SYNOPSIS
With the coming of the “Silver Tsunami,” expanding our knowledge about how a variety of intrinsic and extrinsic factors affect the immune system in the elderly is both timely and of immediate clinical need. It is clear that the global population is increasing in age. By the year 2030, over 20% of the population of the United States will be over 65 years of age. In this chapter, we will focus on how advanced age alters the immune systems and how this, in turn, modulates the ability of the aging lung to deal with the infectious challenges from both the outside world and from within the host.
Keywords: inflamm-aging, elderly, infection, host defense, macrophage, neutrophils, inflammation, immunosenescence
Introduction
With advanced age, there are changes in multiple biologic systems 1, including the immune system. Alterations in both innate and adaptive immune cells in the aged have been noted 2,3. In brief, the age-dependent effects on the innate immune response include diminished pathogen recognition, chemotaxis, and phagocytosis, and in adaptive immunity, declining numbers of naïve T lymphocytes and reduced cytotoxicity and antibody quality and quantity 2. Vaccine efficacy is reduced in the elderly, as are increases in autoimmunity and cancer 2. Overall, these immune defects, referred to collectively as immunosenescence, render the host less able to withstand injury or infection relative to younger individuals.
Among the hallmarks of the aging immune system is the persistent low-grade pro-inflammatory state characterized by heightened basal levels of pro-inflammatory mediators in the blood 4. Because of this association of advanced age and inflammation, Claudio Franceschi coined the term “inflamm-aging” in ~2000 4. Franceschi and others have reported that, even in healthy aged subjects without confirmed ailments, there is an elevated basal level of pro-inflammatory mediators, including interleukin-1 beta (IL-1®), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF〈) 4. The elevated levels of these and other pro-inflammatory factors in the aged can have both local and systemic consequences, none of which are ultimately beneficial to the host. This rise in circulating levels of pro-inflammatory cytokines and other factors is thought by some to be a driving factor in the development and maintenance of immunosenescence 4,5 and contribute to chronic diseases of the lung and other organs 6–8. In this review, we will focus on inflamm-aging, immunosenescence, and the lung, but it should be noted that 1) many of the age-dependent changes are neither limited to nor likely to be caused by changes in the aging lung itself and 2) the majority of these changes are not observed unless the host is challenged by some form of stressor, such as an injury or infection.
Changes in the lung with advanced age
A wide range of pulmonary parameters that influence lung immunity are altered with advanced age as described in Table 1.
Table 1.
Aging of the Lung
| Lung functions that are changed with age | Reference |
|---|---|
| ↓ Mucociliary escalator: reduced ability to clear microbes and debris from the airway | 9,10 |
| ↑ Expression of proteins associated with bacteria attachment and infiltration in the pulmonary epithelial cells, including polymeric immunoglobulin receptor and platelet-activating factor receptor | 11,12 |
| ↑ Expression of markers of cellular senescence | 6,13 |
| ↓ Epithelial expression of anti-microbial peptides | 14,15 |
| ↑ Levels of complement and surfactant proteins | 15 |
| ↑ Proteostasis (and the loss of ability of cells from the aged to properly control protein abundance, proper folding and degradation) | 16, 17 |
| ↑ Susceptibility to pulmonary infections | 11,12,18–23 |
| Dysbiosis (or the imbalance) of the pulmonary microbiome in the absence of infection and after infection | 24–29 |
Innate immune cells of the lung and changes with advanced age
Macrophages
The primary resident innate immune cell in the airway is the alveolar macrophage. This multifaceted cell serves as the first line of defense against invading pathogens and plays a critical role in lung immunologic homeostasis. Macrophages are capable of both initiating and resolving an inflammatory response 30–32. This ability to play divergent roles is due to macrophage plasticity. Macrophages can adapt and even change phenotype in response to environmental cues, enabling them to adapt to varying conditions and perform a plethora of diverse functions 33–36. Historically, this stimulus-induced shift in macrophage phenotype was referred to as M1 and M2 phenotypes with M1 being pro-inflammatory and M2 anti-inflammatory. 37,38 However, because of poor definition and inconsistencies in the cell surface markers defining these two phenotypes, a group of expert macrophage research investigators recently redefined macrophage classification terminology so that they are more narrowly classified based on the source of the macrophages and activation stimuli, as well as the specific group of markers associated with the particular activation phenotype 39. Regardless of nomenclature, under resting conditions, alveolar macrophages maintain an anti-inflammatory profile to keep the pulmonary airway in check and are capable of rapidly springing into action, becoming strongly pro-inflammatory when alerted by the presence of foreign material (Figure 1). After pathogen clearance, the ability of alveolar macrophages to promote resolution and return to an anti-inflammatory resting phenotype is equally important for maintenance of lung homeostasis.
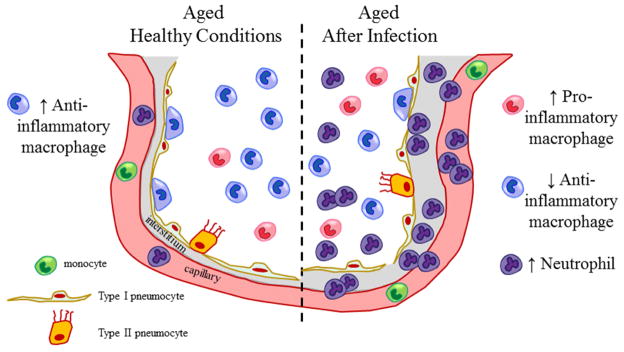
Figure 1. Innate immune phenotype of the aged lung.
Regardless of age, under healthy conditions, the major leukocyte of the distal lung is the alveolar macrophage. These multifaceted cells exist in an anti-inflammatory state to limit inflammation and maintain pulmonary homeostasis. A variety of pathogenic conditions alter alveolar macrophage function. In the young, alveolar macrophages can rapidly respond to external stimuli such as bacteria, clear infections and return to their anti-inflammatory state. In contrast, in the elderly, alveolar macrophages fail to mount an adequate response infectious insult, are slow at recruiting neutrophils to help combat the respiratory pathogens and are unable to return to their anti-inflammatory phenotype, thus leaving the lung in a compromised state.
Multiple factors are involved in the resolution of inflammation in the lung. These include but are not limited to 1) clearance of the pathogen or debris, 2) reduced production of neutrophil chemokines and 3) removal of apoptotic cells, including effete neutrophils. All of these processes are orchestrated by alveolar macrophages 40. It should be noted that the inability of macrophages to perform these functions can result in prolonged inflammation which, if left unchecked, can result in damage to lung tissue 41. Central to the restoration of pulmonary homeostasis is the removal of neutrophils which is associated with a shift in alveolar macrophages phenotype to an anti-inflammatory profile 40.
With advanced age, it is clear that the ability of macrophages to perform their normal functions is impaired and that inflamm-aging plays a role in this altered response despite the lack of change in macrophage number. A comprehensive review of macrophage function and aging is available.37. In brief, both in vivo and in vitro studies conducted in humans and in various animal models suggest that many but not all of the functions of macrophages are slowed or diminished in magnitude in the aged, leaving the host unable to shift between phenotypes when needed 18,34,42. Some of the better documented age-dependent changes in macrophage function are highlighted in Table 2.
Table 2.
Aging and Macrophages
| Alterations in macrophage function with advanced age | Reference |
|---|---|
| ↓ Toll-like Receptor (TLR) expression (both mRNA and protein) and downstream signaling (in most but not all studies) | 24,43–48 |
| ↓ Production of pro-inflammatory and immunomodulatory cytokines, including TNFα, IL-6, IL-1β, and CCL2 (MCP-1) after stimulation by a varity of agonists. | 46–52 |
| ↓ Telomere length | 53 |
| ↑ Regulators of immune signaling, such as A20, a de-ubiquitinase which, in turn, inhibits TLR signaling and NF-κB activation | 11, 12 |
| ↓ Phagocytosis and pathogen clearance | 7,51,54–59 |
Neutrophils
The neutrophil is a key innate immune cell that is often the first cell type to be recruited to sites of injury and infection. Neutrophils are capable of performing a variety of anti-microbial functions that play a critical role in removing pathogens from tissues during the early stages of lung infections. Within minutes after recognition of foreign material, macrophages become activated and initiate a cascade of events which includes the release of chemoattractant cytokines that recruit neutrophils. Working together, macrophages and neutrophils join forces to remove and destroy infectious organisms 60,61. Neutrophil functions that are altered with advanced age are shown in Table 3.
Table 3.
Aging and Neutrophils
| Alterations in neutrophil function with advanced age | Reference |
|---|---|
| ↓ Chemotaxis | 62–69 |
| No change in chemokinesis | 62 |
| ↓ Phagocytosis | 64, 66, 70–77 |
| ↓ Production of reactive oxygen species (ROS) | 63, 64, 66, 72, 73, 78–80 |
| ↓ Generation of neutrophil extracellular traps (NETs) | 81–83 |
| ↓ Production of pro-inflammatory cytokines and mediators, including IL-6, IL-8, myeloperoxidase, elastase and ↑ Production of anti- inflammatory cytokines, IL-10 | 78, 84, 85 |
| No increase in lifespan following stimulation | 85–89 |
A paradox: Aging causes higher cytokine levels in vivo, yet reduced production by inflammatory cells in vitro
The cellular sources of the mediators responsible for inflamm-aging remain unknown. Interestingly, there is a disconnect between the in vivo and in vitro effects of stimulation on the inflammatory response in young adult and older subjects and in cells isolated from those subjects. From both human and rodent studies in which an inflammatory stimulus, like lipopolysaccharide (LPS), is given in vivo, it is clear that the inflammatory response is of greater magnitude and duration in older subjects relative to younger 90–92. In contrast, in vitro stimulation of certain cell subsets, including blood monocytes, lung or peritoneal macrophages from aged subjects, yields lower levels of cytokines relative to cells from younger individuals, 46–48,93,94 suggesting either that monocyte/macrophages are not a major source of these mediators in vivo or that there are additional factors responsible for this discrepancy. The effects of aging on monocyte/macrophage functions were comprehensively reviewed elsewhere 42.
What causes inflamm-aging?
There are multiple theories about the origin and perpetuation of inflamm-aging. Ones that have gained press over time include classical ideas about increased oxidative stress, DNA damage and telomere shortening.1,18 In brief, it is believed that with advanced age there is 1) an increase in post-translational modification of macromolecules including DNA, proteins and lipids that stimulate leukocytes and other cells to secrete pro-inflammatory cytokines and 2) senescence of immune and non-immune cells leading to an increased release of inflammatory mediators via a senescence associated secretory phenotype 1,18. Additionally, a complementary and newer theory about the initiation of inflamm-aging is emerging and gaining support in the literature. This theory revolves around changes in intestinal permeability that allows bacteria and bacterial products (e.g. endotoxin and peptidoglycan) to translocate into the lymphatic system and ultimately the bloodstream where they can trigger the low systemic inflammation in the elderly.
In brief, changes in aged intestine include: dysbiosis of intestinal microbiota in animal models of aging and in elderly humans; 95–98 and decreased integrity of the intestinal epithelial cell barrier in mice and man 99–104
Aging, Dysbiosis of Intestinal Microbiome and the Gut-Liver-Lung Axis
Extensive clinical and experimental evidence reveals that the intestinal barrier integrity plays a role in inflamm-aging which in turn alters pulmonary inflammation. The gut hypothesis states that heightened intestinal permeability, along with changes in immune function of the gut, results in increased translocation of bacteria and bacterial products 105–107.
Like the lung, the intestine is an organ that is exposed to the outside environment with a large surface area. While the lung and intestine provide very different biological functions, they share in common the feature of needing to maintain compartmental barriers which must remain intact to 1) permit normal organ function to occur and 2) protect the host from invading pathogens. Those barriers are created by the epithelium lining the lumen of the respiratory and gastrointestinal tract. The integrity of tight junctions between adjacent epithelial cells is an essential part of these barriers. In both young and the aged, this barrier is maintained in part by the complex interactions between the multiple proteins making up tight junctions (TJ), including occludins and claudins, along with multiple adaptor and scaffolding proteins. Under normal conditions, the epithelium maintains a semi-permeable barrier permitting passage of smaller molecules while preventing the movement of other materials to its underlying mucosal tissue. Regardless of the organ, breach of the epithelial barrier allows inappropriate access of microbial organisms and debris to the underlying mucosa, which can cause inflammation and tissue damage 108–111. The integrity of this barrier can be perturbed in a plethora of disease states, such as reflux esophagitis, cancer, and inflammatory bowel disease, and are discussed elsewhere 110,111. One mechanism of altering the epithelial status quo is mediated by the enzyme myosin light chain kinase (MLCK), the long 210 kDa form, which remains inactive in the cytoplasm of epithelial (and endothelial) cells. When activated, MLCK phosphorylates myosin regulatory light-chain (MLC) at serine 19, allowing it to interact with actin. The interaction between actin and myosin light-chain causes cytoskeletal sliding, which disrupts tight junctions and creates a gap in the epithelial barrier, 112,113 thus permitting the uncontrolled flow of fluid, bacteria, bacterial products and other materials across the epithelial lining 114,115. Of interest to research on the elderly, the same set of pro-inflammatory mediators that are elevated in the circulation of the aged and serve as hallmarks of inflamm-aging, namely IL-1β, IL-6 and TNF–α, can trigger the activation of MLCK. Additionally, in the lung, when MLCK is activated in the capillary lining endothelial cells, it results in paracellular permeability, which can lead to pulmonary edema 112. As noted above, one of the consequences of the leakiness of the intestinal epithelium is the translocation of bacteria from the intestinal lumen to the underlying mucosal tissue and to regional lymph nodes. Subsequently, these products can traffic to the liver where they can stimulate production of pro-inflammatory cytokines (Figure 2). If not appropriately contained by the aging immune system, the dissemination of bacteria and/or release of bacteria and bacterial products such as endotoxins throughout the body. This can occur leading to prolonged and exacerbated inflammation in all organs and likely contributes to increased morbidity and mortality in the aged. Hence, the intestine and its microbial contents can play a critical role in inducing or exacerbating complications in various patient populations 113,116 and in the aged 103,104,117–119.
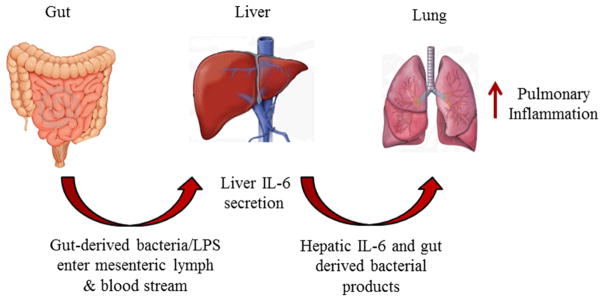
Figure 2. The gut, liver, lung axis.
Under healthy conditions, the epithelial cells lining the intestine maintain tight junctions preventing luminal contents from invading the underlying mucosal tissues. In the aged, it is thought that epithelial cell tight junctions loosen, possibly in response to the presence of the pro-inflammatory cytokines associated with inflamm-aging. This loosening of junctional complexes and subsequent increase in paracellular permeability allows gut-derived bacteria, bacterial products and endotoxins to enter the mesenteric lymph and the bloodstream. Bacteria and their products then trigger Kupffer cells and other cells in the liver to produce and secrete pro-inflammatory cytokines, including IL-6. Hepatic-derived IL-6, along with the gut-derived bacteria products in the circulation, promotes baseline lung inflammation which can then be further exacerbated in the aged after injury or infection.
Summary and Future Directions
Factors or treatments that reduce inflamm-aging are of interest to basic and clinical researchers as they may be able to dampen the prolonged and heightened inflammation seen in the elderly after attempting to combat an infection. Thoughts about the design of therapeutic interventions to reduce inflamm-aging can be directed either at cells themselves or the pro-inflammatory environment in which they reside. Animal studies involving adoptive transfer of subsets of leukocytes are in progress, as are numerous clinical and basic research studies investigating anti-oxidant and anti-inflammatory agents to attenuate the over exuberant inflammatory response in the aged. Some believe that taking the indirect approach of reducing intestinal inflammation or restoring the intestinal microbiota may have benefit, but this is not without controversy. 120–122 It would be of interest to determine if patients receiving anti-inflammatory therapies for other conditions have restored intestinal barrier function and if this, in turn, improves systemic responses to the injury or infection in the aged population. Further exploration of these direct and indirect avenues of therapeutic manipulation may be of benefit to the overall health of the aged and with that will likely improve overall lung health of the elderly.
KEY POINTS.
Age-dependent changes in immune responses cause increased morbidity and mortality in the elderly.
Inflamm-aging causes immunosenescence.
Intestinal permeability in the elderly may be responsible for inflamm-aging.
The ability of alveolar macrophages to maintain pulmonary homeostasis following clearance of infection is reduced in the aged.
Acknowledgments
This work was supported by the National Institutes of Health R01 AG018859 and T32 AG000279.
Footnotes
Disclosure: This work was funded in part by grants from the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2015 doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nature reviews Immunology. 2013;13(12):875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 5.Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Seminars in immunology. 2012;24(5):331–341. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 7.Hearps AC, Martin GE, Angelovich TA, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging cell. 2012;11(5):867–875. doi: 10.1111/j.1474-9726.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 8.Murray MA, Chotirmall SH. The Impact of Immunosenescence on Pulmonary Disease. Mediators of inflammation. 2015;2015:692546. doi: 10.1155/2015/692546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho JC, Chan KN, Hu WH, et al. The Effect of Aging on Nasal Mucociliary Clearance, Beat Frequency, and Ultrastructure of Respiratory Cilia. American journal of respiratory and critical care medicine. 2001;163(4):983–988. doi: 10.1164/ajrccm.163.4.9909121. [DOI] [PubMed] [Google Scholar]
- 10.Svartengren M, Falk R, Philipson K. Long-term clearance from small airways decreases with age. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2005;26(4):609–615. doi: 10.1183/09031936.05.00002105. [DOI] [PubMed] [Google Scholar]
- 11.Hinojosa CA, Akula Suresh Babu R, Rahman MM, Fernandes G, Boyd AR, Orihuela CJ. Elevated A20 contributes to age-dependent macrophage dysfunction in the lungs. Experimental gerontology. 2014;54:58–66. doi: 10.1016/j.exger.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. The Journal of infectious diseases. 2009;200(4):546–554. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivshankar P, Boyd AR, Le Saux CJ, Yeh IT, Orihuela CJ. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging cell. 2011;10(5):798–806. doi: 10.1111/j.1474-9726.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simell B, Vuorela A, Ekstrom N, et al. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine. 2011;29(10):1929–1934. doi: 10.1016/j.vaccine.2010.12.121. [DOI] [PubMed] [Google Scholar]
- 15.Moliva JI, Rajaram MV, Sidiki S, et al. Molecular composition of the alveolar lining fluid in the aging lung. Age. 2014;36(3):9633. doi: 10.1007/s11357-014-9633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaushik S, Cuervo AM. Proteostasis and aging. Nature medicine. 2015;21(12):1406–1415. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- 17.Meiners S, Eickelberg O, Konigshoff M. Hallmarks of the ageing lung. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2015;45(3):807–827. doi: 10.1183/09031936.00186914. [DOI] [PubMed] [Google Scholar]
- 18.Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17(1):7–19. doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd AR, Shivshankar P, Jiang S, Berton MT, Orihuela CJ. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Experimental gerontology. 2012;47(7):507–518. doi: 10.1016/j.exger.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verschoor CP, Johnstone J, Loeb M, Bramson JL, Bowdish DM. Anti-pneumococcal deficits of monocyte-derived macrophages from the advanced-age, frail elderly and related impairments in PI3K-AKT signaling. Human immunology. 2014;75(12):1192–1196. doi: 10.1016/j.humimm.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Kline KA, Bowdish DM. Infection in an aging population. Current opinion in microbiology. 2016;29:63–67. doi: 10.1016/j.mib.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Chen MM, Palmer JL, Plackett TP, Deburghgraeve CR, Kovacs EJ. Age-related differences in the neutrophil response to pulmonary pseudomonas infection. Experimental gerontology. 2014;54:42–46. doi: 10.1016/j.exger.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackaman C, Radley-Crabb HG, Soffe Z, Shavlakadze T, Grounds MD, Nelson DJ. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging cell. 2013;12(3):345–357. doi: 10.1111/acel.12062. [DOI] [PubMed] [Google Scholar]
- 24.Stearns JC, Davidson CJ, McKeon S, et al. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. The ISME journal. 2015;9(5):1246–1259. doi: 10.1038/ismej.2014.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Steenhuijsen Piters WA, Huijskens EG, Wyllie AL, et al. Dysbiosis of upper respiratory tract microbiota in elderly pneumonia patients. The ISME journal. 2016;10(1):97–108. doi: 10.1038/ismej.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krone CL, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Respiratory microbiota dynamics following Streptococcus pneumoniae acquisition in young and elderly mice. Infection and immunity. 2014;82(4):1725–1731. doi: 10.1128/IAI.01290-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thevaranjan N, Whelan FJ, Puchta A, et al. Streptococcus pneumoniae Colonization Disrupts the Microbial Community within the Upper Respiratory Tract of Aging Mice. Infection and immunity. 2016;84(4):906–916. doi: 10.1128/IAI.01275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krone CL, Trzcinski K, Zborowski T, Sanders EA, Bogaert D. Impaired innate mucosal immunity in aged mice permits prolonged Streptococcus pneumoniae colonization. Infection and immunity. 2013;81(12):4615–4625. doi: 10.1128/IAI.00618-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whelan FJ, Verschoor CP, Stearns JC, et al. The loss of topography in the microbial communities of the upper respiratory tract in the elderly. Ann Am Thorac Soc. 2014;11(4):513–521. doi: 10.1513/AnnalsATS.201310-351OC. [DOI] [PubMed] [Google Scholar]
- 30.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Frontiers in immunology. 2011;2:65. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. American journal of physiology Lung cellular and molecular physiology. 2014;306(8):L709–725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porcheray F, Viaud S, Rimaniol AC, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clinical and experimental immunology. 2005;142(3):481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glezeva N, Horgan S, Baugh JA. Monocyte and macrophage subsets along the continuum to heart failure: Misguided heroes or targetable villains? Journal of molecular and cellular cardiology. 2015 doi: 10.1016/j.yjmcc.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Malyshev I, Malyshev Y. Current Concept and Update of the Macrophage Plasticity Concept: Intracellular Mechanisms of Reprogramming and M3 Macrophage “Switch” Phenotype. BioMed research international. 2015;2015:341308. doi: 10.1155/2015/341308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das A, Sinha M, Datta S, et al. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. The American journal of pathology. 2015;185(10):2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. The Journal of pathology. 2013;229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 37.Albright JM, Dunn RC, Shults JA, Boe DM, Afshar M, Kovacs EJ. Advanced Age Alters Monocyte and Macrophage Responses. Antioxidants & redox signaling. 2016;25(15):805–815. doi: 10.1089/ars.2016.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ariel A, Maridonneau-Parini I, Rovere-Querini P, Levine JS, Muhl H. Macrophages in inflammation and its resolution. Frontiers in immunology. 2012;3:324. doi: 10.3389/fimmu.2012.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. The Journal of clinical investigation. 2011;121(3):985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albright JM, Dunn RC, Shults JA, Boe DM, Afshar M, Kovacs EJ. Advanced Age Alters Monocyte and Macrophage Responses. Antioxidants & redox signaling. 2016 doi: 10.1089/ars.2016.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw AC, Panda A, Joshi SR, Qian F, Allore HG, Montgomery RR. Dysregulation of human Toll-like receptor function in aging. Ageing research reviews. 2011;10(3):346–353. doi: 10.1016/j.arr.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Nardo D. Toll-like receptors: Activation, signalling and transcriptional modulation. Cytokine. 2015;74(2):181–189. doi: 10.1016/j.cyto.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 45.Kaparakis M, Philpott DJ, Ferrero RL. Mammalian NLR proteins; discriminating foe from friend. Immunology and cell biology. 2007;85(6):495–502. doi: 10.1038/sj.icb.7100105. [DOI] [PubMed] [Google Scholar]
- 46.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. Journal of immunology. 2002;169(9):4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 47.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. Journal of leukocyte biology. 2004;75(2):342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 48.Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mechanisms of ageing and development. 2005;126(12):1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Chelvarajan RL, Collins SM, Van Willigen JM, Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. Journal of leukocyte biology. 2005;77(4):503–512. doi: 10.1189/jlb.0804449. [DOI] [PubMed] [Google Scholar]
- 50.Chelvarajan RL, Liu Y, Popa D, et al. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. Journal of leukocyte biology. 2006;79(6):1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- 51.Liang S, Domon H, Hosur KB, Wang M, Hajishengallis G. Age-related alterations in innate immune receptor expression and ability of macrophages to respond to pathogen challenge in vitro. Mechanisms of ageing and development. 2009;130(8):538–546. doi: 10.1016/j.mad.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2012;32(1):18–26. doi: 10.1089/jir.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arai Y, Martin-Ruiz CM, Takayama M, et al. Inflammation, But Not Telomere Length, Predicts Successful Ageing at Extreme Old Age: A Longitudinal Study of Semi-supercentenarians. EBioMedicine. 2015;2(10):1549–1558. doi: 10.1016/j.ebiom.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Laboratory investigation; a journal of technical methods and pathology. 1999;79(12):1479–1487. [PubMed] [Google Scholar]
- 55.Albright JF, Albright JW. Senescence of Natural/Innate Resistance to Infection. Totowa, NY: Humana press, Inc; 2003. [Google Scholar]
- 56.Lynch AM, Murphy KJ, Deighan BF, et al. The impact of glial activation in the aging brain. Aging and disease. 2010;1(3):262–278. [PMC free article] [PubMed] [Google Scholar]
- 57.Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging cell. 2014;13(4):699–708. doi: 10.1111/acel.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clinical and experimental immunology. 2008;152(3):448–455. doi: 10.1111/j.1365-2249.2008.03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arnardottir HH, Dalli J, Colas RA, Shinohara M, Serhan CN. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines. Journal of immunology. 2014;193(8):4235–4244. doi: 10.4049/jimmunol.1401313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. Journal of leukocyte biology. 2010;87(1):93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 61.Silva MT, Correia-Neves M. Neutrophils and macrophages: the main partners of phagocyte cell systems. Frontiers in immunology. 2012;3:174. doi: 10.3389/fimmu.2012.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sapey E, Greenwood H, Walton G, et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123(2):239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Lorenzo G, Balistreri CR, Candore G, et al. Granulocyte and natural killer activity in the elderly. Mechanisms of ageing and development. 1999;108(1):25–38. doi: 10.1016/s0047-6374(98)00156-0. [DOI] [PubMed] [Google Scholar]
- 64.Polignano A, Tortorella C, Venezia A, Jirillo E, Antonaci S. Age-associated changes of neutrophil responsiveness in a human healthy elderly population. Cytobios. 1994;80(322):145–153. [PubMed] [Google Scholar]
- 65.McLaughlin B, O’Malley K, Cotter TG. Age-related differences in granulocyte chemotaxis and degranulation. Clinical science. 1986;70(1):59–62. doi: 10.1042/cs0700059. [DOI] [PubMed] [Google Scholar]
- 66.Antonaci S, Jirillo E, Ventura MT, Garofalo AR, Bonomo L. Non-specific immunity in aging: deficiency of monocyte and polymorphonuclear cell-mediated functions. Mechanisms of ageing and development. 1984;24(3):367–375. doi: 10.1016/0047-6374(84)90121-0. [DOI] [PubMed] [Google Scholar]
- 67.Nomellini V, Brubaker AL, Mahbub S, Palmer JL, Gomez CR, Kovacs EJ. Dysregulation of neutrophil CXCR2 and pulmonary endothelial icam-1 promotes age-related pulmonary inflammation. Aging and disease. 2012;3(3):234–247. [PMC free article] [PubMed] [Google Scholar]
- 68.Nomellini V, Faunce DE, Gomez CR, Kovacs EJ. An age-associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. Journal of leukocyte biology. 2008;83(6):1493–1501. doi: 10.1189/jlb.1007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. Journal of immunology. 2013;190(4):1746–1757. doi: 10.4049/jimmunol.1201213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Butcher SK, Chahal H, Nayak L, et al. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. Journal of leukocyte biology. 2001;70(6):881–886. [PubMed] [Google Scholar]
- 71.Butcher SK, Killampalli V, Chahal H, Kaya Alpar E, Lord JM. Effect of age on susceptibility to post-traumatic infection in the elderly. Biochemical Society transactions. 2003;31(2):449–451. doi: 10.1042/bst0310449. [DOI] [PubMed] [Google Scholar]
- 72.Fulop T, Jr, Foris G, Worum I, Leovey A. Age-dependent alterations of Fc gamma receptor-mediated effector functions of human polymorphonuclear leucocytes. Clinical and experimental immunology. 1985;61(2):425–432. [PMC free article] [PubMed] [Google Scholar]
- 73.Wenisch C, Patruta S, Daxbock F, Krause R, Horl W. Effect of age on human neutrophil function. Journal of leukocyte biology. 2000;67(1):40–45. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]
- 74.Amaya RA, Baker CJ, Keitel WA, Edwards MS. Healthy elderly people lack neutrophil-mediated functional activity to type V group B Streptococcus. Journal of the American Geriatrics Society. 2004;52(1):46–50. doi: 10.1111/j.1532-5415.2004.52009.x. [DOI] [PubMed] [Google Scholar]
- 75.Butcher S, Chahel H, Lord JM. Review article: ageing and the neutrophil: no appetite for killing? Immunology. 2000;100(4):411–416. doi: 10.1046/j.1365-2567.2000.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alonso-Fernandez P, Puerto M, Mate I, Ribera JM, de la Fuente M. Neutrophils of centenarians show function levels similar to those of young adults. Journal of the American Geriatrics Society. 2008;56(12):2244–2251. doi: 10.1111/j.1532-5415.2008.02018.x. [DOI] [PubMed] [Google Scholar]
- 77.Esparza B, Sanchez H, Ruiz M, Barranquero M, Sabino E, Merino F. Neutrophil function in elderly persons assessed by flow cytometry. Immunological investigations. 1996;25(3):185–190. doi: 10.3109/08820139609059301. [DOI] [PubMed] [Google Scholar]
- 78.Dalboni TM, Abe AE, de Oliveira CE, et al. Activation profile of CXCL8-stimulated neutrophils and aging. Cytokine. 2013;61(3):716–719. doi: 10.1016/j.cyto.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 79.Tortorella C, Ottolenghi A, Pugliese P, Jirillo E, Antonaci S. Relationship between respiratory burst and adhesiveness capacity in elderly polymorphonuclear cells. Mechanisms of ageing and development. 1993;69(1–2):53–63. doi: 10.1016/0047-6374(93)90071-x. [DOI] [PubMed] [Google Scholar]
- 80.Fu YK, Arkins S, Li YM, Dantzer R, Kelley KW. Reduction in superoxide anion secretion and bactericidal activity of neutrophils from aged rats: reversal by the combination of gamma interferon and growth hormone. Infection and immunity. 1994;62(1):1–8. doi: 10.1128/iai.62.1.1-8.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kruger P, Saffarzadeh M, Weber AN, et al. Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury. PLoS pathogens. 2015;11(3):e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tseng CW, Kyme PA, Arruda A, Ramanujan VK, Tawackoli W, Liu GY. Innate immune dysfunctions in aged mice facilitate the systemic dissemination of methicillin-resistant S. aureus. PloS one. 2012;7(7):e41454. doi: 10.1371/journal.pone.0041454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hazeldine J, Harris P, Chapple IL, et al. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging cell. 2014;13(4):690–698. doi: 10.1111/acel.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qian F, Guo X, Wang X, et al. Reduced bioenergetics and toll-like receptor 1 function in human polymorphonuclear leukocytes in aging. Aging (Albany NY) 2014;6(2):131–139. doi: 10.18632/aging.100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schroder AK, von der Ohe M, Kolling U, et al. Polymorphonuclear leucocytes selectively produce anti-inflammatory interleukin-1 receptor antagonist and chemokines, but fail to produce pro-inflammatory mediators. Immunology. 2006;119(3):317–327. doi: 10.1111/j.1365-2567.2006.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fulop T, Jr, Fouquet C, Allaire P, et al. Changes in apoptosis of human polymorphonuclear granulocytes with aging. Mechanisms of ageing and development. 1997;96(1–3):15–34. doi: 10.1016/s0047-6374(96)01881-7. [DOI] [PubMed] [Google Scholar]
- 87.Tortorella C, Simone O, Piazzolla G, Stella I, Cappiello V, Antonaci S. Role of phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways in granulocyte macrophage-colony-stimulating factor failure to delay fas-induced neutrophil apoptosis in elderly humans. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61(11):1111–1118. doi: 10.1093/gerona/61.11.1111. [DOI] [PubMed] [Google Scholar]
- 88.Fortin CF, Lesur O, Fulop T., Jr Effects of aging on triggering receptor expressed on myeloid cells (TREM)-1-induced PMN functions. FEBS letters. 2007;581(6):1173–1178. doi: 10.1016/j.febslet.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 89.Wessels I, Jansen J, Rink L, Uciechowski P. Immunosenescence of polymorphonuclear neutrophils. ScientificWorldJournal. 2010;10:145–160. doi: 10.1100/tsw.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gomez CR, Goral J, Ramirez L, Kopf M, Kovacs EJ. Aberrant acute-phase response in aged interleukin-6 knockout mice. Shock. 2006;25(6):581–585. doi: 10.1097/01.shk.000029553.39081.ec. [DOI] [PubMed] [Google Scholar]
- 91.Gomez CR, Hirano S, Cutro BT, et al. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Critical care medicine. 2007;35(1):246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 92.Gomez CR, Nomellini V, Baila H, Oshima K, Kovacs EJ. Comparison of the effects of aging and IL-6 on the hepatic inflammatory response in two models of systemic injury: scald injury versus I.p. LPS administration. Shock. 2009;31(2):178–184. doi: 10.1097/SHK.0b013e318180feb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging cell. 2004;3(4):161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 94.Gomez CR, Karavitis J, Palmer JL, et al. Interleukin-6 contributes to age-related alteration of cytokine production by macrophages. Mediators of inflammation. 2010;2010:475139. doi: 10.1155/2010/475139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim KA, Jeong JJ, Yoo SY, Kim DH. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC microbiology. 2016;16(1):9. doi: 10.1186/s12866-016-0625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 98.Langille MG, Meehan CJ, Koenig JE, et al. Microbial shifts in the aging mouse gut. Microbiome. 2014;2(1):50. doi: 10.1186/s40168-014-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Man AL, Bertelli E, Rentini S, et al. Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clinical science. 2015;129(7):515–527. doi: 10.1042/CS20150046. [DOI] [PubMed] [Google Scholar]
- 100.Cesar Machado MC, da Silva FP. Intestinal Barrier Dysfunction in Human Pathology and Aging. Current pharmaceutical design. 2016;22(30):4645–4650. doi: 10.2174/1381612822666160510125331. [DOI] [PubMed] [Google Scholar]
- 101.Pasternak JA, Kent-Dennis C, Van Kessel AG, Wilson HL. Claudin-4 undergoes age-dependent change in cellular localization on pig jejunal villous epithelial cells, independent of bacterial colonization. Mediators of inflammation. 2015;2015:263629. doi: 10.1155/2015/263629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Valentini L, Ramminger S, Haas V, et al. Small intestinal permeability in older adults. Physiological reports. 2014;2(4):e00281. doi: 10.14814/phy2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mabbott NA. A breakdown in communication? Understanding the effects of aging on the human small intestine epithelium. Clinical science. 2015;129(7):529–531. doi: 10.1042/CS20150364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mabbott NA, Kobayashi A, Sehgal A, Bradford BM, Pattison M, Donaldson DS. Aging and the mucosal immune system in the intestine. Biogerontology. 2015;16(2):133–145. doi: 10.1007/s10522-014-9498-z. [DOI] [PubMed] [Google Scholar]
- 105.Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. The Journal of burn care & rehabilitation. 2005;26(5):383–391. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- 106.Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings? Surgery. 2002;131(3):241–244. doi: 10.1067/msy.2002.116408. [DOI] [PubMed] [Google Scholar]
- 107.Deitch EA. Gut-origin sepsis: Evolution of a concept. Surgeon. 2012 doi: 10.1016/j.surge.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ivanov AI. Structure and regulation of intestinal epithelial tight junctions: current concepts and unanswered questions. Advances in experimental medicine and biology. 2012;763:132–148. doi: 10.1007/978-1-4614-4711-5_6. [DOI] [PubMed] [Google Scholar]
- 109.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. The Journal of nutrition. 2011;141(5):769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 110.Hallstrand TS, Hackett TL, Altemeier WA, Matute-Bello G, Hansbro PM, Knight DA. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clinical immunology. 2014;151(1):1–15. doi: 10.1016/j.clim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 111.Oshima T, Miwa H. Gastrointestinal mucosal barrier function and diseases. Journal of gastroenterology. 2016;51(8):768–778. doi: 10.1007/s00535-016-1207-z. [DOI] [PubMed] [Google Scholar]
- 112.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. Journal of applied physiology. 2001;91(4):1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 113.Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013;11(9):1075–1083. doi: 10.1016/j.cgh.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. The American journal of pathology. 2006;169(6):1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Turner JR, Rill BK, Carlson SL, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. The American journal of physiology. 1997;273(4 Pt 1):C1378–1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 116.Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends in molecular medicine. 2014;20(4):214–223. doi: 10.1016/j.molmed.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Man AL, Gicheva N, Nicoletti C. The impact of ageing on the intestinal epithelial barrier and immune system. Cellular immunology. 2014;289(1–2):112–118. doi: 10.1016/j.cellimm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 118.Nicoletti C. Age-associated changes of the intestinal epithelial barrier: local and systemic implications. Expert review of gastroenterology & hepatology. 2015;9(12):1467–1469. doi: 10.1586/17474124.2015.1092872. [DOI] [PubMed] [Google Scholar]
- 119.Schiffrin EJ, Morley JE, Donnet-Hughes A, Guigoz Y. The inflammatory status of the elderly: the intestinal contribution. Mutation research. 2010;690(1–2):50–56. doi: 10.1016/j.mrfmmm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 120.Arboleya S, Watkins C, Stanton C, Ross RP. Gut Bifidobacteria Populations in Human Health and Aging. Frontiers in microbiology. 2016;7:1204. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salazar N, Valdes-Varela L, Gonzalez S, Gueimonde M, de Los Reyes-Gavilan CG. Nutrition and the gut microbiome in the elderly. Gut microbes. 2016:1–16. doi: 10.1080/19490976.2016.1256525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Beek AA, Sovran B, Hugenholtz F, et al. Supplementation with Lactobacillus plantarum WCFS1 Prevents Decline of Mucus Barrier in Colon of Accelerated Aging Ercc1-/Delta7 Mice. Frontiers in immunology. 2016;7:408. doi: 10.3389/fimmu.2016.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]