, Harlan Laboratories) or regulator food for 6 weeks. Tumor establishment and progression were monitored every two weeks by assessing luciferase bioluminescence.
In vivo imaging for tumor growth monitoring
At the indicated time points, 100 mL of D-luciferin (30 mg/mL, Biosynth) was delivered by intraperitoneal injection to each mouse. Mice were anesthetized with isoflurane, and bioluminescence images were acquired 10 to 15 minutes after D-luciferin administration using the Intravital Imaging System (IVIS Spectrum, Caliper Life Sciences). Tumor bioluminescence was quantified with Living Image Software (Caliper Life Sciences), and data were expressed as total photon flux.
Ex vivo imaging and histopathology evaluation
Seven weeks after cancer cell implantation, the mice were sacrificed and tissue samples, including xenograft tumor, lung, and lymph nodes, were collected. Detailed methods of ex vivo imaging and histopathology evaluation (H&E and IHC) are described in the Supplementary Material.
cDNA microarrays
RNA samples were prepared from three independent experiments with FTC236-Notch1 cells treated with or without doxycycline. Quality and quantity of RNA were assessed on an Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA) using the RNA Nano Labchip. Similar as described previously (27), total RNA was reverse-transcribed into cDNA using specific primers containing a Cy-3 “capture sequence.” The cDNA was then hybridized onto a microarray for eighteen hours. The arrays were washed according to the manufacturer’s specifications. After hybridization, slides were scanned on a DNA Microarray Scanner (Agilent). Images were processed and the data was extracted using Agilent Feature Extractor software. All microarray experiments discussed adhere to the Minimum Information about Microarray Experiment (MIAME) guidelines. All microarray data are publicly available through the GEO database using accession number GSE70627 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE70627).
Knock-down Notch1 and SERPINE1 by RNA interference assay
siRNA against Notch1, SERPINE1, or nonspecific siRNA (NS siRNA; all from Santa Cruz Biotechnology) were delivered into DTC cell lines using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s instructions. After 24-hour incubation, the cells were trypsinized and counted for cell migration assay (as described above). Protein andmRNA were isolated from the remaining cells for Western blot and real-time PCR analyses, respectively.
Statistical analysis
The data was analyzed by the Statistical Package for the Social Sciences (IBM SPSS, version 22). Due to the skewed distribution, continuous data of Notch1 expression in patients’ samples were presented as median value (interquartile range). Mann-Whitney U test and Wilcoxon signed-rank test were used to evaluate differences between unpaired and paired observations, respectively. Pearson χ2 test was done to analyze categorical data as appropriate. Correlations between continuous variables were evaluated using the Spearman rank test. Continuous data from the rest of the experiments was expressed as mean ± SD. One-way ANOVA or two-tailed Student’s t test was used to determine statistical significance. P < 0.05 was considered as statistically significant.
RESULTS
Aberrantly-regulated expression of Notch1 in aggressive DTCs
DTCs, contra-lateral non-tumorous tissues, and benign thyroid specimens were analyzed by real-time PCR for the expression levels of Notch1. Out of the 38 DTC tumor specimens examined, the median expression level was 0.366 (interquartile range, 0.257 to 0.687), which showed a significant difference from the other two groups. The median levels of Notch1 expression in contra-lateral and benign thyroid specimens were 0.657(range, 0.207 to 2.016; P = 0.014) and 0.695 (range, 0.341 to 1.464; P = 0.008), respectively (Figure 1A). Among twenty paired DTC and contra-lateral samples, 12 pairs demonstrated higher Notch1 in cancer samples while the other 8 showed the reverse (P = 0.086). Additionally, we examined Notch1 expression in recurrent or metastatic lesions from five DTC patients. Compared with their corresponding primary tumors, all recurrent/metastatic tumors (100%) revealed much lower Notch1 levels.
Figure 1.
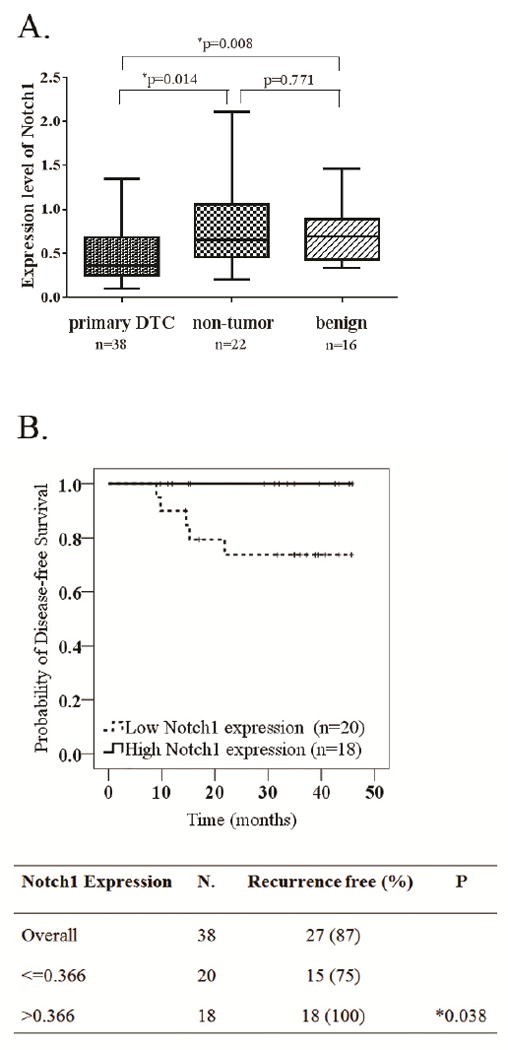
Clinical relevance of Notch1 expression level in human thyroid cancer tissues.(A). Box plot of Notch1 expression levels comparing among primary thyroid cancer, contra-lateral non-tumor and benign tissues. The horizontal line within the box of each individual group indicates the median value; (B). Kaplan-Meier estimate was used to determine the correlation between Notch1 expression in cancer tissue and recurrence-free survival.
Aberrant expression of Notch1 has shown to be associated with cancer progression and metastasis. We therefore compared Notch1 expression in DTC with designated clinicopathologic variables of thyroid cancer, such as age, gender, tumor size, cancer stage, and the presence of nodal or distant metastasis (Table 1). Notch1 tumor expression level was significantly associated with age older than 45 years (P = 0.032) and the presence of extrathyroidal extension in histologic examination (P = 0.005). Furthermore, there was a negative linear correlation between Notch1 expression level and patient age in DTC samples (r = -0.495, P = 0.002; Supplementary Figure S1A). However, such correlation with age was not observed in contra-lateral thyroid tissues or benign thyroid tissues (p=0.721 and 0.149, respectively; Supplementary Figure S1B and S1C). A trend of reduced Notch1 expression was also observed in patients with advanced tumor stages including stage II, III and IV (P = 0.066).
Table 1.
Correlation between Notch1 expression levels and clinicopathological variables in DTC
| Variables | No. | Median | 25~75 percentile | p-value | |
|---|---|---|---|---|---|
| Age | |||||
| <45yr | 17 | 0.505 | 0.323 – 0.843 | ||
| >=45yr | 21 | 0.320 | 0.227 – 0.476 | *0.032 | |
| Gender | |||||
| Male | 9 | 0.326 | 0.255 – 0.514 | ||
| Female | 29 | 0.435 | 0.255 – 0.826 | 0.257 | |
| Tumor size(mm) | |||||
| <20 | 26 | 0.417 | 0.243 – 0.739 | ||
| >=20 | 12 | 0.326 | 0.285 – 0.633 | 0.790 | |
| §1Multifocality | |||||
| No | 20 | 0.498 | 0.271 – 0.769 | ||
| Yes | 18 | 0.317 | 0.254 – 0.453 | 0.260 | |
| §2Extrathyroidal extension | |||||
| No | 30 | 0.471 | 0.313 – 0.805 | ||
| Yes | 8 | 0.260 | 0.236 – 0.314 | *0.005 | |
| Lymph node metastases | |||||
| No | 23 | 0.399 | 0.242 – 0.784 | ||
| Yes | 15 | 0.362 | 0.298 – 0.545 | 0.976 | |
| Distant metastases | |||||
| No | 37 | 0.366 | 0.256 – 0.699 | ||
| Yes | 1 | 0.320 | N.A | 0.789 | |
| pTNM stage | |||||
| I | 29 | 0.449 | 0.278 – 0.826 | ||
| II,III,IV | 9 | 0.308 | 0.243 – 0.384 | 0.066 |
Multifocality: the presence of additional focus of thyroid carcinoma in non-tumorous part of thyroid gland
Extrathyroidal extension: the extension of tumor beyond the capsule of thyroid gland
indicates p value is less than 0.05 which reaches statistically significant.
The median follow-up time for this cohort of DTC patients was 35 months. The median of Notch1 expression in 38 PTC tumor samples (median = 0.366) was used as a cutoff value to define high- and low-Notch1-expression group. Five patients out of 38 had confirmed recurrent thyroid cancer by ultrasound and/or whole body scan. These five patients with recurrence were all in the low-Notch1-expression group. No DTC patient with higher Notch1 expression (n=18) during our follow-up time had recurrent disease. Thus, patients of low-Notch1-expression group had significantly shorter disease-free survival (25% vs. 0%, P =0.038; Figure 1B).
Comparison of NICD levels among different DTC cell lines
To further understand these clinical observations, we first examined Notch1 expression in human DTC cell lines. Using Western blot analysis, we demonstrated that both papillary cancer lines TPC1 and BCPAP showed moderate expression of NICD. FTC133 and FTC236 were derived from a single patient’s follicular thyroid carcinoma (28). The metastatic FTC236 thyroid cancer line had much lower endogenous NICD levels compared with primary FTC133 (Figure 2A).
Figure 2.
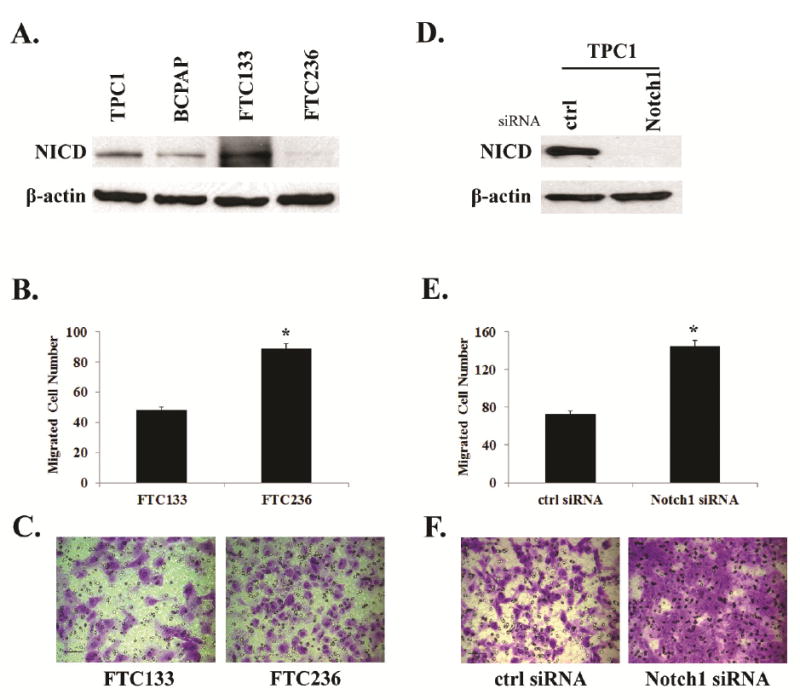
Comparison among four different thyroid cancer cell lines for their Notch1 expression and evaluation of cell migration associated with Notch1. (A). Notch1 intracellular domain (NICD) was determined in different cell lines by Western blot; (B)-(C). Cell migration of FTC133 and FTC 236 was evaluated by a Boyden chamber assay; (D)-(F). TPC1 was transfected either with control siRNA or Notch1 specific siRNA and the cell migration was then evaluated by a Boyden chamber assay. Histograms show the average cell counts of each cell line with the representative fields of migrated cells on the membrane; Each bar represents mean±SEM;*indicates p<0.05; Scale bar:50μm.
Since FTC133 and FTC236 are from the same patient and probably share a similar genetic background, we first examined the association between NICD level and cell migration using these two paired cell lines. Using a Boyden chamber assay, FTC236, which showed lower NICD level, had significantly more migrated cells than FTC133 (87/field vs. 48/field; Figure 2B and C). To further determine the effect of NICD knock-down, we transfected TPC1 cells with either Notch1 siRNA or scramble siRNA. TPC1 cells with transient NICD knock-down (Figure 2D) showed significantly enhanced cell migration (144/field vs.73/field; Figure 2E and 2F). These findings suggested that the down-regulation or low levels of Notch1 may be associated with increased potential for cancer cell migration and metastases.
Restoration of Notch1 in DTC cells by an inducible system
Based on the above observation, we were interested in determining whether restoration or increase of Notch1 in the FTC236 cancer cells may alter the metastatic phenotype. A stable cell line with a doxycycline-inducible NICD was generated from FTC236 parental cells and named as FTC236-Notch1. We also established a control cell line (FTC236-TRE) using empty vector. The NICD inducibility of FTC236-Notch1 cells was analyzed by Western blot after treatment of various concentrations of doxycycline. As shown in Figure 3A, in the absence of doxycycline, there was no detectable Notch1 protein in both FTC236-Notch1 and FTC236-TRE cells. Treatment with increasing concentrations of doxycycline led to a dose-dependent induction of Notch1 expression in FTC236-Notch1 cells but not in FTC236-TRE cells.
Figure 3.
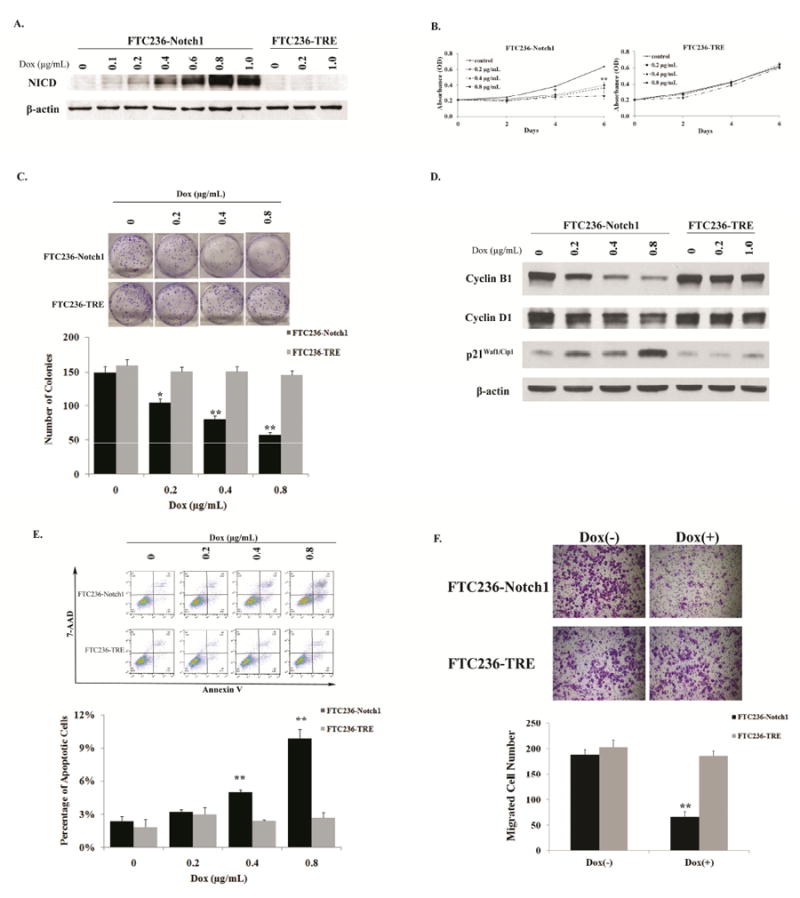
Induction of NICD inhibited DTC cell growth and migration. (A). Whole cell lysate was prepared from both FTC236-Notch1 and FTC236-TRE cells treated with or without doxycycline. The expression level of NICD was analyzed by Western blot; Cell viability of both cell lines with (0-0.8 μg/mL)or without doxycycline treatment was measured by MTT assay (B) and cell proliferation was measured by clonogenic assay (C); (D). Detection of cyclin B1, cyclin D1 and p21Waf1/Cip1 protein expression by Western blot in both FTC236-Notch1 and -TRE cells treated with different concentrations of doxycycline. Equal loading was confirmed with β-actin; (E). Both cell lines were exposed to different concentrations of doxycycline for 48 hours before they were double stained with Annexin V and 7 AAD for flow cytometry analysis. Percentages at right lower quadrant denote the cells in early apoptotic phase. Results of one representative experiment. Data from three repeated experiments are summarized in bar graph; (F). Cell migration was evaluated by a Boyden chamber assay on FTC236-Notch1 and FTC236-TRE with (0.8 μg/mL) or without doxycycline treatment. Histograms show the average cell counts of each cell line with the representative fields of migrated cells on the membrane. Each dot or bar represents mean± SEM; “Dox” indicates doxycycline; * indicates p<0.05 and ** indicates p< 0.01, compared with non-doxycycline treatment groups;Scale bar: 100 μm.
We then assessed the effect of Notch1 induction on DTC cellular proliferation. We treated FTC236-Notch1and FTC236-TRE cells with varying concentrations of doxycycline as shown in Figure 3B and measured cell viability by MTT assay. In the absence of doxycycline, FTC236-Notch1 cells demonstrated the highest growth rate, while, in the presence of 0.2μg/mL doxycycline, there was significant growth inhibition. Notably, the highest concentration of doxycycline (0.8μg/mL) in this experiment resulted in a nearly complete cessation of tumor cell growth. In contrast, the dose-dependent growth reduction was not observed in the control cell line. As shown in Figure 3B, there were no significant differences in growth rate between FTC236-TRE cells with and without doxycycline treatment. To understand the possible mechanism(s) contributing to the Notch1-dependent growth inhibition, we then performed a clonogenic assay to measure cell proliferation of FTC236-Notch1 and the control cell line. Notch1 induction in FTC236-Notch1 cells resulted in decreased ability to form colonies (Figure 3C). Doxycycline treatment of 0.2μg/mL and 0.8μg/mL reduced over 30% and 60% of colony formation, respectively, compared with those without. Meanwhile, similar colony numbers were observed among the control cells treated with different concentrations of doxycycline. Furthermore, the levels of representative cell cycle regulators were assessed 48 hours after doxycycline treatment in both cell lines. Notch1 induction led to an increase in protein levels of cyclin-dependent kinase inhibitor p21Waf1/Cip1, while the levels of cell cycle promoter of cyclin B1 and cyclin D1 were decreased (Figure 3D). We next assessed the potential effect of Notch1 induction on thyroid cancer cell death by quantifying number of cells undergoing apoptosis. FTC236-Notch1 and -TRE cells were double stained with Annexin V and 7-AAD after 48 hours treatment with different concentrations of doxycycline. Early apoptotic cells were positive for Annexin V and negative for 7-AAD when analyzed by flow cytometry. As shown in Figure 3E, the number of apoptotic cells increased significantly during Notch1 induction of higher amount. The above observations suggested that the Notch1 suppressed DTC cell growth by inhibition of proliferation and induction of apoptosis.
To further evaluate the effect of Notch1 induction on cell migration, a wound healing assay was carried out using Notch1 inducible DTC cells. FTC236-Notch1 cells in the absence of doxycycline exhibited an average of 66% gap closure after 24 hours, while those with NICD induction only showed an average of less than 20% gap closure (P <0.01; Supplementary Figure S2). Additionally, by a Boyden chamber assay, significant decrease of migrated cells was observed in FTC236-Notch1 cells with NICD induction compared with non-induced cells (66 cells/ field vs. 188 cells/ field). The control cell line FTC236-TRE, with or without doxycycline treatment, showed similar amount of migrated cells (Figure 3F). These findings further suggested that induction of NICD inhibited cancer cell migration.
Activation of Notch1 signaling in orthotopic xenografts
As cell migration ability, which is important for tumor metastases, could be regulated by Notch1 signaling, we next studied the functional consequences of Notch1 induction using an in vivo orthotopic thyroid cancer model. This model provides more clinical relevant information especially on tumor progression and metastases, which is superior to the subcutaneous xenograft model (26,29-31). Since the orthotopic tumor cannot be palpable especially during the initial stage of development, the FTC236-Notch1 and FTC236-TRE cells were stably labeled with both luciferase and RFP to facilitate tumor monitoring using the optical imaging system. Over 97% of the FTC236-Notch1 cells were confirmed to be positive for luciferase and RFP in both early and late passages by flow cytometry (Supplementary Figure S3). The mice in the treatment groups were given food containing doxycycline one week after the tumor cell injection. By measuring the bioluminescence intensity during different time points, Notch1 induction by docycycline chow in the animals bearing FTC236-Notch1 xenografts led to significantly slower growth of the primary tumors compared with FTC236-Notch1 xenografts without Notch1 induction (no doxycycline chow) (Figure 4A and B). The average tumor volume of FTC236-Notch1 xenografts measured at day 42 by total flux (photons/sec) was 1.5E+9 in Notch1 induction group (Dox+), versus 5.6E+9 in the group without Notch1 induction (Dox-) (P=0.047). In addition, tumor volume of FTC236-TRE xenografts did not differ dramatically between the two groups fed either with doxycycline chow or with normal chow (4.6E+9 vs.4.9E+9 total flux at day 42; P>0.05). When all mice were euthanized at the end point, both the primary tumors and lungs were dissected for further examinations. We confirmed by both ex-vivo optical imaging and pathological evaluation that 80% of the FTC236-Notch1 tumors developed lung metastases without Notch1 induction (Figure 4C). In the Notch1-induced group, the rate for lung metastases was 20%. The average volume of lung metastases measured by total flux was 1.1E+4 for the Notch1-induced group, versus 7.4E+3 for the group without induction. Furthermore, the frequency for lung metastases in the groups of FTC236-TRE did not show significant difference between those fed with and without doxycycline (100% vs. 80%).
Figure 4.
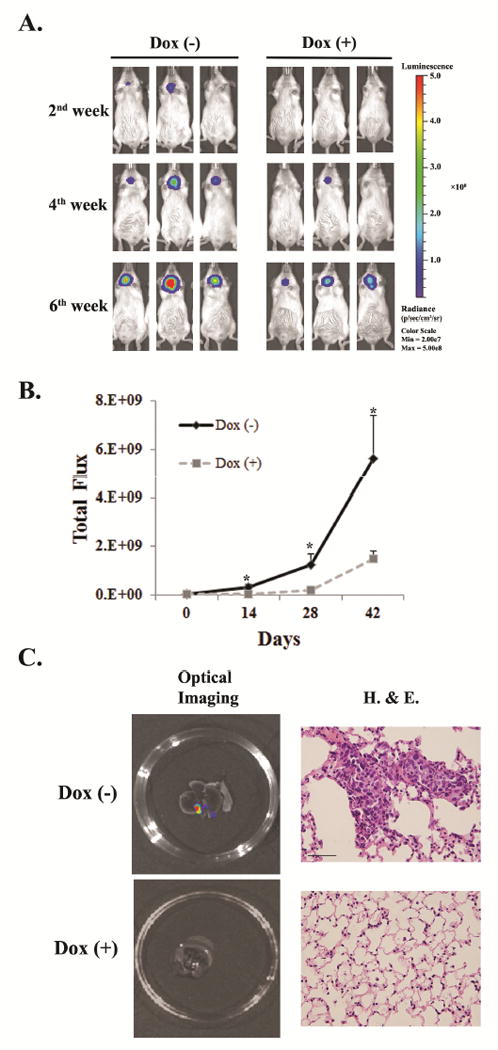
Notch1 induction caused reduced tumor growth and pulmonary metastases in thyroid cancer orthotopic mouse model. (A). Representative imaging for tumor growth in both Notch-induction group (Dox+) and control group (Dox-) during different time points using IVIS. (B). Total flux (photons/sec) was obtained by IVIS for each animal. Each dot represents mean± SEM; * indicates p <0.05. (C). Representative optical imaging and microscopic view (400X) of pulmonary metastases in thyroid cancer without Notch1 induction (Dox-), which was less frequently observed in tumors with Notch1 induction (Dox+). “Dox” indicates doxycycline;Scale bar: 20μm.
SERPINE1, a down-stream target of Notch1 signaling in DTC
Since little is known about the down-stream target(s) which may mediate Notch1-dependent cellular migration, we then carried out cDNA microarray analysis using RNA samples from FTC236-Notch1 cells with or without doxycycline treatment to find out the potential effector (s). Among the top 50 most significant hits (sequences), 8 represented the same gene SERPINE1 (see Supplementary Table S2). SERPINE1 showed consistent down-regulation in microarray when Notch1 was activated. To further confirm our findings, SERPINE1 expression was assessed in both FTC236-Notch1 and FTC236-TRE cells treated with doxycycline. Induction of Notch1 was associated with reduced levels of SERPINE1 in a dose-dependent manner; while treatment with doxycycline without activation of Notch1 did not affect the expression of SERPINE1 in control FTC236-TRE cells (Figure 5A). By quantitative real-time PCR, SERPINE1 mRNA was shown to be significantly down-regulated in FTC236-Notch1 cells with Notch1 induction while it remained similar in FTC236-TRE cells before and after treatment of doxycycline (Figure 5A). Withdrawal of doxycycline treatment in FTC236-Notch1 cells resulted in reduced expression of NICD back to the minimal level and restoration of a relative higher level of SERPINE1 (Figure 5B). We then knocked Notch1 down using Notch1 siRNA in three other DTC cell lines including TPC1, BCPAP and FTC133, which have shown relatively higher NICD. Increased expression of SERPINE1 was observed in all three cell lines treated with Notch1 siRNA but not control siRNA (Figure 5C). These data implied that SERPINE1 might be one of the down-stream effectors regulated by Notch1 signaling.
Figure 5.
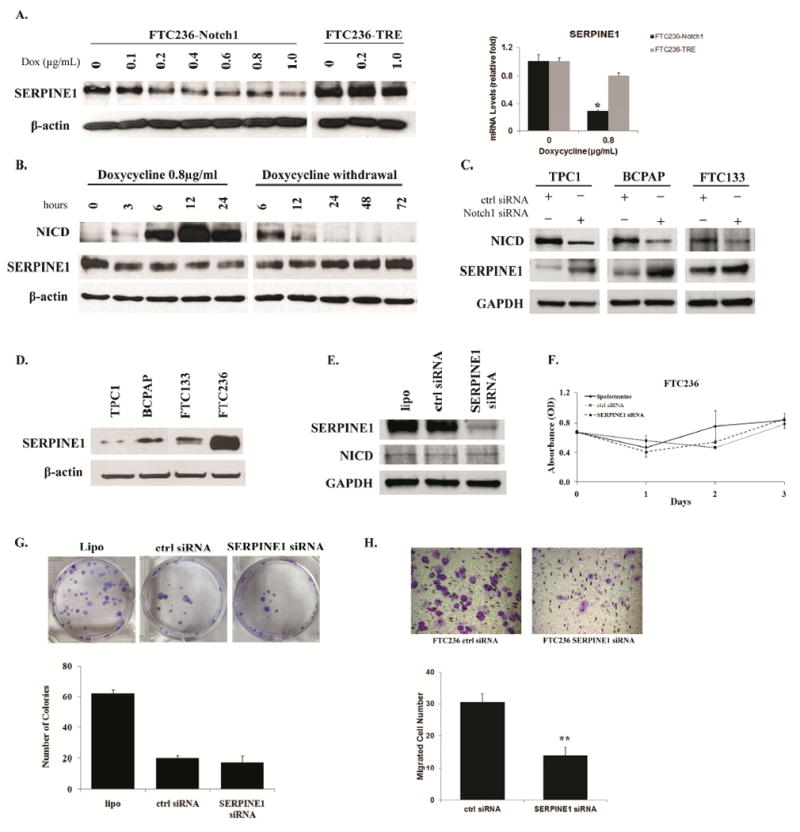
SERPINE1 was regulated by Notch1 signaling. (A). Both FTC236-Notch1 and FTC236-TRE cells were treated with different concentrations of doxycycline. SERPINE1 levels were measured by Western blot and real-time PCR; (B). Dynamic changes of SERPINE1 levels were detected by Western blot during different time points with or withdrawal NICD induction; (C). NICD and SERPINE1 expression was detected by western blot in TPC1, BCPAP and FTC133 transfected with control siRNA or Notch1 siRNA; (D). The endogenous SERPINE1 expression was evaluated by western blot in four different thyroid cancer cell lines; (E) NICD and SERPINE1 expression was detected by western blot in FTC236 cells transfected with lipofectamine alone, control siRNA or SERPINE1 siRNA; Cell viability of FTC236 transfected with lipofectamine alone, control siRNA or SERPINE1 siRNA was measured by MTT assay (F) while cell proliferation was measured by clonogenic assay (G); (H).FTC236 cells, which showed the highest expression of SERPINE1, were transfected with either SERPINE1 siRNA or control siRNA. Cell migration was measured by Boyden chamber assay. Each bar represents mean± SEM; “Dox” indicates doxycycline; “ctrl” indicates control; “lipo” indicates lipofectamine; * indicates p <0.05; **indicates p <0.01; Scale bar: 50 μm.
The basal expression of SERPINE1 was also compared among four thyroid cancer cell lines. Inverse correlation between basal expression of NICD and SERPINE1 was observed; FTC236 which showed minimal NICD level (Figure 2A) revealed the highest expression of SERPINE1 while the other three cell lines with moderate NICD levels presented with much less SERPINE1 (Figure 5D). To investigate whether SERPINE1 is involved in the process of cell growth or migration, we knocked down the expression of SERPINE1 in parental FTC236 cells by specific siRNA. Down-regulation of SERPINE1 did not affect the expression of Notch1 (Figure 5E). FTC236 cells treated with SERPINE1 siRNA demonstrated with a similar growth curve as those with either control siRNA or lipofactamine over a 3-day-incubation (Figure 5F). The numbers of colonies formed during clonogenic assay were similar in FTC236 cells transfected with SERPINE1 siRNA or control siRNA (17 vs. 20 colonies, P>0.05, Figure 5G). Importantly, significantly fewer migrated cells were observed with the SERPINE1 siRNA transfection compared with those transfected with control siRNA (30 cells/field vs. 14 cells/field; Figure 5H). These data suggested that SERPINE1 were actively involved in cancer cell migration but not cell proliferation. SERPINE1 expression was also evaluated in FTC236-Notch1 xenograft samples by IHC. The average score for SERPINE1 staining was 1+ in samples with Notch1 induction, versus 3+ in those without induction (Supplementary Figure S4).
DISCUSSION
Despite the excellent prognosis for most of the DTC patients, those with high risk profiles may still suffer from disease progression including tumor recurrence and metastases, which poses a major challenge to the current treatment modality (14). During the last several decades, various genetic or molecular alterations have been studied to unveil the possible mechanisms which contribute to the aggressiveness of DTC (32). Notch1 signaling, as a fundamental pathway for cell development, has been involved in the initiation and progression of different cancer types including colon cancer, breast cancer, pancreatic ductal adenocarcinoma, and small cell lung cancer (5,33-36). However, in DTC, the role of Notch1 has not been well elucidated. Here, we characterized the biological function of the Notch1 signaling pathway using an inducible system, especially to restore the active intracellular domain. In addition, SERPINE1, an important member in the urokinase plasminogen activating system (uPAS)(37), was first documented in the current study as a Notch1 signaling pathway effector capable of regulating cell migration in a DTC context.
The complexity of Notch1 signaling in cancer biology has been reflected by its dual function either as tumor suppressor or oncogene (38). In DTC, there have been several studies trying to reveal the expression pattern of Notch1 but showing contradictory results (16,17,21,39), which further endorse the complex role of Notch1. To gain a better understanding of the role of Notch1, in the current study, we not only assessed the expression levels of Notch1 in DTC patients but also characterized the function of NICD. Over 60% of the primary tumors from DTC patients demonstrated reduced expression levels of Notch1 compared with their corresponding contra-lateral non-tumor samples. Furthermore, all of the five recurrent/metastatic tissue samples showed much lower expression of Notch1 than the primary tumors. Although the sample size may be too small to draw definitive conclusions, these results suggest that loss of Notch1 could be involved in DTC disease progression. When we compared Notch1 with clinicopathological parameters and outcomes, down-regulation of Notch1 was associated with older age, presence of extrathyroidal invasion, and a higher recurrence rate. Age more than 45 years and extrathyroidal extension both have been used in many DTC risk profiling systems as unfavorable predictive factors including AMES (40) and MACIS scoring (41). In particular, for AJCC pTNM staging (42), age is the first risk factor to consider with: patients younger than 45 years even with distant metastases will still be categorized in stage II. The inverse correlation between Notch1 levels and age may serve as one of the biological explanations.
The dual function of Notch1 signaling can be dependent upon cellular context (6,43) or on the stage of tumor progression (10). Genetic alterations can also affect the function of Notch1 cascade through various kinds of crosstalk (21,22,43). To delineate the Notch1 function in a more specific cellular context, we first carried out gain- and loss- of function studies in BRAF-mutation-negative DTC cells. We observed that knock down Notch1 in TPC1 cells resulted in increased ability of cell migration, which implies that Notch1 may regulate the aggressive behavior of DTC. We then overexpressed NICD using an inducible system in FTC236 cells. The parental FTC236 cells inherit the metastatic phenotype with a strong migration potential and lack detectable endogenous NICD expression. Restoration of NICD, the active fragment of Notch1 receptor(1), is an effective way to activate the signaling as evidenced by enhanced CBF1 binding affinity as well as elevated levels of the canonical pathway components like Hairy Enhancer of Split-1(HES1)(4,35,44). As in many other cancer types where Notch1 serves as a tumor suppressor, we found that Notch1 activation inhibited DTC cell proliferation and tumor growth. As we observed in this study, this proliferation inhibition could be mainly due to the alterations in cell cycle regulators including cyclin B1, cyclin D1 and p21Waf1/Cip1. Similar results have been reported in another study that, in DTC cell lines, transient transfection of Notch1 DNA plasmid causes up-regulation of p21Waf1/Cip1 and down-regulation of cyclin D1(16). Since p21Waf1/Cip1 is a universal inhibitor of cyclin-dependent kinases and its expression is normally regulated by the p53 tumor suppressor protein (45), the results, together with our observations, suggest that growth inhibition on Notch1 expression could be mainly due to cell cycle arrest. Additionally, we also found that Notch1 induction to a higher level led to increased apoptotic cells. This indicated that apoptosis might be another process contributing to growth suppression during higher Notch1 induction. Several Notch1 activating compounds, like resveratrol and chrysin, have demonstrated both growth inhibitory effects on DTC cells via both cell cycle arrest and apoptosis (19,46,47), which are consistent with our current observations.
In addition to the regulatory role in cell proliferation and tumor growth, we found that Notch1 was involved in cancer cell migration and tumor metastatic behavior. Since this function of Notch1 has not been widely reported, little is known about the down-stream target(s) which may mediate Notch1-dependent cellular migration. To find out the potential effector (s), we carried out microarray analysis and identified SERPINE1 as a putative target down regulated by Notch1 activation. SERPINE1 is one of the important members in the urokinase plasminogen activating system (uPAS). Previous studies have shown each uPAS members acts as multitasking factors involved in all steps of tumor progression, from the local growth and spreading of malignant cells to migration and invasion to distant sites (37,48). Clinically, SERPINE1 and its family member urokinase-type plasminogen activator (uPA) both have been accepted as important prognostic markers for breast cancer (49). High expression of SERPINE1 has also been observed in DTC tissue samples, and uPA has been associated with tumor advanced stages as well as reduced disease-free interval (50). In our study, we found that Notch1 activation inhibited SERPINE1 expression on both mRNA and protein levels while withdrawal of Notch1 induction increased expression of SERPINE1. Though the correlation between Notch1 and SERPINE1 has not been previously reported, a recent study on breast cancer showed that uPA is a direct transcriptional target of Notch1 signaling (51), which supports the linkage between Notch1 signaling and uPAS family members. Additionally, we have shown that knock down of SERPINE1 inhibited migration in DTC cells. Similar as in two other studies focusing on pharmacological inhibition of SERPINE1 by two synthesized compounds namely tiplaxtinin and SK-216, both showed the effective suppression on angiogenesis and the inhibition of tumor growth (52,53). These lines of evidence and our studies strongly suggest that SERPINE1 could be an important regulator of cancer progression.
Despite the above findings, this study does possess some limitation. We have shown the biological function of Notch1/SERPINE1 signaling in both PTC and FTC cells lines. However, due to the lack of FTC patient samples, the clinical correlation of Notch1 was observed in a cohort of PTC patients only. Future studies would be worthwhile especially on the detailed expression profiles of Notch1/SERPINE1 in different types of DTC.
Taken together, Notch1 suppresses the aggressive phenotypes of DTC. Restoration of NICD inhibits DTC cell proliferation and migration. More importantly, activation of Notch1 signaling in a DTC xenograft model suppresses tumor growth and pulmonary metastases. Our findings provide the first documentation that SERPINE1, a novel down-stream effector of Notch1, can mediate Notch1-dependent DTC cell migration. Therefore, the Notch1/SERPINE1 axis warrants further investigation as a novel therapeutic target for advanced DTC.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Well-differentiated thyroid cancer (DTC) accounts for the majority of the rapid increase in the general incidence of thyroid carcinoma. Despite the overall excellent prognosis, aggressive DTCs with recurrent or metastatic diseases can be clinically challenging and molecular mechanisms that drive progression remain largely unclear. The results of this study demonstrated that the aberrant expression of Notch1was associated with aggressive phenotypes of DTC including advanced patient age, the presence of extrathyroidal invasion, and higher recurrence rate. Restoration of the intracellular domain of Notch1 in DTC cells inhibited growth of xenograft tumor and reduced cancer metastases, which could be mediated by SERPINE1, a potential down-stream effector responsible for tumor cell migration. These findings revealed a better understanding of Notch1/SERPINE1 signaling as potential therapeutic targets, which may help the drug development and biomarker discovery for advanced thyroid cancer.
Acknowledgments
Financial Support: American Cancer Society Research Scholar Grant; American Cancer Society MEN2 Thyroid Cancer Professorship; Caring for Carcinoid Foundation-AACR Grants for Carcinoid Tumor and Pancreatic Neuroendocrine Tumor Research; NIH R01 CA121115 (HC); The Paul LoGerfo Research Award from The American Association of Endocrine Surgeons (RSS); A postdoctoral traineeship T32 CA157322 (BPJ)
Footnotes
There were no potential conflicts of interest in preparing this manuscript.
References
- 1.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–61. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 3.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–5. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281:39819–30. doi: 10.1074/jbc.M603578200. [DOI] [PubMed] [Google Scholar]
- 5.Hanlon L, Avila JL, Demarest RM, Troutman S, Allen M, Ratti F, et al. Notch1 functions as a tumor suppressor in a model of K-ras-induced pancreatic ductal adenocarcinoma. Cancer Res. 2010;70:4280–6. doi: 10.1158/0008-5472.CAN-09-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12:535–42. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]
- 7.Zweidler-McKay PA, He Y, Xu L, Rodriguez CG, Karnell FG, Carpenter AC, et al. Notch signaling is a potent inducer of growth arrest and apoptosis in a wide range of B-cell malignancies. Blood. 2005;106:3898–906. doi: 10.1182/blood-2005-01-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klinakis A, Lobry C, Abdel-Wahab O, Oh P, Haeno H, Buonamici S, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473:230–3. doi: 10.1038/nature09999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it’s NOTCH what you think. J Exp Med. 2011;208:1931–5. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viatour P, Ehmer U, Saddic LA, Dorrell C, Andersen JB, Lin C, et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011;208:1963–76. doi: 10.1084/jem.20110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–11. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaferri EL, Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981;70:511–8. doi: 10.1016/0002-9343(81)90573-8. [DOI] [PubMed] [Google Scholar]
- 14.Baudin E, Schlumberger M. New therapeutic approaches for metastatic thyroid carcinoma. Lancet Oncol. 2007;8:148–56. doi: 10.1016/S1470-2045(07)70034-7. [DOI] [PubMed] [Google Scholar]
- 15.Ning L, Jaskula-Sztul R, Kunnimalaiyaan M, Chen H. Suberoyl bishydroxamic acid activates notch1 signaling and suppresses tumor progression in an animal model of medullary thyroid carcinoma. Ann Surg Oncol. 2008;15:2600–5. doi: 10.1245/s10434-008-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao X, Ning L, Chen H. Notch1 mediates growth suppression of papillary and follicular thyroid cancer cells by histone deacetylase inhibitors. Mol Cancer Ther. 2009;8:350–6. doi: 10.1158/1535-7163.MCT-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferretti E, Tosi E, Po A, Scipioni A, Morisi R, Espinola MS, et al. Notch signaling is involved in expression of thyrocyte differentiation markers and is down-regulated in thyroid tumors. J Clin Endocrinol Metab. 2008;93:4080–7. doi: 10.1210/jc.2008-0528. [DOI] [PubMed] [Google Scholar]
- 18.Hsu KT, Yu XM, Audhya AW, Jaume JC, Lloyd RV, Miyamoto S, et al. Novel approaches in anaplastic thyroid cancer therapy. Oncologist. 2014;19:1148–55. doi: 10.1634/theoncologist.2014-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu XM, Jaskula-Sztul R, Ahmed K, Harrison AD, Kunnimalaiyaan M, Chen H. Resveratrol induces differentiation markers expression in anaplastic thyroid carcinoma via activation of Notch1 signaling and suppresses cell growth. Mol Cancer Ther. 2013;12:1276–87. doi: 10.1158/1535-7163.MCT-12-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel PN, Yu XM, Jaskula-Sztul R, Chen H. Hesperetin activates the Notch1 signaling cascade, causes apoptosis, and induces cellular differentiation in anaplastic thyroid cancer. Ann Surg Oncol. 2014;21(Suppl 4):S497–504. doi: 10.1245/s10434-013-3459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita AS, Geraldo MV, Fuziwara CS, Kulcsar MA, Friguglietti CU, da Costa RB, et al. Notch pathway is activated by MAPK signaling and influences papillary thyroid cancer proliferation. Transl Oncol. 2013;6:197–205. doi: 10.1593/tlo.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuziwara CS, Kimura ET. High iodine blocks a Notch/miR-19 loop activated by the BRAF(V600E) oncoprotein and restores the response to TGFbeta in thyroid follicular cells. Thyroid. 2014;24:453–62. doi: 10.1089/thy.2013.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–90. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardin H, Guo Z, Shan W, Montemayor-Garcia C, Asioli S, Yu XM, et al. The roles of the epithelial-mesenchymal transition marker PRRX1 and miR-146b-5p in papillary thyroid carcinoma progression. Am J Pathol. 2014;184:2342–54. doi: 10.1016/j.ajpath.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G245–54. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 26.Nucera C, Nehs MA, Mekel M, Zhang X, Hodin R, Lawler J, et al. A novel orthotopic mouse model of human anaplastic thyroid carcinoma. Thyroid. 2009;19:1077–84. doi: 10.1089/thy.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes KR, Zastrow GM, Nukaya M, Pande K, Glover E, Maufort JP, et al. Hepatic transcriptional networks induced by exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chem Res Toxicol. 2007;20:1573–81. doi: 10.1021/tx7003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoelting T, Siperstein AE, Duh QY, Clark OH. Tamoxifen inhibits growth, migration, and invasion of human follicular and papillary thyroid cancer cells in vitro and in vivo. J Clin Endocrinol Metab. 1995;80:308–13. doi: 10.1210/jcem.80.1.7829632. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Park YW, Schiff BA, Doan DD, Yazici Y, Jasser SA, et al. An orthotopic model of anaplastic thyroid carcinoma in athymic nude mice. Clin Cancer Res. 2005;11:1713–21. doi: 10.1158/1078-0432.CCR-04-1908. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Prichard CN, Younes MN, Yazici YD, Jasser SA, Bekele BN, et al. Cetuximab and irinotecan interact synergistically to inhibit the growth of orthotopic anaplastic thyroid carcinoma xenografts in nude mice. Clin Cancer Res. 2006;12:600–7. doi: 10.1158/1078-0432.CCR-05-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nahari D, Satchi-Fainaro R, Chen M, Mitchell I, Task LB, Liu Z, et al. Tumor cytotoxicity and endothelial Rac inhibition induced by TNP-470 in anaplastic thyroid cancer. Mol Cancer Ther. 2007;6:1329–37. doi: 10.1158/1535-7163.MCT-06-0554. [DOI] [PubMed] [Google Scholar]
- 32.Smith N, Nucera C. Personalized therapy in patients with anaplastic thyroid cancer: targeting genetic and epigenetic alterations. J Clin Endocrinol Metab. 2015;100:35–42. doi: 10.1210/jc.2014-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sikandar SS, Pate KT, Anderson S, Dizon D, Edwards RA, Waterman ML, et al. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010;70:1469–78. doi: 10.1158/0008-5472.CAN-09-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530–7. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- 35.Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 2001;61:3200–5. [PubMed] [Google Scholar]
- 36.Demehri S, Turkoz A, Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell. 2009;16:55–66. doi: 10.1016/j.ccr.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulisse S, Baldini E, Sorrenti S, D’Armiento M. The urokinase plasminogen activator system: a target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9:32–71. doi: 10.2174/156800909787314002. [DOI] [PubMed] [Google Scholar]
- 38.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–67. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 39.Geers C, Colin IM, Gerard AC. Delta-like 4/Notch pathway is differentially regulated in benign and malignant thyroid tissues. Thyroid. 2011;21:1323–30. doi: 10.1089/thy.2010.0444. [DOI] [PubMed] [Google Scholar]
- 40.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–53. [PubMed] [Google Scholar]
- 41.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–7. discussion 7-8. [PubMed] [Google Scholar]
- 42.Greene FL. AJCC Cancer Staging Manual. 6. New York: Springer-Verlag; 2002. [Google Scholar]
- 43.Dotto GP. Notch tumor suppressor function. Oncogene. 2008;27:5115–23. doi: 10.1038/onc.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, De Marco MA, Graziani I, Gazdar AF, Strack PR, Miele L, et al. Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res. 2007;67:7954–9. doi: 10.1158/0008-5472.CAN-07-1229. [DOI] [PubMed] [Google Scholar]
- 45.Kaufmann WK, Paules RS. DNA damage and cell cycle checkpoints. FASEB J. 1996;10:238–47. doi: 10.1096/fasebj.10.2.8641557. [DOI] [PubMed] [Google Scholar]
- 46.Phan T, Yu XM, Kunnimalaiyaan M, Chen H. Antiproliferative effect of chrysin on anaplastic thyroid cancer. J Surg Res. 2011;170:84–8. doi: 10.1016/j.jss.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu XM, Phan T, Patel PN, Jaskula-Sztul R, Chen H. Chrysin activates Notch1 signaling and suppresses tumor growth of anaplastic thyroid carcinoma in vitro and in vivo. Cancer. 2013;119:774–81. doi: 10.1002/cncr.27742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–43. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 49.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 50.Ulisse S, Baldini E, Sorrenti S, Barollo S, Gnessi L, Catania A, et al. High expression of the urokinase plasminogen activator and its cognate receptor associates with advanced stages and reduced disease-free interval in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2011;96:504–8. doi: 10.1210/jc.2010-1688. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu M, Cohen B, Goldvasser P, Berman H, Virtanen C, Reedijk M. Plasminogen activator uPA is a direct transcriptional target of the JAG1-Notch receptor signaling pathway in breast cancer. Cancer Res. 2011;71:277–86. doi: 10.1158/0008-5472.CAN-10-2523. [DOI] [PubMed] [Google Scholar]
- 52.Masuda T, Hattori N, Senoo T, Akita S, Ishikawa N, Fujitaka K, et al. SK-216, an inhibitor of plasminogen activator inhibitor-1, limits tumor progression and angiogenesis. Mol Cancer Ther. 2013;12:2378–88. doi: 10.1158/1535-7163.MCT-13-0041. [DOI] [PubMed] [Google Scholar]
- 53.Gomes-Giacoia E, Miyake M, Goodison S, Rosser CJ. Targeting plasminogen activator inhibitor-1 inhibits angiogenesis and tumor growth in a human cancer xenograft model. Mol Cancer Ther. 2013;12:2697–708. doi: 10.1158/1535-7163.MCT-13-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


