Abstract
Nephropathy due to BK virus infection is an evolving challenge in patients undergoing hematopoietic stem cell transplantation. We hypothesized that BKV infection was a marker of Kidney Function Decline and a poor prognostic factor in HSCT recipients who experience this complication.
In this retrospective study, we analyzed all patients who underwent their first allogeneic hematopoietic stem cell transplantation at our institution between 2004 and 2012. We evaluated the incidence of persistent kidney function decline, which was defined as a confirmed reduction in estimated glomerular filtration rate of at least 25% from baseline using the CKD-EPI equation. Cox proportional hazard regression was used to model the cause-specific hazard of kidney function decline and Fine and Gray’s method was used to account for the competing risks of death.
Among 2477 recipients of a first allogeneic hematopoietic stem cell transplantation, BK viruria was detected in 25% (n=629) and kidney function decline in 944 (38.1%). On multivariate analysis, after adjusting for age, sex, acute graft-versus-host disease, chronic graft versus host disease, preparative conditioning regimen, and graft source, BK viruria remained a significant risk factor for kidney function decline (P <0.001). In addition, patients with BKV infection and kidney function decline experienced worse overall survival.
Post-allogeneic hematopoietic stem cell transplantation, BKV infection was strongly and independently associated with subsequent kidney function decline and worse patient survival after HSCT.
Introduction
In the 1980s, BK virus (BKV), a non-enveloped virion that is a member of the Polyomavirus family, emerged as an important pathogen in hematopoietic stem cell transplant (HSCT) recipients following introduction of increasingly potent immunosuppressive drugs (e.g. cyclosporine and antithymocyte globulin)(1, 2). BKV replication is cytopathic and the release of its viral and cellular constituents elicits a non-specific inflammatory reaction, which induces specific humoral and cellular immune responses (3). In SCT population, the proposed mechanism of hemorrhagic cystitis (HC) secondary to BK infection involves interaction between BKV replication and host immune responses (4). HC is induced by immune reconstitution with the initial urothelial damage secondary to conditioning regimen and radiation, allows a permissive environment for BKV replication (5). However, a cytopathic phenomenon post SCT is proposed where BKV viruria would progress to viremia with further renal dysfunction and biopsy proven BKV nephropathy (6). In the absence of sufficient T-cell immunity, BKV reactivation can lead to prolonged hospital stays and increased mortality secondary to late hemorrhagic cystitis and ureteral stenosis(7). The risk factors for BKV reactivation following HSCT include haploidentical or cord blood transplantation(1), acute graft versus host disease (GVHD)(8), and cytomegalovirus (CMV) co-infection(9).
A systematic review and meta-analysis of published literature found that approximately 16.6% of HSCT patients developed chronic kidney disease (CKD), although its definition varied greatly among different reports(10). However, most studies included a limited number of patients (the second largest had 301 patients) and the largest study covered an older time period, ranging from 1991 to 2002. Some of the factors reported to be associated with KFD were age, acute and chronic GVHD, the use of total body irradiation and nephrotoxic therapies such as calcineurin inhibitors (e.g. tacrolimus) and antimicrobials(10, 11).
We conducted the present study to determine the incidence of clinical BKV infection and its association with subsequent KFD and patient survival in a large and contemporary cohort of patients undergoing HSCT.
Materials and methods
Study design and patient population
This retrospective study was approved by the institutional review board at the University of Texas MD Anderson Cancer Center in accordance with the principles of the declaration of Helsinki, protocol number RCR05-0495. Patients were identified by querying the MD Anderson bone marrow and transplant website (BMTWeb) database for subjects who underwent HSCT between January 2004 and December 2012. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. To be considered for transplant at our institution, patients had to have a baseline creatinine level less than 1.4 mg/dL before the start of conditioning chemotherapy. Patients with graft failure after HSCT and those with a prior history of allogeneic HSCT were excluded from the study. Prior autologous SCT recipients were included in the study population.
BKV testing
At our institution, there is no routine screening for BKV; testing is performed almost exclusively in patients with gross hematuria or urinary symptoms if no other etiologies were found. BKV positivity is defined as the detection of any BKV in the urine by polymerase chain reaction (PCR). BK virus quantitative PCR was performed through the Mayo Clinic reference laboratories. The assay does not cross-react with JC or SV40 polyoma virus (12). Not all patients with BK viruria were checked for viremia; therefore, serum BKV values were not included in this study.
Data collection
Detailed demographic information (age, sex, race (Black, Hispanic, Asian and White)), HSCT-related factors (e.g., donor type, stem cell source, conditioning regimen), and post-transplantation events (e.g., acute and, chronic GVHD, relapse, death) were available from the BMTWeb database. Immune panels, tacrolimus levels, and creatinine concentrations were collected at the following time points after HSCT: 30, 60, 100, 180, 365, 750, 1095, and 1825 days. The highest values of BK viruria for each patient were recorded. The time to platelet engraftment was defined as the first of 7 consecutive days of an unsupported (without platelet transfusion) platelet count of at least 50,000/microliter. The neutrophil engraftment time was defined as the first day of three consecutive days where the neutrophil count was 500 cells/microliter or greater.
The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation (13). Patients with tacrolimus levels above 10 ng/ml at any time point during the study period were included in the analysis as elevated.
Outcomes
Overall survival was calculated from the day of stem cell infusion to the day of death. For each post-baseline eGFR, the relative difference (in %) to the pre-HSCT value was determined. Time to first clinically meaningful kidney function decline (KFD; TTKFD) was defined as the time between HSCT and the first drop in eGFR by at least 25%, which had to be persistent, i.e. no evidence of subsequent improvement in eGFR for up to five years post-transplant. For events that had not occurred by the time of data analysis, times were censored at the last contact at which the patient was known not to be diagnosed with KFD for TTKFD or the last time at which the patient was known to be alive. Chronic kidney disease stage 3 or higher was classified in the population based on KDOQI classification using eGFR <60 mL/min per 1.73m2(14).
Statistical analysis
Time to KFD and survival were estimated using the actuarial Kaplan-Meier method(15) and log-rank tests(16) were performed to test differences in survival among the different groups. We used Cox proportional hazards regression(17) models to estimate unadjusted and adjusted cause-specific hazards ratios (HR) and the corresponding 95% confidence intervals (CI) of risk factors. We first included an initial set of candidate variables with a univariate p-value < 0.1. Backward stepwise elimination (BSE) was then performed, using p<0.05 for the significance level in order for a variable to remain in the model. Once the list of variables to be used in our final model was determined, the functional form of each variable and multi co-linearity among the variables were examined. Age at transplant, sex, race, tumor diagnosis, disease status (at time of HSCT), preparative conditioning regimen, degree of HLA mismatch and source of graft were included in the analyses as baseline covariates. BKV infection, acute and/or chronic GVHD, tacrolimus level >10ng/ml at any of the study time points, donor chimerism, absolute lymphocyte count (ALC) and absolute neutrophil count (ANC) as well as other viral infections were included in the analyses as time-dependent covariates. Presence of BKV and BK viral load were highly correlated and therefore not included in the same models. A model of baseline covariates was built first. Then the time-dependent covariates were included to obtain a final model using the BSE method. Extended Kaplan-Meier method proposed by Snappin, Jiang and Iglewicz(18) was used for graphical display of survival curves by cohorts defined by a time-varying covariate. The P-values shown in figures 2A, 2C and 2D were obtained from corresponding univariate Cox regression models with BK infection or KFD as a time-varying covariate. Since 738 (30%) patients died before they experienced the kidney function endpoint, we explored the impact of potential informative censoring by conducting a competing risk analysis using sub distribution hazard models with death as a competing risk using Fine and Gray’s method, i.e. by applying decreasing weights to patients who died before they experienced KFD (16, 17). For time-dependent covariates, the landmark competing risk analyses (19, 20) were performed at the set landmark time points of 30, 60, 100, 180, and 365 days post-transplant. Age at transplant, sex, race, underlying diagnosis, disease status at transplant, degree of HLA mismatch, acute GVHD, chronic GVHD, donor chimerism, ALC, platelet >50K were included as covariates in the multivariate model to assess of the association of BKV infection with KFD as defined above. SAS version 9.2 and S-Plus version 8.04 were used to carry out the computations for all analyses.
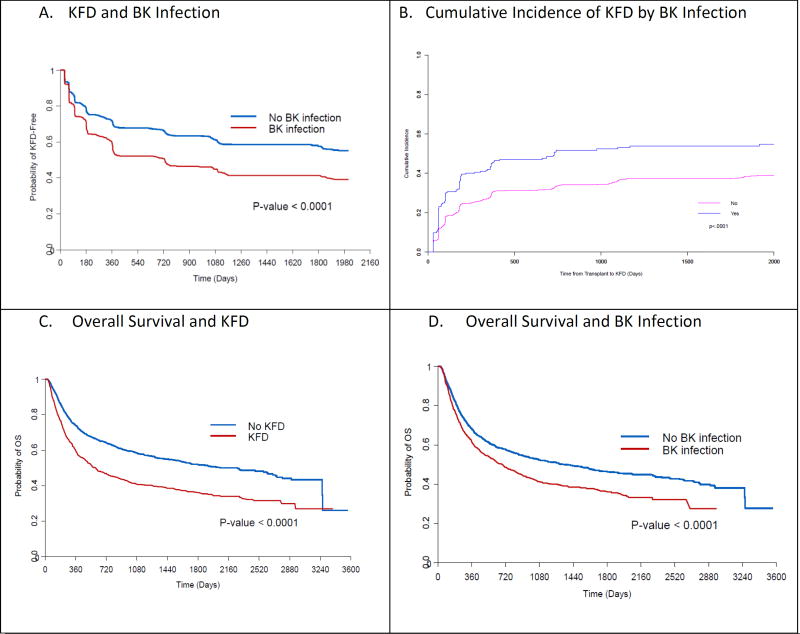
Figure 2.
A, 2C, 2D: Kaplan-Meier curves indicating progression to KFD, overall survival and BK infection. 2B cumulative incidence of KFD. KFD: chronic kidney disease, OS: overall survival.
Results
Characteristics of the study population
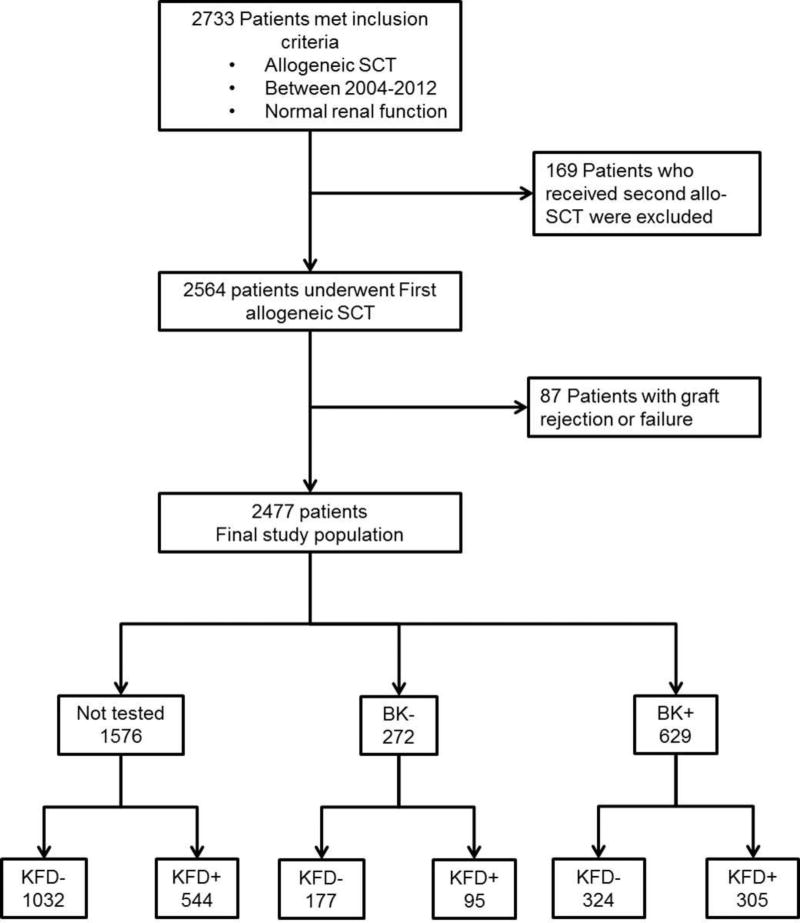
The study population comprised 2477 patients (Figure 1) who were followed for a median 1452 days (interquartile range, IQR: 804–2211 days). The median age at transplantation was 52.3 years, 57.4% were male, and 74.9% were Caucasian (Table 1). The mean kidney function prior to HSCT corresponded to an eGFR of 98.8 (SD = 60.7) mL/min/1.73m2. The main indications for HSCT included acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) (44.7%).
Figure 1.
Population Flow Diagram
Table 1.
Characteristics of first-time allogeneic stem cell transplant recipients, 2004–2012
| Variable | KFD − | KFD + | % |
|---|---|---|---|
|
| |||
| eGFR prior to HSCT, median (interquartile range), in mL/min/1.73m2 | |||
|
| |||
| Age, median (interquartile range), in yr | 50 (0–76) | 54 (7–76) | |
|
| |||
| Sex | |||
| Female | 627 | 429 | 40.6% |
| Male | 906 | 515 | 36.2% |
|
| |||
| Race/Ethnicity | |||
| African-American | 82 | 68 | 45.3% |
| Asian | 49 | 30 | 37.9% |
| Hispanic | 217 | 110 | 33.6% |
| White | 1142 | 712 | 38.4% |
| Other | 10 | 5 | 50.0% |
| Unknown | 33 | 19 | 36.5% |
|
| |||
| Disease diagnosis | |||
| ALL | 161 | 105 | 39.4% |
| AML/MDS | 678 | 431 | 38.8% |
| CLL | 164 | 100 | 37.8% |
| CML/MPD | 114 | 58 | 33.7% |
| Hodgkin’s Lymphoma | 62 | 34 | 35.4% |
| Non-Hodgkin’s Lymphoma | 262 | 162 | 38.2% |
| Myeloma | 37 | 30 | 44.8% |
| Aplastic anemia | 40 | 10 | 20.0% |
| Other hematologic disorders | 2 | 3 | 60.0% |
| Solid tumors | 13 | 11 | 45.8% |
|
| |||
| Previous autologous HSCT | |||
| Yes | 154 | 111 | 41.9% |
| No | 1379 | 833 | 37.7% |
|
| |||
| Disease status at HSCT | |||
| Complete remission | 630 | 338 | 34.9% |
| Partial remission | 267 | 185 | 40.9% |
| Active disease | 616 | 407 | 39.8% |
| Unknown | 20 | 14 | 41.2% |
|
| |||
| Preparative regimen | |||
| Myeloablative | 270 | 189 | 41.2% |
| Non-myeloablative | 228 | 124 | 35.2% |
| Reduced intensity conditioning | 1035 | 631 | 37.9% |
|
| |||
| Graft source | |||
| Peripheral blood | 997 | 578 | 36.7% |
| Bone marrow | 418 | 279 | 40.0% |
| Cord blood | 118 | 87 | 42.2% |
|
| |||
| Allotype | |||
| Matched related | 691 | 345 | 33.3% |
| Matched unrelated | 594 | 397 | 40.1% |
| Mismatched related | 66 | 50 | 43.1% |
| Mismatched unrelated | 182 | 152 | 45.4% |
|
| |||
| Acute GVHD (number of episodes) | |||
| None | 734 | 525 | 41.7% |
| 1–2 | 603 | 420 | 41.5% |
| 3–4 | 131 | 105 | 44.5% |
|
| |||
| Chronic GVHD(number of episodes) | |||
| None | 577 | 360 | 38.4% |
| Limited | 290 | 166 | 36.4% |
| Extensive | 395 | 263 | 39.6% |
|
| |||
| Tacrolimus level >10 ng/ml | |||
| Yes | 792 | 523 | 39.8% |
| No | 736 | 414 | 36.0% |
|
| |||
| Chimerisim | |||
| Donor | 877 | 615 | 41.2% |
| Mixed | 643 | 319 | 33.2% |
|
| |||
| BK Infection | |||
| Yes | 324 | 305 | 48.5% |
| No | 1209 | 639 | 34.6% |
Transplantation characteristics
The stem cell source for transplantation included peripheral blood apheresis (HPC-A) in 63.5%, bone marrow (HPC-BM) in 28.1%, cord blood (HPC-C) in 8.3%, and mixed bone marrow/peripheral blood in 0.04%. The predominant graft source was matched related donor (41%), followed by matched unrelated (40%), mismatched unrelated (13%), and mismatched related (4%) donor; 9.7%. of patients had undergone at least one prior autologous transplantation. Reduced intensity conditioning regimen (RIC) was used in 67.3%. At 30 days post-transplantation, 60% of patients achieved full donor chimerism and 38% had mixed chimerism (23 patients had missing data).
Acute GVHD grade I-IV was diagnosed in 1259 (50.9%) patients and 937 (37.8%) experienced chronic GVHD (limited and extensive). The most common immunosuppressive agent used for GVHD prophylaxis in our study population was tacrolimus (97%), which was targeted at a trough level of 10 ng/dL for the first 6 months after transplant. In 1315 (53%) patients, tacrolimus levels were elevated (above 10ng/ml) at least at one time point during the study.
Incidence of BK Viruria and Kidney Function Decline
BKV viruria was diagnosed in 629 (25%) patients with a median of 42 days from HSCT to the diagnosis of viruria. The median urine BK viral load was 3.9 × 107 (range, 300 to 5 × 109) copies/mL. The one-year cumulative incidence rate of BK infection was 24%. We identified 944 (38.1%) patients who experienced kidney function decline during follow up with the median time from HSCT to this endpoint (KFD) being 101 days (IQR: 60–330 days). In 259 patients who were diagnosed with BKV infection first, and then developed kidney function decline, their median time from HSCT to KFD endpoint was 162 days (IQR: 60–360 days).
Factors associated with Kidney Function Decline
Univariate cause-specific Cox models showed that age (P<0.001), sex (P=0.02), and race (P=0.02), and BK infection (P<0.001) were significantly associated with KFD, as were several other factors (Table 2).
Table 2.
Unadjusted and adjusted associations with kidney function decline
| Parameter | Unadjusted | Multivariable-adjusted | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Hazard Ratio |
95% Hazard Ratio Confidence Limits |
P-Value | Hazard Ratio |
95% Hazard Ratio Confidence Limits |
P-Value | |||
|
| ||||||||
| Age at SCT Per Year Increase | 1.01 | 1.01 | 1.02 | <0.001 | 1.02 | 1.01 | 1.03 | <0.001 |
|
| ||||||||
| Gender | ||||||||
| Female vs. Male | 1.17 | 1.03 | 1.33 | 0.02 | 1.20 | 1.05 | 1.37 | 0.007 |
|
| ||||||||
| Race | ||||||||
| Black vs. Other | 1.36 | 1.06 | 1.74 | 0.02 | 1.37 | 1.06 | 1.77 | 0.02 |
|
| ||||||||
| Diagnosis (ref= Aplastic Anemia) | ||||||||
| ALL | 2.85 | 1.49 | 5.46 | 0.008 | 2.43 | 1.25 | 4.74 | 0.009 |
|
| ||||||||
| AML /MDS | 2.63 | 1.41 | 4.93 | 1.68 | 0.88 | 3.19 | 0.12 | |
|
| ||||||||
| CLL | 2.29 | 1.20 | 4.39 | 1.20 | 0.61 | 2.38 | 0.60 | |
|
| ||||||||
| CML /MPD | 1.92 | 0.98 | 3.76 | 1.09 | 0.54 | 2.20 | 0.80 | |
|
| ||||||||
| Lymphoma | 2.32 | 1.23 | 4.38 | 1.67 | 0.87 | 3.22 | 0.12 | |
|
| ||||||||
| Myeloma | 2.77 | 1.36 | 5.67 | 1.52 | 0.72 | 3.19 | 0.27 | |
|
| ||||||||
| Solid | 3.50 | 1.55 | 7.88 | 2.14 | 0.94 | 4.86 | 0.07 | |
|
| ||||||||
| Disease Status | ||||||||
| Active vs. Remission | 1.34 | 1.16 | 1.55 | <0.001 | 1.30 | 1.11 | 1.53 | 0.001 |
|
| ||||||||
| Partial response vs. Remission | 1.20 | 1.00 | 1.44 | <0.001 | ||||
|
| ||||||||
| Unknown vs. Remission | 1.018 | 0.59 | 1.72 | |||||
|
| ||||||||
| Source of Transplant | 1.34 | 1.07 | 1.68 | 0.01 | ||||
| HPC-C vs. HPC-A | ||||||||
|
| ||||||||
| HPC-M vs. HPC-A | 1.16 | 1.00 | 1.34 | |||||
|
| ||||||||
| Conditioning Regimen | 0.006 | |||||||
| MA vs. NMA | 1.45 | 1.16 | 1.82 | 1.31 | 1.01 | 1.71 | 0.04 | |
|
| ||||||||
| RIC vs. NMA | 1.27 | 1.05 | 1.54 | 1.15 | 0.91 | 1.46 | 0.24 | |
|
| ||||||||
| HLA | <0.001 | |||||||
| MMRD vs. MRD | 1.71 | 1.27 | 2.30 | |||||
|
| ||||||||
| MMUD vs. MRD | 1.74 | 1.44 | 2.11 | |||||
|
| ||||||||
| MUD vs. MRD | 1.38 | 1.20 | 1.60 | |||||
|
| ||||||||
| BK Infection | 1.89 | 1.63 | 2.18 | <0.001 | 1.65 | 1.42 | 1.91 | <0.001 |
|
| ||||||||
| BK Viral Load | 1.03 | 1.02 | 1.03 | <0.001 | 1.06 | 1.04 | 1.09 | <0.001 |
| (Every 10-Fold Increase) | ||||||||
|
| ||||||||
| CHIMERISM | 0.68 | 0.59 | 0.78 | <0.001 | 0.74 | 0.64 | 0.85 | <0.001 |
| Mixed vs. Donor | ||||||||
|
| ||||||||
| ACUTE GVHD | 1.46 | 1.28 | 1.66 | <0.001 | 1.30 | 1.14 | 1.50 | 0.002 |
|
| ||||||||
| Chronic GVHD | 1.57 | 1.30 | 1.90 | <0.001 | 1.44 | 1.19 | 1.75 | 0.002 |
|
| ||||||||
| Tacrolimus | 1.16 | 1.01 | 1.32 | 0.03 | ||||
| Level Above 10 ng/ml | ||||||||
|
| ||||||||
| ALC (Every 2-Fold Increase) | 0.82 | 0.78 | 0.86 | <0.001 | 0.88 | 0.84 | 0.93 | <0.001 |
|
| ||||||||
| ANC (Every 2-Fold Increase) | 1.04 | 0.98 | 1.10 | 0.18 | ||||
|
| ||||||||
| Platelets >50K | 0.44 | 0.37 | 0.51 | <0.001 | 0.52 | 0.44 | 0.62 | <0.001 |
|
| ||||||||
| Adenovirus | 1.14 | 0.47 | 2.74 | 0.78 | ||||
|
| ||||||||
| Influenza | 2.12 | 1.16 | 3.85 | 0.01 | ||||
|
| ||||||||
| RSV | 1.69 | 1.04 | 2.73 | 0.03 | ||||
|
| ||||||||
| CMV | 1.81 | 1.59 | 2.07 | <0.001 | ||||
|
| ||||||||
| HSV | 1.44 | 1.12 | 1.85 | 0.004 | ||||
Abbreviations: ALL: acute lymphocytic leukemia; AML: acute myeloid leukemia; CLL: chronic lymphocytic leukemia; CML: chronic myeloid leukemia; MPD: myeloproliferative disease; HPC-C: hematopoietic progenitor cell-cord blood; HPC-A: hematopoietic progenitor cell-apheresis; HPC-M: hematopoietic progenitor cell-bone marrow; MA: myeloablative conditioning regimen; NMA: nonmyeloablative conditioning regimen; RIC: reduced intensity conditioning regimen; MMRD: mismatched related donor; MMUD: mismatched unrelated donor; MRD: matched related donor; MUD: matched unrelated donor; KFD: kidney function decline; GVHD: graft-vs-host disease; ALC: absolute lymphocyte count (K/ul); ANC: absolute neutrophils count (K/ul); RSV: respiratory syncytial virus; VZV: varicella zoster virus; CMV: cytomegalovirus; HSV: herpes simplex virus.
On multivariate analysis, the factors that were correlated with the development of KFD were age, female sex, African American race, a diagnosis of ALL (reference, aplastic anemia), myeloablative conditioning regimen, active disease at the time of transplantation, BKV infection (HR, 1.65; 95% CI, 1.42–1.91) or high BKV load (HR, 1.06; 95% CI, 1.04–1.09 for every 10-fold increase), full donor chimerism, acute and chronic GVHD. A high ALC (every two-fold increase) and a platelet count of 50,000 and higher were associated with a lower risk of developing KFD (P<0.0001) (Table 2, S1a, S1b & figure 2a). The associations of BKV infection on KFD in the univariate and multivariate cox proportional sub-distribution hazards models, while quantitatively different, were in qualitative agreement with those in the cause-specific hazard models (Table 3, & figure 2b).
Table 3.
Landmark competing risk analyses of the effects of BK viruria on kidney function decline
| Landmark time point post transplant |
Hazard Ratio |
95% Confidence Interval | P-Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| 30 days | 1.58 | 1.29 | 1.93 | <0.001 |
| 60 days | 1.37 | 1.13 | 1.66 | 0.001 |
| 100 days | 1.41 | 1.14 | 1.75 | 0.002 |
| 180 days | 1.56 | 1.22 | 1.99 | <0.001 |
| 365 days | 1.40 | 0.99 | 2.00 | 0.06 |
| 730 days | 1.32 | 0.78 | 2.23 | 0.30 |
| 1095 days | 2.04 | 0.89 | 4.68 | 0.09 |
Note: Associations between BK infection and time-to-KFD with death as competing risk in landmark points adjusting for relevant covariates: age at transplant, gender race, tumor diagnosis, disease status, HLA, acute GVHD, chronic GVHD, conditioning regimen, ALC, platelet count >50K.
We performed a sensitivity analysis with only those patients who were tested for BK infection. The BK infected patients continued to be associated with a higher risk of having KFD (HR1.754, 95% CI, 1.42–2.18). In addition, in the landmark competing risk analysis of the only tested population, patients who were BK infected (BK test positive) had a larger hazard of having KFD than those who were not BK infected (BK test negative). However, the effects of BK infection became statistically insignificant 60 days post-transplant due to insufficient statistical power with a small the sample sizes (S2a,b,&c).
Mortality
Overall survival was 47.2% with nine hundred patients (36.3%) experiencing disease progression. The causes of death included disease progression in 712 (53%), GVHD in 243 (18%), infection in 182 (13%), multiorgan failure in 60 (4%), and other causes in 112 (8%). On univariate analysis, several characteristics were associated with survival as summarized in Table 4. On multivariate analysis, age, all hematologic malignancies (reference, aplastic anemia), myeloablative or RIC (reference non-myeloablative (NMA)), and cord blood transplantation were independently associated with worse overall survival (Table 4 & S3a&b). Most importantly, KFD (HR 4.26; 95% CI, 3.69–4.91), BKV infection (HR, 1.27; 95% CI, 1.11–1.44), or a high BK viral load (for every 10-fold increase, HR, 1.03; 95% CI, 1.02–1.05) were all associated with worse overall survival (Figure 2c&d). In contrast, a platelet count of >50,000 (HR=0.47; 95% CI: 0.40–0.54) and an increase in ALC by a factor of two (HR=0.62; 95% CI: 0.60–0.65) were associated with better overall survival.
Table 4.
Unadjusted and adjusted associations with overall survival
| Parameter | Unadjusted | Multivariable-Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Hazard Ratio |
95% Hazard Ratio Confidence Limits |
P-Value | Hazard Ratio |
95% Hazard Ratio Confidence Limits |
P-Value | |||
|
| ||||||||
| Age Per Year Increase | 1.01 | 1.06 | 1.01 | <.001 | 1.008 | 1.003 | 1.013 | 0.006 |
|
| ||||||||
| Age Group | 1.32 | 1.18 | 1.47 | <.001 | ||||
| >Median (52.28) vs. ≤ Median | ||||||||
|
| ||||||||
| Gender | 0.95 | 0.85 | 1.06 | 0.39 | ||||
| Female vs. Male | ||||||||
|
| ||||||||
| Race | 1.28 | 1.03 | 1.58 | 0.03 | ||||
| Black vs. Other | ||||||||
|
| ||||||||
| Diagnosis (ref= Aplastic Anemia) | 6.21 | 2.91 | 13.25 | <0.001 | 5.34 | 2.47 | 11.55 | <0.001 |
| ALL | ||||||||
|
| ||||||||
| AML / MDS | 5.69 | 2.7 | 11.98 | 4.11 | 1.93 | 8.74 | ||
|
| ||||||||
| CLL | 4.86 | 2.27 | 10.37 | 3.16 | 1.45 | 6.92 | ||
|
| ||||||||
| CML / MPD | 3.59 | 1.66 | 7.78 | 2.23 | 1.01 | 4.93 | ||
|
| ||||||||
| Lymphoma | 3.76 | 1.77 | 7.98 | 2.88 | 1.34 | 6.19 | ||
|
| ||||||||
| Myeloma | 6.11 | 2.75 | 13.54 | 4.04 | 1.77 | 9.20 | ||
|
| ||||||||
| Solid | 10.57 | 4.60 | 24.28 | 7.03 | 3.0 | 16.49 | ||
|
| ||||||||
| Disease Status | 1.90 | 1.68 | 2.15 | <0.001 | 1.65 | 1.43 | 1.89 | <0.001 |
| Active vs. Remission | ||||||||
|
|
||||||||
| Partial response vs. Remission | 1.15 | 0.98 | 1.35 | 1.37 | 1.11 | 1.70 | ||
|
|
||||||||
| Unknown vs. Remission | 1.02 | 0.63 | 1.65 | 1.24 | 0.69 | 2.21 | ||
|
| ||||||||
| Source of Transplant | 1.52 | 1.27 | 1.83 | <0.001 | 1.24 | 0.98 | 1.56 | <0.0730 |
| HPC-C vs. HPC-A | ||||||||
|
| ||||||||
| HPC-M vs. HPC-A | 0.99 | 0.88 | 1.13 | 0.75 | 0.66 | 0.86 | ||
|
| ||||||||
| Conditioning Regimen | 1.72 | 1.41 | 2.10 | <0.001 | 1.49 | 1.15 | 1.93 | 0.005 |
| MA vs. NMA | ||||||||
|
| ||||||||
| RIC vs. NMA | 1.54 | 1.3 | 1.83 | 1.40 | 1.13 | 1.74 | ||
|
| ||||||||
| HLA Status | 1.68 | 1.31 | 2.15 | <0.001 | ||||
| MMRD vs. MRD | ||||||||
|
| ||||||||
| MMUD vs. MRD | 1.58 | 1.35 | 1.86 | |||||
|
| ||||||||
| MUD vs. MRD | 1.17 | 1.03 | 1.32 | |||||
|
| ||||||||
| Previous Autologous Tx >0 vs. 0 | 1.09 | 0.92 | 1.29 | 0.32 | ||||
|
| ||||||||
| KFD (GFR) Time Variable | 3.03 | 2.71 | 3.39 | <0.001 | 4.26 | 3.69 | 4.91 | <0.001 |
|
| ||||||||
| BK Infection | 1.58 | 1.40 | 1.79 | <0.001 | 1.27 | 1.11 | 1.44 | 0.004 |
|
| ||||||||
| BK Viral Load | 1.02 | 1.015 | 1.025 | <0.001 | 1.03 | 1.02 | 1.05 | 0.004 |
| (Every 10-Fold Increase) | ||||||||
|
| ||||||||
| CHIMERISM Status | 0.77 | 0.69 | 0.86 | <0.001 | ||||
| Mixed vs Donor | ||||||||
|
| ||||||||
| ACUTE GVHD | 1.41 | 1.27 | 1.58 | <0.001 | ||||
|
| ||||||||
| Chronic GVHD | 1.06 | 0.93 | 1.21 | 0.38 | ||||
|
| ||||||||
| Tacrolimus | 1.05 | 0.94 | 1.17 | 0.41 | ||||
| Level Above 10 ng/ml | ||||||||
|
| ||||||||
| ALC (Every 2-Fold Increase) | 0.62 | 0.60 | 0.64 | <0.001 | 0.62 | 0.60 | 0.65 | <0.001 |
|
| ||||||||
| ANC (Every 2-Fold Increase) | 0.78 | 0.75 | 0.81 | <0.001 | ||||
|
| ||||||||
| Platelets >50K | 0.77 | 0.69 | 0.86 | <0.001 | 0.47 | 0.40 | 0.54 | <0.001 |
|
| ||||||||
| Adenovirus | 3.01 | 2.01 | 4.51 | <0.001 | ||||
|
| ||||||||
| Influenza | 1.09 | 0.63 | 1.88 | 0.76 | ||||
|
| ||||||||
| RSV | 1.63 | 1.13 | 2.36 | 0.009 | ||||
|
| ||||||||
| VZV | 1.13 | 0.60 | 2.11 | 0.70 | ||||
|
| ||||||||
| CMV | 1.47 | 1.32 | 1.64 | <.001 | ||||
|
| ||||||||
| HSV | 1.83 | 1.53 | 2.19 | <.001 | ||||
Abbreviations: ALL: acute lymphocytic leukemia; AML: acute myeloid leukemia; CLL: chronic lymphocytic leukemia; CML: chronic myeloid leukemia; MPD: myeloproliferative disease; HPC-C: hematopoietic progenitor cell-cord blood; HPC-A: hematopoietic progenitor cell-apheresis; HPC-M: hematopoietic progenitor cell-bone marrow; MA: myeloablative conditioning regimen; NMA: nonmyeloablative conditioning regimen; RIC: reduced intensity conditioning regimen; MMRD: mismatched related donor; MMUD: mismatched unrelated donor; MRD: matched related donor; MUD: matched unrelated donor; KFD: kidney function decline; GVHD: graft-vs-host disease; ALC: absolute lymphocyte count (K/ul); ANC: absolute neutrophils count (K/ul); RSV: respiratory syncytial virus; VZV: varicella zoster virus; CMV: cytomegalovirus; HSV: herpes simplex virus.
Discussion
The 2012 guidelines for screening and preventive practices for autologous and allogeneic HSCT were not derived from randomized or controlled trials but from retrospective, mostly smaller, studies(21). As a result, KFD and its risk factors in long-term survivors of HSCT are under-recognized. Our study of 2477 consecutive allogenic HSCT patients with a median follow-up time of more than 4.0 years shows the association of BKV infection with progression to KFD (P<0.001) and decreased overall survival (P<0.001) when compared to patients without BK infection. In addition, we evaluated several other candidate variables for KFD, confirming previous findings and establishing new associations. In a systematic review of CKD in HSCT recipients, the rate of CKD was reported to be 27.8%, and the prevailing risk factors were female sex, advanced age, total body irradiation, prolonged use of cyclosporine, acute renal failure, and acute and chronic GVHD(10). Our results confirm that advanced age and female sex were independently correlated with KFD in HSCT patients. Variables associated with more intensive therapy such as an underlying diagnosis of ALL, myeloablative conditioning regimen, cord blood transplantation, active disease status at the time of transplantation, mismatched unrelated donor transplant, acute and chronic GVHD, and lower ALC were also found to be associated with an increased risk of KFD. GVHD results in the release of inflammatory cytokines, which in turn can lead to further kidney injury or T cell-mediated organ damage(22–25). Interestingly, patients with mixed donor chimerism were less likely to develop permanent decline in their kidney function (P<0.001) (Table 2). A logical explanation for this observation is that this group of patients is less likely to develop GVHD than those who achieve full donor chimerism and thus, is less likely to be exposed to prolonged immunosuppressive therapy with the associated risks of infections and exposure to nephrotoxins.
We evaluated the immune status of our allogenic transplantation population to determine whether immune suppression played a role in the development of kidney function decline. Lymphocytes (B cells, T cells, and natural killer cells) are major components of the body’s humoral and cellular immunity. An elevated ALC was associated with lower rates of viral infections on both univariate and multivariate analyses: for every two-fold increase in the ALC, there was a significant reduction in the risk of progression to KFD (P=0.001). More recently, platelets have been recognized as key players in the innate and adaptive immune responses(26, 27). Therefore, low platelets count could contribute to poor immune recovery in these patients. This notion is supported by our multivariate analysis, which showed that patients with a platelet count of 50,000 or greater were less likely to experience progression to kidney function decline (P<0.001), likely due to improved immune status and decreased risk of infections including BKV.
The novelty of our study, is in our evaluation of BKV infection and viral load were both associated with KFD and survival post-HSCT. On both univariate and multivariate analyses, BKV infection was associated with KFD (P<0.001). In the kidney transplantation population, BKV infection has been established as a cause of allograft loss, renal impairment and progress to KFD and CKD(28–30). However, in HSCT recipients, most studies have only evaluated hemorrhagic cystitis in the setting of BKV infection,(31) with very few studies evaluating its impact on CKD and long-term renal outcomes(6, 32). In our study, the relationship between BKV infection and development of KFD was further confirmed by evaluation of the BKV load. For every 10 fold increase in the viral load we found a significantly higher risk of progression to KFD (P<0.001). The patients that developed KFD were further stratified into stages of CKD, with the majority (61%) having eGFR <60 ml/min/1.73 m2, reflecting Stage 3 CKD (Table 5).
Table 5.
Stages of CKD (At time of KFD) in population that developed a decrease in GFR 25% or more in comparison to baseline (Time of Transplant). Kidney disease was stratified using the system advocated by the American National Kidney Foundation Kidney Disease Outcomes Initiative3: stage 1, eGFR 90.0 ml/min per 1.73 m2; stage 2, eGFR 60.0–89.9 ml/min per 1.73 m2; CKD3a − GFR 45 to 59 mL/min per 1.73 m2, CKD3b − GFR 30 to 44 mL/min per 1.73 m2; stage 4, eGFR 15.0–29.9 ml/min per 1.73 m2; and stage 5, eGFR <15.0
| Stage | Baseline (n=2477) |
KFD time (n=944) |
|---|---|---|
| 1 | 1534 (62%) | 29 (3%) |
| 2 | 853 (34%) | 334 (35%) |
| 3A | 89 (4%) | 297 (31%) |
| 3B | 1 (0%) | 195 (21%) |
| 4 | - | 79 (8%) |
| 5 | - | 10 (1%) |
In our study, patients with KFD were four times more likely to die compared to those without KFD (P<0.001). The association between BKV infection and BKV load with inferior survival in our study is in keeping with a previous report in a much smaller group of patients (31). The immune panel studies further solidified the relationship between impaired cellular immunity and decreased overall survival in this population. For every two-fold increase in ALC, there was an independent increase in survival (P<0.001). The obvious complications associated with BKV infection, such as BKV hemorrhagic cystitis (BKV-HC) and stenosis, have been studied extensively; patients with BKV-HC have a median of 10 additional days of hospitalization, increasing the cost of their inpatient treatment by 85%(31). Treatment options for BKV infection are limited and the majority of recommendations are derived from the kidney transplant literature. Graft survival rates in kidney transplant recipients with BK virus nephropathy range from 10% to 60% and the only effective treatment for such patients is reduction in systemic immunosuppression (33–35). In both the HSCT and kidney transplant populations leflunomide, fluoroquinolones, and cidofovir have been tried for the treatment of BKV infection with conflicting results (36–38). Although the effectiveness of cidofovir in kidney transplant patients have not been validated by randomized prospective clinical trials; retrospective studies and small case series have reported promising results(39–41). Currently at our institution, we are conducting a phase II randomized study of cidofovir versus best supportive care for BKV-related hemorrhagic cystitis in HSCT recipients to evaluate its efficacy. Brincidofovir (CMX001), a lipid-conjugated version of cidofovir, shows no evidence of nephrotoxicity in rodent models or human studies and has been gaining momentum in the treatment of BKV with promising results in the HSCT population(42, 43)
Thus, with the lack of effective treatment to improve outcomes in this population, it is imperative to develop novel strategies to address factors that affect survival early in the transplantation course, such as GVHD, early immune reconstitution, use of less toxic regimens, and early treatment of late complications such as KFD and BKV infection.
A limitation of this retrospective study is that not all patients were tested for BKV (urine or serum) routinely. It is possible some patients for BKV did indeed have BKV infection, which may have in turn contributed to KFD in this group. Due to the lack of routine testing for most viral infections other than CMV, we were not able to perform a complete analysis of the contribution of other infections to KFD. CMV was evaluated in a multivariate analysis and was strongly correlated with KFD, while BKV also remained significant in the same model. In addition, we did not evaluate the impact of nephrotoxins other than tacrolimus such as aminoglycosides, vancomycin, foscarnet or amphotericin B on KFD. Almost all patients receive antibiotic and antifungal prophylaxis in this high-risk transplant population. In addition, we were faced with the challenge of patients being prescribed antibiotics elsewhere after transplantation, not all of which were captured in our electronic records. In the setting of HC our internal guidelines recommend continuous bladder irrigation and no specific antivirals. Another limitation of our study is that fewer than 10% of our population underwent a kidney biopsy to help delineate the underlying cause of the renal dysfunction and to determine whether BKV nephropathy was the cause of the renal dysfunction. This is likely due to the complicated medical condition of many of these patients post SCT. At all-time points studied GFR was estimated using the CKD EPI equation, which has been shown to be a more accurate estimate at higher GFRs compared to other creatinine-based estimation equations(13). However, it is possible that the GFR in some HSCT recipients was overestimated as it does not take into account the muscle wasting and volume overload commonly seen during the course of their therapy in such patients. Thus, the reported incidence for clinically meaningful kidney function decline may be underestimated. We have initiated a prospective study at our center that will overcome many of the limitations in this manuscript.
Conclusion
BKV screening can identify at least 80%–90% of kidney transplantation patients who are at risk for BKV nephropathy and can thus prevent further renal dysfunction and graft loss(38). Although nephropathy is rare in native kidneys, it has been reported to cause renal failure in immunodeficient patients, solid organ transplantation patients (cardiac, lung, pancreas, liver, and bone marrow), and auto- and allo-HSCT recipients (44). The tremendous costs associated with HSCT transplantation before, during, and after the procedure are increased by the associated complications. Focusing on BKV infections, including protocol screening, will help us formulate better screening techniques, prevention protocols, and most importantly, novel prophylactic and curative immunotherapeutic approaches in both HSCT and renal transplantation recipients.
Supplementary Material
Table S1: (A) A Multivariate Cox PH model of time-to-KFD with only baseline covariates. (B) A Multivariate Cox PH model of time-to-KFD with only time-dependent covariates.
Table S2: (A) Multivariate model of time-to-KFD as shown in Table 2 of the manuscript when only the patients who were urine tested for BK infection are included. (B) Landmark competing risk analyses of the effects of BK viruria on kidney function decline as shown in Table 3 of the manuscript when only the patients who were urine tested for BK infection are included. Associations between BK infection and time-to-KFD with death as competing risk in landmark points adjusting for relevant covariates: age at transplant, gender race, tumor diagnosis, disease status, HLA, acute GVHD, chronic GVHD, conditioning regimen, ALC, platelet count >50K. (C) Multivariate model of OS as shown in Table 4 of the manuscript when only the patients who were urine tested for BK infection are included.
Table S3. (A) A Multivariate Cox PH model of OS with only baseline covariates. (B) A Multivariate Cox PH model of OS with only time-dependent covariates.
Acknowledgments
This research was supported by funds from the University Cancer Foundation and the Duncan Family Institute for Cancer Prevention and Risk Assessment via Cancer Survivorship Research Seed Money Grants at The University of Texas MD Anderson Cancer Center. Dr. Winkelmayer received salary and research support through the endowed Gordon A. Cain Chair in Nephrology at Baylor College of Medicine.
Dr. Winkelmayer reports having served, within the past 36 months, as a scientific advisor to Amgen, Astellas, Astra-Zeneca, Bayer, Fibrogen, GlaxoSmithKline, Keryx, Merck Sharpe & Dohme, Mitsubishi-Tanabe, and Rockwell Pharma, and on data safety monitoring boards for Medgenics and Medtronic. Dr. Roy F. Chemaly reports research grants from Chimerix and having served as a consultant for Chimerix with a brincidofovir clinical study.
Abbreviations
- GVHD
Acute graft versus host disease
- ALC
Absolute lymphocyte count
- ANC
Absolute neutrophil count
- ALL
Acute lymphocytic leukemia
- AML
Acute myeloid leukemia
- BSE
Backward stepwise elimination
- BKV
BK virus
- BKV-HC
BKV hemorrhagic cystitis
- BMTWeb
Bone marrow and transplant website
- HPC-BM
Bone marrow
- CLL
Chronic lymphocytic leukemia
- CML
Chronic myeloid leukemia
- CI
Confidence intervals
- HPC-C
Cord blood
- CMV
Cytomegalovirus
- eGFR
Estimated glomerular filtration rate
- HR
Hazards ratios
- HSCT
Hematopoietic stem cell transplantation
- HPC-C
Hematopoietic progenitor cell-cord blood
- HPC-M
Hematopoietic progenitor cell-bone marrow
- HSV
Herpes simplex virus
- KFD
Kidney function decline
- MAD
Matched related donor
- MUD
Matched unrelated donor
- MMRD
Mismatched related donor
- MMUD
Mismatched unrelated donor
- MA
Myeloablative conditioning regimen
- MDS
Myelodysplastic syndrome
- MPD
Myeloproliferative disease
- NMA
Non-myeloablative
- OS
Overall survival
- HPC-A
Peripheral blood apheresis
- PCR
Polymerase chain reaction
- RIC
Reduced intensity conditioning regimen
- RSV
Respiratory syncytial virus
- TTKFD
Time To Kidney Finction Decline
- VZV
Varicella zoster virus
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. The other authors have no conflicts of interest to disclose.
References
- 1.Arthur RR, Shah KV, Baust SJ, Santos GW, Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. The New England journal of medicine. 1986;315(4):230–4. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- 2.Pappo O, Demetris AJ, Raikow RB, Randhawa PS. Human polyoma virus infection of renal allografts: histopathologic diagnosis, clinical significance, and literature review. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1996;9(2):105–9. [PubMed] [Google Scholar]
- 3.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. The New England journal of medicine. 2002;347(7):488–96. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch HH. BK virus: opportunity makes a pathogen. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41(3):354–60. doi: 10.1086/431488. [DOI] [PubMed] [Google Scholar]
- 5.van Aalderen MC, Heutinck KM, Huisman C, ten Berge IJ. BK virus infection in transplant recipients: clinical manifestations, treatment options and the immune response. Neth J Med. 2012;70(4):172–83. [PubMed] [Google Scholar]
- 6.O'Donnell PH, Swanson K, Josephson MA, Artz AS, Parsad SD, Ramaprasad C, et al. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(9):1038–48. e1. doi: 10.1016/j.bbmt.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva Lde P, Patah PA, Saliba RM, Szewczyk NA, Gilman L, Neumann J, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95(7):1183–90. doi: 10.3324/haematol.2009.016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdanovic G, Priftakis P, Giraud G, Kuzniar M, Ferraldeschi R, Kokhaei P, et al. Association between a high BK virus load in urine samples of patients with graft-versus-host disease and development of hemorrhagic cystitis after hematopoietic stem cell transplantation. Journal of clinical microbiology. 2004;42(11):5394–6. doi: 10.1128/JCM.42.11.5394-5396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erard V, Storer B, Corey L, Nollkamper J, Huang ML, Limaye A, et al. BK virus infection in hematopoietic stem cell transplant recipients: frequency, risk factors, and association with postengraftment hemorrhagic cystitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39(12):1861–5. doi: 10.1086/426140. [DOI] [PubMed] [Google Scholar]
- 10.Ellis MJ, Parikh CR, Inrig JK, Kanbay M, Patel UD. Chronic kidney disease after hematopoietic cell transplantation: a systematic review. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(11):2378–90. doi: 10.1111/j.1600-6143.2008.02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hingorani S. Chronic kidney disease after pediatric hematopoietic cell transplant. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(1 Suppl 1):84–7. doi: 10.1016/j.bbmt.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Viscount HB, Eid AJ, Espy MJ, Griffin MD, Thomsen KM, Harmsen WS, et al. Polyomavirus polymerase chain reaction as a surrogate marker of polyomavirus-associated nephropathy. Transplantation. 2007;84(3):340–5. doi: 10.1097/01.tp.0000275205.41078.51. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Summary of Recommendation Statements. Kidney international supplements. 2013;3(3):263–5. doi: 10.1038/kisup.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–81. [Google Scholar]
- 16.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer chemotherapy reports Part 1. 1966;50(3):163–70. [PubMed] [Google Scholar]
- 17.Cox DR. Regression Models and Life-Tables. J R Stat Soc B. 1972;34(2):187-+. [Google Scholar]
- 18.Snapinn SM, Jiang Q, Iglewicz B. Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat. 2005;59(4):301–7. [Google Scholar]
- 19.van Houwelingen HC. Dynamic prediction by landmarking in event history analysis. Scand J Stat. 2007;34(1):70–85. [Google Scholar]
- 20.Cortese G, Andersen PK. Competing risks and time-dependent covariates. Biometrical journal Biometrische Zeitschrift. 2010;52(1):138–58. doi: 10.1002/bimj.200900076. [DOI] [PubMed] [Google Scholar]
- 21.Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone marrow transplantation. 2012;47(3):337–41. doi: 10.1038/bmt.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couriel D, Caldera H, Champlin R, Komanduri K. Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer. 2004;101(9):1936–46. doi: 10.1002/cncr.20613. [DOI] [PubMed] [Google Scholar]
- 23.Miralbell R, Bieri S, Mermillod B, Helg C, Sancho G, Pastoors B, et al. Renal toxicity after allogeneic bone marrow transplantation: the combined effects of total-body irradiation and graft-versus-host disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14(2):579–85. doi: 10.1200/JCO.1996.14.2.579. [DOI] [PubMed] [Google Scholar]
- 24.Sakellari I, Barbouti A, Bamichas G, Mallouri D, Kaloyannidis P, Fragidis S, et al. GVHD-associated chronic kidney disease after allogeneic haematopoietic cell transplantation. Bone marrow transplantation. 2013;48(10):1329–34. doi: 10.1038/bmt.2013.55. [DOI] [PubMed] [Google Scholar]
- 25.Kersting S, Dorp SV, Theobald M, Verdonck LF. Acute renal failure after nonmyeloablative stem cell transplantation in adults. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(1):125–31. doi: 10.1016/j.bbmt.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Gerdes N, Zhu L, Ersoy M, Hermansson A, Hjemdahl P, Hu H, et al. Platelets regulate CD4(+) T-cell differentiation via multiple chemokines in humans. Thrombosis and haemostasis. 2011;106(2):353–62. doi: 10.1160/TH11-01-0020. [DOI] [PubMed] [Google Scholar]
- 27.Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. Journal of thrombosis and haemostasis : JTH. 2014 doi: 10.1111/jth.12730. [DOI] [PubMed] [Google Scholar]
- 28.Comoli P, Cioni M, Basso S, Gagliardone C, Potenza L, Verrina E, et al. Immunity to Polyomavirus BK Infection: Immune Monitoring to Regulate the Balance between Risk of BKV Nephropathy and Induction of Alloimmunity. Clinical & developmental immunology. 2013;2013:256923. doi: 10.1155/2013/256923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawinski D, Goral S. BK virus infection: an update on diagnosis and treatment. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014 doi: 10.1093/ndt/gfu023. [DOI] [PubMed] [Google Scholar]
- 30.Vasudev B, Hariharan S, Hussain SA, Zhu YR, Bresnahan BA, Cohen EP. BK virus nephritis: risk factors, timing, and outcome in renal transplant recipients. Kidney international. 2005;68(4):1834–9. doi: 10.1111/j.1523-1755.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 31.Gilis L, Morisset S, Billaud G, Ducastelle-Lepretre S, Labussiere-Wallet H, Nicolini FE, et al. High burden of BK virus-associated hemorrhagic cystitis in patients undergoing allogeneic hematopoietic stem cell transplantation. Bone marrow transplantation. 2014;49(5):664–70. doi: 10.1038/bmt.2013.235. [DOI] [PubMed] [Google Scholar]
- 32.Haines HL, Laskin BL, Goebel J, Davies SM, Yin HJ, Lawrence J, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(10):1512–9. doi: 10.1016/j.bbmt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Sood P, Senanayake S, Sujeet K, Medipalli R, Zhu YR, Johnson CP, et al. Management and outcome of BK viremia in renal transplant recipients: a prospective single-center study. Transplantation. 2012;94(8):814–21. doi: 10.1097/TP.0b013e31826690c6. [DOI] [PubMed] [Google Scholar]
- 34.Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, Hopfer H, et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(12):2615–23. doi: 10.1111/j.1600-6143.2010.03310.x. [DOI] [PubMed] [Google Scholar]
- 35.Trofe J, Hirsch HH, Ramos E. Polyomavirus-associated nephropathy: update of clinical management in kidney transplant patients. Transplant infectious disease : an official journal of the Transplantation Society. 2006;8(2):76–85. doi: 10.1111/j.1399-3062.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- 36.Knoll GA, Humar A, Fergusson D, Johnston O, House AA, Kim SJ, et al. Levofloxacin for BK virus prophylaxis following kidney transplantation: a randomized clinical trial. Jama. 2014;312(20):2106–14. doi: 10.1001/jama.2014.14721. [DOI] [PubMed] [Google Scholar]
- 37.Lee BT, Gabardi S, Grafals M, Hofmann RM, Akalin E, Aljanabi A, et al. Efficacy of levofloxacin in the treatment of BK viremia: a multicenter, double-blinded, randomized, placebo-controlled trial. Clinical journal of the American Society of Nephrology : CJASN. 2014;9(3):583–9. doi: 10.2215/CJN.04230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsch HH, Randhawa P, Practice ASTIDCo BK polyomavirus in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(Suppl 4):179–88. doi: 10.1111/ajt.12110. [DOI] [PubMed] [Google Scholar]
- 39.Kuten SA, Patel SJ, Knight RJ, Gaber LW, DeVos JM, Gaber AO. Observations on the use of cidofovir for BK virus infection in renal transplantation. Transplant infectious disease : an official journal of the Transplantation Society. 2014;16(6):975–83. doi: 10.1111/tid.12313. [DOI] [PubMed] [Google Scholar]
- 40.Cabello V, Margarit N, Diaz Pedrero M, Bernal G, Pereira P, Gentil MA. Treatment of BK virus-associated nephropathy with Cidofovir in renal transplantation. Transplantation proceedings. 2008;40(9):2930–2. doi: 10.1016/j.transproceed.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Kuypers DR, Vandooren AK, Lerut E, Evenepoel P, Claes K, Snoeck R, et al. Adjuvant low-dose cidofovir therapy for BK polyomavirus interstitial nephritis in renal transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(8):1997–2004. doi: 10.1111/j.1600-6143.2005.00980.x. [DOI] [PubMed] [Google Scholar]
- 42.Papanicolaou GA, Lee YJ, Young JW, Seshan SV, Boruchov AM, Chittick G, et al. Brincidofovir for Polyomavirus-Associated Nephropathy After Allogeneic Hematopoietic Stem Cell Transplantation. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015 doi: 10.1053/j.ajkd.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rinaldo CH, Gosert R, Bernhoff E, Finstad S, Hirsch HH. 1-O-hexadecyloxypropyl cidofovir (CMX001) effectively inhibits polyomavirus BK replication in primary human renal tubular epithelial cells. Antimicrobial agents and chemotherapy. 2010;54(11):4714–22. doi: 10.1128/AAC.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma SG, Nickeleit V, Herlitz LC, de Gonzalez AK, Stokes MB, Singh HK, et al. BK polyoma virus nephropathy in the native kidney. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28(3):620–31. doi: 10.1093/ndt/gfs537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: (A) A Multivariate Cox PH model of time-to-KFD with only baseline covariates. (B) A Multivariate Cox PH model of time-to-KFD with only time-dependent covariates.
Table S2: (A) Multivariate model of time-to-KFD as shown in Table 2 of the manuscript when only the patients who were urine tested for BK infection are included. (B) Landmark competing risk analyses of the effects of BK viruria on kidney function decline as shown in Table 3 of the manuscript when only the patients who were urine tested for BK infection are included. Associations between BK infection and time-to-KFD with death as competing risk in landmark points adjusting for relevant covariates: age at transplant, gender race, tumor diagnosis, disease status, HLA, acute GVHD, chronic GVHD, conditioning regimen, ALC, platelet count >50K. (C) Multivariate model of OS as shown in Table 4 of the manuscript when only the patients who were urine tested for BK infection are included.
Table S3. (A) A Multivariate Cox PH model of OS with only baseline covariates. (B) A Multivariate Cox PH model of OS with only time-dependent covariates.