Abstract
Adolescence is a period of heightened vulnerability both to addictive behaviors and drug-induced brain damage. Yet, only limited information exists on the brain mechanisms underlying these adolescent-specific characteristics. Moreover, distinctions in brain correlates between predisposition to drug use and effects of drugs in adolescents are unclear. Using cortical thickness and diffusion tensor image analyses, we found greater and more widespread gray and white matter alterations, particularly affecting the frontostriatal system, in adolescent methamphetamine (MA) users compared with adult users. Among adolescent-specific gray matter alterations related to MA use, smaller cortical thickness in the orbitofrontal cortex was associated with family history of drug use. Our findings highlight that the adolescent brain, which undergoes active myelination and maturation, is more vulnerable to MA-related alterations than the adult brain. Furthermore, MA-use-related executive dysfunction was greater in adolescent MA users than in adult users. These findings may provide explanation for the severe behavioral complications and relapses that are common in adolescent-onset drug addiction. Additionally, these results may provide insights into distinguishing the neural mechanisms that underlie the predisposition to drug addiction from effects of drugs in adolescents.
INTRODUCTION
Adolescence is a critical period of brain development as the brain undergoes dynamic synaptic reorganization and myelination.1 Thus, environmental insults during adolescence can derail typical brain development and increase the risk of irreversible damage to the brain.1,2
Methamphetamine (MA) is a highly addictive stimulant, and chronic use of MA may produce persistent neuronal damage, which can be reflected in both micro- and macrostructural changes occurring in multiple brain regions.3,4 Neuroimaging studies in adult MA users have reported that chronic MA use selectively changes the prefrontotemporal areas and frontostriatal pathways,5–8 where smaller cortical thickness, reduced white matter connectivity and lower levels of the neuronal viability marker N-acetylaspartate have been observed.4,5,9–12 Intriguingly, these brain regions, whose functions are associated with inhibitory control and preference for delayed gratification, overlap with the regions where active maturation continues throughout adolescence.13 Thus, if MA exposure in adolescence changes these brain regions, adolescents may be at a greater risk of relapse and intractable MA addiction compared with adults.
Although the developing brain often shows greater neuroplasticity to environmental stimuli,2 it is unknown whether the adolescent brain shows resilience or suffers from MA exposure-related alterations. It is also not known whether inherent regional alterations of the brain predispose adolescents to illicit drug use. Although the annual prevalence of MA use has decreased over the past 10 years in the United States,14 a recent report from the United Nations Office on Drugs and Crime has indicated that the use of amphetamine-type stimulants, including MA, continues to increase in most countries in Asia and remains a major health concern worldwide.15 Therefore, it is important to understand the brain-based mechanisms underlying adolescent MA use.
In the present study, we sought to identify the adolescent-specific attributes of gray and white matter alterations associated with chronic MA use, using cortical thickness and diffusion tensor image analyses. We also examined whether lifetime cumulative dose of MA or onset age of MA use was associated with these adolescent-specific alterations. To distinguish between the brain correlates of predisposition toward adolescent-onset addictive behaviors and the brain alterations induced by long-term exposure to MA, we studied both adolescent and adult MA users and control participants, half of whom had high familial risk for drug dependence.
MATERIALS AND METHODS
Participants
We studied 111 adolescents (MA users, n = 51; controls, n = 60) and 114 adults (MA users, n = 54; controls, n = 60). Detailed information on family history (FH) of alcohol or drug use was obtained from the participants and their biological relatives (authorized legal representatives in the case of adolescent participants) using screening questionnaires that included introductory and symptom-definition questions, which were made by modifying the Family History Screen,16 and history taking. Family pedigrees including first- and second-degree relatives were also collected. Alcohol or drug use problems in first- and second-degree relatives were recorded, and more detailed history taking of parental alcohol or drug use was performed to define positive FH (FH+) participants. The definition of FH+ required having (i) at least one parent who had alcohol or drug dependence and (ii) one or more first- or second-degree relatives with alcohol or drug abuse. In the adolescent groups, 25 MA users and 30 controls had FH+ for drug use, whereas 26 MA users and 30 controls had no FH (FH −). In the adult groups, 27 MA users and 30 controls were FH+, and 27 MA users and 30 controls were FH −.
All participants were enrolled in South Korea and were of East Asian ethnicity. Detailed demographic and clinical characteristics of the participants are presented in Table 1 and Supplementary Methods.
Table 1.
Demographic and clinical characteristics of study participants
| Characteristics | Adolescent group (n = 111) | Adult group (n = 114) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| FH−controls (n = 30) | FH+controls (n = 30) | FH−MA users (n = 26) | FH+MA users (n = 25) | FH− controls (n = 30) | FH+controls (n = 30) | FH− MA users (n = 27) | FH+MA users (n = 27) | |
| Demographics | ||||||||
| Age (years) | 18.0 ±1.4 | 18.1 ±1.0 | 18.1 ±1.4 | 18.0 ±1.3 | 41.2 ±5.5 | 41.8 ±6.3 | 41.3 ±4.1 | 41.8 ±6.8 |
| Male sex, no. (%) | 24 (80.0) | 24 (80.0) | 20 (76.9) | 20 (80.0) | 24 (80.0) | 24 (80.0) | 22 (81.5) | 22 (81.5) |
| Education (years) | 11.4 ±1.2 | 11.5 ±1.0 | 10.6 ±1.9 | 11.2 ±1.4 | 13.8 ±2.1 | 13.7 ±2.4 | 12.6 ±2.1* | 11.7 ±2.0a |
| Right handedness, no. (%) | 25 (83.3) | 26 (86.7) | 22 (84.6) | 22 (88.0) | 25 (83.3) | 26 (86.7) | 23 (85.2) | 24 (88.9) |
| Race/ethnicity, no. (%) | ||||||||
| East Asian | 30 (100) | 30 (100) | 26 (100) | 25 (100) | 30 (100) | 30 (100) | 27 (100) | 27 (100) |
| SES, no. (%) | ||||||||
| Low | 3 (10.0) | 4 (13.3) | 5 (19.2) | 2 (8.0) | 5 (16.7) | 5 (16.7) | 9 (33.3) | 9 (33.3) |
| Middle | 24 (80.0) | 24 (80.0) | 18 (69.2) | 19 (76.0) | 21 (70.0) | 20 (66.7) | 14 (51.9) | 12 (44.4) |
| High | 3 (10.0) | 2 (6.7) | 3 (11.5) | 4 (16.0) | 4 (13.3) | 5 (16.7) | 4 (14.8) | 6 (22.2) |
| Smoking, no. (%) | ||||||||
| Current smoker | 7 (23.3) | 6 (20.0) | 20 (76.9)a | 18 (72.0)a | 12 (40.0) | 12 (40.0) | 22 (81.5)a | 22 (81.5)a |
| Former smoker | 0 (0.0) | 0 (0.0) | 1 (3.9) | 2 (8.0) | 7 (23.3) | 11 (36.7) | 3 (11.1) | 1 (3.7) |
| Never smoked | 23 (76.7) | 24 (80.0) | 5 (19.2) | 5 (20.0) | 11 (36.7) | 7 (23.3) | 2 (7.4) | 4 (14.8) |
| Clinical characteristics | ||||||||
| Onset age of MA use (years) | NA | NA | 15.1 ±1.0 | 14.7 ±1.1 | NA | NA | 24.9 ±5.4 | 26.2 ±7.9 |
| Lifetime cumulative dose (g) | NA | NA | 105.5 ±92.4 | 131.8 ±96.1 | NA | NA | 381.4 ±304.8 | 375.9 ±303.8 |
| Duration of regular use (months)b | NA | NA | 24.0 ±14.5 | 26.1 ±14.0 | NA | NA | 78.1 ±37.3 | 88.1 ±48.4 |
| Route of MA administration, no. (%) | ||||||||
| Intravenous injection | NA | NA | 17 (65.4) | 16 (64.0) | NA | NA | 26 (96.3) | 24 (88.9) |
| Smoking | 7 (26.9) | 9 (36.0) | 1 (3.7) | 3 (11.1) | ||||
| Oral ingestion | 2 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Subjects reported ever having used cannabis, no. (%)c | 0 (0.0) | 0 (0.0) | 8 (30.8) | 7 (28.0) | 0 (0.0) | 0 (0.0) | 10 (37.0) | 13 (48.2) |
| Subjects having a past history of alcohol abuse, no. (%)d | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (8.0) | 0 (0.0) | 0 (0.0) | 2 (7.4) | 2 (7.4) |
| Weekly alcohol consumption-standard drinks | 1.52 (2.56) | 2.20 (2.98) | 3.26 (3.78)* | 5.20 (5.24)a | 4.40 (5.36) | 4.41 (5.75) | 9.28 (9.13)* | 9.73 (9.91)* |
| SDS—total sores | NA | NA | 6.00 (3.64) | 5.76 (3.57) | NA | NA | 7.30 (3.23) | 7.00 (2.20) |
| AWQ—total sores | NA | NA | 9.73 (7.83) | 10.36 (8.67) | NA | NA | 14.89 (8.49) | 13.15 (6.66) |
| VASe—scores | NA | NA | 3.42 (1.06) | 3.32 (1.35) | NA | NA | 3.70 (1.38) | 3.48 (1.12) |
| ACQf—total sores | NA | NA | 114.3 (33.7) | 117.1 (38.1) | NA | NA | 127.4 (39.4) | 125.4 (36.1) |
| Stroop interferencea | 0.394 (0.155) | 0.451 (0.217) | 0.439 (0.202) | 0.589 (0.313)a | 0.473 (0.1 90) | 0.490 (0.214) | 0.546 (0.210) | 0.584 (0.303) |
Means and standard deviation values are denoted as mean ±s.d. FH − stands for a negative family history of drug-related problems. FH+ stands for a positive family history of drug-related problems. A positive family history was defined as having (i) at least one parent who had alcohol or drug dependence and (ii) one or more first- or second-degree relatives with alcohol or drug abuse.
Significant at P<0.05 and significant at **P<0.01 when compared with FH − controls in the respective adolescent or adult group using the Student’s t-test for continuous variables and the χ2 tests or the Fisher’s exact tests for categorical variables.
Abbreviations: ACQ, Amphetamine Craving Questionnaire; AWQ, Amphetamine Withdrawal Questionnaire; MA, methamphetamine; NA, not applicable; SDS, Severity of Dependence Scale; SES, socioeconomic status; VAS, visual analog scale.
Stroop interference was calculated by subtracting time per item of Color task from time per item of Color-Word task. A lower score means less susceptibility to interference thus higher task performance.
Duration was calculated by summing up the time when MA was used more frequently than weekly.
None of MA users had a history of lifetime and current cannabis dependence or abuse.
MA users had a past history of alcohol abuse but did not have a current one.
Subjective craving for MA was measured using a visual analog scale that ranges from 0 to 10.
A modified version of the Cocaine Craving Questionnaire, which substitutes the word ‘MA’ for ‘cocaine,’ was used.
All participants and their parents or authorized legal representatives, if participants were <19 years old, provided written informed consent to participate in the study. Assent from adolescent participants were also obtained in addition to parental consent. This study was approved by the Institutional Review Boards of Ewha Womans University, the Catholic University of Korea College of Medicine, Seoul National University Hospital and the University of Utah.
Clinical assessments
An experienced psychiatrist performed a diagnostic evaluation of addictive behaviors and obtained a detailed history of drug use. In addition, core clinical symptoms of drug addiction including craving and withdrawal were evaluated using a visual analog scale and the Amphetamine Withdrawal Questionnaire,17 respectively. The multidimensional aspects of craving were also assessed using the Amphetamine Craving Questionnaire.18 Stroop interference, which is considered as a general measure of focused attention,19 inhibitory control20 or cognitive flexibility,21 was also assessed using the Color-Word Stroop task.22 Information regarding weekly alcohol consumption was also obtained. Detailed information on the scales used in the current study is presented in the Supplementary Methods.
Cognitive assessments
Neuropsychological tests were administered to assess the participants’ cognitive performance. Each test was classified into the specific cognitive domains according to the criteria suggested by the previous meta-analysis on neurocognitive effects of MA use.23 We assessed cognitive performance in the domains including executive function, memory, learning, verbal fluency, working memory, information processing speed and motor skill. Information on neuropsychological tests for each of the seven cognitive domains is described in the Supplementary Methods and Supplementary Table 1.
Brain imaging
High-resolution T1-weighted and diffusion tensor brain magnetic resonance images were acquired using a 1.5-T whole-body imaging system (Signa HDx; GE Healthcare, Milwaukee, WI, USA). Cortical thickness and fractional anisotropy (FA), which reflect the cortical cytoarchitecture and the integrity of white matter fiber tracts, respectively, were the main outcome variables for the assessment of gray and white matter alterations. Detailed information on acquisition parameters and imaging analyses is presented in the Supplementary Methods.
Statistical analyses
Group differences in demographic and clinical characteristics were examined using independent t-tests, χ2 tests or Fisher exact tests.
Global mean thickness, which was calculated by averaging thickness values over all vertices of the entire cerebrum, was extracted for the measurement of alterations in gray matter cortical thickness. For the comparisons of mean thickness in atlas-based parcellated regions (Supplementary Figure 1), the average thickness over all vertices within each parcellated region was calculated. Global mean FA across the whole white matter skeleton was extracted for the measurement of microstructural alterations within the entire cerebral white matter. For the comparisons of mean FA values in tract-based anatomical regions (Supplementary Figure 1), FA values of association tracts were extracted from each individual’s FA skeleton map. All values of mean cortical thickness and FA were adjusted for age.
To examine whether and where the effects of MA use were greater in adolescents than in adults, standardized Z-scores for average thickness values of parcellated regions and those for the mean FA values across tract-based anatomical regions were compared between the adolescent and adult MA user groups. Using the group means and s.d.’s of adolescent and adult FH − control groups as the reference values, average thickness or FA values were converted into standardized Z-scores. A negative Z-score indicated that age-adjusted average thickness or FA values were below the mean of the corresponding FH − control group in s.d. units. Independent t-tests were used to compare the Z-scores of thickness or FA between groups. Gray and white matter regions, where the effects of MA use were greater in adolescents than in adults, were chosen as the regions of interest (ROIs) for further analyses.
Age- and educational-level-adjusted neuropsychological test scores were converted to standardized Z-scores using the group means and s.d.’s of adolescent and adult FH − control groups as the reference values. Standardized Z-scores for the individual tests in each cognitive domain were then averaged to yield a single composite score that reflected cognitive performance. If necessary, test scores were reversed so that positive standardized Z-scores would indicate better performance. Standardized Z-scores for cognitive domains were compared between the adolescent and adult MA groups using independent t-tests.
The relationships between cortical thickness/FA values of each ROI and MA-use-related executive dysfunction were examined using Pearson’s correlation analyses.
We also conducted Pearson’s correlation analyses between standardized Z-scores for global mean thickness/mean thickness values of each ROI and lifetime cumulative dose of MA or onset age. Similar analyses were conducted for global or ROI mean FA value Z-scores. Bonferroni corrections were used for determining significances of correlation analyses between the multiple ROI data set and clinical measures. P-values for these correlation analyses were Bonferroni-adjusted.
To assess the impact of FH on adolescent-specific brain alterations related to MA use, we also examined a three-way interaction between FH, MA use and age group in ROI mean thickness or FA values using analysis of variance model. The model included main effects, all possible two-way interactions, and a three-way interaction.
Mediation analysis24 was performed to test the hypothesis that thickness alterations in the region of a significant three-way interaction between FH, MA use and age group might have influenced lifetime cumulative dose of MA through core clinical symptoms of addiction.
Detailed information on supplementary vertex- and voxel-wise analyses of thickness and FA values and mediation analysis is presented in the Supplementary Methods.
Two-tailed significance of P<0.05 was considered as statistically significant. Data were analyzed using the Stata SE, v.11.0 (Stata Corp, College Station, TX, USA).
RESULTS
Adolescent-specific brain alterations related to MA use
Adolescent-specific gray matter alterations related to MA use
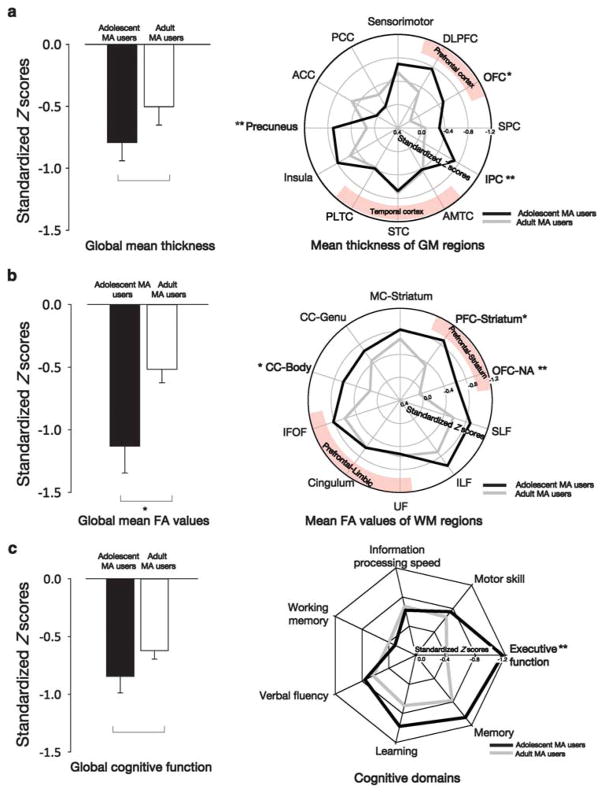
Standardized Z-scores reflecting cortical thickness differences between MA users and FH − controls were greater in adolescents than in adults in the orbitofrontal cortex (OFC) (t = 2.46, P = 0.02), precuneus (t = 2.72, P = 0.008) and inferior parietal cortex (IPC) (t = 3.16, P = 0.002) (Figure 1a and see Supplementary Figure 1 for anatomical locations). These regions where adolescent MA users had greater thickness differences than adult MA users (the OFC, precuneus and IPC) were chosen as the gray matter ROIs for further analyses. Vertex-wise group differences in cortical thickness between the MA user groups and the corresponding FH − control groups are presented as Supplementary Results (Supplementary Figure 2 and Supplementary Table 2). In brief, smaller cortical thickness in the temporolimbic regions was shown in both the adolescent and adult MA user groups than in the corresponding FH − control groups, whereas MA-use-related thickness differences in the prefrontal, parietal and precuneus regions were predominantly found in adolescent MA users. There was no difference in standardized Z-score for global mean thickness between the adolescent and adult MA user groups (t = 1.39, P = 0.17) (Figure 1a).
Figure 1.
Adolescent-specific gray and white matter alterations and cognitive dysfunction related to methamphetamine (MA) use. (a) The bar graph presents standardized Z-scores for global mean thickness in adolescent and adult MA users. The polar plot presents standardized Z-scores for bilateral mean thickness of predefined atlas-based parcellated regions in adolescent and adult MA users. A negative standardized Z-score indicates that the age-adjusted mean thickness was smaller in the MA user groups than in the corresponding FH − (family history) control groups in an s.d. unit. Significant differences in standardized Z-scores between the adolescent and adult MA user groups were found in the orbitofrontal cortex (OFC) (t =2.46, P =0.02), precuneus (t =2.72, P =0.008) and inferior parietal cortex (IPC) (t =3.16, P =0.002). (b) The bar graph presents standardized Z-scores for global mean fractional anisotropy (FA) values in adolescent and adult MA users. The polar plot presents standardized Z-scores for bilateral mean FA values of predefined tract-based anatomical regions in adolescent and adult MA users. A negative standardized Z-score indicates that the age-adjusted mean FA value was smaller in the MA user groups than in the corresponding FH − control groups in an s.d. unit. Significant differences in standardized Z-scores between the adolescent and adult MA user groups were found in the prefrontal cortex (PFC)-striatum tract (t =2.44, P =0.02), OFC-nucleus accumbens (NA) tract (t =2.82, P =0.006) and the body of the corpus callosum (t =2.19, P =0.03). (c) The bar graph presents standardized Z-scores for global cognitive function in adolescent and adult MA users. The radar plot presents standardized Z-scores for the performance in each cognitive domain in adolescent and adult MA users. A negative standardized Z-score indicates that the age- and educational-level-adjusted composite score was smaller in the MA user groups than in the corresponding FH − control groups in a SD unit. Significant differences in standardized Z-scores between the adolescent and adult MA user groups were found in the cognitive domain of executive function (t =2.69, P =0.008). Error bars represent errors. *P<0.05 and **P<0.01. Abbreviations for the atlas-based parcellated gray matter regions and tract-based anatomical regions of white matter are provided in Supplementary Figure 1.
Adolescent-specific white matter alterations related to MA use
Standardized Z-scores of FA differences between MA users and FH − controls were greater in adolescents than in adults in the corticostriatal tracts connecting the prefrontal cortex and striatum (PFC-striatum tract, t = 2.44, P = 0.02) and the OFC and nucleus accumbens (OFC-NA tract, t = 2.82, P = 0.006), and in the body of the corpus callosum (t = 2.19, P = 0.03) (Figure 1b and see Supplementary Figure 1 for anatomical locations). The PFC-striatum tract, OFC-NA tract and the body of the corpus callosum, the regions that showed greater FA differences in adolescent MA users than in adult MA users, were chosen as the white matter ROIs for further analyses. Voxel-wise group comparisons of FA values between the MA user groups and corresponding FH − control groups are provided in Supplementary Results (Supplementary Figure 3 and Supplementary Table 3). In short, both the adolescent and adult MA user groups showed smaller FA values in the prefrontolimbic tracts, superior and inferior longitudinal fasciculi, tract connecting the motor cortex and striatum and corpus callosum, in comparison with the corresponding FH − control groups. Meanwhile, FA alterations related to MA use in the prefrontostriatal tracts were predominantly found in adolescent MA users. The magnitude of differences in global mean FA values between MA users and FH − controls was greater in adolescents than in adults (t = 2.58, P = 0.01) (Figure 1b). Results from other diffusion indices such as mean diffusivity, radial diffusivity and axial diffusivity were consistent with the FA outcomes (Supplementary Result 1).
To ensure the robustness of the current results, we repeated all analyses including potential confounding factors such as smoking history, the route of MA administration or alcohol drinking as additional covariates. The results remained unchanged after adjusting for these potential confounding factors (Supplementary Result 2).
Adolescent-specific cognitive dysfunction related to MA use
Standardized Z-scores of cognitive performance differences in executive function between MA users and FH − controls were greater in adolescents than in adults (t = 2.69, P = 0.008), whereas the magnitude of cognitive dysfunctions in other domains was similar between adolescent and adult MA users (memory, t = 1.28, P = 0.20; learning, t = 1.22, P = 0.23; verbal fluency, t = 0.56, P = 0.58; working memory, t = − 0.50, P = 0.62; information processing speed, t = − 0.30, P = 0.76; motor skill, t = 0.39, P = 0.70) (Figure 1c). There was no difference in standardized Z-scores for global cognitive function between adolescent and adult MA users (t = 1.40, P = 0.17) (Figure 1c).
Relationships between executive function and adolescent-specific brain alterations
Greater differences in global mean thickness (r = 0.44, P = 0.002) and FA values (r = 0.34, P = 0.02) were associated with MA use-related lower performance on executive function in adolescent MA users. These relationships were not significant in adult MA users (global mean thickness, r = 0.25, P = 0.07; global mean FA values, r = 0.03, P = 0.85).
Greater thickness differences of the OFC (r = 0.41, P = 0.01), precuneus (r = 0.37, P = 0.03) and IPC (r = 0.43, P = 0.006) were associated with lower performance on executive function in adolescent MA users. Standardized Z-scores for mean FA values of the white matter ROIs were not associated with lower performance on executive function (OFC-NA, r = 0.29, P = 0.14; PFC-striatum, r = 0.25, P = 0.28; the body of the corpus callosum, r = 0.15, P = 0.90).
Clinical correlates of adolescent-specific brain alterations related to MA use
Relationships with lifetime cumulative dose of MA
Lifetime cumulative dose of MA was negatively associated with standardized Z-scores for global mean thickness in adolescent MA users (r = − 0.47, P = 0.001) and adult MA users (r = − 0.32, P = 0.02), and to a greater degree in adolescent users relative to adults users (P-value for interaction = 0.01) (Supplementary Figure 4).
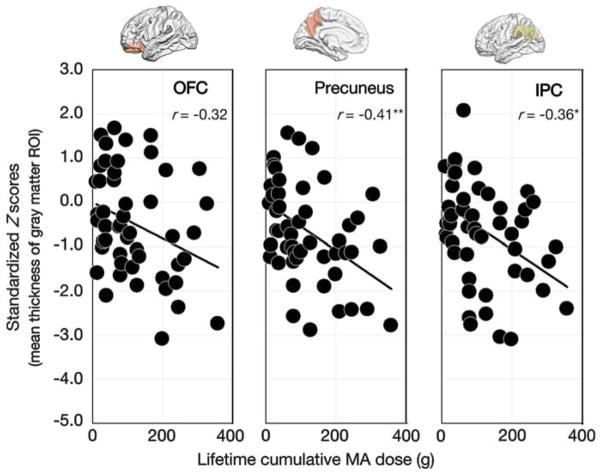
In adolescent MA users, greater lifetime cumulative dose of MA was associated with greater thickness differences of the precuneus (r = − 0.41, P = 0.009) and IPC (r = − 0.36, P = 0.03). The relationship between standardized Z-scores for mean thickness of the OFC (r = − 0.32, P = 0.07) and lifetime cumulative dose of MA did not reach statistical significance (Figure 2).
Figure 2.
Relationships between lifetime cumulative dose of methamphetamine (MA) and standardized Z-scores for mean thickness of gray matter regions of interest (ROIs) in adolescent MA users. Scatter plots and regression lines indicate the relationships between lifetime cumulative dose of MA and standardized Z-scores for thickness of gray matter ROIs (OFC, r =− 0.32, P =0.07; precuneus, r =− 0.41, P =0.009; inferior parietal cortex (IPC), r =− 0.36, P =0.03) in adolescent MA users. *P<0.05; **P<0.01.
Lifetime cumulative dose of MA was not associated with standardized Z-scores for global mean FA values in adolescent MA users (r = − 0.26, P = 0.08) or in adult MA users (r = 0.16, P = 0.27). In adolescent MA users, lifetime cumulative dose of MA was not associated with standardized Z-scores for mean FA values of these regions (PFC-striatum, r = − 0.14, P = 1.00; OFC-NA, r = − 0.14, P = 1.00; the body of the corpus callosum, r = − 0.23, P = 0.34).
Relationships with onset age of MA use
Onset age of MA use was not associated with standardized Z-scores for global mean thickness both in adolescent (r = 0.18, P = 0.21) and in adult MA users (r = 0.10, P = 0.49). In adolescent MA users, onset age of MA use was not associated with standardized Z-scores for mean thickness of the OFC (r = 0.24, P = 0.31), precuneus (r = − 0.10, P = 1.00) and IPC (r = 0.06, P = 1.00).
Onset age of MA use was not associated with standardized Z-scores for global mean FA values both in adolescent (r = 0.22, P = 0.13) and adult MA users (r = 0.02, P = 0.91). In adolescent MA users, earlier onset age of MA use was not associated with greater FA differences in the PFC-striatum (r = 0.34, P = 0.06), OFC-NA (r = 0.30, P = 0.12) tracts or the body of the corpus callosum (r = 0.17, P = 0.72).
Repeated correlation analyses including potential confounding factors such as smoking history, the route of MA administration or alcohol drinking as additional covariates yielded results similar to the main findings (Supplementary Result 2).
Contribution of FH to adolescent-specific brain alterations related to MA use
A three-way interaction between FH, MA use and age group was significant in the OFC (F = 4.38, P = 0.04) but not in the precuneus (F = 1.86, P = 0.17) and IPC (F = 0.19, P = 0.67) gray matter ROIs. There was no significant three-way interaction between FH, MA use and age group in white matter ROIs (PFC-striatum tract, F = 0.78, P = 0.38; OFC-NA tract, F = 1.35, P = 0.25; the body of the corpus callosum, F = 0.58, P = 0.45).
A three-way interaction in the OFC ROI originated from a trend toward greater interaction between FH and MA use in adolescents (F = 3.09, P = 0.08) than in adults (F = 1.39, P = 0.24). Vertex-wise analysis also indicated a significant three-way interaction in the right OFC cluster at corrected P<0.05 (Supplementary Figure 5 and Supplementary Table 4).
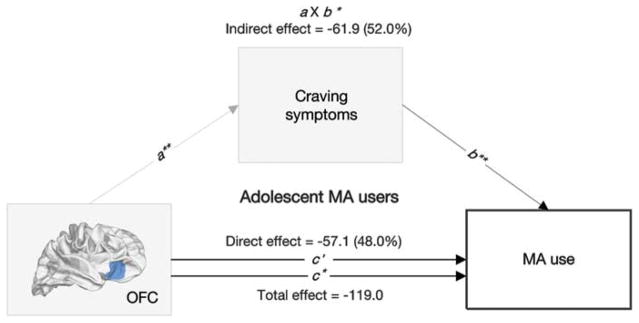
Mediation analysis was conducted based on the assumption that premorbid thickness alterations in the OFC cluster, where a significant three-way interaction among FH, MA use and age group was observed, might have influenced subsequent MA use not only directly but also indirectly via core symptoms of addiction. Structural alterations in the OFC cluster had significant indirect effects on MA intake, through craving symptoms measured using the visual analog scale (percent mediation = 52.0%, indirect effect coefficient = − 61.9, P-value from the bootstrap test = 0.04, 95% confidence interval from the bootstrap test = − 120.2 to − 3.6) (Figure 3). Similar results were found when the total Amphetamine Craving Questionnaire scores were used as a measure for craving symptoms (percent mediation = 49.7%, indirect effect coefficient = − 59.1, P-value from the bootstrap test = 0.04, 95% confidence interval from the bootstrap test = − 115.3 to − 2.9). Indirect effects mediated by the Stroop interference and withdrawal symptoms were not significant. The relationships between adolescent-specific brain alterations related to MA use and clinical symptoms related to drug use including craving, withdrawal and the Stroop interferences are presented below.
Figure 3.
Mediation analysis testing the relationships between orbitofrontal cortex (OFC) thickness differences, craving symptoms and methamphetamine (MA) use. The negative association between OFC thickness differences (X) and lifetime cumulative dose of MA (Y) was partially mediated by increased craving symptoms (M). c, c′ and a × b indicate total, direct and indirect effects, respectively. *P<0.05; **P<0.01.
A significant association between standardized Z-scores for thickness of OFC and craving symptoms was found in adolescent MA users (r = − 0.38, P = 0.02). MA-related thickness alterations in the precuneus (r = − 0.48, P = 0.003) and IPC (r = − 0.37, P = 0.03) were associated with Stroop interference. Otherwise, there were no significant associations between standardized scores for thickness of ROIs and clinical symptoms of craving (precuneus, r = − 0.14, P = 1.00; IPC, r = − 0.20, P = 0.53), withdrawal (OFC, r = − 0.32, P = 0.08; precuneus, r = − 0.33, P = 0.06; IPC, r = − 0.24, P = 0.28) and the Stroop interference (OFC, r = − 0.28, P = 0.15) in adolescent MA users.
No significant associations between standardized Z-scores for FA values and clinical symptoms including craving (global mean FA values, r = − 0.23, P = 0.12; PFC-striatum, r = − 0.20, P = 0.55; OFC-NA, r = − 0.18, P = 0.68; the body of the corpus callosum, r = − 0.10, P = 1.00), withdrawal (global mean FA values, r = − 0.07, P = 0.63; PFC-striatum, r = − 0.009, P = 1.00; OFC-NA, r = − 0.10, P = 1.00; the body of the corpus callosum, r = − 0.07, P = 1.00) and the Stroop interference (global mean FA values, r = − 0.19, P = 0.19; PFC-striatum, r = − 0.07, P = 1.00; OFC-NA, r = − 0.28, P = 0.17; the body of the corpus callosum, r = − 0.16, P = 0.86) were found in adolescent MA users.
DISCUSSION
The current study is, to our knowledge, the first to report that the adolescent brain is more susceptible to MA exposure than the adult brain, even with smaller doses and shorter durations of use. Furthermore, the current results suggest that the adolescent-specific brain characteristics are related to a high risk of developing MA dependence.
Global gray matter thickness differences were dose-dependently associated with chronic MA use both in adolescent and adult users, and the extent of global thickness reductions was comparable across groups (t = 1.39, P = 0.17). However, as adolescent users tend to use much lower doses of MA in comparison with adult users (t = 5.90, P<0.001), the influences of MA on gray matter may be even greater in adolescents than in adults. A recent animal study using high-resolution imaging has also shown evidence of adolescent-specific brain vulnerability to the effects of repeated stimulant use.25 Adolescent rodents, in comparison with adult ones, exhibited more pronounced cocaine-induced structural change in the frontal, striatal and insular regions of the brain.25
Notably, in the OFC, the impact of FH may be larger in adolescence than in adulthood. A significant three-way interaction in the OFC indicated a greater interaction between FH and MA use in adolescents than in adults. As the heritability of problematic substance use is high in adolescence,26 the premorbid OFC thickness alteration related to FH effects may potentially precede the adolescent onset of MA dependence. It has been shown that premorbid OFC alterations contribute to an increased likelihood of drug use in adolescents.27,28 A recent large-scale imaging genetic study of adolescents has also suggested that the reduction of lateral OFC activity may precede drug use.29 Furthermore, increased gray matter volumes in the OFC may be a protective factor against the development of addiction.30 In addition, previous human and animal data suggest that the OFC is simultaneously a substrate for enduring alterations induced by psychostimulants.31–33 Since the OFC has a role in conditioned reinforcement and craving,31 OFC dysfunction, regardless of its origin, is likely to be related to continued drug use by adolescents. The current mediation analysis implies that craving symptoms may mediate the association between the extent of OFC alterations and the amount of MA intake. However, readers should be cautious in interpreting indirect effects of the mediation due to modest effect sizes.
The current results provide new insights into the interplay of familial risk for drug use and effects of drug on the adolescent brain. However, causal and temporal relations among these effects are difficult to address in cross-sectional human studies. It will be essential to conduct longitudinal studies with systematic observations to disentangle genetically determined brain predispositions from environmental effects.
MA-use-related FA differences in the prefrontostriatal tracts and the body of the corpus callosum were more pronounced in adolescents than in adults. In these white matter regions, there was no significant three-way interaction between FH, MA use and age group. This may indirectly suggest that the greater MA-use-associated alterations within these areas in adolescents may be related to MA-induced effects rather than FH-related white matter alterations.
A post-mortem study on the human brain has demonstrated that white matter myelination continues during adolescence.34 Given that there is a critical period of brain development for certain functions,35 it is possible that MA exposure during adolescence may cause a critical derailment from the normal trajectory of white matter myelination. As regional alterations in the frontostriatal fiber tracts were not affected by MA use in a dose-dependent manner, the developmental stage during which drug exposure occurs appears to have a more significant role in determining magnitude of MA-induced white matter changes. For instance, during adolescence, a potentially time-limited window of active myelination, the developing white matter may show a greater vulnerability to alterations induced by MA exposure.
Substantial maturation of fiber tracts constituting the frontostriatal pathway, which facilitates increased preference for delayed reward,36 takes place during adolescence.13 In light of this observation, the current findings have considerable clinical relevance to the clinical course of adolescent-onset drug dependence. Unsuccessful rewiring of the involved white matter regions after MA exposures may contribute to a prolonged inability to inhibit prepotent addictive responses to drugs in the adolescent population. Epidemiological evidence appears to support this speculation, as adolescent-onset drug users may have a higher risk for lifelong drug use as well as a faster transition to dependence than their adult-onset counterparts.37
There was a significant three-way interaction between FH, MA use and age group in the OFC. Factors that influence this three-way interaction cannot be clearly identified with this cross-sectional design. However, while the effect size in adults for the effects of FH on the OFC was small (mean standardized Z-score for thickness of combined adult FH+ controls and FH+ MA users = − 0.08, s.d. = 1.42), the effect size in adolescents for the effects of FH on the OFC (mean standardized Z-score for thickness of combined adolescent FH+ controls and FH+ MA users = − 0.43, s.d. = 1.34) was large and similar to that for the effects of MA use (mean standardized Z-score for thickness of combined adolescent FH − MA users and FH+ MA users = − 0.50, s.d. = 1.20) in adolescents. Future longitudinal studies with a larger sample will be needed to further examine the FH effects on the adolescent brain, which may precede the MA exposure.
There was MA-use-related cognitive dysfunction both in adolescent and adult MA users. This is in line with the findings of a previous meta-analysis.23 The magnitude of MA-use-related executive dysfunction was greater in adolescent MA users than in adult MA users. Interestingly, MA-use-associated gray and white matter alterations were related to the executive dysfunction in adolescents. However, cognitive dysfunction that may predispose adolescents to use MA from those that may result from MA use will need to be differentiated in future studies.38
It should be noted that adolescent MA users were more likely to smoke MA rather than inject intravenously as the route of administration, whereas adult users predominantly used intravenous injection. We have repeated all analyses including this potential confounding factor as an additional covariate and obtained results similar to the current findings (Supplementary Result 2). In view of their similar bioavailability and pharmacokinetics, intravenous injection and smoking of MA are likely to exert psychoactive effects at a comparable speed and intensity with high levels of rapid drug delivery to the brain.39,40
Previous literature has suggested that tobacco smoking may be associated with structural alterations in gray and white matter.41–45 Although individuals in the current sample did not have other comorbid drug dependence, most MA users were current smokers. It would therefore be difficult to examine the independent effects of MA apart from those of tobacco smoking on the brain in this sample. Although results from repeated analyses including smoking history as an additional covariate remained similar (Supplementary Result 2), future studies with a larger sample size would be needed to define the shared and independent effects of MA abuse and tobacco smoking on the developing brain.
Although participants of the present study did not have a current diagnosis of alcohol abuse or dependence and the estimated average amount of weekly alcohol consumption was below the World Health Organization criteria for hazardous or harmful drinking,46 the potential effects of concurrent alcohol use need to be considered in interpreting the data. For example, heavy alcohol use has been reported to be associated with volume loss in the prefrontal and parietal brain regions.47–49 Despite the fact that the analysis results with alcohol drinking as an additional covariate were similar to those without in the current study (Supplementary Result 2), the potential interactive effects between MA and heavy alcohol use on the developing brain should be investigated in future studies with participants who have both conditions.
The current results were obtained from an ethnically homogeneous population. Considering the large gene-by-environment interactions that are common in addiction,50 the present results need to be replicated in a larger and more ethnically diverse sample.
Although this is the largest multimodal brain imaging study to examine the relationships between gray and white matter alterations and MA-use-related clinical and cognitive characteristics in both adolescents and adults with MA exposure, the current study has a clear limitation of being a cross-sectional design. Studies with a longitudinal design that examine factors predicting future drug use are likely to provide important information as to the temporal and causal relationship between brain vulnerability and behavioral outcomes associated with MA use in adolescents.
In summary, the current study suggests that the effects of MA on the brain were greater and more widespread in adolescence than in adulthood. This poses even greater problems since the top-down control over risky addictive behavior is less mature in adolescence than in adulthood. The current findings provide a line of neurobiological evidence elucidating the danger of drug use in adolescence even at low doses.
Supplementary Material
Acknowledgments
This work was supported by NIDA Grants 1R01 DA024070 (to IK Lyoo and PF Renshaw), K05 DA031247 (to PF Renshaw), Grant KRF-2008-220-E00021 from the Global Research Network Program funded by the Korea Research Foundation (to IK Lyoo), the Global Top 5 program from the Ewha W University (to IK Lyoo) and Grant 2012R1A2A2A01010739 from the National Research Foundation of Korea (to IK Lyoo). We thank Y Cheon, SY Won, YS Kim and YS Kwon for assistance with data collection and recruitment and JS Kim, KM Kim and KI Yang for technical assistance.
Footnotes
CONFLICT OF INTEREST
Professor IK Lyoo has received research support from Lundbeck, Eli Lilly, AstraZeneca, GSK, Nongshim and Pulmuone Holdings and Professor PFR has been a consultant for Ridge Diagnostics and Kyowa Hakko. The other authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 2.Rapoport JL, Gogtay N. Brain neuroplasticity in healthy, hyperactive and psychotic children: insights from neuroimaging. Neuropsychopharmacology. 2008;33:181–197. doi: 10.1038/sj.npp.1301553. [DOI] [PubMed] [Google Scholar]
- 3.Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102:5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 5.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, et al. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, et al. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. NeuroImage. 2010;50:1392–1401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alicata D, Chang L, Cloak C, Abe K, Ernst T. Higher diffusion in striatum and lower fractional anisotropy in white matter of methamphetamine users. Psychiatry Res. 2009;174:1–8. doi: 10.1016/j.pscychresns.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, et al. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10:765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- 11.Salo R, Nordahl TE, Buonocore MH, Natsuaki Y, Waters C, Moore CD, et al. Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: a diffusion tensor imaging study. Biol Psychiatry. 2009;65:122–128. doi: 10.1016/j.biopsych.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobias MC, O’Neill J, Hudkins M, Bartzokis G, Dean AC, London ED. White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacology. 2010;209:13–24. doi: 10.1007/s00213-009-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future: National Results on Adolescent Drug Use: Overviewof Key, Findings, 2011. Institute for Social Research. The University of Michigan; Ann Arbor, USA: 2012. [Google Scholar]
- 15.United Nations Office on Drugs and Crime (UNODC) Patterns and Trends of Amphetamine-Type Stimulants and other Drugs: Challenges for Asia and the Pacific 2013. A Report from the Global SMART Programme. UNODC; Vienna, Austria: 2013. [Google Scholar]
- 16.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57:675–682. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- 17.Srisurapanont M, Jarusuraisin N, Jittiwutikan J. Amphetamine withdrawal: I. Reliability, validity and factor structure of a measure. Aust N Z J Psychiatry. 1999;33:89–93. doi: 10.1046/j.1440-1614.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- 18.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- 19.Eidels A, Townsend JT, Algom D. Comparing perception of Stroop stimuli in focused versus divided attention paradigms: evidence for dramatic processing differences. Cognition. 2010;114:129–150. doi: 10.1016/j.cognition.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uttl B, Graf P. Color-Word Stroop test performance across the adult life span. J Clin Exp Neuropsychol. 1997;19:405–420. doi: 10.1080/01688639708403869. [DOI] [PubMed] [Google Scholar]
- 22.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 23.Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 24.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheeler AL, Lerch JP, Chakravarty MM, Friedel M, Sled JG, Fletcher PJ, et al. Adolescent cocaine exposure causes enduring macroscale changes in mouse brain structure. J Neurosci. 2013;33:1797–1803. doi: 10.1523/JNEUROSCI.3830-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- 27.Lotfipour S, Ferguson E, Leonard G, Perron M, Pike B, Richer L, et al. Orbitofrontal cortex and drug use during adolescence: role of prenatal exposure to maternal smoking and BDNF genotype. Arch Gen Psychiatry. 2009;66:1244–1252. doi: 10.1001/archgenpsychiatry.2009.124. [DOI] [PubMed] [Google Scholar]
- 28.Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry. 2012;71:684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- 30.Ersche KD, Jones PS, Williams GB, Smith DG, Bullmore ET, Robbins TW. Distinctive personality traits and neural correlates associated with stimulant drug use versus familial risk of stimulant dependence. Biol Psychiatry. 2013;74:137–144. doi: 10.1016/j.biopsych.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15:358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- 33.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 34.Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- 35.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- 38.Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013;38:259–274. doi: 10.1038/npp.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The National Institute on Drug Abuse. National Institute on Drug Abuse Methamphetamine Abuse: Epidemiologic Issues and Implications. Department of Health and Human Services; Rockville, MD, USA: 1991. Available at: http://archives.drugabuse.gov/pdf/monographs/115.pdf. [Google Scholar]
- 40.Degenhardt L, Mathers B, Guarinieri M, Panda S, Phillips B, Strathdee S, et al. The Global Epidemiology of Methamphetamine Injection: A Review of the Evidence of Use and Associations with HIV and Other Harm. National Drug and Alcohol Research Centre, University of New South Wales; Sydney, Australia: 2007. [Google Scholar]
- 41.Morales AM, Ghahremani D, Kohno M, Hellemann GS, London ED. Cigarette exposure, dependence, and craving are related to insula thickness in young adult smokers. Neuropsychopharmacology. 2014;39:1816–1822. doi: 10.1038/npp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morales AM, Lee B, Hellemann G, O’Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 2012;125:230–238. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almeida OP, Garrido GJ, Lautenschlager NT, Hulse GK, Jamrozik K, Flicker L. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:92–98. doi: 10.1097/JGP.0b013e318157cad2. [DOI] [PubMed] [Google Scholar]
- 44.Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- 45.Hudkins M, O’Neill J, Tobias MC, Bartzokis G, London ED. Cigarette smoking and white matter microstructure. Psychopharmacology. 2012;221:285–295. doi: 10.1007/s00213-011-2621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization. International Guide for Monitoring Alcohol Consumption And Related Harm. Department of Mental Health and Substance Dependence, World Health Organization; Geneva, Switzerland: 2000. [Google Scholar]
- 47.Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- 48.Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- 49.Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- 50.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.