Abstract
Purpose of review
Optical coherence tomography (OCT) has become the cornerstone technology for clinical ocular imaging in the past few years. The technology is still rapidly evolving with newly developed applications. This manuscript reviews recent innovative OCT applications for glaucoma diagnosis and management.
Recent findings
The improvements made in the technology have resulted in increased scanning speed, axial and transverse resolution, and more effective use of the OCT technology as a component of multimodal imaging tools. At the same time, the parallel evolution in novel algorithms makes it possible to efficiently analyze the increased volume of acquired data.
Summary
The innovative iterations of OCT technology have the potential to further improve the performance of the technology in evaluating ocular structural and functional characteristics and longitudinal changes in glaucoma.
Keywords: adaptive optics OCT, glaucoma, OCT angiography, polarization-sensitive OCT, swept-source OCT
INTRODUCTION
The progress made in ophthalmic imaging technologies in the past two decades has revolutionized clinical approaches and improved the efficiency of eye care. The ability to visualize ocular structures in vivo and in real time at a micron scale level and automatically quantify these structures provide clinicians important information in the process of clinical decisions. Among the imaging technologies, optical coherence tomography (OCT) has a leading role in eye care. This technology has been shown to provide reproducible quantification of ocular structures [1–3], allowing precise detection of structural damage, monitoring of disease progression, and assessment of the effectiveness of treatment in glaucoma and retinal pathologies.
Since the first publication of OCT in the early nineties [4], the technology has evolved substantially with enhancements both in imaging method and image analysis. The developmental path started with time-domain (TD)-OCT, followed by spectral-domain (SD)-OCT and other OCT techniques with higher scan aquisition speed [5,6] and higher axial and transverse resolution [7,8].
Optical coherence tomography provides high-resolution cross-sectional images of areas of interest using the principle of low-coherence interferometry. The current commercially available generation, SD-OCT, projects a near infrared broad-bandwidth light to the eye and the frequency information of the back reflected light is used to generate an image [9]. The image acquisition rate of the device ranges between 25 000 and 75 000 axial scans/second, enabling the aquisition of three-dimensional (3D) data from the area of interest [10,11]. OCT technology is still evolving, and at the time of this writing, OCT has been shown to be capable of scanning speeds of up to 20.8 million axial scans/second in nonophthalmic applications [12].
The purpose of this manuscript is to discuss new developments in OCT technology and its clinical application.
SWEPT-SOURCE OPTICAL COHERENCE TOMOGRAPHY
Swept-source (SS)–OCT is a form of Fourier domain OCT that employs a fast sweeping scan pattern through a broad bandwidth. Instead of a broad-bandwidth light source that is projected at once as in SD-OCT, the SS-OCT uses a single tunable laser that sweeps through different frequencies to rapidly cover the entire broad spectrum. The reflectance of the light from the eye is captured by a photodetector, which is much faster than the charge-coupled device camera used in SD-OCT technology [6,13,14]. This allows a faster scanning speed of up to 400 000 axial scans/second, and eliminates the typical depth dependent signal drop-off observed with the SD-OCT technology [15]. Increased scanning speed results in shorter scanning time and the reduction of image distortions caused by eye movement. This has been shown to improve scan quality and allow better visualization of fine structures [5,16]. In addition, many SS-OCT systems use a light source centered at an approximately 1050 nm wavelength, allowing better tissue penetration than SD-OCT, which typically uses a light source centered at approximately 840 nm. This allows visualization of structures such as the choroid [17,18,19■] and lamina cribrosa (Fig. 1) [20■,21■■], along with structures at the anterior chamber angle [22■,23■].
FIGURE 1.
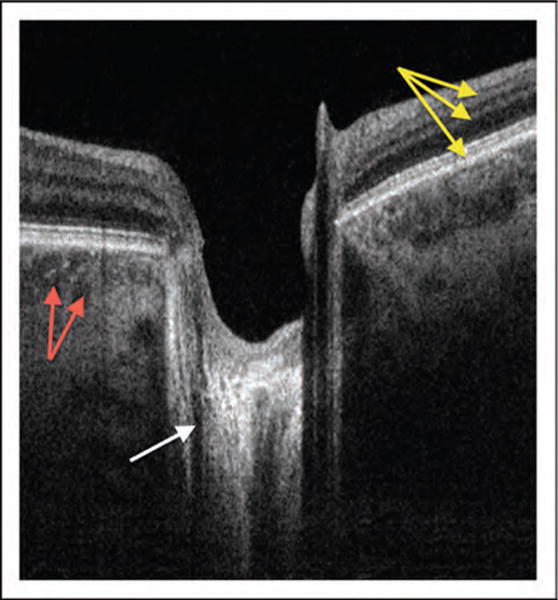
Swept-source OCT cross-section. The same scan captures retinal layers (yellow arrows), nerve fiber fascicles passing through lamina cribrosa (white arrow), and choroidal vessels (red arrows) because this technology is less prone to signal drop-off in comparison with other OCT iterations. OCT, optical coherence tomography.
Studies that used SS-OCT to examine the lamina cribrosa and the optic nerve head reported that full-thickness and focal lamina cribrosa defects corresponded with neuroretinal rim thinning, concurrent or previous disc hemorrhages, thinning of the circumpapillary retinal nerve fiber layer (RNFL) thickness, and visual field defects. The lamina cribrosa defects were significantly associated with disc hemorrhages and longer axial lengths. SS-OCT can provide further detailed information on the microstructure of the lamina cribrosa. In a study that used an automated segmentation software for delineating the beams and pores of the lamina cribrosa, the authors evaluated the differences in the lamina cribrosa microstructure between healthy and glaucomatous eyes [24■]. The findings demonstrated an increase in beam thickness and a decrease in pore thickness with severe disease [25■]. The authors concluded that the changes reflected the remodeling of the eye as part of the glaucomatous process.
The deep signal penetration of SS-OCT has been used to measure the scleral thickness in highly myopic eyes with glaucoma [26■]. The authors identified a negative correlation between the scleral thickness and axial length in normal-tension glaucoma (NTG) eyes, and no significant correlation in primary open-angle glaucoma (POAG) eyes. This finding suggests that scleral thickness has a role in NTG, which might explain the difference between NTG and POAG pathogenesis.
The advantages of SS-OCT in anterior segment imaging have been demonstrated in a study that examined the anterior chamber angle topography [27]. The authors demonstrated reproducible identification and quantification of the scleral spur and Schwalbe’s line. They reported trabecular meshwork width differences by quadrants, with the inferior trabecular meshwork being the widest compared to other quadrants. Moreover, another study that evaluated the angle in patients with angle closure glaucoma reported that SS-OCT outperforms gonioscopy in measuring the area and the degree of peripheral anterior synechia involvement [28■]. Anterior-segment SS-OCT is shown to detect a much higher prevalence of iridotrabecular contact in eyes with a shallow peripheral anterior chamber (AC) compared with ultrasound biomicroscopy [29■]. These findings indicate that SS-OCT scanning might be useful for angle closure glaucoma diagnosis and to determine the best surgical approach before laser or surgical procedures at the angle.
The only commercially available anterior-segment SS-OCT at the time of this writing (CASIA SS-1000, Tomey, Nagoya, Japan) provides built-in software that measures the iris–trabecular contact (ITC) index. A study of 108 healthy individuals and 32 patients with angle closure reported good diagnostic performance of the ITC index and moderate agreement on the measured extent of angle closure when compared with gonioscopy [30].
ADAPTIVE OPTICS OPTICAL COHERENCE TOMOGRAPHY
The axial resolution of OCT images depends on the coherence properties of the light source. Currently used light sources provide axial resolution at the range of 5 μm, which is sufficient to resolve the axial dimensions of most retinal cells. Meanwhile, the transverse resolution of an OCT image is limited by the spot size of the light beam when focused on the tissue. The laser beam is prone to optical aberrations when passing through different media in the eye, which limits the transverse resolution to the range of 20 μm. Adaptive optics was introduced to correct the optical aberrations, reduce the projected spot size, and improve the transverse resolution. Adaptive optics systems measure the monochromatic aberrations occurring in the eye and corrects them using a wave-front sensor and deformable mirrors. The improved resolution provided by the adaptive optics system allows the acquisition of high-quality images, enabling visualization of fine details such as the retinal microvasculature, photoreceptors, lamina cribrosa (Fig. 2), and microstructures within the RNFL and ganglion cell layer.
FIGURE 2.
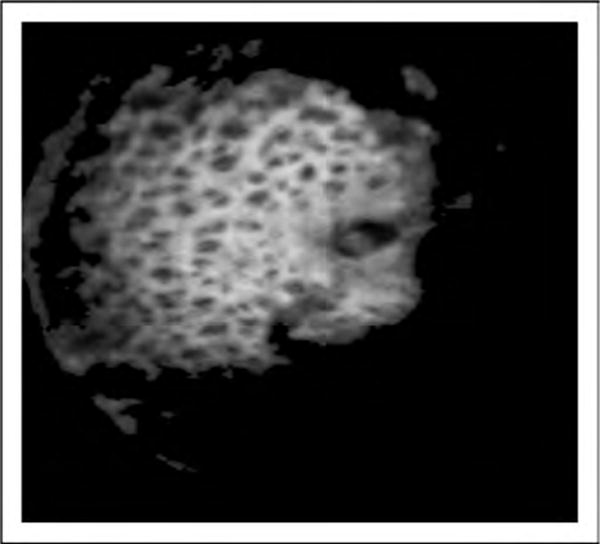
Adaptive optics OCT en-face image of lamina cribrosa that clearly demonstrates the mesh-like structure with pores (dark) and beams (white). OCT, optical coherence tomography.
In a study that examined the photoreceptors in glaucomatous eyes with an adaptive optics OCT (AO-OCT) system, the authors identified evidence of outer retinal changes along with the expected thinning of the inner retina in locations corresponding to the visual field (VF) loss [31]. This was the first in-vivo imaging study to demonstrate the involvement of the outer retina in glaucoma, exhibiting the importance of the highly detailed information provided by this system.
A recent study demonstrated the ability of AO-OCT to detect fine structures inside the lamina cribrosa [32]. An AO-SDOCT system with a 1050 nm wavelength light source was used for healthy, glaucoma suspects, and glaucomatous eyes. Utilizing an automated segmentation software [24■], the authors reported good repeatability of lamina cribrosa microstructure measurements. AO-OCT also allowed discerning the RNFL axonal bundles with higher detail than possible with other OCT systems [33].
POLARIZATION-SENSITIVE OPTICAL COHERENCE TOMOGRAPHY
Another modification of the OCT technology is the polarization-sensitive OCT (PS-OCT) that is based on the state of polarized light, which changes through various light–tissue interactions. This allows the differentiation of different tissues and generates images with tissue-specific contrast. The technology can distinguish ocular structures based on properties that alter polarization state, such as birefringence (sclera, RNFL), polarization-preservation (photoreceptors), and depolarization (RPE). Initially introduced in TD-OCT technology, polarization sensitivity can be incorporated into all known OCT systems, such as SD-OCT [34], SS-OCT [35], and AO-OCT [36].
Animal models of experimental glaucoma have shown that changes in the RNFL polarization precede thickness changes [37]. A new normalized RNFL reflectance index was tested in a recent study that used two custom-built PS-OCT systems and a commercial OCT system. The proposed index outperformed RNFL thickness in the ability to distinguish glaucoma suspects from healthy eyes with all utilized OCT devices [38■■]. This study and others suggest that PS-OCT could be a very attractive candidate for early detection of glaucoma, but at the time of this writing further research is warranted.
A study of eyes that had undergone a trabeculectomy or an Ex-Press tube shunt placement examined bleb morphology using anterior-segment PS-OCT. The registered internal phase retardation was correlated with the functionality of the blebs, which is the most important factor in trabeculectomy success. The study showed that PS-OCT is able to noninvasively estimate bleb fibrosis by measuring birefringence in the filtrating blebs. This information may improve the monitoring of glaucoma-related surgical outcomes [39■].
Another recent study demonstrated the ability of PS-OCT to assess the birefringence properties of peripapillary sclera in the rat eye [40]. This in-vivo assessment enables the detection of collagen orientation in the posterior sclera, which might be useful in the assessment of the biomechanical aspects of glaucoma pathogenesis.
A PS-OCT system with retinal tracking, which operates at up to 60 Hz, has been demonstrated to reduce motion artifacts in healthy and diseased eyes, improving the reliability of acquired images [41].
OPTICAL COHERENCE TOMOGRAPHY BLOOD FLOW
In addition to tissue structure examination, the OCT technique is capable of estimating functional characteristics of scanned tissue such as tissue blood flow using Doppler OCT (Fig. 3) and OCT angiography. The optical frequency of light shifts when it scatters from moving red blood cells. The amount of the shift is related to flow velocity, and this information provides a noncontrast method to visualize and quantify retinal blood flow. The in-vivo estimation of the retinal blood flow can provide insight on the pathogenesis of ocular diseases, such as diabetic retinopathy, macular degeneration, and glaucoma.
FIGURE 3.
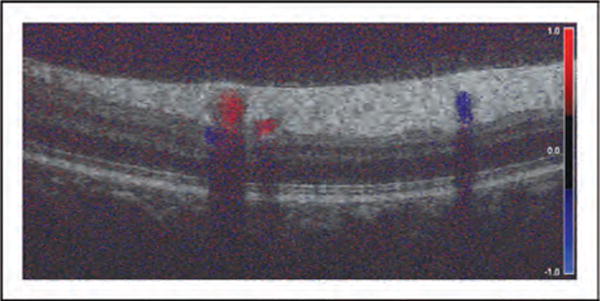
Doppler OCT cross-section of the retina. The velocity and direction of retinal blood flow is presented as color-coded filling of the vessels. Vertically elongated shadows are the result of the light beam blockage. OCT, optical coherence tomography.
A study of retinal blood flow with Doppler OCT in normal and glaucoma patients demonstrated that in glaucomatous eyes with single-hemifield damage, the retinal blood flow was significantly reduced in the hemisphere corresponding with the visual field abnormality [42■]. In addition, reduced retinal blood flow was associated with thinner RNFL and ganglion cell complex in the corresponding abnormal hemisphere.
While Doppler OCT provides data on total retinal blood flow, it is not sensitive enough to examine the microcirculation with low-velocity blood flow. A study reported the use of OCT angiography to quantify human optic disc perfusion in glaucoma [43■]. The authors utilized a split-spectrum amplitude-decorrelation angiography algorithm to differentiate between static and dynamic tissues. They reported that normal discs had a denser microvascular network compared with the glaucomatous disc. They also introduced a new parameter, the optic disc flow index, which was significantly lower in glaucomatous eyes (wide range of disease severity). Another study that used the same image-processing analysis showed the superiority of this method in comparison with fluorescein angiography in imaging all layers of retinal vasculature without the need of injectable dye [44■]. These findings strongly demonstrate the potential of OCT in assessing retinal function by imaging in-vivo blood flow.
EMERGING INNOVATIONS
Optical coherence tomography technology is still rapidly evolving with innovative applications presented frequently. At the time of this writing, two interesting innovations have been recently introduced whose ophthalmic utility is currently under investigation. Photoacoustic ophthalmoscopy technology can detect the ultrasonic waves induced by the tissue when laser light is projected into it. These waves are the results of tissue-specific optical absorption properties. Combining this technology with OCT has been shown in animal models to generate strong signals from the hemoglobin in the blood vessels and the melanin in the RPE while all other retinal layers had very low optical absorption [45]. These properties can enable in-vivo assessment of retinal blood volume, oxygen saturation, and pigment distribution in the RPE.
Using an innovative light source, a vertical-cavity surface emitting laser (VCSEL) technology that sweeps through the bandwidth, the OCT’s properties have been shown to accomplish new imaging levels. This device can reach an ultrahigh imaging rate of up to 580 000 axial scans/second, an axial resolution of 9–12 μm in the tissue, and an imaging depth of up to 38 mm [46]. These exceptional properties allow, for the first time, imaging of the entire eyeball in a single scan. The clinical utility of this device is yet to be determined.
CONCLUSION
In this manuscript, we reviewed recent publications on the main directions of research dedicated to the development and improvement of OCT technology. The ongoing and future trends in the research are promising, and advances in OCT technology are likely to improve detection and monitoring of ocular pathologies.
KEY POINTS.
Advancement in the OCT technology offers new venues for disease detection and a better understanding of the disease pathophysiology.
SS-OCT employs a fast sweeping scan pattern through a broad bandwidth, allowing faster scanning speed.
Adaptive optics coupled with OCT corrects for optical aberrations, thus enabling the acquisition of in-vivo images of the retinal microvasculature, photoreceptors, ganglion cells, and the lamina cribrosa at near the cellular level.
Polarization-sensitive OCT provides cross-sectional tissue-specific contrast information using the polarization status of the scanned tissue.
OCT can provide retinal function information by quantifying ocular blood flow.
Acknowledgments
Supported in part by National Institutes of Health contracts R01-EY013178, P30-EY008098 (Bethesda, MD); Eye and Ear Foundation (Pittsburgh, PA); Research to Prevent Blindness (New York, NY).
Footnotes
Financial support and sponsorship
None.
Conflicts of interest
Dr Schuman receives royalties for an optical coherence tomography patent owned and licensed by the Massachusetts Institute of Technology and Massachusetts Eye & Ear Infirmary to Zeiss (Dublin, CA).
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Menke MN, Knecht P, Sturm V, et al. Reproducibility of nerve fiber layer thickness measurements using 3D Fourier-domain OCT. Invest Ophthalmol Vis Sci. 2008;49:5386–5391. doi: 10.1167/iovs.07-1435. [DOI] [PubMed] [Google Scholar]
- 2.González-García AO, Vizzeri G, Bowd C, et al. Reproducibility of RTVue retinal nerve fiber layer thickness and optic disc measurements and agreement with Stratus optical coherence tomography measurements. Am J Ophthalmol. 2009;147:1067–1074. doi: 10.1016/j.ajo.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francoz M, Fenolland J-R, Giraud J-M, et al. Reproducibility of macular ganglion cell-inner plexiform layer thickness measurement with cirrus HD-OCT in normal, hypertensive and glaucomatous eyes. Br J Ophthalmol. 2014;98:322–328. doi: 10.1136/bjophthalmol-2012-302242. [DOI] [PubMed] [Google Scholar]
- 4.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potsaid B, Gorczynska I, Srinivasan VJ, et al. Ultrahigh speed spectral/Fourier domain OCT ophthalmic imaging at 70,000 to 312,500 axial scans per second. Opt Express. 2008;16:15149–15169. doi: 10.1364/oe.16.015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potsaid B, Baumann B, Huang D, et al. Ultrahigh speed 1050 nm swept source/Fourier domain OCT retinal and anterior segment imaging at 100,000 to 400,000 axial scans per second. Opt Express. 2010;18:20029–20048. doi: 10.1364/OE.18.020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godara P, Dubis AM, Roorda A, et al. Adaptive optics retinal imaging: emerging clinical applications. Optom Vis Sci. 2010;87:930–941. doi: 10.1097/OPX.0b013e3181ff9a8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong IY, Koizumi H, Lai WW. Enhanced depth imaging optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2011;42(Suppl):S75–84. doi: 10.3928/15428877-20110627-07. [DOI] [PubMed] [Google Scholar]
- 9.Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res. 2008;27:45–88. doi: 10.1016/j.preteyeres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Kim JS, Ishikawa H, Sung KR, et al. Retinal nerve fibre layer thickness measurement reproducibility improved with spectral domain optical coherence tomography. Br J Ophthalmol. 2009;93:1057–1063. doi: 10.1136/bjo.2009.157875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahlers C, Schmidt-Erfurth U. Three-dimensional high resolution OCT imaging of macular pathology. Opt Express. 2009;17:4037–4045. doi: 10.1364/oe.17.004037. [DOI] [PubMed] [Google Scholar]
- 12.Wieser W, Biedermann BR, Klein T, et al. Multimegahertz OCT: high quality 3D imaging at 20 million A-scans and 4.5 GVoxels per second. Opt Express. 2010;18:14685–14704. doi: 10.1364/OE.18.014685. [DOI] [PubMed] [Google Scholar]
- 13.Huber R, Adler DC, Fujimoto JG. Buffered Fourier domain mode locking: unidirectional swept laser sources for optical coherence tomography imaging at 370,000 lines/s. Opt Lett. 2006;31:2975–2977. doi: 10.1364/ol.31.002975. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Brezinski ME. Theoretical and practical considerations on detection performance of time domain, Fourier domain, and swept source optical coherence tomography. J Biomed Opt. 2007;12:044007. doi: 10.1117/1.2753410. [DOI] [PubMed] [Google Scholar]
- 15.Schuman JS. Spectral domain optical coherence tomography for glaucoma (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:426–458. [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan VJ, Adler DC, Chen Y, et al. Ultrahigh-speed optical coherence tomography for three-dimensional and en face imaging of the retina and optic nerve head. Invest Ophthalmol Vis Sci. 2008;49:5103–5110. doi: 10.1167/iovs.08-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol. 2013;58:387–429. doi: 10.1016/j.survophthal.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Adhi M, Liu JJ, Qavi AH, et al. Enhanced visualization of the choroido-scleral interface using swept-source OCT. Ophthalmic Surg Lasers Imaging Retina. 2013;44(Suppl):S40–42. doi: 10.3928/23258160-20131101-08. [DOI] [PubMed] [Google Scholar]
- 19■.Adhi M, Liu JJ, Qavi AH, et al. Choroidal analysis in healthy eyes using swept-source optical coherence tomography compared to spectral domain optical coherence tomography. Am J Ophthalmol. 2014;157:1272–1281. doi: 10.1016/j.ajo.2014.02.034. The performance of prototype SS-OCT device was compared with a commercially available SD-OCT for a detailed analysis of the choroid in healthy eyes. [DOI] [PubMed] [Google Scholar]
- 20■.Wang B, Nevins JE, Nadler Z, et al. Reproducibility of in-vivo OCT measured three-dimensional human lamina cribrosa microarchitecture. PLoS One. 2014;9:e95526. doi: 10.1371/journal.pone.0095526. Authors proposed an automated segmentation for assessing 3D LC microarchitecture using SS-OCT device. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21■■.Takayama K, Hangai M, Kimura Y, et al. Three-dimensional imaging of lamina cribrosa defects in glaucoma using swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:4798–4807. doi: 10.1167/iovs.13-11677. This cross-sectional study assessed full-thickness lamina cribrosa defects in glaucomatous eyes using a prototype SS-OCT system. Optic disc hemorrhages and longer axial length were significantly associated with lamina cribrosa defects. [DOI] [PubMed] [Google Scholar]
- 22■.Römkens HCS, Beckers HJM, Frusch M, et al. Reproducibility of anterior chamber angle analyses with the swept-source optical coherence tomography in young, healthy Caucasians. Invest Ophthalmol Vis Sci. 2014;55:3999–4004. doi: 10.1167/iovs.13-12904. This study demonstrated the importance of observers’ training for analyzing SS-OCT images of anterior chamber angle. [DOI] [PubMed] [Google Scholar]
- 23■.Mak H, Xu G, Leung CK-S. Imaging the iris with swept-source optical coherence tomography: relationship between iris volume and primary angle closure. Ophthalmology. 2013;120:2517–2524. doi: 10.1016/j.ophtha.2013.05.009. Iris volume changes associated with pupil size were studied with commercially available swept-source OCT device in healthy and glaucomatous eyes. [DOI] [PubMed] [Google Scholar]
- 24■.Nadler Z, Wang B, Wollstein G, et al. Automated lamina cribrosa microstructural segmentation in optical coherence tomography scans of healthy and glaucomatous eyes. Biomed Opt Express. 2013;4:2596–2608. doi: 10.1364/BOE.4.002596. The performance of automated segmentation was compared with manual segmentation to determine lamina cribrosa microstructural differences between glaucomatous and healthy eyes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25■.Wang B, Nevins JE, Nadler Z, et al. In vivo lamina cribrosa micro-architecture in healthy and glaucomatous eyes as assessed by optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:8270–8274. doi: 10.1167/iovs.13-13109. LC micro-architectural changes were observed in glaucoma eyes compared with healthy eyes with SS-OCT imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26■.Lopilly Park H-Y, Lee NY, Choi JA, Park CK. Measurement of scleral thickness using swept-source optical coherence tomography in patients with open-angle glaucoma and myopia. Am J Ophthalmol. 2014;157:876–884. doi: 10.1016/j.ajo.2014.01.007. The difference in subfoveal scleral thickness was demonstrated between NTG and POAG eyes using SS-OCT. [DOI] [PubMed] [Google Scholar]
- 27.Tun TA, Baskaran M, Zheng C, et al. Assessment of trabecular meshwork width using swept source optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2013;251:1587–1592. doi: 10.1007/s00417-013-2285-8. [DOI] [PubMed] [Google Scholar]
- 28■.Lai I, Mak H, Lai G, et al. Anterior chamber angle imaging with swept-source optical coherence tomography: measuring peripheral anterior synechia in glaucoma. Ophthalmology. 2013;120:1144–1149. doi: 10.1016/j.ophtha.2012.12.006. The authors showed the usefulness of SS-OCT in estimation of the degree of peripheral anterior synechia in eyes with chronic angle-closure glaucoma. [DOI] [PubMed] [Google Scholar]
- 29■.Mishima K, Tomidokoro A, Suramethakul P, et al. Iridotrabecular contact observed using anterior segment three-dimensional OCT in eyes with a shallow peripheral anterior chamber. Invest Ophthalmol Vis Sci. 2013;54:4628–4635. doi: 10.1167/iovs.12-11230. SS-OCT outperformed ultrasound biomicroscopy in estimating iridotrabecular contact in eyes with shallow anterior chamber. [DOI] [PubMed] [Google Scholar]
- 30.Baskaran M, Ho S-W, Tun TA, et al. Assessment of circumferential angle-closure by the iris–trabecular contact index with swept-source optical coherence tomography. Ophthalmology. 2013;120:2226–2231. doi: 10.1016/j.ophtha.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Choi SS, Zawadzki RJ, Lim MC, et al. Evidence of outer retinal changes in glaucoma patients as revealed by ultrahigh-resolution in vivo retinal imaging. Br J Ophthalmol. 2011;95:131–141. doi: 10.1136/bjo.2010.183756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadler Z, Wang B, Wollstein G, et al. Repeatability of in vivo 3D lamina cribrosa microarchitecture using adaptive optics spectral domain optical coherence tomography. Biomed Opt Express. 2014;5:1114–1123. doi: 10.1364/BOE.5.001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kocaoglu OP, Cense B, Jonnal RS, et al. Imaging retinal nerve fiber bundles using optical coherence tomography with adaptive optics. Vision Res. 2011;51:1835–1844. doi: 10.1016/j.visres.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotzinger E, Pircher M, Baumann B, et al. Three-dimensional polarization sensitive OCT imaging and interactive display of the human retina. Opt Express. 2009;17:4151–4165. doi: 10.1364/oe.17.004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamanari M, Makita S, Lim Y, Yasuno Y. Full-range polarization-sensitive swept-source optical coherence tomography by simultaneous transversal and spectral modulation. Opt Express. 2010;18:13964–13980. doi: 10.1364/OE.18.013964. [DOI] [PubMed] [Google Scholar]
- 36.Cense B, Gao W, Brown JM, et al. Retinal imaging with polarization-sensitive optical coherence tomography and adaptive optics. Opt Express. 2009;17:21634–21651. doi: 10.1364/OE.17.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dwelle J, Liu S, Wang B, et al. Thickness, phase retardation, birefringence, and reflectance of the retinal nerve fiber layer in normal and glaucomatous nonhuman primates. Invest Ophthalmol Vis Sci. 2012;53:4380–4395. doi: 10.1167/iovs.11-9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38■■.Liu S, Wang BB, Yin BM, et al. Retinal nerve fiber layer reflectance for early glaucoma diagnosis. J Glaucoma. 2014;23:45–52. doi: 10.1097/IJG.0b013e31829ea2a7. The study demonstrates that changes in RNFL reflectance can be detected earlier than RNFL thinning in early stages of glaucoma. Authors proposed a new index, which has the potential for preperimetric glaucoma detection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39■.Fukuda S, Beheregaray S, Kasaragod D, et al. Noninvasive evaluation of phase retardation in blebs after glaucoma surgery using anterior segment polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:5200–5206. doi: 10.1167/iovs.14-14474. PS-OCT has demonstrated the ability to noninvasively estimate postsurgical filtration bleb functionality. [DOI] [PubMed] [Google Scholar]
- 40.Baumann B, Rauscher S, Glösmann M, et al. Peripapillary rat sclera investigated in vivo with polarization sensitive optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:7686–7696. doi: 10.1167/iovs.14-15037. [DOI] [PubMed] [Google Scholar]
- 41.Sugita M, Zotter S, Pircher M, et al. Motion artifact and speckle noise reduction in polarization sensitive optical coherence tomography by retinal tracking. Biomed Opt Express. 2013;5:106–122. doi: 10.1364/BOE.5.000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42■.Sehi M, Goharian I, Konduru R, et al. Retinal blood flow in glaucomatous eyes with single-hemifield damage. Ophthalmology. 2014;121:750–758. doi: 10.1016/j.ophtha.2013.10.022. Reduced retinal blood flow (RBF) was detected by Doppler SD-OCT in perimetric glaucoma patients in both affected and perimetrically nonaffected hemispheres. Decrease in RBF was associated with RNFL thinning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43■.Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121:1322–1332. doi: 10.1016/j.ophtha.2014.01.021. Authors suggested that noninvasive assessment of optic disc perfusion with OCT angiography can be used in glaucoma diagnosis and estimation of disease progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44■.Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. J Am Med Assoc Ophthalmol. 2014 doi: 10.1001/jamaophthalmol.2014.3616. [Epub ahead of print] The study compared performance of fluorescein angiography and OCT angiography in visualization of the radial peripapillary capillary network. [DOI] [PubMed] [Google Scholar]
- 45.Jiao S, Jiang M, Hu J, et al. Photoacoustic ophthalmoscopy for in vivo retinal imaging. Opt Express. 2010;18:3967–3972. doi: 10.1364/OE.18.003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grulkowski I, Liu JJ, Potsaid B, et al. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers. Biomed Opt Express. 2012;3:2733–2751. doi: 10.1364/BOE.3.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]


