Abstract
Objective
To study the effect of organ allocation system in the era of continuous flow pumps.
Background
Left Ventricular Assist Devices (LVAD) are increasingly used as a bridge to transplant (BTT) for patients with advanced congestive heart failure (CHF) and are assigned United Network for Organ Sharing (UNOS) high priority status (1B or 1A).
Methods
A retrospective chart review was performed of all patients transplanted between 1/2001–1/2011 at Columbia University Medical Center.
Results
726 adult heart transplantations were performed. 274 BTT patients were implanted with LVAD; of which 227 patients were transplanted. 63 patients were transplanted as UNOS-1B, while 164 were transplanted as UNOS-1A (72%). Of these 164 patients, 65 were transplanted during their 30-day 1A period (43%) and 96 after upgrading to UNOS-1A for device complication (56%). For 452 non-device patients 139 (31%) were transplanted as UNOS-1A, 233 as UNOS-1B (52%), and 80 as UNOS-2 (17%). The percentage of patients bridged with LVAD increased from 19% in 2001 to 64% in 2010 while the number transplanted during their 30 day 1A grace period declined from 57% in 2005 to 16% in 2011; i.e. 84% of BTT patients in 2011 needed more than 30 days 1A time to be transplanted. Most LVAD patients are now transplanted while suffering device complication. There was no difference in post transplant survival between LVAD patients transplanted as UNOS 1B, 1A grace period or for a device complication
Conclusions
As wait time for cardiac transplantation increased the percentage of patients being bridged to transplant with an LVAD has increased with the majority of them transplanted in the setting of device complication.
Introduction
Heart transplantation remains the treatment of choice for end stage heart failure. With the scarcity of donor organs, the waiting time to transplant continues to lengthen (1–3). As a result, left ventricular assist devices (LVAD) are increasingly used as bridge to transplant (BTT). Given the limited durability of earlier generation devices, (1–3) LVAD implantation automatically triggers United Network for Organ Sharing (UNOS) status upgrade (1B) to increase the likelihood of cardiac transplantation as well as a 30 day period of upgrade of priority status to the highest level (1A) at the discretion of the transplant center (4–6). This urgency status can also be upgraded in the event of device malfunction or device associated complication (6). Newer generation mechanical devices using axial continuous-flow pumps have been increasingly used as a bridge to transplantation (7). These smaller pumps have increased durability, and reduced infections compared to earlier generation pumps but other complications inherent to continuous flow physiology such as aortic insufficiency, and recurrent bleeding due to von Willebrand factor deficiency are common (8,9). The aim of this study was to evaluate the evolution of the transplant recipient in the era of increasing use of LVADs and to assess the impact of the UNOS status listing policy on these candidates.
Methods
Study Population
A retrospective chart review was performed from 1/1/01 through 1/1/11. All patients that underwent LVAD as BTT on the waitlist were identified and were included in the study (n=274). Patients with amyloidosis, those undergoing re-transplantation and/or double organ transplants were excluded from analysis. Only long-term left ventricular devices were included in this analysis (Heartmate I, Heartmate II, Duraheart, DeBakey, Toyobo and Ventrassist devices)(Table 1) with all short-term and biventricular assist devices excluded. All patients were followed until death, orthotopic heart transplant (OHT), delisting from the UNOS registry, or “still waiting” status on the UNOS registry on the day of the last observation on 6/1/11. Patient data was obtained from electronic medical records and UNOS data was retrieved from the United Network for Organ Sharing (UNOS) Registry, (beginning from initial listing and following each change, as well as reason for the status change) until the time of the ultimate disposition. The sub-categorization of Status 1A patients is as follows: a) 30 day VAD time; b) Device complication; c) on respirator; d) in CCU on inotropes; e) other (6). The sub-categorization at time of transplant was included in analysis of device outcome post transplant.
Table 1.
Distribution of Left Ventricular Assist Types
| Type of Assist Device | # |
|---|---|
| N= 274 | |
| Debakey | 9 |
| DuraHeart | 7 |
| HMI | 128 |
| HMII | 116 |
| Jarvic2000 | 1 |
| Novacor | 3 |
| Toyobo | 2 |
| Ventra Assist | 8 |
Patients with chronic LVAD support and short-term RVAD support
Outcome data of the BTT population was compared to all patients undergoing their first single organ heart transplant at Columbia University at the same time period without mechanical support prior to the OHT (n=452)
Statistical Analysis
Data was collected using Excel software (2007 Microsoft Corp., Redmond, Washington). All data was analyzed using the Stata version 11.0 (StataCorp, College Station, Texas). Categorical variables were summarized by frequencies and percentages, and were analyzed using the chi-square test. Student’s t-tests for independent samples were used to determine differences in normally distributed data. The Wilcoxon rank sum test was used to determine differences in non-normal distributions. Kaplan-Meier analysis was used to assess differences between survival functions.
Results
Study Population
A total of 274 patients underwent long term LVAD BTT implantation at Columbia University Medical Center. 45% of patients were supported by HMI as bridge to transplant, 42% HM II and the remaining 14% a combination of Duraheart, DeBakey, VentrAssist or Toyobo devices. 40 patients expired prior to transplant and one patient was delisted leaving a study population of 234 patients (Figure 1). Six patients are still awaiting transplant. The clinical characteristics of the patients are shown in Table 2. Mean age was 53±12 years. LVAD patients were mostly males, (82%), Eighty three percent (n=227) were transplanted, 40 died, and 6 are still awaiting transplant. 452 adult patients were transplanted during this period without the benefit of any type of mechanical support.
Figure 1. Outcomes of 274 Bridge to Transplant LVAD Patients.

227 patients were successfully transplanted during the study period. 40 patients expired prior to transplant and one patient was delisted.
Table 2.
Patient Characteristics
| BTT (n=274) | De Novo Tx (n=452) | |
|---|---|---|
|
| ||
| Mean±SD;(n) | Mean±SD;(n) | |
| # Transplanted | 228 | 452 |
| Time VAD to Transplant (days) | 208±199 | |
| Time List to Transplant (days) | 281±440 | 272±479 |
| Dead without OHTx | 40 | |
| # Waiting | 6 | |
| Age (yrs) | 53 ±12 | 52 ±12 |
| UNOS status at List | ||
| 1a | 62 (22.6%) | 22 (4.9%) |
| 1b | 153 (55.8%) | 186 (41.1%) |
| 2 | 59 (21.5%) | 205 (45.4%) |
| UNOS Status at Transplant | ||
| 1a | 170 (64.4%) | 139 (30.8%) |
| 1b | 94 (35.6%) | 233 (51.5%) |
| 2 | 0 | 80 (17.7%) |
| 7 | 10 (3.8%) | |
| Gender | ||
| Female | 50 (18.9%) | 120 (26.5%) |
| Male | 224 (91.1%) | 332 (73.5%) |
Bridge to Transplant
The overall outcome of our BTT cohort is shown in figure 2: 85% of the patient were alive on device or transplanted after a mean follow up time of 25 months. 83% were transplanted after a mean wait time of 288±440 days. Only 15% of the patients were delisted or died.
Figure 2. Competing Risks Analysis of Bridge to Transplant Patients.

The overall outcome bridging patients with LVAD to heart transplantation at our institution was 85.4% of the listed patients are alive or transplanted after a mean follow up time of 25 months.
UNOS Status at the Time of Transplantation
UNOS status at the time of transplantation suggests increasing urgency as the vast majority are now UNOS 1A (Figure 3a). This can be partially explained by the increasing number of mechanical assist device patients who are being transplanted: Of 227 LVAD patients the majority was UNOS 1A (72%) at time of transplant (Figure 1).
Figure 3a. UNOS 1A at Time of Transplant Proportion by year in All Transplant Patients.

Status of current transplant recipients over the past decade demonstrates the increasing urgency such that now the vast majority is in UNOS 1A status.
The proportion of bridged transplant candidates in priority status 1A has remained between 60 – 90% throughout the observation period in our center (Figure 3b), however, while UNOS status 1A patients were traditionally those on inotropic support in the Intensive Care Unit, most 1A patients in our institution qualified for status upgrade because of the LVAD grace period and/or LVAD related complications: 65 patients were transplanted using subcategory (a) i.e. 30 day wait time, 1 patient was transplanted on mechanical ventilation. The high percentage of 1A exception in the years 2001–2004 reflects the use of 30-day grace period for devices whereas most Status 1A listing in the current years is due to device complications (figure 3c). 96 of the 164 pts transplanted as 1A during the observation period were upgraded to UNOS 1A due to device malfunction/complication i.e. infection, refractory arrhythmia, thromboembolic events, bleeding, aortic insufficiency.
Figure 3b. UNOS 1A at Time of Transplant Proportion by year in Bridge to Transplant Patients.

The proportion of bridged transplant candidates in priority status 1A has remained between 60 – 90% throughout the observation period in our center.
Figure 3c. Reason for UNOS 1A at Time of Transplant by year in Bridge to Transplant Patients.

The high percentage of 1A exception in the years 2001–2004 reflects the use of 30-day grace period for devices whereas most Status 1A listing in the current years is due to device complications.
In our retrospective review we found the etiology of UNOS upgrade to 1A in 90 out of 96 patients. Device related Infection was the most common cause 39/90, follow by device failure 25/90, heart failure need for inotropes/RVAD 12/90, recurrent bleeding 5/90, arrhythmia 5/90, aortic insufficiency 1/90 and Others 3/90.
30% of patients with blood type O needed more than 30 days 1A time (i.e. were not transplanted in the 30 day grace period) while this was true for only 10% of the patients in blood type B and 20% of patients with blood type A. In contrast, in transplant recipients without mechanical support, though the percent of patients in the highest urgency category has increased in recent years it is still less than the mechanically supported patients (figure 3d).
Figure 3d. UNOS 1A at Time of Transplant Proportion by year in Transplant Patients without Prior Device.

In transplant recipients without mechanical support, though the percent of patients in the highest urgency category has increased in recent years it is still less than the mechanically supported patients.
Post Heart Transplant Survival
There was no difference in short or long term post transplant survival in patients bridged with devices or medications (Figure 4). Similarly, survival post transplant when categorized into priority groups was not significant for those transplanted with or without prior LVAD support (Figures 5a, 5b). When this was further broken down in the mechanically supported patients, there was no difference in survival between patients who were transplanted as UNOS 1A 30-Day versus patients who were transplanted as UNOS 1A Device Complication or UNOS 1B (1yr: 88% vs. 83% vs. 88%; 3yr: 81% vs. 81% vs. 86%; 5yr: 72% vs. 72% vs. 76%; p = 0.57, Figure 6).
Figure 4. Survival Post Transplant BTT vs Patient without Prior Device.

No difference in short or long term post transplant survival in patients bridged with devices or medications.
Figure 5a. Survival Post Transplant by UNOS Status of Transplant Patients without Prior Device.

Post transplant survival for transplant patients without prior was not significant different when categorized into priority groups.
Figure 5b. Survival Post Transplant by UNOS Status of BTT Patients.
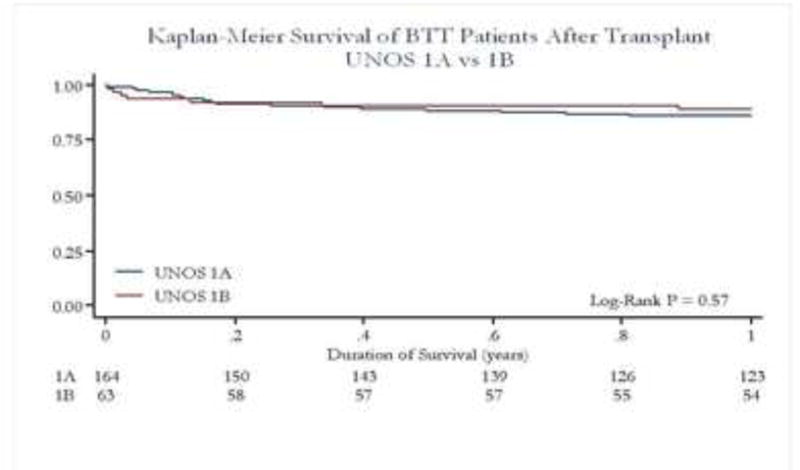
Post transplant survival for the mechanically bridged patients was not significant different when categorized into priority groups.
Figure 6. Survival Post Transplant by UNOS 1A reason of BTT Patients.

No difference in survival between patients who were transplanted as UNOS 1A 30-Day versus patients who were transplanted as UNOS 1A Device Complication or UNOS 1B.
Driveline Infection and its Effect on Heart Transplant Survival
The most common cause for upgrade from UNOS 1B to1A in the device patients was drive line/device infection. Out of 227 transplanted patients 56 had a history of drive-line infection but only 22 had an on going drive-line infection at the time of transplant. Post transplant survival of patients with device infection was not different from patients bridged with device and no infection (Fig 7a) or UNOS 1A patients without prior LVAD (Figure 7b).
Figure 7a. Survival Post Transplant of BTT Patients by History of Driveline Infection vs non Driveline Infection.
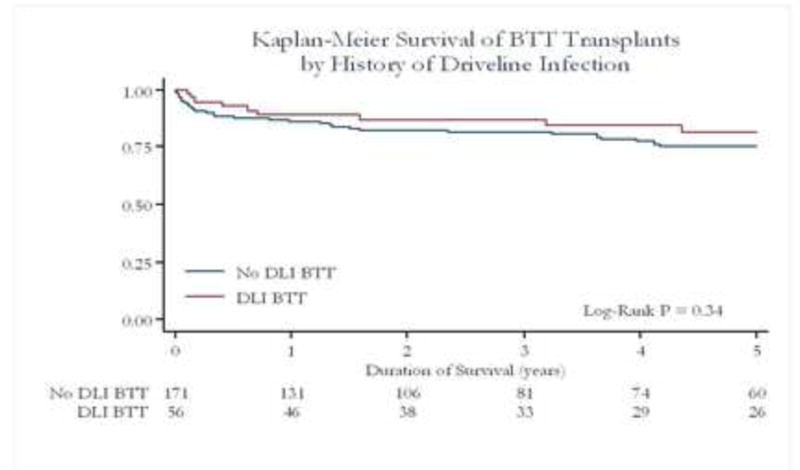
Post transplant survival of patients with device infection was not different to patients bridged with device and no infection.
Figure 7b. Survival Post Transplant BTT Patients Drive line infection vs UNOS 1A Transplant Patients without Prior Device.

Post transplant survival of patients with device infection was not different to non device 1A patients.
Discussion
We examined the UNOS status at the time of heart transplantation at our institution between 2000 and 2010 and the effect of UNOS status policy conceived in the early VAD era (before 2005) on organ allocation in the modern era. Our principal findings are as follows;
The percentage of patients receiving mechanical circulatory support as a bridge to transplant has increased dramatically from 19 to 64 percent over the past decade in our institution.
UNOS 1A status, traditionally justified by parenteral inotropic use and invasive hemodynamic monitoring in the Intensive Care Unit setting, in the current era is most often justified by the LVAD 30 day grace period and/or device complication.
There was no significant difference in post heart transplant survival between patients that were bridged to transplant with an LVAD and those who were transplanted without LVAD support.
Heart transplant remains the gold standard for the treatment of end stage heart failure. In the past decade, the number of heart transplantations in the United States plateau at 2200 heart transplants/year while the need for heart transplantation continues to grow. Due to the limited amount of donor hearts, the waiting time for heart transplant is increasing. The United Network for Organ Sharing (UNOS) is contracted by the federal government to provide a system for equitable distribution of all organs available for transplantation in the US. To achieve this objective in heart and lung transplantation, UNOS created the Thoracic Organ Committee, which includes a multidisciplinary group of professionals responsible for the design and monitoring of thoracic organ allocation algorithms. This algorithm has evolved over time. An allocation policy change in 1999 introduced Status 1A and Status 1B priority listings. Today, organ allocation in heart transplantation is based on the severity of heart failure defined by UNOS group (1A, 1B, 2), duration of listing and geographic location. The most recent thoracic organ allocation policy change was implemented in 2006 and provides for regional sharing of organs for the most medically urgent adult patients. Before 2006, donor hearts were offered first to local Status 1A patients, then 1B and 2 sequentially. If an appropriate recipient could not be located among those waiting locally, then the heart was offered out of UNOS region. The new policy was designed to reduce waiting-list mortality. Recent reports have conflicted as to the efficacy of this policy change (10,11). What has been a consistent feature of the UNOS allocation policy has been designation of LVAD recipients as 1B and assignment of 30-day 1A time as well as permanent upgrade to 1A in the setting of device associated complications. The current heart allocation policy is based on prior experience with pulsatile pumps, primarily Heartmate I (HMI) as a long term bridging device. The HM I provided reliable support for only about 1 year, and its use was complicated by frequent and severe infections. Accordingly, the UNOS policy allowed “30 day 1A time in an effort to circumvent device complications. As device technology improved, with extended device durability and lower rate of device infection, no change has occurred in the allocation system. Whether successful LVAD candidates should be given automatic UNOS 1B status and/or a 30-day 1A grace period can be debated particularly in light of near 90% 1 year survival in current Heartmate II bridge to transplant patients. Indeed, in Europe, stable HF patients on device support are not prioritized.
Given the persistent donor shortage and the growing list of prospective transplant candidates with more requiring LVAD support, the revision of our allocation system needs to be addressed. This large single center experience in a region where the transplant rate is low per capita, suggests that there will be an increasing shift to device use prior to transplant and that those bridged patients who will be transplanted are those with serious complications. The original intent of LVAD placement was to improve the patient’s overall status and thus enhance post transplant recovery. Although the 30 day grace period provides a welcome benefit to our device patients and was justified given the clinical results with earlier generation devices, it should not be overlooked that this policy has increased the wait time needed for all patients and, at least in our region, has practically deprived blood type 0 patients from receiving a transplant without LVAD bridging. The latter was clearly not the intent of the policy.
Survival post transplant in device patients has been the subject of several reports as multivariate analysis of large registry data has suggested that mechanical support is a predictive factor for poor transplant survival (12). More recent reports, which focused exclusively on long-term left ventricular assist device use as a bridge to transplant has refuted these observations (13,14). Nevertheless if the trend continues that complicated LVAD patients comprise the majority of transplant candidates it may only be a matter of time when post transplant survival will suffer. The task of sifting out which LVAD complications need to be prioritized is clearly at hand. Not all device complications and malfunctions are equal in severity and outcome. Currently, patients with driveline infections, systemic infections related to device infection, severe aortic insufficiency or recurrent severe bleeding are all equally upgraded to UNOS 1A. However, our analysis suggests that patient transplanted with a device complication or malfunction after 2006 have a trend toward worse post transplant survival. LVAD patients with device infection not localized to the pocket are at increased risk for septic and vasodilatory shock post transplant in the setting of intense immunosuppression. A severe neurologic deficient as a complication of a devastating stroke during LVAD support will preclude transplant; similarly there may be infections that are too extensive or coagulopathies too profound to make transplant tenable.
New Organ Allocation Policy Recommendation for LVAD Patients
The authors believe that changes are needed in the organ allocation system to facilitate equitable use of organs with best possible post transplant outcomes. Whether LVAD implantation merits a separate category should be considered. The 30 day 1A grace period should be reconsidered and probably removed as outcomes and quality of life afforded by current generation LVADs do not seem to justify a”grace” period. This is particularly true in light of the differences in waitlist time by UNOS regions. Upgrade of patients with LVADs should be stratified by the specific device complication; i.e. systemic infection with bacteremia should have higher urgency than a superficial driveline exit site infection.
Limitation
The study is a retrospective analysis and carry limitations inherent to this method. Specifically, patients reported as UNOS 1A 30 days grace period may actually have a coexistent device related complication or malfunction. In order to overcome the above limitation our data was collected both from UNOS and the electronic medical records.
Conclusion
The percentage of OHT patients requiring BTT LVADs is increasing. Most transplants are now performed in patients categorized as UNOS 1A and wait times have increased. More patients are categorized as UNOS 1A because of device related complications and/or malfunction. With the dramatic improvement in device technology and substantial heterogeneity in device complications with variable effect on prognosis, current allocation policies for cardiac transplantation for LVADs should be adjusted to reflect these changes.
Acknowledgments
No financial support was given for this work
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stehlink J, Edwards L, Kucheryavaya A, et al. The registry of the international society for heart and lung transplantation: twenty eighth adult heart transplant report-2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 2.hhtp://optn.transplant.hrsa.gov/r2009. The 2009 Annual Report of the OPTN and SRTR: Transplant Data 1999–2008; Chapter VI: Heart Transplantation in the US 1999–2008
- 3.Kirklin J, Naftel D, Kormos R, Stevenson L, Pagani F, Miller M, Baldwin J, Young J. The fourth INTERMACS annual report:4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Rose E, Gelijns A, Moskowitz A, Heitjan D, Stevenson L, Dembitsky W, Long J, Ascheim D, Tierney A, Levitan R, Watson J, Meier P, Ronan N, Shapiro P, Lazar R, Miller L, Gupta L, Frazier OH, Desvigne-Nickens P, Oz M, Poirier V, Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group Long term use of a left ventricular assist device for end stage heart failure. New Engl J of Med. 2001;45(20):1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 5.Lietz K, Long J, Kfoury A, Slaughter M, Silver M, Milano C, Rogers J, Naka Y, Mancini D, Miller L. Outcomes of Left Ventricular Assist Device Implantation as Destination Therapy in the Post-REMATCH Era: Implications for Patient Selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 6.http://optn.transplant.hrsa.gov/Policies
- 7.Slaughter M, Rogers J, Milano C, Russell S, Conte J, Feldman D, Sun B, Tatooles A, Delgado R, Long J, Wozniak T, Ghumman W, Farrar D, Frazier OH. Advanced Heart Failure Treated with Continuous flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 8.Pak SW, Uriel N, Takayama H, Cappleman S, Song R, Colombo PC, Charles S, Mancini D, Gillam L, Naka Y, Jorde UP. Prevalence of de novo aortic insufficiency during long-term support with left ventricular assist devices. Journal of Heart & Lung Transplantation. 2010;29(10):1172–6. doi: 10.1016/j.healun.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Uriel N, Pak S, Jorde U, Suen S, Vincentelli A, Ennezzat P, Cappleman S, Naka Y, Mancini D. Acquired von Willebrand Syndrome after Continuous flow mechanical device support contributes to a high prevalence of bleeding during long term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Nativi J, Kfoury A, Myrick C, Peters M, Renlund D, et al. Effects of the 2006 US Thoracic organ allocation change: analysis of local impact on organ procurement and heart transplantation. J Heart Lung Tx. 2010;29:235–239. doi: 10.1016/j.healun.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Singh T, Almond C, Taylor D, Graham Decline in heart transplant waitlist mortality in the United States following broader regional sharing of Donor hearts. Circ Heart Failure. 2012 Jan 13; doi: 10.1161/CIRCHEARTFAILURE.111.964247. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Patolla V, Patten R, Denofrio D, Konstam M, Krishnamani R. The effect of ventricular assist devices on post transplant mortality in analysis of the United Network for organ sharing thoracic registry. J Am Coll Cardiol. 2009;53:264–271. doi: 10.1016/j.jacc.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 13.Osaki S, Edwards N, Johnson M, et al. Improved survival after heart transplantation in patients with bridge to transplant in the recent era: a 17 year single center experience. J Heart Lung Transplant. 2009;28:591–597. doi: 10.1016/j.healun.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Russo M, Hong K, Davies R, Chen J, Sorabella R, Ascheim D, Williams M, Gelijns A, Stewart A, Argenziano M, Naka Y. Posttransplant survival is not diminished in heart transplant recipients bridged with implantable left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;138(6):1425–32. doi: 10.1016/j.jtcvs.2009.07.034. [DOI] [PubMed] [Google Scholar]


