Abstract
Respiratory syncytial virus (RSV) is an important and ubiquitous respiratory pathogen for which no vaccine is available notwithstanding more than 50 years of effort. It causes the most severe disease at the extremes of age and in settings of immunodeficiency. Although RSV is susceptible to neutralizing antibody, it has evolved multiple mechanisms of immune evasion allowing it to repeatedly infect people despite relatively little genetic diversity. Recent breakthroughs in determining the structure and antigenic content of the fusion (F) glycoprotein in its metastable untriggered prefusion form (pre-F) and the stable rearranged postfusion form (post-F) have yielded vaccine strategies that can induce potent neutralizing antibody responses and effectively boost pre-existing neutralizing activity. In parallel, novel live-attenuated and chimeric virus vaccine candidates and other novel approaches to deliver vaccine antigens have been developed. These events and activities have aroused optimism and a robust pipeline of potential vaccine products that promise to provide a means to reduce the public health burden of RSV infection.
Epidemiology and vaccine target populations
Respiratory syncytial virus (RSV) is a pneumovirus in the paramyxoviridae family, and is the leading viral cause of severe respiratory disease and hospitalization in young children. The peak age of hospitalization is between 2 and 3 months of age, but risk of severe disease continues until about 5 years of age. In hospitalized children there is an increased frequency of childhood wheezing [1]. RSV infects nearly all people globally by the end of the 2nd year of life and everyone by 3 years of age [2]. People continue to be infected throughout life every 3–10 years [3]. In people over 5 years of age RSV infection rarely leads to hospitalization until they become susceptible through aging or immune deficiency. The frail elderly experience substantial increased mortality following RSV infection, in many years comparable to that of influenza [4], and it generally manifests as a complication of underlying heart and lung disease and a weakening constitution. People who have diminished CD8T cell function in lung because of severe combined immunodeficiency [5], allogenic bone marrow transplant [6], lung transplant [7], or aging [8,9] also experience severe disease from RSV infection. The goals for vaccination are to prevent severe disease and its subsequent complications. Therefore, the major target populations for protection by an RSV vaccine are children under 6 months of age and the frail elderly. While RSV infection is ubiquitous, the different population structure in high income (HIC) vs. low and middle income countries (LMIC), and the higher risk of infant mortality from RSV in LMIC [10], influences the emphasis on target populations. In LMIC the major focus is on protecting young infants and in HIC both young infants and the elderly have equivalent priority.
History
RSV was discovered in 1955 as Chimpanzee Coryza Agent [11], and associated with bronchiolitis in children in 1956 [11]. The first written description of the syndrome appears to be in 1826 [12], although it is likely RSV is an ancient disease and was not easily discriminated from other causes of acute respiratory disease in children. Goodpasture et al. described the pathology in 1939 [13] and Adams provided the first clinical description of the disease in the microbial era [14,15].
Why has RSV eluded vaccine development when the disease burden is so high; the identity of the virus has been known for 60 years; it is an acute self-limited infection; there is relatively little genetic variation; and there is no zoonotic reservoir? In addition, everyone is infected early in life so there is no ‘antigen-naïve’ population without pre-existing adaptive immunity other than the annual infant cohort which is no more than 2% of the total population. These features of an infectious disease would typically indicate that conventional intervention strategies are likely to be successful. Here I will describe the biological rationale for current RSV vaccine development efforts, and provide some thoughts on why RSV has been a successful pathogen when it occupies what seems to be a very vulnerable ecological niche.
Pathogenesis
What is associated with susceptibility to severe disease? Only about 2–3% of infants develop severe disease requiring hospitalization. The rest either have mild or subclinical disease sometimes with complications of otitis media or sinusitis. Factors most associated with infant hospitalization include prematurity especially with bronchopulmonary dysplasia, congenital heart disease, family history or genetic predisposition to allergic inflammation, being male, and environmental factors like smoke exposure. Disease severity is highest in some ethnic populations like Native Americans [16,17]. These individuals are also highly susceptible to encapsulated bacteria, but it is not known whether the immunological basis for this vulnerability is the same. Another factor that complicates RSV vaccine development is the history of vaccine-enhanced disease that occurred when a heat and formalin-inactivated whole virus vaccine was administered to children in the 1960s. During the season subsequent to vaccination, infection was not prevented, and disease was more severe with 80% hospitalization rate among vaccinees and two deaths in the youngest age cohort immunized between 2 and 7 months of age [18]. Pre-existing host factors including prior antigen exposure contribute to disease severity for different reasons. In thinking about how vaccine-induced immunity might reduce disease it is helpful to separate disease of the upper respiratory tract, lower airways, and the lung. It is also, useful to consider the role of viral cytopathology, lung and airway physiology, and immune response patterns in each compartment, and the special circumstances relevant to infants and the elderly (Table 1).
Table 1.
Pathogenesis of RSV-induced disease
| Upper respiratory tract | Lower airways | Lung | |
|---|---|---|---|
| Pathology | Pharyngitis, otitis, sinusitis | Bronchiolitis, mucous and fibrin production, inflammatory debri | Interstitial pneumonia |
| Symptoms and signs | Coryza, congestion, rhinorrhea | Dyspnea, tachypnea, wheezing, chest wall retractions | Hypoxia, shortness-of-breath, tachypnea |
| Tropism | Ciliated epithelium | Ciliated, polarized bronchiolar epithelium | Type 1 alveolar pneumocytes |
| Airway physiology | Reduced air flow through nasal and sinus passages | Obstructive airway disease, reactive airwaysa | Pulmonary hypertension (PHT) |
| Immune response | Intraepithelial T cells and mucosal antibodyb | Neutrophils in airway; eosinophils and additional mucous stimulated by allergic inflammationc | Serum antibodyb, peribronchioloar, perivascular and interstitial CD8 T cellsd |
| Infant | Secondary otitis media, mouth-breathing complicates breast-feeding | Small airways easy to obstruct; infection of developing airways may cause long-term physiological or anatomic effects | PHT complicates congenital heart disease |
| Elderly | Secondary bacterial sinusitis | Inflammation contributes to obstruction from underlying COPD | PHT complicates underlying heart disease |
Complicated by other irritants like smoke or other stimuli that increase mucous production or increase airways hypersensitivity.
RSV is not more frequent or severe in people with IgA deficiency. Parenterally delivered antibody is less able to protect upper airway than lower airway presumably because of gradient for transudation from serum is greater in upper airway than lung.
Exaggerated by certain genetic polymorphisms [50].
CD8T cells required for clearance but depending on quality, magnitude, antigen load, and timing of response, they can diminish or exacerbate illness [23].
Immunity
Antibody is the principle immune mediator associated with protection from viral infections. The best evidence that antibody plays an important role in RSV immunity are studies showing that passively administered antibody (either polyclonal or monoclonal) can protect infants from severe disease [19–21]. The irony is that people with immunoglobulin deficiency do not experience more frequent or severe RSV infections. It is the children and adults with diminished CD8T cell function because of SCID, allogenic bone marrow transplantation, or lung transplantation that have the most lethal RSV disease. These are conditions in which the CD8T cells cannot be produced at all or where the antigen presenting cells in the lung are not perfectly matched to the effector T cells. Therefore antibody neutralizing activity can diminish the number of infected cells from the initial inoculum and delay spread of virus into the lower airway, but once infection has been established, T cells are critical for viral clearance and bringing the infection to a close. There are some antibody Fc-mediated antibody functions that could contribute to viral clearance, but in most settings they likely play an ancillary role to CD8T cells. We are learning more about the role of local mucosal induction of intraepithelial T cells and their role in viral clearance [22] and about the selection of effector cells with the optimal phenotype for accomplishing viral clearance without undue pathology [22,23], but we do not yet have enough basic knowledge to rationally design a T-cell based vaccine that can rapidly respond and clear infection without risk of disease. It is possible that knowledge will come from other vaccine development programs on HIV, malaria, or tuberculosis, and it would be valuable to include CD8 T cell immunity in a vaccine especially if one of the goals is to interrupt transmission by preventing or reducing the period of viral shedding in infected people.
Mechanisms of immune escape
RSV has multiple mechanisms of evading immunity, which may explain how it can be a ubiquitous pathogen that reinfects people throughout life, yet has relatively little genetic variation relative to other RNA viruses. There are three major categories of evasion that include anatomical, conformational evasion of neutralizing antibody, and direct modulation of immune function. RSV is the HPV of the respiratory tract. It infects superficial epithelium of the airway and is even more superficial and protected from systemic immunity that than HPV because its tropism does not include basal epithelium. In the airway the virus enters and buds almost exclusively from highly differentiated, polarized, ciliated epithelium [24,25], and RSV antigen is not displayed basolaterally. Occasionally, dendritic cells must be infected or otherwise carry antigens to local lymph nodes to initiate immune responses. Therefore, the virus evades much of the systemic immune mechanisms by residing primarily outside the body.
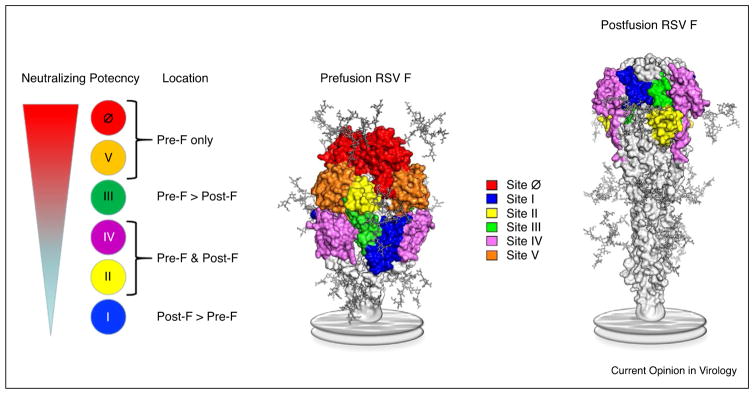
The virus itself, while easily transmitted by aerosol, is susceptible to high temperatures and dies in a few hours on fomites at room temperature ( . . . ref . . . ). In part this is due to instability of the F glycoprotein that spontaneously rearranges and transitions from the prefusion conformation of the trimer (pre-F) to the postfusion form (post-F) [26]. The pre-F conformation is required for viral entry and mediates membrane fusion between virus and cell or between an infected cell and an uninfected cell. In shed virus that is no longer part of the budding filament from the infected cell, the matrix eventually becomes fragmented and the virus assumes a pleomorphic and eventually a round shape. As this happens, the pre-F flips into the post-F conformation. The post-F is taller (~16 nm) than the functional pre-F (~11 nm) and can shield pre-F from neutralizing antibodies (Figure 1). Thus, the virus has to make a calculation of how easily triggered the F protein should be. Being easily triggered may make the virus more fusogenic and potentially better suited for cell-to-cell spread, and may provide some cover for pre-F and inhibit access of neutralizing antibodies. However, if it is too easily triggered, rearrangement may occur before the virus is in proximity to a susceptible cell and could lead to premature fusion incompetence and death of the virus. When the virus selects the optimal level of pre-F instability to maintain infectiousness while successfully avoiding pre-existing antibody to the pre-F surfaces then it has achieved conformational evasion.
Figure 1.
Antigenic sites on the RSV F. Prefusion and postfusion RSV F (pre-F and post-F) structures are shown as molecular surfaces, with N-linked glycans modeled as sticks and the viral membrane represented as a gray disc. There are two pre-F-specific antigenic sites (Ø and V) and two sites that are present on both conformations (II and IV). Antibodies against site III generally bind tighter to the pre-F conformation, whereas antibodies against site I bind tighter to the post-F conformation. The neutralization sensitivity of each antigenic site is directly related to exclusive or preferential binding to the pre-F conformation. The most potent monoclonal antibodies (mAbs) bind to the apex of the pre-F trimer at sites Ø and V, and mAbs to those sites compete with antibodies that account for the large majority of neutralizing activity in human sera. Images prepared by Morgan S. Gilman, PhD, Department of Biochemistry and Cell Biology, Giesel School of Medicine at Dartmouth.
RSV has evolved multiple mechanisms for directly modulating innate and adaptive immune responses. First, the primary virus infection targets young infants who have immunological features like immature dendritic cell function and lack of B cell somatic hypermutation that inherently limit magnitude and repertoire of responses [27–29]. In addition, the NS1 and NS2 proteins which assume the dominant position in the gene order, have many mechanisms for inhibiting Type I IFN [30], and the shed portion of the G glycoprotein can alter dendritic cell signaling [31] and serve as a decoy for antibody responses [32].
Vaccine approaches to protect infants
As noted from the features of RSV biology noted above, particularly the vaccine-enhanced illness phenomenon, the impact of RSV infection on airway function, and the location of infection, and immunological consequences of first infection in the very young infant, the primary immunization event with RSV antigens is all important in determining the type of life-long immunity a person will have against RSV infection. The specificity of the B cell and T cell repertoire and the prevailing phenotypic distribution of effector cells is strongly influenced by the first antigen exposure. Therefore, it would be ideal to design a vaccine that could be effectively used as the first RSV immunizing event. However, protecting against the peak of severe disease that occurs at 2–3 months with an effective primary vaccination is challenging for logistical and biological reasons. Consequently, a major strategy for protecting the young infant is the use of passive antibody. This has been done historically through the use of polyclonal serum with high neutralizing activity (RSVIG) or a monoclonal antibody (palivizumab) given to premature infants at high risk of severe RSV disease and reaches a very small population [19,20]. Recently, a potent human monoclonal antibody specific for antigenic site Ø at the apex of the pre-F trimer (Figure 1) has been modified with a YTE mutation in the Fc region to extend the half-life, and this new product is being proposed as a single birth dose or as a single seasonal dose for young infants regardless of severe disease risk category [33]. This could potentially augment the passive immunity naturally transferred during gestation and allow active vaccination to begin at an age when the immune system is more mature. Another major strategy for protecting infants is to immunize pregnant women to boost pre-existing memory B cells leading to an increased transfer of maternal antibody can provide infant protection through the first 5–6 months of life. The leading vaccine approaches for maternal immunization are subunit proteins based on the pre-F structure that will access the greatest number of relevant B cell precursors [34] and boost antibody responses with the greatest neutralizing potency [35] (Figure 1). Initially these candidate subunit protein vaccines will be tested with either no adjuvant or with conventional alum formulations.
In addition to the biological challenge of effectively immunizing the young infant, there is a safety concern that will have to be addressed for any vaccine being proposed as a primary immunization event in an antigen-naïve child because of the legacy of vaccine-enhanced illness. The only vaccine approaches proven to not induce enhanced disease are live-attenuated or live chimeric virus vaccines [36]. Recent advances in the development of vaccines within this category that either improve immunogenicity despite greater vaccine virus attenuation [37,38], improve manufacturing capacity [39], or improve stability of surface proteins and immunogenicity [38,40,41] will provide potential solutions for immunizing the antigen-naïve infant. Other approaches that deliver vaccine antigens through gene-based vectors [42–44] or nucleic acid [45,46] are another possible avenue towards the goal of infant immunization. These approaches induce a pattern of immune responses analogous to live virus and have not been associated with enhanced pathology in animal models of RSV infection, and so are likely to be safe in antigen-naïve infants. Immunizing this age group with proteins or other approaches that have obligate MHC class II processing and that are unlikely to induce CD8T cell responses will require greater justification if proposed as a primary immunization for RSV.
Vaccine approaches for the elderly
The basis for severe disease in the frail elderly is more complex than the disease that occurs during primary infection in the infant. It is nearly always associated with underlying chronic cardiac or pulmonary disease or an event accompanied by immunodeficiency. The immunological factors needed to supplement pre-existing RSV immunity in the elderly are not well understood, and the immunological vulnerabilities are more variable between individuals and influenced by prior exposure history more so than in young infants. Pre-existing immunity in adults will preclude the use of live-attenuated or live chimeric virus approaches because replication will be too limited to generate sufficient immunogenicity. The primary approaches being advanced involve subunit proteins based on the pre-F conformation or more complex virus-like particles or virosomes. Recent failures of vaccines based on post-F antigens along with prior studies based on post-F antigen [47,48], support the use of antigens in the pre-F conformation that can induce more potent neutralizing antibody responses. Adjuvant formulations such as AS01 are available for this population and can provide significant benefits for magnitude and durability of antibody responses as demonstrated by recent success using recombinant gD protein for varicella zoster [49]. It is also possible that gene-based delivery with vectors or nucleic acids would stimulate both CD8T cells and antibody responses in pre-immune adults that may benefit the aging immune system.
This brief commentary attempts to integrate observations from many aspects of RSV biology and provide a point-of-view on the current understanding of RSV pathogenesis and immunity. It is not a comprehensive review and my apologies for the many references and fascinating biological features of RSV that have been neglected. The intent was to highlight advances in antigen design and vaccine delivery approaches in the context of current knowledge to explain the rapid expansion of RSV vaccine development activity and the sense of hope that a solution for RSV disease prevention is possible and may be available in the not too distant future.
Acknowledgments
I thank Kaitlyn M. Morabito, PhD for critical review and comments and Morgan S. Gilman, PhD for preparation of images. I also thank the investigators at the NIAID Vaccine Research Center, and members of the VRC Viral Pathogenesis Laboratory for their discussions and work that provide the basis for this commentary. The project was supported by intramural funding from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References
- 1.Martinez FD. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr Infect Dis J. 2003;22(Suppl 2):S76–S82. doi: 10.1097/01.inf.0000053889.39392.a7. [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 4.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 5.Milner ME, de la Monte SM, Hutchins GM. Fatal respiratory syncytial virus infection in severe combined immunodeficiency syndrome. Am J Dis Child. 1985;139:1111–1114. doi: 10.1001/archpedi.1985.02140130049028. [DOI] [PubMed] [Google Scholar]
- 6.Hertz MI, Englund JA, Snover D, Bitterman PB, McGlave PB. Respiratory syncytial virus-induced acute lung injury in adult patients with bone marrow transplants: a clinical approach and review of the literature. Medicine (Baltimore) 1989;68:269–281. doi: 10.1097/00005792-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Wendt CH. Community respiratory viruses: organ transplant recipients. Am J Med. 1997;102:31–36. doi: 10.1016/s0002-9343(97)80008-3. discussion 42–43. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Wang Y, Gilmore X, Xu K, Wyde PR, Mbawuike IN. An aged mouse model for RSV infection and diminished CD8(+) CTL responses. Exp Biol Med (Maywood) 2002;227:133–140. doi: 10.1177/153537020222700208. [DOI] [PubMed] [Google Scholar]
- 9.Malloy AM, Falsey AR, Ruckwardt TJ. Consequences of immature and senescent immune responses for infection with respiratory syncytial virus. Curr Top Microbiol Immunol. 2013;372:211–231. doi: 10.1007/978-3-642-38919-1_11. [DOI] [PubMed] [Google Scholar]
- 10.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blount RE, Jr, Morris JA, Savage RE. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med. 1956;92:544–549. doi: 10.3181/00379727-92-22538. [DOI] [PubMed] [Google Scholar]
- 12.Parrish J. Observations on a peculiar catarrhal complaint in children. North Am Med Surg J. 1826;1:24–31. [Google Scholar]
- 13.Goodpasture EWA, Swanson HS, Cotter EF. Virus pneumonia of infants secondary to epidemic infections. Am J Dis Child. 1939;57:997–1011. [Google Scholar]
- 14.Adams JM. Primary virus pneumonitis in infants. Pediatrics. 1948;1:398. [PubMed] [Google Scholar]
- 15.Adams JM. Primary virus pneumonitis with cystoplasmic inclusion bodies: study of epidemic involving 32 infants with 9 deaths. JAMA. 1941;116:9. [Google Scholar]
- 16.Bockova J, O’Brien KL, Oski J, Croll J, Reid R, Weatherholtz RC, et al. Respiratory syncytial virus infection in Navajo and White Mountain Apache children. Pediatrics. 2002;110(2 Pt 1):e20. doi: 10.1542/peds.110.2.e20. [DOI] [PubMed] [Google Scholar]
- 17.Karron RA, Singleton RJ, Bulkow L, Parkinson A, Kruse D, DeSmet I, et al. Severe respiratory syncytial virus disease in Alaska native children. RSV Alaska Study Group. J Infect Dis. 1999;180:41–49. doi: 10.1086/314841. [DOI] [PubMed] [Google Scholar]
- 18.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 19.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102(3 Pt 1):531–537. [PubMed] [Google Scholar]
- 20.Groothuis JR, Simoes EA, Hemming VG. Respiratory syncytial virus (RSV) infection in preterm infants and the protective effects of RSV immune globulin (RSVIG). Respiratory Syncytial Virus Immune Globulin Study Group. Pediatrics. 1995;95:463–467. [PubMed] [Google Scholar]
- 21.O’Brien KL, Chandran A, Weatherholtz R, Jafri HS, Griffin MP, Bellamy T, et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis. 2015;15:1398–1408. doi: 10.1016/S1473-3099(15)00247-9. [DOI] [PubMed] [Google Scholar]
- 22.Morabito KM, Ruckwardt TR, Redwood AJ, Moin SM, Price DA, Graham BS. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Haddad EK, Marceau J, Morabito KM, Rao SS, Filali-Mouhim A, et al. A numerically subdominant CD8 T cell response to matrix protein of respiratory syncytial virus controls infection with limited immunopathology. PLoS Pathog. 2016;12:e1005486. doi: 10.1371/journal.ppat.1005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76:5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 26.Killikelly AM, Kanekiyo M, Graham BS. Pre-fusion F is absent on the surface of formalin-inactivated respiratory syncytial virus. Sci Rep. 2016;6:34108. doi: 10.1038/srep34108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruckwardt TJ, Morabito KM, Graham BS. Determinants of early life immune responses to RSV infection. Curr Opin Virol. 2016;16:151–157. doi: 10.1016/j.coviro.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu C, Openshaw PJ. Antiviral B cell and T cell immunity in the lungs. Nat Immunol. 2015;16:18–26. doi: 10.1038/ni.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambert L, Sagfors AM, Openshaw PJ, Culley FJ. Immunity to RSV in early-life. Front Immunol. 2014;5:466. doi: 10.3389/fimmu.2014.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barik S. Respiratory syncytial virus mechanisms to interfere with type 1 interferons. Curr Top Microbiol Immunol. 2013;372:173–191. doi: 10.1007/978-3-642-38919-1_9. [DOI] [PubMed] [Google Scholar]
- 31.Johnson TR, McLellan JS, Graham BS. Respiratory syncytial virus glycoprotein G interacts with DC-SIGN and L-SIGN to activate ERK1 and ERK2. J Virol. 2012;86:1339–1347. doi: 10.1128/JVI.06096-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bukreyev A, Yang L, Fricke J, Cheng L, Ward JM, Murphy BR, et al. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J Virol. 2008;82:12191–121204. doi: 10.1128/JVI.01604-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffin MP, Khan AA, Esser MT, Jensen K, Takas T, Kankam MK, et al. Safety, tolerability, and pharmacokinetics of MEDI8897, the respiratory syncytial virus prefusion F-targeting monoclonal antibody with an extended half-life, in healthy adults. Antimicrob Agents Chemother. 2017:61. doi: 10.1128/AAC.01714-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med. 2015;7:309ra162. doi: 10.1126/scitranslmed.aac4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilman MS, Castellanos CA, Chen M, Ngwuta JO, Goodwin E, Moin SM, et al. Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci Immunol. 2016:1. doi: 10.1126/sciimmunol.aaj1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright PF, Karron RA, Belshe RB, Shi JR, Randolph VB, Collins PL, et al. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007;25:7372–7378. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karron RA, Luongo C, Thumar B, Loehr KM, Englund JA, Collins PL, et al. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci Transl Med. 2015;7:312ra175. doi: 10.1126/scitranslmed.aac8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stobart CC, Rostad CA, Ke Z, Dillard RS, Hampton CM, Strauss JD, et al. A live RSV vaccine with engineered thermostability is immunogenic in cotton rats despite high attenuation. Nat Commun. 2016;7:13916. doi: 10.1038/ncomms13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt AC, McAuliffe JM, Murphy BR, Collins PL. Recombinant bovine/human parainfluenza virus type 3 (B/HPIV3) expressing the respiratory syncytial virus (RSV) G and F proteins can be used to achieve simultaneous mucosal immunization against RSV and HPIV3. J Virol. 2001;75:4594–4603. doi: 10.1128/JVI.75.10.4594-4603.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang B, Surman S, Amaro-Carambot E, Kabatova B, Mackow N, Lingemann M, et al. Enhanced neutralizing antibody response induced by respiratory syncytial virus prefusion F protein expressed by a vaccine candidate. J Virol. 2015;89:9499–9510. doi: 10.1128/JVI.01373-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang B, Ngwuta JO, Herbert R, Swerczek J, Dorward DW, Amaro-Carambot E, et al. Packaging and prefusion stabilization separately and additively increase the quantity and quality of respiratory syncytial virus (RSV)-neutralizing antibodies induced by an RSV fusion protein expressed by a parainfluenza virus vector. J Virol. 2016;90:10022–10038. doi: 10.1128/JVI.01196-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson TR, Rangel D, Graham BS, Brough DE, Gall JG. Genetic vaccine for respiratory syncytial virus provides protection without disease potentiation. Mol Ther. 2014;22:196–205. doi: 10.1038/mt.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor G, Thom M, Capone S, Pierantoni A, Guzman E, Herbert R, et al. Efficacy of a virus-vectored vaccine against human and bovine respiratory syncytial virus infections. Sci Transl Med. 2015;7:300ra127. doi: 10.1126/scitranslmed.aac5757. [DOI] [PubMed] [Google Scholar]
- 44.Widjojoatmodjo MN, Bogaert L, Meek B, Zahn R, Vellinga J, Custers J, et al. Recombinant low-seroprevalent adenoviral vectors Ad26 and Ad35 expressing the respiratory syncytial virus (RSV) fusion protein induce protective immunity against RSV infection in cotton rats. Vaccine. 2015;33:5406–5414. doi: 10.1016/j.vaccine.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 45.Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci U S A. 2012;109:14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardi N, Weissman D. Nucleoside modified mRNA vaccines for infectious diseases. Methods Mol Biol. 2017;1499:109–121. doi: 10.1007/978-1-4939-6481-9_6. [DOI] [PubMed] [Google Scholar]
- 47.Langley JM, Sales V, McGeer A, Guasparini R, Predy G, Meekison W, et al. A dose-ranging study of a subunit respiratory syncytial virus subtype A vaccine with and without aluminum phosphate adjuvantation in adults > or = 65 years of age. Vaccine. 2009;27:5913–5919. doi: 10.1016/j.vaccine.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 48.Falsey AR, Walsh EE, Capellan J, Gravenstein S, Zambon M, Yau E, et al. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (RSV) vaccines – nonadjuvanted vaccine or vaccine adjuvanted with alum – given concomitantly with influenza vaccine to high-risk elderly individuals. J Infect Dis. 2008;198:1317–1326. doi: 10.1086/592168. [DOI] [PubMed] [Google Scholar]
- 49.Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 50.Miyairi I, DeVincenzo JP. Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev. 2008;21:686–703. doi: 10.1128/CMR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]