Abstract
The 10th International Conference on Hantaviruses, organized by the International Society on Hantaviruses, was held from May 31–June 3, 2016 at Colorado State University, Fort Collins, CO, USA. These conferences have been held every three years since 1980. The current report summarizes research presented on all aspects of hantavirology: ecology and epidemiology, virus replication, phylogeny, pathogenesis, immune response, clinical studies, vaccines and therapeutics.
Keywords: Hantavirus, Hemorrhagic fever with renal syndrome, Hantavirus pulmonary syndrome
1. Introduction
To facilitate the exchange of information, promote collaborations and further the understanding of the biology of hantaviruses and the diseases they cause, an International Conference on Hantaviruses has been held every three years since 1980, when Professor Ho Wang Lee, the discoverer of Hantaan virus (HTNV), organized the first conference directly following a World Health Organization meeting on hemorrhagic fever with renal syndrome (HFRS) in Seoul, Korea (Lee et al., 2014). The next eight conferences took place in Beijing, China; Helsinki, Finland; Atlanta, U.S.A.; Annecy, France; Seoul, Korea; Buenos Aires, Argentina; Athens, Greece; and Beijing, China. At the Fifth International Conference in Annecy, France, in 2001, the delegates voted to create the International Society for Hantaviruses (ISH), which has organized the meetings since that time.
The many unanswered questions about hantaviruses, their distribution in nature and how to lessen their impact on human health have resulted in continued research on all aspects of these important human pathogens. The current report summarizes research presented at the 10th International Conference on Hantaviruses, which was held at Colorado State University, in Fort Collins, CO, USA from May 31–June 3, 2016.
2. Hantaviruses: current knowledge
The genus Hantavirus, family Bunyaviridae, includes numerous viruses isolated from or detected in rodents, shrews, moles, and bats (Fig. 1). The ecology of hantaviruses and their native hosts is complex and our understanding of virus/host interactions is still incomplete. Along with other approaches, next generation sequencing studies of both viruses and hosts are expected to help fill this knowledge gap.
Fig. 1.
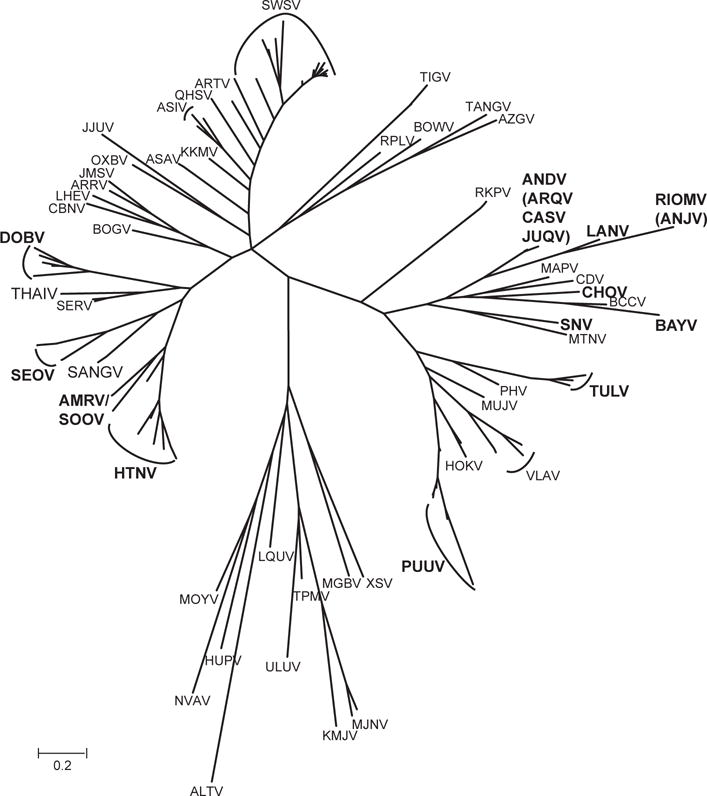
Phylogenetic tree of rodent-, shrew-, and bat-borne hantaviruses (modified after Krüger et al., 2015). Viruses with evident human pathogenicity are highlighted by boldface in the case that infections were serologically and molecularly proven or by use of italics if there is serological evidence only. AMRV/SOOV, Amur/Soochong virus; ALTV, Altai virus; ANDV, Andes virus; ANJV, Anajatuba virus; ARQV, Araraquara virus; ARRV, Ash River virus; ARTV, Artybash virus; ASAV, Asama virus; ASIV, Asikkala virus; AZGV, Azagny virus; BAYV, Bayou virus; BCCV, Black Creek Canal virus; BOGV, Boginia virus; BOWV, Bowé virus; CADV, Cano Delgadito virus; CASV, Castelo dos Sonhos virus; CBNV, Cao Bang virus; CHOV, Choclo virus; DOBV, Dobrava-Belgrade virus; HOKV, Hokkaido virus; HUPV, Huangpi virus; HTNV, Hantaan virus; JJUV, Jeju virus; JMSV, Jemez Springs virus; JUQV, Juquitiba virus; KMJV, Kilimanjaro virus; KKMV, Kenkeme virus; LHEV, Lianghe virus; LANV, Laguna Negra virus; LQUV, Longquan virus; MAPV, Maporal virus; MGBV, Magboi virus; MJNV, Imjin virus; MOYV, Mouyassué virus; MTNV, Montano virus; MUJV, Muju virus; NVAV, Nova virus; OXBV, Oxbow virus; PHV, Prospect Hill virus; PUUV, Puumala virus; QHSV, Qian Hu Shan virus; RIOMV, Rio Mamore virus; RKPV; Rockport virus; RPLV, Camp Ripley virus; SANGV, Sangassou virus; SEOV, Seoul virus; SERV, Serang virus; SWSV, Seewis virus; SNV, Sin Nombre virus; THAIV, Thailand virus; TGNV, Tanganya virus; TPMV, Thottapalayam virus; TULV, Tula virus; ULUV, Uluguru virus; VLAV, Vladivostok virus; XSV, Xuan Son virus.
All known pathogenic hantaviruses are carried by persistently infected rodents and are transmitted to humans by aerosols derived from the rodents’ urine, feces, and saliva, or occasionally by bite. Humans are usually dead-end hosts for hantaviruses. Old World rodents transmit viruses causing HFRS, while New World rodents transmit viruses causing hantavirus (cardio-) pulmonary syndrome (HPS). Both diseases are characterized by vascular leakage as a significant pathogenic consequence of infection. The underlying mechanisms leading to increased blood vessel permeability after hantavirus infections have not been clearly identified and remain the subject of intense research.
Thousands of cases of HFRS occur each year in Asia and Europe, with most due to infection with HTNV, Seoul (SEOV), Puumala (PUUV), or Dobrava-Belgrade (DOBV) viruses. The prevalence of HPS is much lower than HFRS, with only a few dozen cases reported in the Americas each year. Although several HPS-causing hantaviruses have been discovered throughout the New World, the best known are Sin Nombre (SNV) and Andes (ANDV) viruses in North and South America, respectively. Case fatality rates for HFRS range from <1% for PUUV infections to as high as 5–15% for HTNV and DOBV infections. HPS is much more lethal with a fatality rate approaching 40–50%.
Effective vaccines and therapeutics for hantavirus infections have been difficult to develop and evaluate, in part because of the absence of useful animal models. No animal model of disease has been found for HFRS in non-primate hosts, but Syrian hamsters infected with ANDV have been shown to develop clinical signs and pathologies similar to those observed for human HPS. This hamster model has proven useful for evaluating medical countermeasures for HPS. Efforts to develop animal models for other hantaviruses are in progress.
3. Opening lecture
The opening lecture was given by Antti Vaheri (Finland), who was President of the Society from 2004 to 2010. His lecture was entitled “New pathogenicity markers, vascular leakage, tissue plasminogen activator and galectin-3 binding protein as therapeutic targets in hantavirus infections”. He first reviewed the pathobiology of hantavirus infections, and mentioned that endothelial cells and monocyte/macrophages are the main targets of the virus, and that vascular leakage of endothelial cells (increased capillary permeability) is a key element, mediated largely by bradykinin. He also stated that additional contributing mechanisms include thrombocytopenia, complement activation, certain cytokines (such as interleukin 6 and tumor necrosis factor -alpha), the host genotype, neutralizing antibodies and cytotoxic and regulatory T cells play a role in the pathogenesis of hantavirus diseases (Charbonnel et al., 2014; Koivula et al., 2014; Mustonen et al., 2013; Vaheri et al., 2013).
Antti mentioned that the Helsinki group has recent evidence that also tissue plasminogen activator and galectin-3 binding protein (AKA Mac-2 binding protein) play a role and cited evidence suggesting that the latter may be part of the innate immunity system (Hepojoki et al., 2014; Strandin et al., 2016). He reported that icatibant, a bradykinin receptor antagonist, has been successfully used in the treatment of severe cases of hantavirus disease (Antonen et al., 2013; Laine et al., 2015; Vaheri et al., 2014) and reported that studies are under way to establish the involvement of bradykinin in several other diseases in which endothelial leakage leads to shock and/or hemorrhage. In view of the role of complement activation in hantavirus disease (Sane et al., 2012), eculizumab, a humanized monoclonal antibody which inhibits the terminal complement membrane attack complex (MAC, C5b-9), may be also beneficial in treatment of severe hantavirus disease.
4. Award lectures
Two awards were given: the Ho-Wang Lee Award, honoring the discoverer of HTNV, who set the basis for all subsequent scientific advances in the field of hantavirus research, and the Dalrymple Award, dedicated to the memory of Joel McKeith Dalrymple, a brilliant and innovative scientist and hantavirus pioneer. The Ho-Wang Lee and the Dalrymple awards were given respectively to James W. Le Duc and Irina Gavrilovskaya, in recognition of their lifetime achievements in the field of hantavirology.
4.1. The Ho-Wang Lee award lecture
James LeDuc is the Director of the Galveston National Laboratory and a Professor at the University of Texas Medical Branch in Galveston, Texas (USA). Previously he served as Director of the Division of Viral and Rickettsial Diseases at the Centers for Disease Control and Prevention, as medical officer at the World Health Organization, and as an Officer in the US Army. His presentation, on The power of “We”, focused on his work with many collaborators from around the world at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) during the decade immediately following the discovery of HTNV. His early studies identified what is now known to be SEOV as present in domestic rats in many parts of the United States and subsequently globally. The seminal studies conducted by James Childs, Greg Glass and George Korch, in partnership with Le Duc, led to the discovery that SEOV was commonly found in Baltimore rats, and through a decade of study, the team clearly documented the dynamics of virus transmission between rats and the possible infection of resident humans.
Their discovery that past hantavirus infection was associated with an increased risk of chronic kidney disease remains an intriguing observation worthy of additional investigation. Other collaborations with partners in Latin America, China, Sweden, Greece and countries of the former Yugoslavia further advanced the global appreciation of hantaviruses as important emerging pathogens. Le Duc’s overarching message was the importance of collaboration and how, through partnerships, each of our contributions can be significantly leveraged to everyone’s benefit. Further, the investments made to build capacity and nurture junior colleagues will pay a lifetime of dividends in terms of future scientific discoveries.
4.2. The Dalrymple Award lecture
Irina Gavrilovskaya is an Emeritus Professor at Stony Brook University (USA). Until 1994, she was Professor at the Institute of Poliomyelitis and Viral Encephalitis AMS, in Moscow where she studied the etiology, epidemiology, and epizootology of HFRS and served as an ad hoc WHO advisor on HFRS. In her lecture, Irina described her studies on the role of immunity in HFRS patients and the role of human neutralizing antibodies in HFRS treatment. Since 1994, Irina has worked at Stony Brook University, where she studied hantavirus interactions with human endothelial cells. She identified hantavirus receptors on endothelial cells and described the potential mechanisms of capillary permeability during hantavirus infection (Gavrilovskaya et al., 1998, 2013).
5. Scientific presentations
5.1. Ecology and epidemiology
Roger Hewson (UK) reported that hantaviruses are likely to be of public health importance in regions of the UK since 3 human HFRS cases have been diagnosed serologically. Molecular studies on rodents showed evidence of SEOV, while a virus strain (strain Humber) was isolated from Rattus norvegicus around the farm of one of the patients (Jameson et al., 2013a, 2013b). Among people living or working on farms in Northern England, 7.6% had antibodies to hantaviruses while the seroprevalence among pet rat breeders was 33%.
Detlev Krüger (Germany) said that following the isolation of Sangassou virus (SANGV), the first indigenous African hantavirus from rodents in Guinea (Klempa et al., 2012), there is an urgent need to assess the public health relevance of hantaviruses in Africa. Seroprevalence rates of 1.2%, 3.9% and 2.4%, were found in humans in Guinea, Côte d’Ivoire, and Democratic Republic of Congo, respectively, while 4.7% of patients with fever of unknown origin from Guinea were IgG-positive, including 1 patient with specific IgM and SANGV neutralizing antibodies (Klempa et al., 2010). For specific detection of infections by novel viruses associated with non-rodent hosts, they developed a panel of serological tests (ELISA, Western blot, immunofluorescence) based on recombinantly expressed nucleocapsid proteins of African shrew-associated hantaviruses. Since antibodies to non-rodent borne hantaviruses were detected in patients with acute renal or lung disorders, it was suggested that these viruses can also infect humans.
Genetically distinct hantaviruses were recently found in African and Asian bats. Boris Klempa (Germany and Slovakia) reported the identification of a novel hantavirus, provisionally named Makokou virus (MAKV), in Noack’s Roundleaf Bat in Gabon, central Africa; ancestral state reconstruction based on a tree of L gene sequences combined with phylogenetic fossil host hypotheses testing, indicated the mammalian superorder Laurasiatheria (including shrews, moles, and bats) as potential hosts of ancestral hantaviruses at most basal tree nodes (Witkowski et al., 2016).
Barry Rockx (Netherlands) reported that 36% of the rodents in the Netherlands are carrying Tula virus (TULV) and that the Dutch TULV strains are closely related to strains from Germany and Belgium. Since no human TULV cases have been reported in the country, he made the suggestion that diagnostics in humans should include specific assays for TULV to assess the pathogenic ability of TULV.
Angela Luis (USA) described a “susceptible-infected” epidemiological model for SNV to find out the drivers of the so-called ‘dilution effect’ in deer mice, in which the SNV prevalence is lower in communities with higher diversity of small mammals. Application of the model showed that the ‘dilution effect’ is driven by reduced deer mouse density, and once this is taken into account, there is a positive effect of small mammal diversity on transmission (a component ‘amplification effect’).
Lies Laenen (Belgium) reported that 7% of European moles (Talpa europaea) are infected by a novel hantavirus, Bruges virus (BRGV), which was detected in moles near Bruges, Belgium. This is another hantavirus in the European mole, in addition to Nova virus (NVAV), suggesting that two genetically distinct hantavirus species share the same reservoir host. Moreover, co-infection with NVAV was detected in renal tissues from 22 of 24 BRGV-infected European moles suggesting that investigations are needed into possible reassortment of these viruses.
Claudia Filippone (Madagascar) reported the results of a serological study in Madagascar following the recent detection of Anjozorobe virus (ANJV), a novel hantavirus in rats, which is a variant of Thailand virus (THAIV) present in southeastern Asia (Reynes et al., 2014). A seroprevalence of 1.7% was found, and further studies are planned to confirm the results with specific assays and to assess the zoonotic risk of hantaviruses in the human population of the island.
Heikki Henttonen (Finland) described the results of a 7-year longitudinal capture-mark-recapture study of PUUV infection in bank vole populations in Finland, where approx. 35,000 human HFRS cases have been diagnosed during 1995–2015. The study was conducted at monthly intervals to monitor seasonal and multi-annual patterns of the PUUV infection rate exhibiting a 3-year cycle in the highly endemic area in boreal taiga in Central Finland. It was based on a long-term dataset which can serve as a basis for model development to predict the dynamic public health threat posed by PUUV in northern Europe (Voutilainen et al., 2016).
Joerg Hofmann (Germany) reported that hantavirus disease in not an exotic disease in Germany as its average annual incidence rate is 1.15/100,000. Hantavirus disease in Germany is caused by PUUV and DOBV that occur in West/South and North/East Germany, respectively. PUUV infections are responsible for large outbreaks with up to 3000 cases as in 2012. Molecular identification of hantaviruses in humans and rodents revealed several distinct PUUV clades, with molecular identity between local human and rodent-derived virus strains. Molecular clustering was also identified with DOBV-Kurkino virus in patients and local Apodemus mice (Hofmann et al., 2014). To improve hantavirus routine serological diagnostics, an external quality assessment (EQA) scheme was performed in German laboratories that demonstrated reasonable results.
Mifang Liang (China) reported that hantaviral infections present a serious public health problem in China; therefore, in 2004 the China CDC has established the National Disease Reporting System to strengthen the surveillance of HFRS. During the past ten years, a total of 112,177 cases and 1116 deaths have been reported in the country. Farmers are at the highest risk. A peak of cases is observed in November. The majority of cases are reported from eight provinces. The same provinces have also the highest rodent density with Apodemus agrarius and Rattus norvegicus being the dominant hantavirus reservoir species. Besides the known hantaviruses, several new strains have been isolated. Mifang concluded that surveillance and prevention and control strategies should be improved and strengthened in order to reduce the incidence of the disease in China.
SEOV, associated with brown rats, is responsible for the increasing number of urban hantavirus infections worldwide. Lorraine McElhinney (UK) reported about severe HFRS cases with acute renal failure caused by SEOV found recently in UK. She has also described an unusual outbreak of SEOV-associated cases in South Wales, which occurred in domestic and commercial rat breeding facilities.
Charlotte Robin (UK) presented the interactions and relationships between rats and humans among three at-risk groups: pet rat owners, farmers and pest control workers. In order to explore the study participant understanding of illness, health, risk and their perceptions of rats, semi-structured interviews were performed. With this study, a fast and successful communication of public health interventions that reduce the risk of hantavirus infection within these at-risk communities was achieved.
Kumiko Yoshimatsu (Japan) reported about the first three serologically confirmed HFRS patients from Sri Lanka. They have examined serum samples from febrile patients for hantavirus and leptospira infections since several clinical symptoms in both diseases are alike. However, none of the rodents and shrews investigated was hantavirus positive.
Liudmilla Yashina (Russia) provided insights into the Sorex-borne hantaviruses in Far-eastern Russia. Genetic analysis revealed the presence of three viruses co-circulating in one forest located in Far-eastern Russia: Artybash, Altai-like and Yakeshi viruses. The first two highly divergent hantaviruses were detected in the Laxmann’s shrew (Sorex caecutiens) and two lineages of Yakeshi virus were detected in the taiga shrew (Sorex isodon).
5.2. Hantavirus replication
Nicole Tischler (Chile) reported on the establishment of a cell-free system to study fusion processes of ANDV with its host cells (Acuña et al., 2015). Fusion was found to be non-reversibly activated by acidification involving lipid interaction and viral glycoprotein Gc multimerization led to a stable post-fusion homotrimer. The fusion activity was blocked by addition of exogenous fusion protein fragments spanning domain III and peptides representing the glycoprotein stem region, suggesting that hantavirus Gc shares not only structural, but also mechanistic similarity with class II viral fusion proteins.
A replication-competent recombinant vesicular stomatitis virus (VSV) system for studying Old World hantavirus entry was presented by Rohit Jangra (USA). In this system, they were able to enhance the replication and spread of the early passage rVSV-HTNV glycoprotein by introduction of two key amino acid changes in the viral protein (one each in Gn and Gc glycoproteins).
Adelaïde Dubois (France) and colleagues tested the hypothesis that variations in bank vole (Myodes glareolus) immune responses to PUUV could affect virus replication and excretion into the environment. They emphasized genetic signatures of selection along bank vole genomes that could be linked to the adaptation to the virus and compared them between two geographical transects. Among these SNP ‘outliers’, some included genomic regions associated with proinflammatory responses.
Matthias Schade (Germany) and co-authors studied the intracellular packaging of PUUV genomic segments by multicolor fluorescence in situ hybridization. In infected cells, a comparison of all three vRNA segments (S, M, L) at 3 dpi and 11 dpi resolved vRNA monomers, heterodimers, and heterotrimers. They found an absence of a preferred class of vRNA heterodimers in infected cells pointing towards an assembly process lacking a specific segment assembly order. With over 62% of all detected mature virions lacking at least one of three vRNA segments, the data imply a suboptimal, non-segment specific assembly process of hantaviruses.
5.3. Phylogenetic studies
Undertaking phylogenetic analyses of HTNV tripartite genomes recovered from HFRS patients, Won-Keun Kim (Korea) showed geographic clustering of these strains with virus strains circulating in rodents in different geographical regions of South Korea. The authors concluded that multiplex PCR-based NGS should be useful to obtain the whole genome sequence of HTNV from HFRS patients and, together with genome sequences of HTNV from the natural reservoir (Apodemus agrarius), the phylogeographic analysis may provide a robust tool to develop risk analyses as well as preventive and therapeutic strategies against hantavirus diseases.
Satoru Arai (Japan) presented the whole genome analysis of Dakrong virus, a novel hantavirus harbored by the Stoliczka’s Asian trident bat (Aselliscus stoliczkanus) in Vietnam. With the discovery of this virus, the number of bat-borne hantaviruses reached a total of eight (5 from Asia and 3 from Africa). All 8 hantaviruses are harbored by insectivorous bats, in keeping with the conjecture that primordial hantaviruses may have originated as insect viruses.
Whereas most novel non-rodent associated hantaviruses were detected by molecular methods only, Richard Yanagihara (USA) and colleagues were able to isolate the mole-associated NVAV in cell culture. Molecular phylogenetic analyses showed that NVAV was most closely related to hantaviruses harbored by insectivorous bats, consistent with an ancient evolutionary origin. The authors were able to infect infant Swiss Webster mice by the intraperitoneal route. Mice developed neurological disease and exhibited high NVAV RNA copies in lung, liver, kidney, spleen and brain.
5.4. Pathogenesis of hantavirus disease
Miša Korva (Slovenia) presented the dynamics of VEGF and sVEGFR2 levels of plasma and urine in patients infected with PUUV or DOBV to examine the relation to severity of disease. The results suggested a dual role of VEGF in hantavirus pathogenesis.
Carles Sola Riera (Sweden) reported that induction of apoptosis was strongly inhibited in HUVEC cells infected with both pathogenic and non-pathogenic hantaviruses and therefore the inhibiting effect was considered as important feature of hantaviruses in general. Further, hantavirus infection induced IL-15/ILR expression in endothelial cells.
Matthew Simons (USA) presented that ANDV nucleocapsid protein binds to E3 ubiquitin ligase TRIM21. Further, induction of interferon (IFN)-beta was inhibited synergistically by TRIM21 and N protein. These results demonstrated TRIM 21 as a novel target of ANDV-regulated IFN induction.
Günther Schönrich (Germany) examined the immunopathological mechanisms of hantavirus infection by using a humanized mouse model that harbors neutrophils and functional HLA-A2-restricted CD8+ T cells. The results suggest that activation of neutrophils and CD8+ T lymphocytes play an important role in hantavirus-associated immunopathogenesis (Kobak et al., 2015).
Jonas Klingström (Sweden) reported that hantaviruses induce several anti-apoptotic mechanisms (some of them may be unique for hantaviruses) which deregulate inflammatory responses-affecting bystander cells and potentially contribute to the strong inflammatory state observed in patients. The findings of their study indicate that hantaviruses have possible direct and indirect effects on carcinogenesis, which might explain the increased risk for lymphoma observed in Swedish HFRS patients.
To explore the mechanism of endothelial cells dysfunction that permits hantavirus-induced vascular permeability, Erich Mackow (USA) and his team found that ANDV infection of primary human pulmonary microvascular endothelial cells activates the vascular permeability inducer RhoA and increases cell size by preventing de-repression of mTOR signaling responses. The nucleocapsid protein interacts with TSC2 and Rac1/RhoA regulatory proteins and controls the endothelial cell permeability responses, suggesting potential therapeutic approaches for resolving hantavirus-induced vascular leakage (Gavrilovskaya et al., 2013).
Hiroaki Kariwa (Japan) pointed out that virulence of HTNV strain AA57 in mouse model is reduced after 30 serial passages in Vero E6 cells. One and 4 amino acid substitutions were found in nucleocapsid protein and RNA polymerase, respectively. The low virulence strain grows faster in Vero E6 cells than the high virulence strain.
Peter Witkowski (Germany) presented the susceptibility of the human small intestine epithelium for hantavirus infection by using human intestinal epithelial (Caco-2) cell culture. Results indicated that PUUV and DOBV are able to survive stomach passage and to grow in Caco-2 cells. Thus, a possible alimentary tract route of hantavirus infection was proposed.
5.5. Innate and adaptive immune responses
To examine the role of cytokines in pathogenesis of hantavirus infection, Katerina Tsergouli (Greece) presented the levels of 27 cytokines in 24 hospitalized patients during the acute phase of DOBV infection. The results demonstrated differences in the cytokine patterns seen in severe and non-severe DOBV cases. In all patient groups IP-10 was increased and RANTES was decreased. Multivariate logistic regression analysis showed VEGF as positively associated with disease severity. Th1 response was high in non-severe cases and low in the fatal case, while a mixed Th1/Th2 immune response was seen in the survivors from severe disease.
Myriam Ermonval (France) compared by proteome array and flow cytometry analysis the effect of the pathogenic PUUV and two other hantaviruses, TULV and Prospect Hill virus (PHV), on activation of epithelial cells and neutrophils. Different pathways were activated either leading to recruitment of immune cells or to pro-inflammatory responses. PUUV, unlike TULV and PHV, promoted survival of purified human neutrophils and led to a delay in apoptosis.
Ivan-Christian Kurolt (Croatia) and co-workers measured the levels of selected micro (mi)RNAs in urinary samples of HFRS patients after PUUV infection. The results showed the miRNA abundance in the urine of HFRS patients and acute pyelonephritis patients. This study revealed the possibility of miRNA in urine as HFRS biomarkers of progression and severity of HFRS.
Marina Garcia (Argentina) reported that ANDV causes a massive and transiently circulating plasmablasts response, corresponding to what has been previously morphologically defined as “immunoblasts”. The massive plasmablast response could be explained by a polyclonal activation of B cells that takes place during the acute phase of HPS in ANDV patients.
Lidija Cvetko Krajinović (Croatia) and collaborators explored the components of innate and adaptive immunity important in the peripheral immune response and showed the regulatory effect of miRNA on early immunoreactions during the hantavirus infection. Using real-time PCR array technology, they observed down-regulation of genes encoding the synthesis of pattern recognition receptors, chemokines and their receptors, cytokines, transcription factors, and signaling molecules, suggesting that the early immune response to PUUV is suppressed and this is more pronounced in the more severe cases.
5.6. Clinical studies
Jan Clement (Belgium) presented 3 retrospective cases of clinical pathology (“HFRS”), fallen ill shortly after overt exposure to soricomorph hantaviruses. Case A was bitten by a water shrew (Neomys fodiens), case B was splashed in the face and eyes by blood of a mole (Talpa europaea) while killing the animal, and case C manipulated a dead mole. All cases sero-reacted clearly both to HTNV in a high density-particle agglutination assay, and to PUUV in IFA. However, PRNT was negative for SEOV, and showed insignificant low titers (10–40) for HTNV and/or for PUUV. Some so-called HFRS or NE cases in the past may have been misinterpreted as being caused by HTNV, respectively PUUV, in classical serological screening.
Tomas Strandin (Finland) analyzed the regulation of tissue-type plasminogen activator (tPA), best-known for its capability to induce fibrinolysis, and plasminogen activator inhibitor (PAI)-1, the main inhibitor of tPA, in the acute stage of PUUV disease. Enhanced levels of tPA in the acute versus convalescent stage of PUUV infection were detected. In contrast, the levels of PAI-1 were not regulated to similar extent, thus facilitating enhanced activity of tPA during acute PUUV infection. Upregulated levels of tPA mRNA and protein were found also in hantavirus-infected human microvascular endothelial cells, with additional evidence that interferons (type I and II) were able to induce tPA in endothelial cells by activating signal transducer and activator of transcription (STAT)-1 (Strandin et al., 2016).
Jukka Mustonen (Finland) presented a retrospective study in 205 HFRS patients on the predictive value of different forms of proteinuria (24-h proteinuria, overnight albuminuria, and urinary IgG excretion), and particularly of simple albumin dipstick test upon hospitalization. Significant correlations were found between these forms of proteinuria, between three categories (+, ++, and +++) of dipstick albuminuria, and different indices of clinical severity in PUUV infections, such as median values of peak plasma creatinine during illness, higher blood leukocyte count, higher maximum hematocrit, lower minimum serum sodium, weight gain, and duration of hospitalization. Moreover, the maximal degree of proteinuria occurred at day 5 post onset of fever, whereas the peak median plasma creatinine was reached only on day 9, suggesting that both forms of pathophysiology might not be entirely identical. Both, however, have a good prognostic value (Outinen et al., 2015).
Clas Ahlm (Sweden) focused his presentation on endothelial dysfunction during PUUV infection. The levels of studied endothelial markers [e.g. endothelial glycocalyx degradation (syndecan-1), soluble vascular cellular adhesion molecule, sVCAM-1, intercellular adhesion molecule 1, sICAM-1, and endothelial selectin, sE-selectin], some cytokines associated with vascular repair (vascular endothelial growth factor, VEGF, erythropoietin, EP, angiopoietin, Ang-2, and stromal cell-derived factor 1, SDF-1, including marker for hypoxia, as insulin-like growth factor binding protein 1, IGFBP-1), were highest during the earliest phase of hantaviral disease and associated with clinical and laboratory surrogate markers for disease outcome. In particular, the marker for glycocalyx degradation, syndecan-1, was significantly associated with levels of thrombocytes, albumin, IGFBP-1, decreased blood pressure and disease severity (Connolly-Andersen et al., 2014).
Kimia Maleki (Sweden) in her study aimed to determine expression of pro-inflammatory cytokines and acute phase reactants, including markers of gastrointestinal inflammation, during acute and convalescent PUUV-caused HFRS. Longitudinal plasma samples from Swedish patients with confirmed PUUV infection were analyzed for different inflammatory cytokines and acute phase reactants, using ELISA. Preliminary data confirm a strong inflammatory response during the acute phase of HFRS and indicate the existence of a potential gastrointestinal inflammation during the disease. The mechanisms behind the gastrointestinal involvement, as well as its consequences, remain to be elucidated.
Johan Rasmuson (Sweden) investigated the association between viral load and immune responses in the lungs, and their relation to disease severity. For that purpose, bronchoscopy with sampling of bronchoalveolar lavage (BAL) fluid was performed in 17 patients with acute PUUV infection and 16 healthy volunteers acting as control group. Lymphocyte subsets, granzyme concentrations, and viral load were determined by flow cytometry, ELISA and quantitative RT-PCR, respectively. The results indicate that the magnitude of the pulmonary immune response was associated with disease severity in hantavirus infection. Patients with a pronounced CD8+-response were more severely ill in terms of impaired alveolar gas exchange and systemic organ dysfunction, implicating the significance of these cells in the pathogenesis (Rasmuson et al., 2016).
Jan Clement (Belgium) presented a personal hypothesis on the hitherto unexplained mechanics of rapidly progressive but transient acute kidney injury in hantavirus infections. The main culprit would be an imbalance of intraglomerular Starling forces, consisting of markedly increased hydrostatic pressure in Bowman’s capsule (Pbc), due to inflammatory interstitial edema, and counteracting the glomerular net ultrafiltration force Kf, which is normally only of about 10 mmHg. If Pbc becomes greater than Kf, the renal ultrafiltration stops, and oliguria or anuria installs. On the other hand, if renal interstitial edema diminishes again, due to spontaneous resolution or to anti-edema agents like icatibant S.C., Pbc diminishes likewise, and ultrafiltration resumes again in a kidney without other sequelae (Clement, 2015).
5.7. Vaccine development
Jay Hooper (USA) updated the progress on hantavirus vaccines. He reported that both the HFRS and HPS DNA vaccines delivered by various technologies including gene gun, intramuscular electroporation, and needle-free disposable syringe jet injection elicit high titer neutralizing antibodies in animals, including nonhuman primates. He reported that the HTNV and PUUV DNA vaccines delivered by intramuscular electroporation are safe and immunogenic in humans, and that the seroconversion rate (neutralizing antibodies) in the Phase 1 testing was sufficiently high to warrant a Phase 2a dose ranging study (in progress) (Hooper et al., 2014b).
5.8. Therapeutics
Greg Mertz (USA) and collaborators from Chile reviewed the antiviral, anti-inflammatory and supportive treatment for HPS including placebo-controlled trials of intravenous ribavirin and methylprednisolone, an open trial of immune plasma that had been collected by plasmapheresis, and a retrospective case series in patients treated with extracorporeal membrane oxygenation (ECMO). He concluded that ECMO appeared to reduce the risk of death in persons with severe HPS, but it is expensive, carries risk of morbidity and mortality, and most HPS patients do not have access to centers with ECMO. Parenteral ribavirin and methylprednisolone were found to be ineffective. Passive administration of neutralizing antibody appears promising and deserves further evaluation, ideally on the basis of a product with potential for commercial development and with neutralizing activity against both SNV and ANDV.
Rebecca Brocato (USA) reported that transchromosomal bovine- and anseriform avian-based approaches are effective to develop polyclonal antibody-based antivirals targeting hantaviruses since they are capable to protect animal models of lethal hantavirus disease. This work provides a partial proof of concept that these novel platforms can be used to develop candidate next-generation polyclonal immunoglobulin-based medical products, without the need for human donors, despeciation protocols, or inactivated/attenuated vaccine antigen (Hooper et al., 2014a).
6. Concluding remarks
Hantaviruses continue to cause morbidity and mortality in many areas of the world. The global detection of numerous novel hantaviruses and their natural hosts suggests that these viruses may have ecological and epidemiological impacts that we have yet to recognize. Progress toward understanding hantaviruses and the diseases that they cause has resulted in better detection and treatment methods but the specific events leading to vascular leakage remain to be fully explained. Likewise, more effective vaccines, diagnostics and therapeutics are still required. Participants felt that this Conference provides a singular opportunity for hantavirus researchers to meet and share information that might lead to the accomplishment of these goals. Consequently, the Advisory Council of the ISH recommended continuation of the triennial Conference on Hantaviruses with the next conference expected to be held in 2019 in Leuven, Belgium.
Acknowledgments
We thank all participants who presented their data during the 10th International Conference on Hantaviruses. We are thankful to Richard Yanagihara for the constructive comments and Tony Schountz, the local organizer from Colorado State University, for the excellent organization of the conference. Support for the conference was provided in part by National Institutes of Health grant 1R13AI124621-01 to C.B.J.
References
- Acuña R, Bignon EA, Mancini R, Lozach PY, Tischler ND. Acidification triggers Andes hantavirus membrane fusion and rearrangement of Gc into a stable post-fusion homotrimer. J Gen Virol. 2015;96:3192–3197. doi: 10.1099/jgv.0.000269. [DOI] [PubMed] [Google Scholar]
- Antonen J, Leppanen I, Tenhunen J, Arvola P, Makela S, Vaheri A, Mustonen J. A severe case of Puumala hantavirus infection successfully treated with bradykinin receptor antagonist icatibant. Scand J Infect Dis. 2013;45:494–496. doi: 10.3109/00365548.2012.755268. [DOI] [PubMed] [Google Scholar]
- Charbonnel N, Pages M, Sironen T, Henttonen H, Vapalahti O, Mustonen J, Vaheri A. Immunogenetic factors affecting susceptibility of humans and rodents to hantaviruses and the clinical course of hantaviral disease in humans. Viruses. 2014;6:2214–2241. doi: 10.3390/v6052214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement J. Acute kidney injury and hantavirus disease. In: Turner N, Lameire N, Goldsmith D, Winearls C, Himmelfarb J, Remuzzi G, editors. Oxford Textbook of Clinical Nephrology. Oxford University Press; Oxford, UK: 2015. pp. 2059–2066. [Google Scholar]
- Connolly-Andersen AM, Thunberg T, Ahlm C. Endothelial activation and repair during hantavirus infection: association with disease outcome. Open Forum Infect Dis. 2014;1 doi: 10.1093/ofid/ofu027. ofu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Gorbunova EE, Mackow ER. Hypoxia induces permeability and giant cell responses of Andes virus-infected pulmonary endothelial cells by activating the mTOR-S6K signaling pathway. J Virol. 2013;87:12999–13008. doi: 10.1128/JVI.02103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER. beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc Natl Acad Sci U S A. 1998;95:7074–7079. doi: 10.1073/pnas.95.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepojoki J, Strandin T, Hetzel U, Sironen T, Klingstrom J, Sane J, Makela S, Mustonen J, Meri S, Lundkvist A, Vapalahti O, Lankinen H, Vaheri A. Acute hantavirus infection induces galectin-3-binding protein. J Gen Virol. 2014;95:2356–2364. doi: 10.1099/vir.0.066837-0. [DOI] [PubMed] [Google Scholar]
- Hofmann J, Meier M, Enders M, Fuhrer A, Ettinger J, Klempa B, Schmidt S, Ulrich RG, Krüger DH. Hantavirus disease in Germany due to infection with Dobrava-Belgrade virus genotype Kurkino. Clin Microbiol Infect. 2014;20:O648–O655. doi: 10.1111/1469-0691.12543. [DOI] [PubMed] [Google Scholar]
- Hooper JW, Brocato RL, Kwilas SA, Hammerbeck CD, Josleyn MD, Royals M, Ballantyne J, Wu H, Jiao JA, Matsushita H, Sullivan EJ. DNA vaccine-derived human IgG produced in transchromosomal bovines protect in lethal models of hantavirus pulmonary syndrome. Sci Transl Med. 2014a;6:264ra162. doi: 10.1126/scitranslmed.3010082. [DOI] [PubMed] [Google Scholar]
- Hooper JW, Moon JE, Paolino KM, Newcomer R, McLain DE, Josleyn M, Hannaman D, Schmaljohn C. A Phase 1 clinical trial of Hantaan virus and Puumala virus M-segment DNA vaccines for haemorrhagic fever with renal syndrome delivered by intramuscular electroporation. Clin Microbiol Infect. 2014b;20(Suppl 5):110–117. doi: 10.1111/1469-0691.12553. [DOI] [PubMed] [Google Scholar]
- Jameson LJ, Logue CH, Atkinson B, Baker N, Galbraith SE, Carroll MW, Brooks T, Hewson R. The continued emergence of hantaviruses: isolation of a Seoul virus implicated in human disease, United Kingdom, October 2012. Euro Surveill. 2013a;18:20415. pii. [PubMed] [Google Scholar]
- Jameson LJ, Taori SK, Atkinson B, Levick P, Featherstone CA, van der Burgt G, McCarthy N, Hart J, Osborne JC, Walsh AL, Brooks TJ, Hewson R. Pet rats as a source of hantavirus in England and Wales, 2013. Euro Surveill. 2013b;18:20415. pii. [PubMed] [Google Scholar]
- Klempa B, Koivogui L, Sylla O, Koulemou K, Auste B, Krüger DH, ter Meulen J. Serological evidence of human hantavirus infections in Guinea, West Africa. J Infect Dis. 2010;201:1031–1034. doi: 10.1086/651169. [DOI] [PubMed] [Google Scholar]
- Klempa B, Witkowski PT, Popugaeva E, Auste B, Koivogui L, Fichet-Calvet E, Strecker T, Ter Meulen J, Krüger DH. Sangassou virus, the first hantavirus isolate from Africa, displays genetic and functional properties distinct from those of other murinae-associated hantaviruses. J Virol. 2012;86:3819–3827. doi: 10.1128/JVI.05879-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivula TT, Tuulasvaara A, Hetemaki I, Makela SM, Mustonen J, Sironen T, Vaheri A, Arstila TP. Regulatory T cell response correlates with the severity of human hantavirus infection. J Infect. 2014;68:387–394. doi: 10.1016/j.jinf.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Kobak L, Raftery MJ, Voigt S, Kühl AA, Kilic E, Kurth A, Witkowski P, Hofmann J, Nitsche A, Schaade L, Krüger DH, Schönrich G. Hantavirus-induced pathogenesis in mice with a humanized immune system. J Gen Virol. 2015;96:1258–1263. doi: 10.1099/vir.0.000087. [DOI] [PubMed] [Google Scholar]
- Krüger DH, Figueiredo LT, Song JW, Klempa B. Hantaviruses–globally emerging pathogens. J Clin Virol. 2015;64:128–136. doi: 10.1016/j.jcv.2014.08.033. [DOI] [PubMed] [Google Scholar]
- Laine O, Leppanen I, Koskela S, Antonen J, Makela S, Sinisalo M, Vaheri A, Mustonen J. Severe Puumala virus infection in a patient with a lymphoproliferative disease treated with icatibant. Infect Dis. 2015;47:107–111. doi: 10.3109/00365548.2014.969304. [DOI] [PubMed] [Google Scholar]
- Lee HW, Vaheri A, Schmaljohn CS. Discovery of hantaviruses and of the hantavirus genus: personal and historical perspectives of the Presidents of the International Society of hantaviruses. Virus Res. 2014;187:2–5. doi: 10.1016/j.virusres.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Mustonen J, Makela S, Outinen T, Laine O, Jylhava J, Arstila PT, Hurme M, Vaheri A. The pathogenesis of nephropathia epidemica: new knowledge and unanswered questions. Antivir Res. 2013;100:589–604. doi: 10.1016/j.antiviral.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Outinen TK, Makela S, Clement J, Paakkala A, Porsti I, Mustonen J. Community acquired severe acute kidney injury caused by hantavirus-induced hemorrhagic fever with renal syndrome has a favorable outcome. Nephron. 2015;130:182–190. doi: 10.1159/000433563. [DOI] [PubMed] [Google Scholar]
- Rasmuson J, Pourazar J, Mohamed N, Lejon K, Evander M, Blomberg A, Ahlm C. Cytotoxic immune responses in the lungs correlate to disease severity in patients with hantavirus infection. Eur J Clin Microbiol Infect Dis. 2016;35:713–721. doi: 10.1007/s10096-016-2592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynes JM, Razafindralambo NK, Lacoste V, Olive MM, Barivelo TA, Soarimalala V, Heraud JM, Lavergne A. Anjozorobe hantavirus, a new genetic variant of Thailand virus detected in rodents from Madagascar. Vector Borne Zoonotic Dis. 2014;14:212–219. doi: 10.1089/vbz.2013.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane J, Laine O, Makela S, Paakkala A, Jarva H, Mustonen J, Vapalahti O, Meri S, Vaheri A. Complement activation in Puumala hantavirus infection correlates with disease severity. Ann Med. 2012;44:468–475. doi: 10.3109/07853890.2011.573500. [DOI] [PubMed] [Google Scholar]
- Strandin T, Hepojoki J, Laine O, Makela S, Klingstrom J, Lundkvist A, Julkunen I, Mustonen J, Vaheri A. Interferons induce STAT1-dependent expression of tissue plasminogen activator, a pathogenicity factor in Puumala hantavirus disease. J Infect Dis. 2016;213:1632–1641. doi: 10.1093/infdis/jiv764. [DOI] [PubMed] [Google Scholar]
- Vaheri A, Strandin T, Hepojoki J, Sironen T, Henttonen H, Makela S, Mustonen J. Uncovering the mysteries of hantavirus infections. Nat Rev Microbiol. 2013;11:539–550. doi: 10.1038/nrmicro3066. [DOI] [PubMed] [Google Scholar]
- Vaheri A, Strandin T, Jaaskelainen AJ, Vapalahti O, Jarva H, Lokki ML, Antonen J, Leppanen I, Makela S, Meri S, Mustonen J. Pathophysiology of a severe case of Puumala hantavirus infection successfully treated with bradykinin receptor antagonist icatibant. Antivir Res. 2014;111:23–25. doi: 10.1016/j.antiviral.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Voutilainen L, Kallio ER, Niemimaa J, Vapalahti O, Henttonen H. Temporal dynamics of Puumala hantavirus infection in cyclic populations of bank voles. Sci Rep. 2016;6:21323. doi: 10.1038/srep21323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski PT, Drexler JF, Kallies R, Lickova M, Bokorova S, Mananga GD, Szemes T, Leroy EM, Krüger DH, Drosten C, Klempa B. Phylogenetic analysis of a newfound bat-borne hantavirus supports a laurasiatherian host association for ancestral mammalian hantaviruses. Infect Genet Evol. 2016;41:113–119. doi: 10.1016/j.meegid.2016.03.036. [DOI] [PubMed] [Google Scholar]


