Abstract
Purpose
Current normal tissue complication probability modeling using logistic regression suffers from bias and high uncertainty in the presence of highly correlated radiation therapy (RT) dose data. This hinders robust estimates of dose-response associations and, hence, optimal normal tissue—sparing strategies from being elucidated. Using functional data analysis (FDA) to reduce the dimensionality of the dose data could overcome this limitation.
Methods and Materials
FDA was applied to modeling of severe acute mucositis and dysphagia resulting from head and neck RT. Functional partial least squares regression (FPLS) and functional principal component analysis were used for dimensionality reduction of the dose-volume histogram data. The reduced dose data were input into functional logistic regression models (functional partial least squares—logistic regression [FPLS-LR] and functional principal component—logistic regression [FPC-LR]) along with clinical data. This approach was compared with penalized logistic regression (PLR) in terms of predictive performance and the significance of treatment covariate—response associations, assessed using bootstrapping.
Results
The area under the receiver operating characteristic curve for the PLR, FPC-LR, and FPLS-LR models was 0.65, 0.69, and 0.67, respectively, for mucositis (internal validation) and 0.81, 0.83, and 0.83, respectively, for dysphagia (external validation). The calibration slopes/intercepts for the PLR, FPC-LR, and FPLS-LR models were 1.6/−0.67, 0.45/0.47, and 0.40/0.49, respectively, for mucositis (internal validation) and 2.5/−0.96, 0.79/−0.04, and 0.79/0.00, respectively, for dysphagia (external validation). The bootstrapped odds ratios indicated significant associations between RT dose and severe toxicity in the mucositis and dysphagia FDA models. Cisplatin was significantly associated with severe dysphagia in the FDA models. None of the covariates was significantly associated with severe toxicity in the PLR models. Dose levels greater than approximately 1.0 Gy/fraction were most strongly associated with severe acute mucositis and dysphagia in the FDA models.
Conclusions
FPLS and functional principal component analysis marginally improved predictive performance compared with PLR and provided robust dose-response associations. FDA is recommended for use in normal tissue complication probability modeling.
Introduction
Normal tissue complication probability (NTCP) modeling uses radiation therapy (RT) dose data, often in combination with clinical and biological data, to construct statistical models of RT-induced toxicity. There are 2 distinct aims of NTCP modeling: (1) accurate prediction of toxicity outcomes for individual patients; and (2) estimates of associations between treatment covariates and toxicity. Accurate prediction enables clinical decision support (1), treatment plan comparison, treatment modality selection (2), and personalized dose prescription (3). Robust estimates of associations between covariates and toxicity can inform the design of RT planning interventions aimed at reducing toxicity.
A major weakness of many NTCP models is suboptimal dimensionality reduction of the RT dose distribution (reducing the number of variables used to describe the dose distribution from all of the points on the 3-dimensional [3D] dose grid to a small number of summary metrics). To input dose data into statistical models, the 3D dose distribution delivered to an organ at risk (OAR) is reduced to a single or series of scalar metrics, for example, maximum dose or mean dose, or multiple points sampled from the dose-volume histogram (DVH), such as the volume of an OAR receiving at least x cGy (Vx). Ideally, information from each dose level should be explicitly input into the model to prevent loss of potentially important information. However, given the nature of the dose deposition within the patient, adjacent dose levels are very highly correlated (4). This is problematic for many statistical modeling methods, such as the commonly used logistic regression, which often exhibit biased regression coefficients with large standard errors in the presence of collinearity (5). The structure of the correlations is often consistent between patients because the volumes of an OAR receiving adjacent dose levels are highly correlated for all patients. Therefore, if the same or similar treatment techniques are used, this does not necessarily prevent the models from being able to accurately predict outcomes prospectively for new patients. However, it does result in the regression coefficients of the dosimetric covariates being biased and having large standard errors. The apparent regression co-efficients of the dosimetric covariates do not generalizewell to new patients and, hence, should not be used to determine the strength of associations between correlated dose metrics and toxicity, as is commonly done (6).
A small number of studies have attempted to address this issue through using principal component analysis (PCA) to reduce the dimensionality of the DVH data (7-12). However, PCA has been shown to perform poorly when the number of predictors (DVH points) is comparable to, or larger than, the number of observations (patients), as is often the case in NTCP modeling (9, 11). Functional data analysis (FDA) is a statistical framework for analyzing continuous curves rather than discrete measurements (13). Treating an entire curve, for example, a DVH curve, as a single entity removes the problem of correlation (14) and explicitly retains the relationship between points on the DVH curve, which standard, nonfunctional statistical techniques do not capture. Data are represented as curves through the use of basis functions. There are different types of basis functions including a priori fixed bases, such as splines or wavelets, and data-driven bases, for example, functional principal component analysis (FPCA). Functional logistic regression uses functional data to predict binary outcomes. It is well suited to NTCP modeling because of the continuous nature of DVH curves and the binary nature of toxicity endpoints. Functional logistic regression has recently been applied to NTCP modeling by Benadjaoud et al (15), using FPCA (16) for dimensionality reduction of the DVH data. However, FPCA (and nonfunctional PCA) is unsupervised (it does not use outcome data), which may be a limitation for NTCP modeling. The FPCA components with the most variance in the RT dose data may not be the ones that are most strongly associated with the toxicity outcome of interest. Functional partial least squares regression (FPLS) (17, 18) is a supervised analogue of FPCA. It overcomes this limitation through generating uncorrelated covariates (FPLS components) in the linear space of the predictors, accounting for the correlation between those predictors and outcome, in this case toxicity. As partial least squares regression (and FPLS) uses the outcome (toxicity) data in establishing the components, it often outperforms PCA (and FPCA) in prediction tasks (19). However, because of the inclusion of outcome data, it is more susceptible to overfitting.
In this study we applied FPLS and FPCA to NTCP modeling of severe acute mucositis and dysphagia. We compared our novel application of FDA with nonfunctional penalized logistic regression (PLR) models. The aims of this study were to (1) determine whether using FPLS or FPCA to reduce the DVH data would improve predictive performance compared with PLR; and (2) assess whether FPLS or FPCA would lead to more robust estimates of associations between DVH data and toxicity than PLR.
Methods and Materials
Patient data
Data from 351 head and neck RT patients, enrolled in 1 of 6 different clinical trials (20-24) (International Standard Randomised Controlled Trial Number 81772291), were used to train and internally validate severe acute mucositis and dysphagia NTCP models. Data from the same patients were used for the modeling of both toxicities. This dataset is described in Appendix A (available online at www.redjournal.org) and the publication (25). Mucositis and dysphagia were both consistently scored for all studies using the Common Terminology Criteria for Adverse Events version 2.0 instrument (26) or version 3.0 instrument (27). The mucositis and dysphagia grading systems are nearly equivalent in both versions. Both toxicities were recorded, prospectively, for all patients prior to the start of RT, weekly during RT, and at 1 to 4 and 8 weeks after RT by head and neck cancer specialists trained in the use of the scoring systems, using standard trial protocols. The toxicity outcome was defined as the peak grade of toxicity, dichotomized into grade 3 or worse (severe) and less than grade 3 (non-severe). Grade 3 mucositis corresponds to confluent mucositis and grade 3 dysphagia corresponds to feeding-tube dependence for >24 hours. Patients with baseline toxicity were excluded from the analysis. To attempt to reduce bias at the expense of statistical power, patients with any missing toxicity scores and a peak score below 3 were excluded from the analysis. A detailed justification for this approach is provided in Appendix B (available online at www.redjournal.org). Of the 351 patients, 183 met the inclusion criteria for mucositis modeling (severe mucositis incidence, 73%) and 179 met the inclusion criteria for dysphagia modeling (severe dysphagia incidence, 66%). Ninety head and neck RT patients treated at Washington University School of Medicine in St Louis with acute dysphagia data available were used as an external validation cohort for the dysphagia models (severe dysphagia incidence, 48%). In this cohort severe acute dysphagia was defined as the requirement for percutaneous endoscopic gastrostomy (PEG) tube insertion. It should be noted that there was a slight difference in the scoring systems because of the data available. All centers involved in treating patients included in this study used a reactive approach to PEG insertion, that is, delaying insertion until deemed clinically necessary.
Induction chemotherapy (yes or no), concurrent chemotherapy regimen (cisplatin, carboplatin, 1 cycle of cisplatin followed by 1 cycle of carboplatin, or none), definitive versus postoperative RT, primary disease site (grouped into oropharynx or oral cavity, nasopharynx or nasal cavity, hypopharynx or larynx, parotid gland, and unknown primary), age, and sex were also included as covariates in the models. Concurrent chemotherapy was administered in 2 cycles, on day 1 and day 29 of RT. A comparison of the clinical covariate data in the training and external validation datasets is provided in Appendix C (available online at www.redjournal.org).
RT dose data
The extended oral cavity (25) and pharyngeal mucosa (described in Appendix D) were contoured by clinical oncologists and used as OARs in the mucositis and dysphagia models, respectively. The physical dose distribution was converted to the fractional dose distribution (physical dose delivered in each fraction). This has been shown to be appropriate for NTCP modeling of acute toxicity (28) as the toxicities often develop before the total dose is administered. The fractional dose distribution was described by the normalized cumulative DVH. Preliminary work indicated that corrections for different fractionation regimens based on radiobiological models made negligible difference to the results. This is because the fractionation regimens used (Appendix A; available online at www.redjournal.org) were similar. An alternative approach would be to use the cumulative dose delivered up to the appearance of the toxicity endpoint. However, treating clinicians’ subjective choice of when to initiate a feeding-tube intervention would lead to substantial noise in the cumulative dose delivered up to the time of intervention.
PLR model
For the nonfunctional model, the fractional DVH curves were discretely sampled from 0.2 Gy to 2.6 Gy at 0.2 Gy intervals. This sampling was chosen to encompass the entire range of OAR doses with enough granularity to capture the shapes of the DVHs. These DVH measurements were input into a PLR model along with the clinical covariates. Penalization was performed using the least absolute shrinkage and selection operator (LASSO) (29). LASSO reduces the magnitude of the regression co-efficients, setting some to 0, to prevent overfitting. In the context of correlated variables, it reduces the impact of multicollinearity. The penalization strength was selected by 10-fold cross validation with the value producing the highest average (over all of the cross validation folds) area under the receiver operating characteristic curve (AUC) on cross validation selected.
Functional data analysis
The fractional DVH curves (sampled from 0 Gy to 2.60 Gy in 0.01-Gy intervals) were represented using penalized FPCA (16, 30) and penalized FPLS (17, 31) basis functions. FPCA is a dimensionality reduction technique that represents the functional DVH data as orthonormal vector components explaining the maximum variance between patients in the DVH curves. The orthonormality constraint removes the collinearity in the dose metrics used for subsequent modeling and, hence, overcomes the limitations associated with modeling collinear data. The functional principal components represent the functional DVH data (normalized volume as a function of dose, d for patient i), Vi(d), as the sum of the eigenfunctions, ξk(d), weighted by their coefficients, cik:
| (1) |
where μ(d) is the mean V(d) and cik describes the score for functional principal component k for the DVH of patient i and is given by:
| (2) |
The eigenfunctions, , and their corresponding eigenvalues (describing the amount of variance explained by each eigenfunction), λ1 ≥ λ2 ≥ …, are determined by eigendecomposition (factorization into eigenvalues and eigenvectors) of the covariance operator, Σ, where:
| (3) |
in which d1 and d2 are two different dose levels and E is the expected value. V(d) can be approximated by a small number of principal components, kn, assuming that cik = 0 for k > kn, to achieve dimensionality reduction to a small number of basis functions efficiently describing the variation between patients in the DVH data:
| (4) |
The eigenfunctions and their coefficients can then be used in subsequent analyses. The FPCA components can be used to estimate a toxicity outcome for patient i, yi, using a functional linear model (30, 32):
| (5) |
where α is the intercept and εi is a centered random error.
When FPCA is used to describe the DVH data, β(d) represents a “weighting function” describing the amount of variation between patients at all dose levels on the DVH. It can be approximated by kn eigenfunctions:
| (6) |
An estimate of the response, ŷi, can be made using the following [with the derivation described in a previous publication (30)]:
| (7) |
where
| (8) |
The model was fit to the data, placing penalization on the curvature (second derivative) of the eigenfunctions, by:
| (9) |
where r is the amount of penalization, P is the vector (0, 0, 1) that defines the penalty matrix such that the second derivative (curvature) is penalized, and yi is the actual outcome (toxicity) data for patient i. The choice of which components to include (within the first 5 components) and the magnitude of the roughness penalty, r, to apply (selected from a set of values in the range from 0 to 1350) to best estimate the toxicity outcomes were determined using model selection criteria (MSC) (16) with the Bayesian information criterion:
| (10) |
where n is the number of patients. This penalizes the model complexity to reduce overfitting. Models with different values of r and kn were generated, and the combination of values that minimized MSC was selected. The FPCA or FPLS components included affect the smoothness of the estimate of the β(d) function as the dominant mode of variation tends to be smooth and roughness tends to increase for subsequent modes of variation, in part because of the orthogonality constraint.
FPLS is similar to FPCA but uses the response (toxicity) data in constructing the FPLS components (17), , to establish orthogonal components that have maximum covariance to the response. This is achieved through maximizing the squared covariance between Vi(d) and the response, yi, with the constraint that all components are mutually orthogonal (31). This takes the place of the eigendecomposition used for FPCA, described in equation 3. The iterative algorithm used to compute the FPLS components was described previously (33). When FPLS is used for dimensionality reduction of the DVH data, β(d) can be interpreted as a data-driven weighting function for the importance of each dose level in causing severe toxicity. It is important to consider that, as this is a data-driven approach, the weighting function is an estimate of the “true weighting function” over the range of available data and is influenced by the structure (ie, distribution in dose-volume space) of the available data. MSC was performed for the FPLS analysis in the same manner as for FPCA. The FPCA and FPLS analyses were bootstrapped with 2000 replicates to assess the uncertainty in the shapes of the components.
The optimal FPCA and FPLS components (those producing the lowest MSC) were used as basis functions as input into functional logistic regression (34, 35) models (functional principal component—logistic regression [FPC-LR] and functional partial least squares—logistic regression [FPLS-LR]) along with the (nonfunctional) clinical covariates. The functional logistic regression model describes the probability of patient i having severe toxicity, P(yi = 1), and is given by:
| (11) |
using the substitution for the functional linear model described in equation 7, where α is the intercept and are the nonfunctional covariates with regression coefficients . Maximum likelihood estimation of the regression coefficients was performed using iteratively reweighted least squares.
Model comparisons
The predictive performance and generalizability of the models (addressing aim 1) were assessed in terms of discrimination, calibration, and overall performance on internal validation, as well as additionally for the dysphagia models on external validation. The discriminative abilities of the models were assessed using the AUC. Calibration was evaluated by the slope and intercept of a logistic regression model of the actual toxicity against the predicted probability of severe toxicity (36, 37). Overall model performance was measured using the Brier score (BS) (38). It is defined as:
| (12) |
where pt is the predicted probability, yt is the actual outcome, and N is the number of predictions. The score takes a value between 0 and 1, with lower values indicating better performance.
For the internal validation, the performance metrics were “corrected for optimism” using bootstrapping with 2000 replicates (39). The optimism-corrected performance metrics, Mcorrected, were calculated by:
| (13) |
where Mapparent is the performance metric, for example, AUC, calculated using all of the training data to both fit the model and evaluate its performance, and the optimism, O, is given by:
| (14) |
where B is the number of bootstrap replicates, Mb,boot is the performance metric calculated using the bootstrap dataset b to both fit and evaluate model performance, and Mb,orig is the performance of the model fit using the bootstrap dataset b evaluated on the original dataset. This provides an unbiased estimate of internal validity, penalizing for over-fitting. Model hyper-parameter tuning, such as the selection of the amount of penalization for the PLR models and the selection of components and penalization for the FDA models, was performed for each bootstrap replicate. This prevents any “data leakage” from the training data into the internal validation data. The dysphagia models were used to predict severe dysphagia probability for the external validation cohort. Those predictions were compared to the actual PEG-dependence data for the cohort and the same performance metrics calculated. The uncertainties of the odds ratios (addressing aim 2) were assessed by calculating bootstrapped 95% confidence intervals with 2000 replicates. Statistical analysis was performed using the statistical computing R language version 3.2.4 (40) and the fda.usc version 1.2.2 (41), glmnet version 2.0 (42), rms version 4.5 (43) and val.prob.ci.2 (44) packages.
Results
For FPCA, the variances in the DVH data explained by the first 5 FPCA components were 80.8%, 12.5%, 3.7%, 1.2%, and 0.6% for mucositis and 70.8%, 14.5%, 5.6%, 4.4%, and 1.6% for dysphagia. For FPLS, the variances explained by the first 5 FPLS components were 78.1%, 16.9%, 2.0%, 2.5%, and 0.6% for mucositis and 76.2%, 8.6%, 11.2%, 2.7%, and 1.3% for dysphagia. The model selection resulted in the first 2 components being selected for the FPCA and FPLS mucositis models and only the first component being selected in both of the dysphagia FDA models. Penalization of 1342 was chosen by the model selection for the mucositis FPCA model, 0 for the mucositis FPLS model, and 1350 for both of the dysphagia FDA models.
Figure 1 shows the first FPCA and FPLS components for the mucositis and dysphagia models. Bootstrapping the FPCA and FPLS indicated that the shapes of the first FPCA and FPLS components were very similar irrespective of the random sample of patients selected. There was a general trend that the FPCA and FPLS loadings increased with increasing dose and sharply decreased to 0 at the maximum dose. The FPCA components indicate that the variation between patients in the volume of OAR irradiated to a certain dose level increased with increasing dose level. The same trend in the FPLS components indicates that higher doses were more strongly associated with severe toxicity. The decrease in the first FPCA and FPLS component loadings at around 1.8 Gy (Fig. 1) for the dysphagia training data is indicative of reduced variation in this region of the DVHs between patients. This is likely to be because most of the variation in the pharyngeal mucosa dose distribution between patients is related to the variation in volume of overlap of the 2 different planning target volumes (whose prescription dose levels correspond to the positions of the 2 peaks in the FPCA and FPLS components) with the pharyngeal mucosa.
Fig. 1.
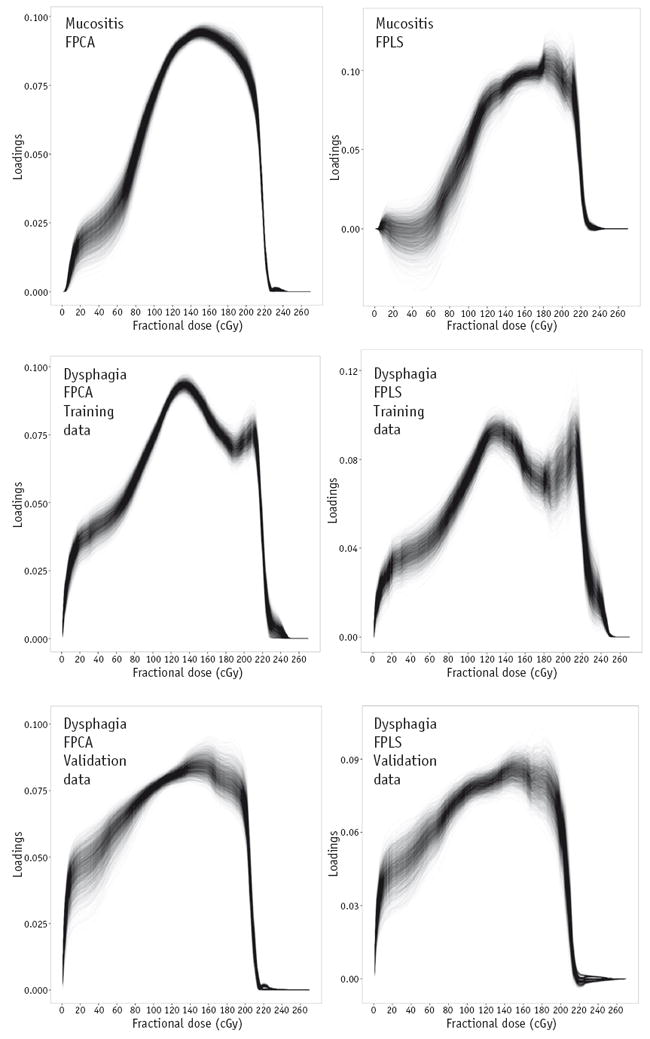
First functional principal component (left column) and first functional partial least squares component (right column) for mucositis training (top row), dysphagia training (middle row), and dysphagia external validation (bottom row) data bootstrapped with 2000 replicates. Each line represents 1 bootstrap sample. The functional principal components show the variance in the patient dose-volume histograms over the range of dose levels. The functional partial least squares components show the covariance between the patient dose-volume histograms and toxicity outcomes over the range of dose levels. Note that the components for the validation data set are shown for comparison with the training data and were not used in any of the model training or validation tasks.
Abbreviations: FPCA = functional principal component analysis; FPLS = functional partial least squares regression.
For the PLR, FPC-LR, and FPLS-LR modeling, oropharynx or oral cavity and no concurrent chemotherapy were removed as covariates to prevent perfect collinearity (correlation matrices are shown in Appendix E; available online at www.redjournal.org). Odds ratios for other primary disease sites are thus relative to oropharynx or oral cavity, and odds ratios for concurrent chemotherapy are relative to no concurrent chemotherapy.
Regarding aim 1, the predictive performance of the 3 different mucositis and dysphagia models, as assessed by internal and external (for dysphagia) validation, is displayed in Table 1. The mucositis models had modest (PLR and FPLS-LR) or modest to good (FPC-LR) discriminative ability [using the interpretation previously described (45)] on internal validation. The discriminative abilities and overall performances of the FPC-LR and FPLS-LR models were marginally better than the PLR model. Calibration was relatively poor for all of the models, with the FPC-LR and FPLS-LR models overfitting the data (calibration slope <1) and the PLR model underfitting the data (calibration slope >1). The underfitting exhibited by the PLR models was likely due to over-shrinkage of the regression coefficients by the LASSO penalization caused by high multicollinearity. It should be noted that the “correction for optimism” may have improved the calibration of the PLR models, as they underfit the data.
Table 1.
Predictive performance of mucositis and dysphagia models on internal validation (corrected for optimism by bootstrapping with 2000 replicates) and external validation (for dysphagia models)
| Model | AUC | Brier score | Calibration slope | Calibration intercept |
|---|---|---|---|---|
| Mucositis | ||||
| PLR | 0.65 | 0.21 | 1.6 | −0.67 |
| FPC-LR | 0.69 | 0.19 | 0.45 | 0.47 |
| FPLS-LR | 0.67 | 0.20 | 0.40 | 0.49 |
| Dysphagia* | ||||
| PLR | 0.74/0.81 | 0.20/0.18 | 1.2/2.5 | −0.15/−0.96 |
| FPC-LR | 0.76/0.83 | 0.19/0.18 | 0.59/0.79 | 0.21/−0.04 |
| FPLS-LR | 0.75/0.83 | 0.20/0.18 | 0.56/0.79 | 0.22/0.00 |
Abbreviations: AUC = area under receiver operating characteristic curve; FPC-LR = functional principal componentelogistic regression; FPLS-LR = functional partial least squares—logistic regression; PLR = penalized logistic regression.
For the dysphagia models, the metrics of predictive performance are given as internal validation/external validation.
The discrimination and calibration of the dysphagia models were better than the mucositis models. All 3 dysphagia models had good discriminative ability on internal validation. The discriminative abilities of all 3 models increased on external validation, with the PLR model showing good to excellent discrimination and the FPC-LR and FPLS-LR models showing excellent discrimination. The overall performance of all of the models was similar, both on internal validation and on external validation. Calibration of all of the models on internal validation was modest, with the PLR model under-fitting the data and the FDA models overfitting the data. The FPC-LR and FPLS-LR models had substantially better calibration than the PLR model on external validation. The FPLS-LR model had marginally better calibration than the FPC-LR model on external validation. A logistic calibration curve for the external validation of this model is shown in Figure 2. The curve lies close to the identity line, indicating good model calibration on external validation.
Fig. 2.
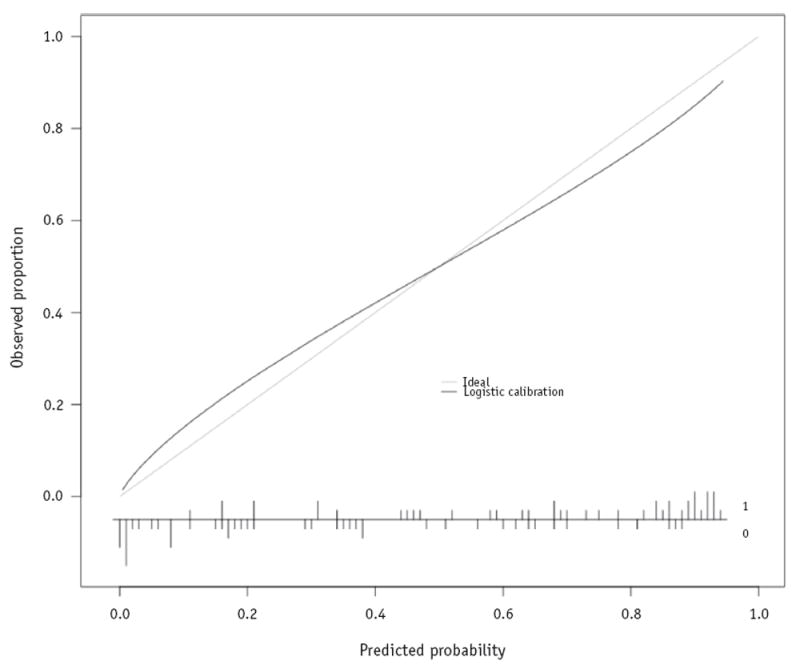
Logistic calibration curve of the functional partial least squares—logistic regression dysphagia model predictions against actual toxicity outcome for the external validation data. The relative frequency distribution of the raw predicted probabilities, along with the actual outcome (where 0 indicates non-severe dysphagia and 1 indicates severe dysphagia), is displayed at the bottom of the figure.
Concerning aim 2, the results of the bootstrapped penalized and functional logistic regression odds ratios are shown in Tables 2-4. The odds ratios for the covariates in the PLR models were often set to 1 by the LASSO penalization. In the mucositis and dysphagia PLR models, none of the covariates was significantly associated with severe toxicity. Conversely, there was a significant association between the first FPLS component and severe toxicity in the mucositis and dysphagia FPLS-LR models. The first FPCA components were not significantly associated with severe mucositis or dysphagia. Compared with the first FPLS components, slightly less weight was given to the higher doses (Fig. 1). It should be noted that the sign of the FPCA component loadings is arbitrary, so the fact that the odds ratios are <1 does not indicate that there is an inverse correlation between RT dose and severe toxicity.
Table 2.
Odds ratios for penalized logistic regression models
| Covariate | Mucositis model
|
Dysphagia model
|
||
|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Intercept | 2.512 | 0.016-12.43 | 0.360 | 0.007-2.583 |
| Male | 1.000 | 1.000-2.554 | 1.000 | 1.000-1.945 |
| Age | 1.000 | 0.971-1.006 | 1.000 | 0.980-1.000 |
| Definitive RT | 1.000 | 0.110-1.000 | 1.000 | 0.544-1.000 |
| Induction chemotherapy | 1.000 | 0.410-1.166 | 1.000 | 1.000-2.089 |
| Cisplatin | 1.000 | 1.000-3.464 | 1.277 | 1.000-3.230 |
| Carboplatin | 1.000 | 0.361-4.015 | 1.000 | 1.000-4.278 |
| Cis-carbo | 1.000 | 0.136-1.769 | 1.000 | 0.989-2.930 |
| Hypopharynx or larynx | 1.000 | 1.000-14.71 | 1.000 | 1.000-2.203 |
| Nasopharynx or nasal cavity | 1.000 | 0.905-6.190 | 1.000 | 0.247-1.000 |
| Unknown primary | 1.000 | 0.022-1.000 | 1.000 | 0.945-1.210 |
| Parotid | 0.814 | 0.231-2.546 | 0.600 | 0.208-1.000 |
| V020 | 1.000 | 1.000-1.119 | 1.000 | 1.000-1.031 |
| V040 | 1.000 | 0.891-1.000 | 1.000 | 1.000-1.014 |
| V060 | 1.000 | 1.000-1.032 | 1.000 | 1.000-1.003 |
| V080 | 1.000 | 1.000-1.050 | 1.000 | 1.000-1.023 |
| V100 | 1.000 | 0.934-1.000 | 1.000 | 1.000-1.029 |
| V120 | 1.000 | 1.000-1.084 | 1.019 | 1.000-1.044 |
| V140 | 1.000 | 0.917-1.000 | 1.000 | 1.000-1.019 |
| V160 | 1.000 | 1.000-1.038 | 1.000 | 1.000-1.011 |
| V180 | 1.002 | 1.000-1.085 | 1.000 | 0.997-1.009 |
| V200 | 1.000 | 0.949-1.007 | 1.000 | 1.000-1.019 |
| V220 | 1.000 | 1.000-1.098 | 1.008 | 1.000-1.031 |
| V240 | 1.000 | 0.616-1.154 | 1.000 | 1.000-1.025 |
| V260 | 1.000 | 1.000-1.000 | 1.000 | 1.000-1.000 |
Abbreviations: Cis-carbo = 1 cycle of cisplatin followed by 1 cycle of carboplatin; RT = radiation therapy; 95% CI = 95% confidence interval calculated by bootstrapping model fitting with 2000 replicates; Vx = volume of organ receiving x cGy of radiation per fraction.
Table 4.
Odds ratios for functional partial least squares—logistic regression models
| Covariate | Mucositis model
|
Dysphagia model
|
||
|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Intercept | 12.90 | 0.961-2.424×1010 | 1.634 | 0.128-104.4 |
| Male | 1.539 | 0.620-4.757 | 1.661 | 0.472-4.719 |
| Age | 0.991 | 0.947-1.033 | 0.988 | 0.942-1.029 |
| Definitive RT | 0.260 | 7.707×10−11-1.245 | 0.975 | 0.046-7.831 |
| Induction chemotherapy | 0.484 | 0.064-2.442 | 1.100 | 0.222-7.866 |
| Cisplatin | 2.246 | 0.728-11.33 | 4.235 | 1.083-20.88* |
| Carboplatin | 1.315 | 0.110-1.051×108 | 4.393 | 0.580-8.424×107 |
| Cis-carbo | 0.313 | 8.668×10−9-3.303×107 | 2.245 | 0.324-4.247×107 |
| Hypopharynx or larynx | 4.169 | 0.506-484.8 | 1.677 | 0.168-1.998×107 |
| Nasopharynx or nasal cavity | 2.336 | 0.350-1.457×108 | 0.266 | 0.028-1.250 |
| Unknown primary | 0.132 | 2.020×10−9-95.47 | 0.903 | 0.092-2.895×106 |
| Parotid | 1.408 | 0.097-56.81 | 1.196 | 0.071-27.80 |
| DVH FPLS1 | 1.004 | 1.002-1.017* | 1.005 | 1.001-1.016* |
| DVH FPLS2 | 1.002 | 1.000-1.047 | - | 1.000-1.041 |
| DVH FPLS3 | - | 1.000-1.110 | - | 1.000-1.009 |
| DVH FPLS4 | - | 1.000-1.107 | - | 1.000-1.009 |
| DVH FPLS5 | - | 1.000-1.085 | - | 1.000-1.009 |
Abbreviations: Cis-carbo = 1 cycle of cisplatin followed by 1 cycle of carboplatin; DVH FPLSx = functional partial least squares component x of dose-volume histogram data; RT = radiation therapy; 95% CI = 95% confidence interval calculated by bootstrapping model fitting with 2000 replicates.
Statistically significant at α = .05 level.
None of the clinical covariates was significantly associated with toxicity in the mucositis models. Concurrent cisplatin was significantly associated with severe acute dysphagia in the FPC-LR and FPLS-LR models but not in the PLR model. None of the clinical covariates was significantly associated with severe toxicity in either of the PLR models.
Discussion
Our results show that FPC-LR and FPLS-LR produced models with marginally better discrimination and overall performance than PLR and superior calibration (aim 1). They also show that FPCA and FPLS are appropriate methods to provide robust estimates of dose-response associations, to inform RT planning, in the presence of highly correlated DVH data (aim 2). We, therefore, encourage the use of FDA methods in future NTCP modeling studies. We suggest that our externally validated dysphagia FPLS-LR model is suitable for clinical decision support. To our knowledge, it represents the severe acute dysphagia model with the best predictive performance to date. Previous models of severe dysphagia during or shortly after RT that measured discrimination had AUC values of 0.62 (46) and 0.74 (47). These studies did not perform external validation. The mucositis FPC-LR model had the best performance on internal validation and should be externally validated to determine its potential to aid clinical decision making. Both models are available at https://github.com/jamiedean/fda-ntcp-models.
The shapes of the first FPLS components indicate that both severe mucositis and dysphagia are most strongly associated with the volume of the oral cavity or pharyngeal mucosa receiving high and intermediate fractional doses (greater than approximately 1.0 Gy). Therefore, RT planning interventions aiming to minimize the incidence of severe acute mucositis and dysphagia should minimize the volumes of oral cavity and pharyngeal mucosa receiving high and intermediate fractional doses, without compromising other aspects of the plan, such as target coverage. Although this is intuitively unsurprising, many RT planning protocols, such as Radiation Therapy Oncology Group (RTOG) 0912, RTOG 0920, and RTOG 1216, set planning objectives based on OAR mean doses, which give equal importance to low doses and high doses. This suboptimal approach is likely taken because of the common use of mean dose to achieve dimensionality reduction in studies aiming to elucidate dose-response relationships. The first FPCA components, which are unsupervised, had similar shapes to the first FPLS components, which are supervised, suggesting that, for this dataset, the variation in severity of toxicities is related to the variations in the DVHs. This suggestion is further supported by the fact that the MSC for FPCA selected the first FPCA component (the one describing the most variation in the DVH data). This will not necessarily be the case for all datasets. The variations in the bootstrapped first FPLS components are slightly wider than those of the first FPCA components (Fig. 1) because of the presence of patients who did not follow the general dose-response trend (ie, received lower doses but had severe toxicity and vice versa). The substantial penalization of the PLR odds ratios (many often being set to 1) shows the limitations of using PLR models to infer associations between correlated dosimetric covariates and toxicity, and hence, we do not recommend its use in this context. Unlike the FDA models, the PLR models were unable to identify that high doses, greater than approximately 1.0 Gy/fraction, had higher correlations with toxicity than low doses, as would be intuitively expected.
The FDA models were also able to identify an association between concurrent cisplatin and severe acute dysphagia. The associations between cisplatin and dysphagia in the PLR model were not significant. This may be due to the fact that concurrent chemotherapy was correlated with the DVH metrics because of patients with parotid gland primary tumors (who receive unilateral, rather than bilateral, irradiation and, hence, lower pharyngeal mucosa doses) not receiving concurrent chemotherapy. The number of patients receiving concurrent carboplatin or a combination of cisplatin and carboplatin was low (Appendix C; available online at www.redjournal.org), leading to large uncertainties in the odds ratios for those covariates. The FDA models featured large uncertainties for the odds ratios of clinical covariates that were highly correlated with other covariates or which applied to small numbers of patients. It should be noted that the regression coefficients of the clinical covariates were not penalized in the FDA models.
There have been very few previous attempts to apply FDA to NTCP modeling (15, 48, 49). These have used either spline basis functions (48, 49) or FPCA (15). To our knowledge, this study represents the first application of FPLS to NTCP modeling. Many previous NTCP modeling studies have not addressed the problem of the high uncertainties of the model regression coefficients caused by multicollinearity. Investigators who have recognized this limitation have avoided the multicollinearity problem by reducing the data describing heterogeneous dose distributions to simple summary metrics, such as mean or maximum dose. However, this leads to suboptimal recommendations for RT planning. For example, using mean dose to optimize or assess RT plans gives equal weight to all dose levels, whereas preferentially minimizing the volume of an OAR receiving high doses rather than low doses is, in fact, likely to result in a lower toxicity incidence.
A limitation of our approach is that, as the technique is an empirical data—driven method, there are decreases in the weighting function describing the relative importance of each dose level with increasing dose, which does not have a biophysical rationale. This should be carefully considered when interpreting dose-response associations from these components. This limitation could be overcome through adopting a Bayesian approach whereby prior knowledge is provided to the model dictating that, with increasing dose level, the weighting function can only remain constant or increase and not decrease. Mathematically, this would take the form of a monotonically increasing prior function (48). The slight difference in the dysphagia scoring systems between the training and external validation cohorts may have reduced the performances of the models on external validation. However, the models performed at least as well on external validation as internal validation. The relatively small size of the external validation cohort should also be considered a potential limitation.
In the future, FPCA or FPLS could be applied to the 3D dose distribution (rather than the DVH) (15), either to a single OAR or to the entire dose grid, encompassing multiple OARs. This would allow associations between spatial aspects of the dose distribution and toxicity to be explored. This would require accurate mapping of the 3D dose distributions onto a common reference.
Conclusions
FPC-LR and FPLS-LR models of severe acute mucositis had marginally better discrimination than PLR on internal validation. FDA models of dysphagia had marginally improved discrimination and substantially superior calibration compared with PLR on external validation, indicating potential advantages for clinical decision support. FPCA and FPLS enable robust estimates of dose-response associations in the context of correlated dose data. This permits understanding of the most beneficial OAR dose-volume levels, Vx to reduce in RT planning. Minimizing the volumes of the oral cavity and pharyngeal mucosa receiving high and intermediate doses is expected to reduce the incidence of severe acute mucositis and dysphagia. We recommend that FDA methods be applied to future NTCP modeling studies.
Supplementary Material
Table 3.
Odds ratios for functional principal component—logistic regression models
| Covariate | Mucositis model
|
Dysphagia model
|
||
|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Intercept | 12.89 | 1.035-1.734×109* | 1.616 | 0.142-77.46 |
| Male | 1.535 | 0.637-4.088 | 1.675 | 0.533-4.880 |
| Age | 0.991 | 0.951-1.029 | 0.988 | 0.943-1.027 |
| Definitive RT | 0.254 | 2.679×10−9-1.773 | 0.997 | 0.080-7.541 |
| Induction chemotherapy | 0.487 | 0.070-1.960 | 1.100 | 0.210-7.670 |
| Cisplatin | 2.251 | 0.745-9.540 | 4.255 | 1.077-19.86* |
| Carboplatin | 1.320 | 0.142-7.314×107 | 4.429 | 0.685-8.332×107 |
| Cis-carbo | 0.311 | 7.815×10−9-2.531×107 | 2.238 | 0.319-4.587×107 |
| Hypopharynx or larynx | 4.371 | 0.512-143.9 | 1.723 | 0.193-1.881×107 |
| Nasopharynx or nasal cavity | 2.370 | 0.308-1.096×108 | 0.263 | 0.026-1.223 |
| Unknown primary | 0.136 | 3.042×10−9-3.707 | 0.859 | 0.077-3.876×106 |
| Parotid | 1.387 | 0.103-40.37 | 1.135 | 0.068-18.72 |
| DVH FPC1 | 0.997 | 0.993-1.007 | 0.996 | 0.990-1.008 |
| DVH FPC2 | 1.003 | 0.992-1.009 | - | 0.992-1.003 |
| DVH FPC3 | - | 0.996-1.003 | - | 0.995-1.000 |
| DVH FPC4 | - | 0.987-1.010 | - | 0.991-1.006 |
| DVH FPC5 | - | 0.971-1.033 | - | 0.991-1.006 |
Abbreviations: Cis-carbo = 1 cycle of cisplatin followed by 1 cycle of carboplatin; DVH FPCx = functional principal component x of dose-volume histogram data; RT = radiation therapy; 95% CI = 95% confidence interval calculated by bootstrapping model fitting with 2000 replicates.
The sign of the functional principal component loadings is arbitrary, so the fact that the odds ratios are <1 does not indicate that there is an inverse correlation between RT dose and severe toxicity.
Statistically significant at α = .05 level.
Summary.
Normal tissue complication probability modeling using logistic regression (LR) suffers from bias and uncertainty in the presence of highly correlated radiation therapy dose data. Consequently, robust estimates of the dose levels most strongly associated with toxicity and, potentially, predictive performance are limited. To overcome this limitation, functional data analysis, which describes the dose-volume histogram as a continuous curve, was applied to modeling of severe acute mucositis and dysphagia and compared with LR. Functional data analysis models showed slightly better predictive performance and more robust dose-response estimates than LR.
Acknowledgments
We wish to thank Hannah Eyles, Emma Wells, James Morden, and Dr Emma Hall at The Institute of Cancer Research Clinical Trials and Statistics Unit for data collation and Dr Cornelis Kamerling, Dr Alex Dunlop, Dr Dualta McQuaid, Dr Simeon Nill, and Professor Uwe Oelfke for general support.
This work was supported by the Engineering and Physical Sciences Research Council, Cancer Research UK Programme Grant A13407, and NHS funding to the National Institute for Health Research Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research. The PARSPORT and COSTAR trials were supported by Cancer Research UK (trial reference No. CRUK/03/005 and CRUK/08/004). None of the funding sources had any role in any part of the study or manuscript preparation and submission.
Footnotes
Supplementary material for this article can be found at www.redjournal.org.
Conflict of interest: none.
References
- 1.Lambin P, Roelofs E, Reymen B, et al. “Rapid learning health care in oncology”—An approach towards decision support systems enabling customised radiotherapy. Radiother Oncol. 2013;109:159–164. doi: 10.1016/j.radonc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Langendijk JA, Lambin P, De Ruysscher D, et al. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach. Radiother Oncol. 2013;107:267–273. doi: 10.1016/j.radonc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 3.van Baardwijk A, Wanders S, Boersma L, et al. Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non-small-cell lung cancer. J Clin Oncol. 2010;28:1380–1386. doi: 10.1200/JCO.2009.24.7221. [DOI] [PubMed] [Google Scholar]
- 4.Yorke ED, Kutcher GJ, Jackson A, et al. Probability of radiation-induced complications in normal tissues with parallel architecture under conditions of uniform whole or partial organ irradiation. Radiother Oncol. 1993;26:226–237. doi: 10.1016/0167-8140(93)90264-9. [DOI] [PubMed] [Google Scholar]
- 5.Slinker BK, Glantz SA. Multiple regression for physiological data analysis: The problem of multicollinearity. Am J Physiol. 1985;249:R1–R12. doi: 10.1152/ajpregu.1985.249.1.R1. [DOI] [PubMed] [Google Scholar]
- 6.Bentzen SM, Constine LS, Deasy JO, et al. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): An introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:3–9. doi: 10.1016/j.ijrobp.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Söhn M, Alber M, Yan D. Principal component analysis-based pattern analysis of dose-volume histograms and influence on rectal toxicity. Int J Radiat Oncol Biol Phys. 2007;69:230–239. doi: 10.1016/j.ijrobp.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 8.Dawson LA, Biersack M, Lockwood G, et al. Use of principal component analysis to evaluate the partial organ tolerance of normal tissues to radiation. Int J Radiat Oncol Biol Phys. 2005;62:829–837. doi: 10.1016/j.ijrobp.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Skala M, Rosewall T, Dawson L, et al. Patient-assessed late toxicity rates and principal component analysis after image-guided radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:690–698. doi: 10.1016/j.ijrobp.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 10.Liang Y, Messer K, Rose BS, et al. Impact of bone marrow radiation dose on acute hematologic toxicity in cervical cancer: Principal component analysis on high dimensional data. Int J Radiat Oncol Biol Phys. 2010;78:912–919. doi: 10.1016/j.ijrobp.2009.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vesprini D, Sia M, Lockwood G, et al. Role of principal component analysis in predicting toxicity in prostate cancer patients treated with hypofractionated intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:415–421. doi: 10.1016/j.ijrobp.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Bauer JD, Jackson A, Skwarchuk M, et al. Principal component, Varimax rotation and cost analysis of volume effects in rectal bleeding in patients treated with 3D-CRT for prostate cancer. Phys Med Biol. 2006;51:5105–5123. doi: 10.1088/0031-9155/51/20/003. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay JO. When the data are functions. Psychometrika. 1982;47:379–396. [Google Scholar]
- 14.Levitin DJ, Nuzzo RL, Vines BW, et al. Introduction to functional data analysis. Can Psychol. 2007;48:135–155. [Google Scholar]
- 15.Benadjaoud MA, Blanchard P, Schwartz B, et al. Functional data analysis in NTCP modeling: A new method to explore the radiation dose-volume effects. Int J Radiat Oncol Biol Phys. 2014;90:654–663. doi: 10.1016/j.ijrobp.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Hall P, Hosseini-Nasab M. On properties of functional principal components analysis. J R Stat Soc Series B Stat Methodol. 2006;68:109–126. [Google Scholar]
- 17.Preda C, Saporta G. PLS regression on a stochastic process. Comput Stat Data Anal. 2005;48:149–158. [Google Scholar]
- 18.Reiss PT, Ogden RT. Functional principal component regression and functional partial least squares. J Am Stat Assoc. 2007;102:984–996. [Google Scholar]
- 19.Worley B, Powers R. Multivariate analysis in metabolomics. Curr Metabolomics. 2013;1:92–107. doi: 10.2174/2213235X11301010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gujral DM, Miah AB, Bodla S, et al. Final long-term results of a phase I/II study of dose-escalated intensity-modulated radiotherapy for locally advanced laryngo-hypopharyngeal cancers. Oral Oncol. 2014;50:1089–1097. doi: 10.1016/j.oraloncology.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Miah AB, Schick U, Bhide SA, et al. A phase II trial of induction chemotherapy and chemo-IMRT for head and neck squamous cell cancers at risk of bilateral nodal spread: The application of a bilateral superficial lobe parotid-sparing IMRT technique and treatment outcomes. Br J Cancer. 2015;112:32–38. doi: 10.1038/bjc.2014.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miah AB, Bhide SA, Del Rosario L, et al. Induction chemotherapy followed by chemo-intensity-modulated radiotherapy for locally advanced nasopharyngeal cancer. Clin Oncol (R Coll Radiol) 2016;28:e61–e67. doi: 10.1016/j.clon.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Richards TM, Bhide SA, Miah AB, et al. Total mucosal irradiation with intensity-modulated radiotherapy in patients with head and neck carcinoma of unknown primary: A pooled analysis of two prospective studies. Clin Oncol (R Coll Radiol) 2016;28:e77–e84. doi: 10.1016/j.clon.2016.04.035. [DOI] [PubMed] [Google Scholar]
- 25.Dean JA, Wong KH, Welsh LC, et al. Normal tissue complication probability (NTCP) modelling using spatial dose metrics and machine learning methods for severe acute oral mucositis resulting from head and neck radiotherapy. Radiother Oncol. 2016;120:21–27. doi: 10.1016/j.radonc.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Common Toxicity Criteria (CTC) Version 2.0. Bethesda, MD: National Cancer Institute; 1999. [Google Scholar]
- 27.National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 28.Tucker SL, Michalski JM, Bosch WR, et al. Use of fractional dose-volume histograms to model risk of acute rectal toxicity among patients treated on RTOG 94-06. Radiother Oncol. 2012;104:109–113. doi: 10.1016/j.radonc.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc Ser B. 1996;58:267–288. [Google Scholar]
- 30.Cardot H, Ferraty F, Sarda P. Functional linear model. Stat Probab Lett. 1999;45:11–22. [Google Scholar]
- 31.Kraemer N, Sugiyama M. The degrees of freedom of partial least squares regression. J Am Stat Assoc. 2011;106:697–705. [Google Scholar]
- 32.Cai TT, Hall P. Prediction in functional linear regression. Ann Stat. 2006;34:2159–2179. [Google Scholar]
- 33.Mevik BH, Wehrens R. The pls package: Principal component and partial least squares regression in R. J Stat Softw. 2007;18 [Google Scholar]
- 34.Escabias M, Aguilera AM, Valderrama MJ. Modeling environmental data by functional principal component logistic regression. Environmetrics. 2005;16:95–107. [Google Scholar]
- 35.Müller HG, Stadtmüller U. Generalized functional linear models. Ann Stat. 2005;33:774–805. [Google Scholar]
- 36.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavlou M, Ambler G, Seaman SR, et al. How to develop a more accurate risk prediction model when there are few events. BMJ. 2015;351:h3868. doi: 10.1136/bmj.h3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brier GW. Verification of forecasts expressed in terms of probability. Mon Weather Rev. 1950;78:1–3. [Google Scholar]
- 39.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 41.Febrero-Bande M, Oviedo de la Fuente M. Statistical computing in functional data analysis: The R package fda.usc. J Stat Softw. 2012;51:1–28. [Google Scholar]
- 42.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 43.Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Cham, Switzerland: Springer International; 2015. [Google Scholar]
- 44.Van Calster B, Nieboer D, Vergouwe Y, et al. A calibration hierarchy for risk models was defined: From utopia to empirical data. J Clin Epidemiol. 2016;74:167–176. doi: 10.1016/j.jclinepi.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: Wiley; 2000. [Google Scholar]
- 46.Otter S, Schick U, Gulliford S, et al. Evaluation of the risk of grade 3 oral and pharyngeal dysphagia using atlas-based method and multivariate analyses of individual patient dose distributions. Int J Radiat Oncol Biol Phys. 2015;93:507–515. doi: 10.1016/j.ijrobp.2015.07.2263. [DOI] [PubMed] [Google Scholar]
- 47.Sanguineti G, Gunn GB, Parker BC, et al. Weekly dose-volume parameters of mucosa and constrictor muscles predict the use of percutaneous endoscopic gastrostomy during exclusive intensity-modulated radiotherapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2011;79:52–59. doi: 10.1016/j.ijrobp.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 48.Schipper M, Taylor JM, Lin X. Bayesian generalized monotonic functional mixed models for the effects of radiation dose histograms on normal tissue complications. Stat Med. 2007;26:4643–4656. doi: 10.1002/sim.2887. [DOI] [PubMed] [Google Scholar]
- 49.Schipper M, Taylor JM, Lin X. Generalized monotonic functional mixed models with application to modelling normal tissue complications. J R Stat Soc Ser C Appl Stat. 2008;57:149–163. doi: 10.1002/sim.2887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


