Abstract
Purpose of review
Sarcopenia or loss of skeletal muscle loss is the major component of malnutrition and occurs in the majority of patients with liver disease. Lower muscle contractile function also contributes to the adverse consequences of sarcopenia. There are no effective therapies to prevent or reverse sarcopenia in liver disease. This review will discuss the advances in diagnosis, pathogenesis and treatment options for sarcopenia in liver disease.
Recent findings
Sarcopenia increases mortality, risk of development of other complications of cirrhosis, and post liver transplant outcomes while quality of life is decreased. Unlike other complications of cirrhosis that reverse after liver transplantation, sarcopenia may not improve and actually worsens. Impaired skeletal muscle protein synthesis and increased proteolysis via autophagy contribute to sarcopenia. Hyperammonemia is the best-studied mediator of the liver-muscle axis. Molecular studies show increased expression of myostatin while metabolic studies show impaired mitochondrial function and tricarboxylic acid cycle intermediates due to cataplerosis of α-ketoglutarate. Impaired skeletal muscle pyruvate and fatty acid oxidation during hyperammonemia suggest amino acids are diverted to acetyl CoA and potentially aggravate hyperammonemia. Nutritional supplementation is of limited or no benefit and suggests that cirrhosis is a state of anabolic resistance. Exercise maybe beneficial but whether it overcomes anabolic resistance is not known.
Summary
The high clinical significance of sarcopenia is well established. Current approaches to nutritional supplementation have not been effective in reversing sarcopenia because of anabolic resistance. Myostatin antagonists, specific amino acid supplementation, mitochondrial protection and combination endurance-resistance exercise are potential future therapeutic options.
Keywords: Sarcopenia, myostatin, hyperammonemia, leucine supplementation, mitochondrial function
Introduction
Malnutrition was a term used nearly universally to describe the phenotype of skeletal muscle loss with or without fat loss in patients with liver disease[1]. Skeletal muscle loss or sarcopenia is the major contributor to adverse clinical outcomes[2–11]. Despite recognition of the clinical impact of sarcopenia in liver disease, there are no effective therapies primarily because of the mechanisms of muscle loss in liver disease are not well understood. Since, most studies to date have been descriptive, the mediators of and molecular targets for the treatment of sarcopenia in liver disease have not identified[12]. It is also interesting that even though clinical outcomes are poorer in sarcopenic patients[4,9], it is unclear how exactly muscle loss contributes to the greater morbidity and mortality. Lower muscle mass can result in either impaired skeletal muscle metabolic or contractile functions that contribute to the adverse outcomes in cirrhosis[13–15]. Since the skeletal muscle functions as a metabolic partner to the liver, it would be intuitively obvious that sarcopenia or muscle loss will result in adverse clinical outcomes in cirrhosis. Skeletal muscle dysfunction in cirrhosis contributes to frailty and deconditioning that have been identified to increase morbidity in cirrhosis[15,16]. Finally, one of the frequent symptoms in patients with cirrhosis is fatigue but the contribution of central fatigue and skeletal muscle contractile dysfunction or fatigue responses to repetitive contraction has not been well characterized[13]. It is still unclear if loss of muscle mass and functional consequences are directly related or independent effects of common mediator(s). The mediator(s) of the liver muscle axis have not been completely established but recent studies involving integrated metabolic-molecular approaches suggest that alterations in multiple signaling pathways converge on protein synthesis and increased proteolysis that result in reduction in muscle mass[17–19]. The mechanisms of functional alterations are less studied but data suggest that reduced ATP synthesis due to mitochondrial dysfunction as well as direct modifications of contractile proteins contribute to decreased muscle function[13,20,21]. Treatment approaches to date have focused on replacing calories and proteins by different routes of administration with little benefit[22,23]. Exercise and physical activity may be beneficial but are limited by fatigue[24–26]. Molecular approaches using direct activation of protein synthesis by L-leucine have shown benefit at least in the acute setting[19]. The present commentary will focus on the contributions of muscle loss to clinical consequences in cirrhosis, novel mechanistic insights based on studies on the skeletal muscle and therapeutic strategies beyond providing supplemental nutrition.
What is malnutrition in cirrhosis?
For decades, malnutrition was the term most often used to describe the clinical phenotype of cirrhotic patients and overt muscle loss with or without fat loss. Since recognition that the term “malnutrition” has been used to refer to decreased nutrient intake, loss of muscle and/or fat mass and reduced muscle strength and function. To overcome this limitation and have a more uniform terminology that allows data from different studies to be compared, the term sarcopenia or loss of muscle mass and disordered energy metabolism were recommended and are now widely used[1,4,9,27]. Despite disordered energy metabolism with increased gluconeogenesis and fatty acid oxidation show that cirrhosis is a state of accelerated starvation that contribute to muscle loss[28]. To ensure uniformity and precision in terminology, skeletal muscle loss, energy dysmetabolism and a combination of muscle and fat loss should be used in future studies addressing nutritional status in cirrhosis and liver disease[12,16]. There is some concern that the term sarcopenia is specific for aging related muscle loss but the term is actually derived from sarcos- flesh, penia- loss of, suggesting that the qualifier of the underlying disease can be used to specify the cause of muscle loss. A review of the majority of publications that describe malnutrition in cirrhosis show that anthropometric measures were used in the majority of studies while some investigators used other methods including bioelectrical impedance analysis, dual energy X ray absorptiometry and impedance plethysmography[14,29–31]. These measurements allow quantification of lean body mass that consists primarily of skeletal muscle mass but are not direct measures of muscle mass. More recently, computed tomography and magnetic resonance imaging are used to precisely quantify the muscle area and derive whole body muscle mass. Most studies use percentile values to define sarcopenia and values between the 20th and 5th percentile of normal values are considered moderate sarcopenia and less than the 5th percentile are believed to be severe sarcopenia[32].
Clinical consequences of sarcopenia in cirrhosis
Sarcopenia in cirrhosis has been reported in 40–60% of patients depending on the population studied and patients with alcoholic cholestatic cirrhosis have the most severe forms of sarcopenia[1,12,14,33]. Mild degree of muscle loss probably occurs in all cirrhotics but the severity of sarcopenia measured by anthropometric assessment worsens with increasing severity of liver disease quantified by Child’s score. The prevalence of functional consequences, reduced grip strength is more frequent but the occurrence of muscle fatigue has not been studied well[13,25]. However, there is recent data that muscle fatigue in response to repetitive contraction is significantly higher in cirrhosis than controls. Both reduced grip strength and sarcopenia contribute to higher mortality in cirrhosis[14,27]. Quality of life, hospitalization, infections and encephalopathy are also higher in cirrhotic patients with sarcopenia[1,4,6,11,12]. However, the contribution of reduced muscle mass and impaired contractile function to these clinical outcomes is not as well described, primarily because of the very limited studies that have determined if the impaired contractile function is due to or independent of sarcopenia. Lower muscle strength normalized to muscle mass has been reported in an animal model of portosystemic shunting but there are no human data in cirrhotics normalizing grip strength to muscle mass[13]. In patients undergoing liver transplantation, pretransplant sarcopenia adversely affects outcomes during liver transplantation including longer duration of hospital stay, duration on the ventilator, intensive care unit stay and number of blood transfusions required. In patients after liver transplantation, sarcopenia before transplantation has been consistently reported to adversely affect survival[9]. Finally, unlike other complications of cirrhosis, lean body mass and sarcopenia do not reverse or may even become worse though a recent paper suggests that in some patients, sarcopenia may reverse after OLT[34–36]. These observations show that sarcopenia adversely affects clinical outcomes in cirrhotics before, during and after liver transplantation.
Mechanisms of sarcopenia in cirrhosis
Even though a large number of descriptive studies show the high clinical significance of sarcopenia in cirrhosis, mechanistic studies to identify how sarcopenia develops and the mediators of the liver muscle axis are much less well understood. Early studies have suggested that patients with cirrhosis have a number of reasons for malnutrition, primarily muscle loss, but the most important reasons are poor oral intake and lack of activity. However, 2 metaanalyses have suggested that supplemental nutrition does not improve nutritional our clinical outcomes[22,23]. This is because patients with liver disease are believed to be in a state of anabolic resistance that prevents appropriate skeletal muscle responses to nutrient administration[37]. Skeletal muscle mass is maintained by a balance between protein synthesis, breakdown and possibly the regenerative potential mediated by the myogenically committed stem cells, satellite cells[1]. Early studies using metabolic tracer kinetics were conflicting and suggested that whole body protein breakdown in cirrhosis was increased, unaltered or decreased depending on the patient population studied. Indirect studies suggested that limb protein synthesis and postprandial protein synthesis response were lower in cirrhotics than controls. Our studies in the portacaval anastomosis rat provided an explanation for these contradictory human findings[17]. Early after the anastomosis, there was increased skeletal muscle proteolysis with unaltered protein synthesis and later in the course, both protein synthesis and proteasome mediated proteolysis were lower but one group did report that expression of genes in the proteasome pathway was increased in the muscle from patients with cirrhosis[18,19,38,39]. Interestingly, autophagy was increased in the muscle of cirrhotics even when ubiquitin mediated proteolysis was unaltered or decreased[18,19,40]. A recent study reported lower protein synthesis markers in the skeletal muscle of patients with cirrhosis with decreased ubiquitin mediated but increased autophagy mediated proteolysis[19]. These data show that reduced protein synthesis and increased autophagy mediated protein breakdown contribute to ongoing skeletal muscle loss in cirrhosis.
Having identified the protein turnover responses, the molecular mechanisms that mediate these have been identified. Our understanding of muscle loss in cirrhosis has advanced mainly due to the rapid advances in the field of skeletal muscle biology[12]. Increased circulating and skeletal muscle expression of myostatin, a TGFβ superfamily member, have been reported in patients with cirrhosis even though one group did not find increased myostatin in cirrhotics undergoing transplantation[19,38,39]. Myostatin inhibits protein synthesis and increases autophagy. There are also reports of myostatin activating proteasome components through the FOXO mediated pathway but direct studies on proteasome activity in the skeletal muscle of patients with cirrhosis showed unaltered proteasome activity[18,19,40]. Extensive studies have been performed on myostatin binding to its receptor on the skeletal muscle membrane and the regulation of protein synthesis by inhibition of mTORC1. In contrast, upstream regulation of myostatin is not as well studied, but hyperammonemia has been recognized as an activator of myostatin via an NFkB mediated mechanism[38]. Hyperammonemia is an activator of reactive oxygen species and this may be another mechanism of activating autophagy. Interestingly, ammonia disposal by the skeletal muscle is mediated via glutamate synthesis in the mitochondria followed by its conversion to glutamine in the cytosol that is transported to the circulation in exchange for leucine that is transported into the muscle[16,19,41,42]. This mechanism can explain the low circulating leucine but relatively unaltered muscle leucine concentrations in cirrhosis. However, within the cell, leucine can either be used for peptide chain elongation or protein synthesis and for activation of mTORC1 by lysosomal transport. An alternate method of leucine metabolism in cirrhosis and potentially hyperammonemia is via mitochondrial oxidation to generate acetyl CoA as a source of energy[19,41,42]. Such compartmentation can explain previous reports of normal or near normal muscle leucine concentrations in cirrhosis with a robust synthetic response to high dose leucine supplementation because the large dose of supplemental leucine can potentially saturate the mitochondrial transport with sufficient leucine available for molecular signaling via direct stimulation of protein synthesis.
Since the primary mechanism of skeletal muscle ammonia disposal is via cataplerosis (loss of TCA cycle intermediates by enzymatic reactions) of α ketoglutarate (αKG)[41,42], one option is to increase anaplerosis (addition of 4 and 5 carbon TCA cycle intermediates). This can be achieved by either isoleucine or valine, both of which provide direct anaplerotic input or recent reports that suggest that leucine carbon is used to form αKG. The deamination/transamination of amino acids as anaplerotic agents results in release of ammonia in a 1:1 stoichiometric ratio with only one net ammonia removed during the formation of glutamine[42]. A novel approach is to provide cell permeable TCA cycle intermediate, αKG that provides the substrate to remove 2 molecules of ammonia in the muscle. A schematic overview of the current concepts in the pathogenesis of sarcopenia and contractile dysfunction in cirrhosis is shown in Figures 1 and 2.
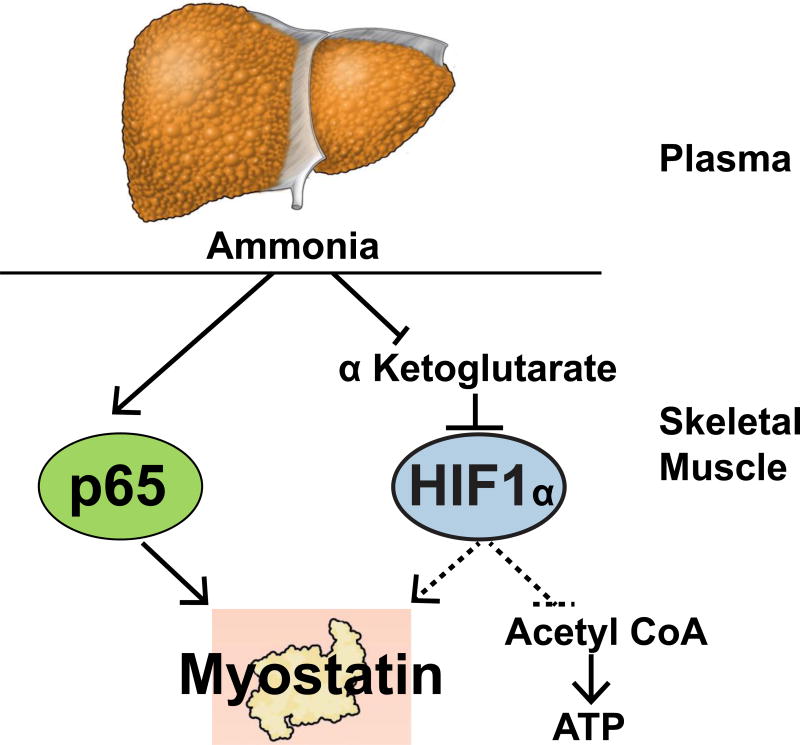
Figure 1. Overview of ammonia induced metabolic and signaling perturbations.
Impaired hepatic ureagenesis results in skeletal muscle hyperammonemia. Ammonia transcriptionally upregulates myostatin via a p65NFkB mediated mechanism and decreases α ketoglutarate by cataplerosis. Reduction in α ketoglurate can stabilize hypoxia inducible factor 1α (HIF1α) that in turn can activate myostatin and inhibit pyruvate to acetyl CoA oxidation (dotted lines are preliminary studies from our laboratory).
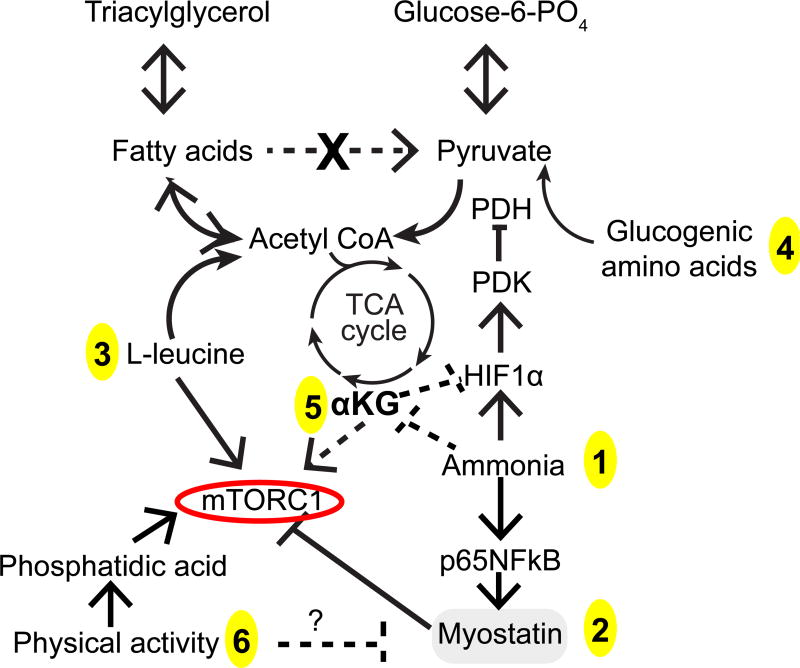
Figure 2. Metabolic abnormalities that contribute to and potential therapeutic targets.
Accelerated lipolysis generates acetyl CoA from fatty acids due to impaired pyruvate dehydrogenase (PDH) by hypoxia inducible factor 1α (HIF1α) via pyruvate dehydrogenase kinase (PDK) and possibly directly by ammonia. Cataplerosis of α-ketoglutarate (αKG) by to form glutamate is a metabolic disposal pathway activated in the muscle during hyperammonemia. Lower αKG results in stabilization of HIF1α, decreased mTORC1 activation and decreased tricarboxylic acid (TCA) cycle flux and lower ATP synthesis. These perturbations contribute to lower protein synthesis. Homeostatic responses include the utilization of branched chain amino acids to provide anaplerosis for generate αKG (isoleucine, valine) and acetyl CoA (leucine, isoleucine) with increased transport of branched chain amino acids from circulation for metabolic disposal. Ammonia via a p65NFkB mediated mechanism also activates myostatin that in turn inhibits mTORC1. These metabolic and molecular perturbations contribute to decreased sensitivity to anabolic stimuli (anabolic resistance) that can be potentially reversed by intervention at targeted sites. 1. Long term ammonia lowering strategies. 2. Myostatin blocking agent including antagomirs. 3. L-leucine provides acetyl-CoA, activates mTORC1 and protein synthesis. 4. Glucogenic amino acids can be a source of anaplerotic input to provide succinyl CoA replacing the loss of (cataplerosis) of αKG that is converted to glutamate during hyperammonemia (since skeletal muscle cannot generate urea). 5. Cell permeable esters of αKG are a potential strategy to reverse cataplerosis and a novel method to increase muscle ammonia disposal. 6. Physical activity stimulates mTORC1 via phosphatidic acid.
Post liver transplantation sarcopenia and sarcopenic obesity have been identified to be of high clinical significance but the mechanisms are even less understood than that in non-transplanted subjects[32]. The contribution of epigenetic alterations due to hyperammonemia before transplantation, use of calcineurin and mTORC1 inhibitors as immunosuppressants and metabolic-molecular perturbations due medications and lack of standard guidelines are not known[12,32].
Therapeutic options
The standard clinical approach to prevent and reverse malnutrition, primary skeletal muscle loss, includes nutritional supplementation to ensure adequate calorie and protein intake because cirrhosis is a state of accelerated starvation [16,28]. With an increasing understanding of skeletal muscle loss and contractile dysfunction that may not necessarily be linked together, it is important to determine the specific outcome desired. Despite the recognized reduction in survival [43] with sarcopenia, data on impact of improved muscle has mass on survival has been reported but is much more limited. If muscle mass is increased, hepatic metabolic support functions including ammonia removal, providing amino acids for gluconeogenesis, glucose disposal and insulin response are improved but contractile function may or may not be normalized. Reversal of contractile dysfunction does not necessarily translate into improved metabolic function especially during accelerated starvation response in cirrhosis but is likely to improve quality of life, hospitalizations, and infection due to greater mobility. Since the current focus is on skeletal muscle wasting and sarcopenia, the major emphasis will be on increasing muscle mass.
Cirrhosis is believed to be a state of anabolic resistance and standard replacement of nutrients are generally ineffective[22,23]. Potential reasons for this include the molecular and metabolic perturbations that result in lower protein synthesis and increased autophagic proteolysis as discussed above. To overcome anabolic resistance, a strategy that has been beneficial is to shorten the postabsorptive or fasting state. Overnight fasting provides the greatest window of anabolic opportunity but frequent overnight feeding is difficult, causes sleep disturbances and has the potential for aggravating both reflux and insulin resistance[37]. To overcome these limitations by avoiding fasting induced proteolysis and lipolysis, frequent feeding and late evening snacks have been used with some benefit. Adequate protein intake (1–1.2g.kg−1.d−1) is tolerated well but due to the ammoniagenic potential and need for hepatic disposal for aromatic amino acids, at least a third of dietary protein intake from plant sources are both tolerated well and provide adequate branched chain amino acids. Whether accelerated lipolysis in cirrhosis is of metabolic benefit is however not clear since acetyl-CoA from fatty acids cannot be converted to glucose. Acetyl CoA from fatty acid oxidation can, however, inhibit pyruvate dehydrogenase that is also inhibited directly by ammonia and by pyruvate dehydrogenase kinase (PDK) that is a hypoxia inducible factor 1α (HIF1α) target. We have noted in preliminary studies that skeletal muscle hyperammonemia activates HIF1α. The consequent impaired conversion of pyruvate to acetyl CoA necessitates gluconeogenesis from amino acids because fatty acid carbon cannot be used for gluconeogenesis. Impaired pyruvate dehydrogenase also impairs the ability of the muscle to completely oxidize glucose via the TCA cycle with increased lactate in the muscle potentially promoting a futile gluconeogenic cycle. Thus, complex metabolic-molecular integration occurs at multiple sites: ammonia and potentially other upstream regulators (TNFα, lipopolysaccharide, reduced testosterone) mediated myostatin activation, ammonia induced hypoxia inducible factor 1 mediated impaired pyruvate dehydrogenase that in turn prevents efficient and complete glucose utilization for energy and accelerated gluconeogenesis necessitating proteolysis, and cataplerosis of αKG that in turn impairs TCA cycle flux, all of which contribute to sarcopenia that cannot be reversed by providing nutrient substrates alone. Instead, a combination of therapies targeting myostatin, long term ammonia lowering to potentially decrease skeletal muscle ammonia and direct activation of regulators of skeletal muscle protein turnover including amino acids like L-leucine and potentially isoleucine.
Physical activity increases skeletal muscle mass and functional capacity[24–26,44,45]. Given subjective fatigue and decreased muscle strength, it is not clear if it is possible to increase muscle mass by resistance exercise and endurance exercise improves functional capacity but may not have an impact on skeletal muscle mass. Phosphatidic acid (PA) has been shown to mediate transduction of mechanical stimuli like exercise and activity to mTORC1 activation[46], but whether the PA response to mechanical stimuli is maintained in cirrhosis is unknown. In the absence of definitive data, it would be appropriate to recommend physical activity to the extent tolerated to improve functional capacity. Exercise increases muscle ammonia concentrations that can potentially compound the functional consequences of skeletal muscle hyperammonemia and impaired muscle ATP synthesis with resultant fatigue and inability to gain muscle mass. Lowering ammonia over the long term maybe a potential strategy even in non-encephalopathic patients with cirrhosis to improve muscle mass. In the post-transplant population, the lowering immunosuppressant dose, intense nutrient and physical activity based recommendations to improve muscle mass are necessary but there are currently no reported studies to reverse/prevent post-OLT sarcopenia. Defining the mechanisms responsible for the same will be a critical step before effective therapies can be developed for post OLT sarcopenia.
Finally, since ammonia mediated cerebral effects clinically manifest with reversal of circadian sleep patterns, it is not clear if hyperammonemia alters skeletal muscle circadian genes also. Emerging data suggests that mTORC1, a critical protein synthesis regulatory molecule, is downstream of clock gene Bmal1[47]. Clinical translation will include lowering muscle ammonia in combination with strategies to regulate muscle circadian genes.
Conclusion
In summary, a combination of low ammoniagenic protein supplementation with branched chain amino acids enriched in leucine, long term ammonia lowering strategies combined with increased physical activity to improve functional capacity should complement frequent feeding with late evening snacks in preventing and potentially reversing muscle loss in cirrhosis (Figure 3). Whether a different strategy is necessary to reverse post-liver transplant sarcopenia is not known but the recent push towards lower doses of calcineurin and mTORC1 inhibitors may be beneficial for the skeletal muscle in the increasing population of post OLT patients.
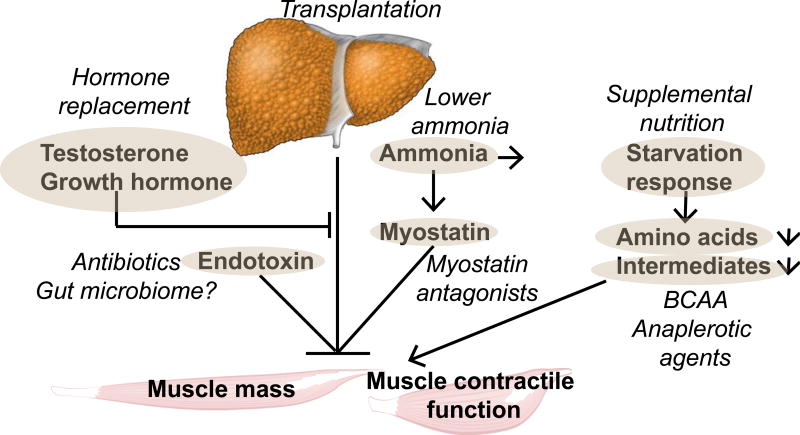
Figure 3. Overview of strategies to reverse sarcopenia and potentially contractile dysfunction in cirrhosis.
Bold, oval encircled targets and putative interventions (italics). Liver transplantation is a definitive therapy but may not reverse sarcopenia. Hyperammonemia is the best studied mediator of the liver-muscle axis but duration of therapy needed to lower muscle ammonia is not known. It is also not known if lowering muscle ammonia will indeed reverse the metabolic and molecular perturbations in the muscle with functional translation into increased muscle mass, improved contractile function and better clinical outcomes. Starvation response has been addressed using frequent feeds, nocturnal meals and late evening snacks with protein supplementation and shown to be of some benefit. Direct myostatin antagonists are in various stages of development and preclinical rodent studies have shown benefit. Amino acid supplementation with branched chain amino acids, anaplerotic substrates including cell permeable tricarboxyclic acid intermediates are exciting novel approaches being evaluated in preclinical studies. Therapies to reverse hormone deficiencies and endotoxemia have not been effective but the impact of alterations in gut microbiome has not been evaluated and may be another potential therapeutic target.
Key points.
Sarcopenia or loss of skeletal muscle mass is the major component of malnutrition in cirrhosis with a prevalence of 40–60%.
Decreased survival, lower quality of life, increased risk of other complications of cirrhosis, and worse post liver transplant outcomes occur in sarcopenic cirrhotic patients.
Methods to quantify body composition have been used extensively but CT and MR image analyses are currently the most accurate methods to measure skeletal muscle area.
Of the putative mediators of the liver-muscle axis, hyperammonemia is the best-studied pathogenic agent but other causal factors include endotoxemia, cytokines, and altered circulating hormone.
Impaired skeletal muscle protein synthesis and autophagic degradation rather than proteasomal proteolysis contribute to muscle loss in liver disease.
Nutritional supplementation alone is not effective but high doses of leucine and potentially other branched chain amino acids and long-term ammonia lowering measures may be beneficial.
Acknowledgments
Funded in part by: NIH RO1 DK 83414; R21 AA 022742, UO1 DK 061732, UO1 AA 021893
Footnotes
Conflicts of interest: The author has no conflict of interest to declare.
Annotated Bibliography
- 1.Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–117. doi: 10.1016/j.nut.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Antar R, Wong P, Ghali P. A meta-analysis of nutritional supplementation for management of hospitalized alcoholic hepatitis. Can J Gastroenterol. 2012;26:463–467. doi: 10.1155/2012/945707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergerson JT, Lee JG, Furlan A, Sourianarayanane A, Fetzer DT, Tevar AD, Landsittel DP, DiMartini AF, Dunn MA. Liver transplantation arrests and reverses muscle wasting. Clin Transplant. 2015;29:216–221. doi: 10.1111/ctr.12506. [DOI] [PubMed] [Google Scholar]
- 4.Dam G, Ott P, Aagaard NK, Vilstrup H. Branched-chain amino acids and muscle ammonia detoxification in cirrhosis. Metab Brain Dis. 2013;28:217–220. doi: 10.1007/s11011-013-9377-3. [DOI] [PubMed] [Google Scholar]
- 5.Dasarathy J, Alkhouri N, Dasarathy S. Changes in body composition after transjugular intrahepatic portosystemic stent in cirrhosis: a critical review of literature. Liver Int. 2011;31:1250–1258. doi: 10.1111/j.1478-3231.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- 6.Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3:225–237. doi: 10.1007/s13539-012-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasarathy S. Posttransplant sarcopenia: an underrecognized early consequence of liver transplantation. Dig Dis Sci. 2013;58:3103–3111. doi: 10.1007/s10620-013-2791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasarathy S. Treatment to improve nutrition and functional capacity evaluation in liver transplant candidates. Curr Treat Options Gastroenterol. 2014;12:242–255. doi: 10.1007/s11938-014-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dasarathy S, McCullough AJ, Muc S, Schneyer A, Bennett CD, Dodig M, Kalhan SC. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54:915–921. doi: 10.1016/j.jhep.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi J, Shiraishi K, Haida M, Matsuzaki S. Abnormality of energy metabolism in the skeletal muscle of patients with liver cirrhosis and changes under administration of glucose and branched-chain amino acids. Tokai J Exp Clin Med. 2004;29:191–198. [PubMed] [Google Scholar]
- 11.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gam CM, Nielsen HB, Secher NH, Larsen FS, Ott P, Quistorff B. In cirrhotic patients reduced muscle strength is unrelated to muscle capacity for ATP turnover suggesting a central limitation. Clin Physiol Funct Imaging. 2011;31:169–174. doi: 10.1111/j.1475-097X.2010.00998.x. [DOI] [PubMed] [Google Scholar]
- *13.Giusto M, Lattanzi B, Albanese C, Galtieri A, Farcomeni A, Giannelli V, Lucidi C, Di Martino M, Catalano C, Merli M. Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol. 2015;27:328–334. doi: 10.1097/MEG.0000000000000274. An elegant study that evaluates different methods to quantify skeletal muscle mass. Direct quantification of skeletal muscle mass is best evaluated by computed tomography. [DOI] [PubMed] [Google Scholar]
- 14.Glass C, Hipskind P, Tsien C, Malin SK, Kasumov T, Shah SN, Kirwan JP, Dasarathy S. Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: a prospective controlled study. J Appl Physiol (1985) 2013;114:559–565. doi: 10.1152/japplphysiol.01042.2012. Even though cirrhosis is known to be a state of accelerated starvation, this study in hospitalized cirrhotic patients showed that reduced respiratory quotient was associated with sarcopenia. Mechanistically, increased fatty acid oxidation is associated with muscle loss and respiratory quotient can potentially be used as a response measure to nutrients or other therapies that have the potential to reverse sarcopenia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193–199. doi: 10.1016/j.nut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Holecek M, Kandar R, Sispera L, Kovarik M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids. 2011;40:575–584. doi: 10.1007/s00726-010-0679-z. [DOI] [PubMed] [Google Scholar]
- 17.Huisman EJ, Trip EJ, Siersema PD, van Hoek B, van Erpecum KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23:982–989. doi: 10.1097/MEG.0b013e32834aa4bb. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen EB, Hamberg O, Quistorff B, Ott P. Reduced mitochondrial adenosine triphosphate synthesis in skeletal muscle in patients with Child-Pugh class B and C cirrhosis. Hepatology. 2001;34:7–12. doi: 10.1053/jhep.2001.25451. [DOI] [PubMed] [Google Scholar]
- 19.Jones JC, Coombes JS, Macdonald GA. Exercise capacity and muscle strength in patients with cirrhosis. Liver Transpl. 2012;18:146–151. doi: 10.1002/lt.22472. [DOI] [PubMed] [Google Scholar]
- 20.Koretz RL, Avenell A, Lipman TO. Nutritional support for liver disease. Cochrane Database Syst Rev. 2012;5:CD008344. doi: 10.1002/14651858.CD008344.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai S, Malani PN. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19:1396–1402. doi: 10.1002/lt.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai JC, Dodge JL, Sen S, Covinsky K, Feng S. Functional Decline in Patients with Cirrhosis Awaiting Liver Transplantation: Results from the Functional Assessment in Liver Transplantation (FrAILT) Study. Hepatology. 2015 doi: 10.1002/hep.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Lipton JO, Yuan ED, Boyle LM, Ebrahimi-Fakhari D, Kwiatkowski E, Nathan A, Guttler T, Davis F, Asara JM, Sahin M. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell. 2015;161:1138–1151. doi: 10.1016/j.cell.2015.04.002. This is a very interesting study that shows that circadian changes alter muscle responses. Hyperammonemia causes reversal of sleep pattern but its impact on skeletal muscle circadian genes may be a contributor to the impaired protein synthesis via altering BMAL1 expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maharshi S, Sharma BC, Srivastava S. Malnutrition in cirrhosis increases morbidity and mortality. J Gastroenterol Hepatol. 2015;30:1507–1513. doi: 10.1111/jgh.12999. [DOI] [PubMed] [Google Scholar]
- **25.McDaniel J, Davuluri G, Hill EA, Moyer M, Runkana A, Prayson R, van Lunteren E, Dasarathy S. Hyperammonemia Results in Reduced Muscle Function Independent of Muscle Mass. Am J Physiol Gastrointest Liver Physiol. 2015 doi: 10.1152/ajpgi.00322.2015. ajpgi 00322 02015. This prospective study used a comprehensive array of models including patients with cirrhosis, the hyperammonemic portacaval anastomosis rat and ex vivo muscle preparations to show that ammonia mediates the liver muscle axis by impaired contractile function and rapid fatigue in the skeletal muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merli M, Giusto M, Gentili F, Novelli G, Ferretti G, Riggio O, Corradini SG, Siciliano M, Farcomeni A, Attili AF, et al. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010;30:208–214. doi: 10.1111/j.1478-3231.2009.02135.x. [DOI] [PubMed] [Google Scholar]
- 27.Merli M, Giusto M, Lucidi C, Giannelli V, Pentassuglio I, Di Gregorio V, Lattanzi B, Riggio O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 28.Merli M, Giusto M, Molfino A, Bonetto A, Rossi M, Ginanni Corradini S, Baccino FM, Rossi Fanelli F, Costelli P, Muscaritoli M. MuRF-1 and p-GSK3beta expression in muscle atrophy of cirrhosis. Liver Int. 2013;33:714–721. doi: 10.1111/liv.12128. [DOI] [PubMed] [Google Scholar]
- 29.Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979–985. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Merli M, Nicolini G, Angeloni S, Riggio O. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition. 2002;18:978–986. doi: 10.1016/s0899-9007(02)00984-x. [DOI] [PubMed] [Google Scholar]
- 31.Merli M, Riggio O, Dally L. Does malnutrition affect survival in cirrhosis? PINC (Policentrica Italiana Nutrizione Cirrosi) Hepatology. 1996;23:1041–1046. doi: 10.1002/hep.510230516. [DOI] [PubMed] [Google Scholar]
- 32.Merli M, Romiti A, Riggio O, Capocaccia L. Optimal nutritional indexes in chronic liver disease. JPEN J Parenter Enteral Nutr. 1987;11:130S–134S. doi: 10.1177/014860718701100521. [DOI] [PubMed] [Google Scholar]
- 33.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173. 173 e161. doi: 10.1016/j.cgh.2011.08.028. One of the most highly cited and early work that demonstrates increased mortality in cirrhotic patients with sarcopenia. [DOI] [PubMed] [Google Scholar]
- 34.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, Narayanan A, Eghtesad B, Mozdziak PE, McDonald C, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-kappaB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110:18162–18167. doi: 10.1073/pnas.1317049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, Eghtesad B, Singh K, Fu X, Dubyak G, et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303:E983–993. doi: 10.1152/ajpendo.00183.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roman E, Torrades MT, Nadal MJ, Cardenas G, Nieto JC, Vidal S, Bascunana H, Juarez C, Guarner C, Cordoba J, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci. 2014;59:1966–1975. doi: 10.1007/s10620-014-3086-6. [DOI] [PubMed] [Google Scholar]
- 38.Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, Baracos V, Montano-Loza AJ, Myers RP. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 39.Thapaliya S, Runkana A, McMullen MR, Nagy LE, McDonald C, Naga Prasad SV, Dasarathy S. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy. 2014;10:677–690. doi: 10.4161/auto.27918. This study showed in a comprehensive array of models that skeletal muscle autophagy was increased in alcoholic liver disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **40.Tsien C, Davuluri G, Singh D, Allawy A, Ten Have GA, Thapaliya S, Schulze JM, Barnes D, McCullough AJ, Engelen MP, et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61:2018–2029. doi: 10.1002/hep.27717. A single dose of branched chain amino acid with leucine content that is double that of isoleucine and valine reversed the molecular perturbations in the skeletal muscle. This is the first study of its kind to show the beneficial effects in direct muscle biopsies. Tracer incorporation into skeletal muscle protein showed that in response to the oral Administration of a single dose of amino acid mixture resulted in similar rates of protein synthesis in the skeletal muscle from patients with cirrhosis and controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsien C, Garber A, Narayanan A, Shah SN, Barnes D, Eghtesad B, Fung J, McCullough AJ, Dasarathy S. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol. 2014;29:1250–1257. doi: 10.1111/jgh.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsien C, Shah SN, McCullough AJ, Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol. 2013;25:85–93. doi: 10.1097/MEG.0b013e328359a759. [DOI] [PubMed] [Google Scholar]
- 43.Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27:430–441. doi: 10.1111/j.1440-1746.2011.06951.x. [DOI] [PubMed] [Google Scholar]
- 44.Underwood PW, Cron DC, Terjimanian MN, Wang SC, Englesbe MJ, Waits SA. Sarcopenia and failure to rescue following liver transplantation. Clin Transplant. 2015 doi: 10.1111/ctr.12629. [DOI] [PubMed] [Google Scholar]
- 45.Wu LJ, Wu MS, Lien GS, Chen FC, Tsai JC. Fatigue and physical activity levels in patients with liver cirrhosis. J Clin Nurs. 2012;21:129–138. doi: 10.1111/j.1365-2702.2011.03900.x. [DOI] [PubMed] [Google Scholar]
- **46.You JS, Lincoln HC, Kim CR, Frey JW, Goodman CA, Zhong XP, Hornberger TA. The role of diacylglycerol kinase zeta and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem. 2014;289:1551–1563. doi: 10.1074/jbc.M113.531392. This is an outstanding work that identified the mechanism of signal transduction by mechanical stimuli. A novel, phosphatidic acid mediated activation of mTORC1 results in increased protein synthesis. Analogs of phosphatidic acid are being developed that make this approach of high translational significance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *47.Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, Ma M, Abraldes JG, Paterson I, Haykowsky MJ, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1920–1926. e1922. doi: 10.1016/j.cgh.2014.04.016. This prospective study showed that exercise is beneficial in a selective group of cirrhotics showed improvement in both muscle mass and function. [DOI] [PubMed] [Google Scholar]