Abstract
A greater understanding of neural mechanisms that contribute to anxiety is needed in order to develop better therapeutic interventions. The current study interrogates a novel molecular mechanism that shapes anxiety-like behavior, demonstrating that the microRNA miR-101a-3p and its target, enhancer of zeste homolog 2 (Ezh2) in the amygdala, contribute to rodent anxiety-like behavior. We utilized rats that were selectively-bred for differences in emotionality and stress reactivity, showing that high novelty responding (HR) rats, which display low trait anxiety, have lower miR-101a-3p levels in the amygdala compared to low novelty responding (LR) rats that characteristically display high trait anxiety. To determine if there is a causal relationship between amygdalar miR-101a-3p and anxiety behavior, we used a viral approach to over-express miR-101a-3p in the amygdala of HR rats and test whether it would increase their typically low levels of anxiety-like behavior. We found that increasing miR-101a-3p in the amygdala increased HRs’ anxiety-like behavior in the open field test and elevated plus maze. Viral-mediated miR-101a-3p over-expression also reduced expression of the histone methyltransferase Ezh2, which mediates gene silencing via tri-methylation of histone 3 at lysine 27 (H3K27me3). Knockdown of Ezh2 with short-interfering RNA (siRNA) also increased HRs’ anxiety-like behavior, but to a lesser degree than miR-101a-3p over-expression. Overall our data demonstrate that increasing miR-101a-3p expression in the amygdala increases anxiety-like behavior and that this effect is at least partially mediated via repression of Ezh2. This work adds to the growing body of evidence implicating miRNAs and epigenetic regulation as molecular mediators of anxiety behavior.
Keywords: microRNA, miR-101a-3p, Ezh2, Polycomb Repressive Complex 2, Amygdala, Anxiety
INTRODUCTION
Anxiety disorders are characterized by excessive fear and anxiety and are both highly prevalent, with a lifetime risk for developing any anxiety disorder estimated at 28.8% in the U.S., and can cause lifelong impairment in many patients (Kessler et al., 2008). A greater understanding of the molecular mechanisms that contribute to anxiety is needed in order to develop more effective treatments. In order to elucidate genetic and environmental factors that produce an anxious phenotype, we developed a rat model of individual differences in emotionality by selectively breeding Sprague Dawley rats for disparate response to novelty (Stead et al., 2006). Rats bred for high response to novelty (HRs) vigorously explore new environments and exhibit low-levels of trait anxiety compared to rats bred for low response to novelty (LRs), which exhibit high levels of trait anxiety (Clinton et al., 2011; Cohen et al., 2015; Glover et al., 2015).
Previous work from our group suggests that HR/LR differences in anxiety-like behavior rely, in part, on differences in the amygdala (Cohen et al., 2015; Cohen et al., 2016). We recently found that low-anxiety HR rats display reduced c-Fos activation in multiple amygdalar subnuclei following electric-shock stress compared to LRs. We then used high-throughput sequencing of mRNAs and microRNAs (miRNAs) in the HR/LR amygdala to begin to interrogate molecular mechanisms that may drive their disparate behavioral and neural responses to stress (Cohen et al., 2016). miRNAs are short non-coding RNAs that control translation and/or stability of mRNA targets. Each miRNA potentially targets several genes, so co-expression of only a few miRNAs exerts powerful control over large gene networks (Mathonnet et al., 2007; Brodersen and Voinnet, 2009). miRNAs are increasingly recognized for their role in human mental illness (O’Connor et al., 2012; Hauberg et al., 2016). Single nucleotide polymorphisms associated with several miRNAs effect risk for anxiety disorders (Muinos-Gimeno et al., 2011; Hommers et al., 2015) and expression levels of other miRNAs have been shown to correlate with anxiety symptoms (Xu et al., 2016). Rodent studies show that acute and chronic stress effect miRNA expression (Meerson et al., 2010; Rinaldi et al., 2010), and rats that are vulnerable vs. resilient to developing learned helpless behavior display baseline miRNA expression differences (Smalheiser et al., 2011). Additionally, several miRNA knockout mice display alterations in anxiety-like behavior (Andolina et al., 2016; Jin et al., 2016).
Our recent transcriptome study identified miR-101a-3p as one of the most abundant miRNAs in the amygdala and showed that HR rats had significantly lower miR-101a-3p levels in the amygdala relative to LRs (Cohen et al., 2016). A major target miR-101a-3p is the Polycomb Repressive Complex 2 (PRC2), a multi-protein complex including histone methyltransferase enhancer of zeste homolog 2 (Ezh2) (Varambally et al., 2008; Friedman et al., 2009), which mediates gene silencing via tri-methylation of histone 3 at lysine 27 (H3K27me3) (Cao et al., 2002; Marchesi and Bagella, 2013). PRC2 critically regulates gene expression during several developmental processes (Marchesi et al., 2014), including formation of brain circuits (Di Meglio et al., 2013; Neo et al., 2014), although a fairly limited number of studies have examined Ezh2 and H3K27me3 in the adult brain (Qi et al., 2014; Zhang et al., 2014; Koo et al., 2015).
The present study builds on our previous findings by measuring Ezh2 and H3K27me3 levels in the amygdala of HR/LR rats (to test whether HR/LR miR-101a expression differences are associated with predicted changes in these targets); and determining whether manipulating amygdalar miR-101a-3p and Ezh2 expression can alter anxiety-like behavior. Because we found significantly lower mi-R101a-3p levels in the HR versus LR amygdala and because miR-101a-3p represses PRC2 signaling, we hypothesized (a) that HRs would exhibit elevated Ezh2 and H3K27me3 levels in the amygdala relative to LRs; and (b) that increasing miR-101a-3p expression in the HR amygdala would increase their anxiety-like behavior through repression of Ezh2 and H3K27me3.
METHODS
All experiments were approved by the local Institutional Animal Care and Use Committee and conducted in accordance with National Institutes of Health guidelines on animal care and experimentation.
Animals
Adult male HR/LR rats were obtained from the 8th generations of our in-house colony (McCoy et al., 2016). Rats were pair-housed in a 12:12 light-dark cycle in a temperature- and humidity-controlled environment with food and water available ad libitum.
Viral Constructs
Adeno-associated viruses (AAVs) engineered to express either pre-miR-101a and GFP or only GFP were created by the University of Alabama at Birmingham, Neuroscience NINDS Protein Interactions Core C. pre-miR-101a was cloned downstream of GFP in the pSLIK series of vectors, MBA253 digested with BfuAI (ATCC) (Shin et al., 2006), and ligated with annealed primers to miR-101a (primers designed based on miRBase Accession Number: MI0000886) with overlapping BfuAI sites (miR-101a-F: 5′-agcgaTGCCCTGGCTCAGTTATCACAGTGCTGATGCTGTCCATTCTAAAGGTACAGTACTGTGATAACTGAAGGATGGCAC and miR-101a-R: 5′-ggcagTGCCATCCTTCAGTTATCACAGTACTGTACCTTTAGAATGGACAGCATCAGCACTGTGATAACTGAGCCAGGGCAT). The resulting MBA253-pre-miR-101a clone was amplified by PCR to include the GFP provided by the MBA253 vector [miR-101a-GFP-F: 5′-AAGCTTggatccGCCGCCACCATGGTGAGCAAGGGCGAG; mir101a-GFP-R: gatatcagatctAATTGAAAAAAGTGATTTAATTTATACC]. It was cloned into the BamHI and BglII sites of AAV-MCS (Agilent, Santa Clara, CA, USA) to obtain the AAV-GFP-miR-101a vector; the clones obtained were in reverse orientation due to the compatibility of the BamHI and BglII overhangs. The vector was digested with BamHI and XbaI and the ~1100 bp GFP-miR-101a fragment was subcloned into the BamHI and XbaI sites of AAV-MCS (Agilent) to achieve proper orientation with respect to the promoter. pAAV-GFP vector was obtained from Agilent. All clones were verified by sequencing.
For AAV production, 293A cells (Agilent) were transfected using calcium phosphate transfection (Clontech. Mountain View, CA, USA) with 10 μg each of pAAV-RC, pAAV-Helper, and AAV-GFP-miR-101a or pAAV-GFP. After 72 h, the transfected cells were harvested, pelleted, resuspended in serum-free DMEM, and subjected to four rounds of freeze/thaw cycles. The AAV-containing supernatants were spun at 10,000 × g for 10 min to pellet cell debris and viral supernatants were stored at −80°C. The AAVs were purified using columns according to the manufacturer’s instructions (Cell Biolabs, San Diego, CA, USA), which included final buffer exchange and concentration. AAV-GFP-pre-miR-101a (referred to henceforth as AAV-miR-101a) and AAV-GFP were tittered by infectivity on HT1080 cells (ATCC) (1.5 × 108 AAV-miR-101a and 1.2 × 108 AAV-GFP infectious units/ml) and by RT-PCR (5.3 × 1011 AAV-miR-101a and 5.2 × 1011 AAV-GFP genome copies/ml).
Intracranial Surgeries to Manipulate miR-101a or Ezh2 Expression
Intracranial surgeries were performed on 71 HR male rats (60–75 days old) as described previously (Nam and Kerman, 2016). One group of rats (N=33) were used in an experiment to overexpress miR-101a via AAV viral infusion in the amygdala; the second group (N=48) was used in an experiment to suppress Ezh2 expression via short-interfering RNA (siRNA) infusion. Rats were anesthetized with a mixture of oxygen (1 l/min) and isoflurane (5% for induction, and 1–3% for maintenance). Following administration of carprofen (5 mg/kg sc; Pfizer Inc., New York, NY, USA) and buprenorphine (0.05–0.1 mg/kg sc; Reckitt Benckiser Pharmaceuticals, Richmond, VA, USA) for post-surgical analgesia, rats were secured in a stereotaxic frame using blunt ear bars. Skin over the skull was shaved, sterilized, and incised. Bregma and lambda were identified in order to determine stereotaxic coordinates using Angle Two software (Leica Biosystems, Richmond, IL, USA). The coordinates A/P: −2.8, M/L: ±4.8, and D/V: −8.43 were used to target the left and right amygdala, respectively. Both left and right amygdala were injected with either 1 μl AAV-miR-101a (n=18; 5.3 × 1011 genome copies/ml) or AAV-GFP (n=15; 5.2 × 1011 genome copies/ml) for the miR-101a overexpression experiment or 1 μl Ezh2 siRNA (n=26; 15μM; GE Dharmacon, Lafayette, CO, USA) or control siRNA (n=22; 15μM) over 5-min. After injections the overlying skin was sutured. Rats were returned to their home cages upon full recovery from anesthesia.
Animals in the miR-101a overexpression experiment were given 2-weeks to allow for surgical recovery and adequate viral expression while animals in the Ezh2 siRNA experiment were given 7-days to recover. After each of these recovery periods, all animals were subjected to two days of behavioral testing.
Behavioral Testing
All behavior was recorded using a computerized analysis program (Ethovision XT 8.0, Noldus, Wageningen, The Netherlands) and all testing was conducted under dim light (30 lux) between 8:00–1:00 pm. Animals were tested first on the Open Field Test (OFT) followed by the Elevated Plus Maze (EPM) 24 h later.
Open Field Test
The OFT was conducted in a 100×100×50 cm black Plexiglas box with a black floor as described (Cohen et al., 2015). At the beginning of the test, a rat was placed in a corner of the box and was permitted to explore the apparatus for 5 min. The latency to enter the center of the OF, the amount of time spent and distance traveled in the center, sides, and corners of the apparatus were quantified. The periphery was defined by a 20 cm zone around the edge of the OF arena that was further subsided into mutually exclusive side (20 × 60 cm) and corner (20 × 20 cm) zones.
Elevated Plus Maze
The EPM test was conducted as described (Cohen et al., 2015) in a black Plexiglas EPM consisting of four elevated arms (70 cm from the floor, 45 cm long, 12 cm wide) arranged in a cross. Two opposite arms are enclosed by 45-cm-high walls (lighting 3–5 lux), and the remaining arms are open (lighting 30 lux). To start the test, rats were placed in a central square platform facing the same closed arm at the intersection of the open and closed arms provided access to all arms. The latency to enter the open arms, the amount of time spent in the open arms, closed arms, and center square, and the total distance traveled over the course of the 5-min test were recorded. An animal was considered to be in the open arm when the rat’s body fully crossed out of the center square onto an open arm platform.
Tissue Collection and Molecular Analyses
For HR/LR comparisons, 75-day old male rats were sacrificed (n=12/phenotype) by rapid decapitation, brains were removed, flash frozen, and stored at −80°C until further processing. For the miR-101a overexpression and Ezh2 siRNA experiments, all animals were sacrificed in the same manner 24 h following the last behavioral test.
RNA Extraction
Amygdala samples were dissected and RNA was extracted using the miRNeasy Mini Kit (Qiagen, Germantown, MD, USA). cDNA was created using the miScript RT Kit II (Qiagen, Germantown, MD, USA) and stored at −20°C until processing for quantitative Real-Time PCR (qRT-PCR).
Protein Extractions
Whole cell protein extractions were performed on amygdala samples in RIPA lysis buffer, quantified by BSA assay (Thermo Scientific, Waltham, MA), and stored at −80°C until processing for Western Blot.
Quantitative Real-Time PCR
cDNA was used for qRT-PCR as described (Clinton et al., 2011) on a StepOne Plus (Applied Biosystems, Grand Island, NY, USA). Primers for the mRNAs Ezh2 (F-GGAGACGATCCTGATGAAAGAG; R-CTTCTGCTGTGCCCTTATCT), Gapdh (F-ACCTTTGATGCTGGGGCTGGC; R-GGGCTGAGTTGGGATGGGGACT), and GFP (F-ATGGTGAGCAAGGGCGAGGA; R-TTTACGTCGCCGTCCAGCTCGA) were purchased from Integrated DNA Technologies (Coralville, Iowa, USA). Primers for the small RNAs rno-miR-101a-3p (Ca. #: MS00012950), pre-miR-101a (Ca. #: MP00006993), and Snord61 (Ca. #: MS00033705) were ordered from Qiagen.
Western Blot
Protein fractions were added to Laemmli sample buffer and loaded onto SDS-PAGE gels (4–20% acrylamide and 12% acrylamide for histones; Bio-Rad, Hercules, CA, USA) for protein gel electrophoresis. Immunoblots were later incubated in primary antibodies overnight at 4 °C followed by 1 h incubation with secondary antibodies and visualization on an Odyssey Infrared Imaging System (LI-COR). Following imaging, blots were put in a stripping buffer if necessary (1:50 NaOH) for 10 min and re-blocked prior to incubation with the primary antibody for the housekeeping protein (actin or histone H3) for 2 h. Secondary antibody incubation and imaging was performed in the same manner. The following primary antibodies (all of rabbit host) were used: anti-H3K27me3 (1:4000; Millipore #07-449), anti-Histone H3 (1:4000; Abcam #ab1791), anti-Ezh2 (1:2000; Abcam #ab3748), and anti-Actin (1:2000; Abcam #ab1801). Secondary antibodies were donkey anti-rabbit IRDye 800CW fluorescent antibodies (1:20,000; LI-COR #925-32213).
GFP Histology
Two-weeks following intracranial surgery, HR rats that were injected with either AAV-miR-101a (N=3) or AAV-GFP (N=3) were not exposed to behavioral testing, but instead were deeply anesthetized with sodium pentobarbital (150 mg/kg i.p.) and then transcardially perfused with ~100 ml of physiological saline followed by ~300 ml of 4% paraformaldehyde. Brains were extracted, post-fixed overnight, cryoprotected in 20% sucrose, and processed for immunohistochemistry as described in previous studies (Kerman et al., 2006). Briefly, brains were sectioned coronally on a freezing microtome at a thickness of 40 μm. Sections were washed in phosphate buffered saline (PBS) (pH 7.2), blocked in a PBS solution containing 1% BSA, 1% normal goat serum, and 0.3% Triton X-100 for 1 h, and incubated overnight in chicken anti-GFP antibody (1:500; Invitrogen A1026) at 4 °C. The next day, sections were again washed in PBS, incubated in secondary antibody (goat anti-chicken IgY, Alexa Fluor 488; Invitrogen A11039) for 2 h at room temperature, and mounted on slides and coverslipped using Fluromount-G (SouthernBiotech, Birmingham, AL, USA). Images were taken using an Olympus BX-UCB microscope (Center Valley, PA, USA) equipped with a motorized stage (96S100-LE; Ludl Electronic Products, Hawthorne, NY, USA), fluorophore-specific fluorescent filter sets, and a cooled mono CCD camera (Orca R2; Hamamtsu, Boston, MA, USA).
Statistical Analyses
Data were analyzed with Prism 6.0 software (GraphPad Software, San Diego, CA). qPCR data was analyzed by comparing ΔCTs, difference in threshold between the gene of interest and housekeeping gene (Gapdh for mRNA and Snord61 for miRNA). Western blots were analyzed using ImageJ software (NIH, Bethesda, MD), where band densities were determined using area under the curve of each lane’s optical density histogram. Bands for H3K27me3 were normalized to total Histone 3, while bands for Ezh2 were normalized to actin; HR/LR, AAV-miR-101a/AAV-GFP, and Ezh2 siRNA/Control siRNA groups were compared via one-way Student’s t-test or Welch’s corrected t-test when there was a significant difference (p<0.05) between the variances of the two groups. OFT and EPM data were compared in the same manner. For all tests, significance was set at p < 0.05 and results are presented as mean ± SEM.
RESULTS
HR rats display reduced expression of miR-101a-3p and increased expression of Ezh2 and H3K27me3 in the amygdala compared to LR rats
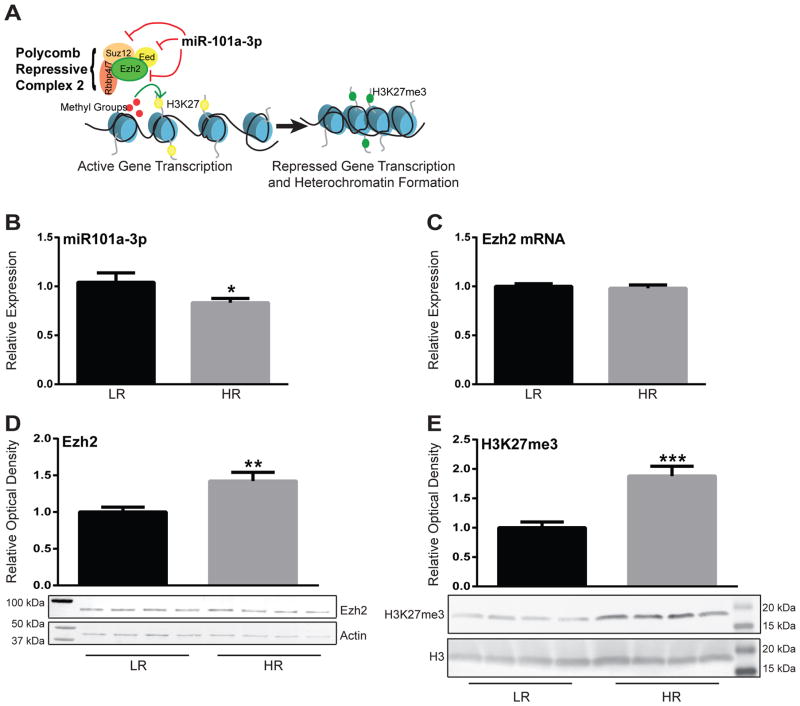
Using a high-throughput sequencing approach, we previously found reduced miR-101a-3p expression in the amygdala of HR rats compared to LRs. miR-101a-3p is a known regulator of PRC2 that represses the function of Ezh2 (Varambally et al., 2008; Friedman et al., 2009), the histone methyltransferase that mediates gene silencing by trimethylating H3K27 (Fig. 1A) (Cao et al., 2002). Using qRT-PCR we confirmed a significant reduction in miR-101a-3p in the amygdala of HR rats compared to LRs (p<0.05; Fig. 1B). Consistent with miR-101a-3p’s reported role in repressing translation of Ezh2, we found that while there was no change in Ezh2 mRNA (Fig. 1C) there was significantly more Ezh2 protein in HR compared to LR amygdala (p<0.01; Fig. 1D). HRs also displayed greater levels of H3K27me3 compared to LRs (p<0.001; Fig. 1E), indicating greater functional output of Ezh2 activity.
Figure 1. High novelty responding (HR) rats display reduced miR-101a-3p, Ezh2, and H3K27me3 expression compared to low novelty responding (LR) rats in the amygdala.
(a) Schematic representation miR-101a-3p’s role in repressing gene transcription. miR-101a-3p inhibits the translation of several proteins that comprise the Polycomb Repressive Complex 2 (PRC2), including the histone-methyltransferase enhancer of zeste homolog 2 (Ezh2), embryonic ectoderm development (Eed), and suppressor of zeste (Suz12). Ezh2 mediates the functional output of the PRC2 since it silences target gene expression via tri-methylation of Histone 3 at Lysine 27 (H3K27me3). (b) Quantitative Real-Time PCR for miR-101a-3p showed that HRs have reduced expression in the amygdala compared to LRs (p<0.05). (b) There was no difference in Ezh2 mRNA between HRs and LRs. (c) HRs displayed greater Ezh2 protein levels compared to LRs (p<0.01). (d) HRs also displayed greater H3K27me3 levels compared to LRs (p<0.001). *p<0.05, **p<0.01, ***p<0.001
Viral-mediated miR-101a overexpression in the amygdala increases HR rats’ anxiety-like behavior
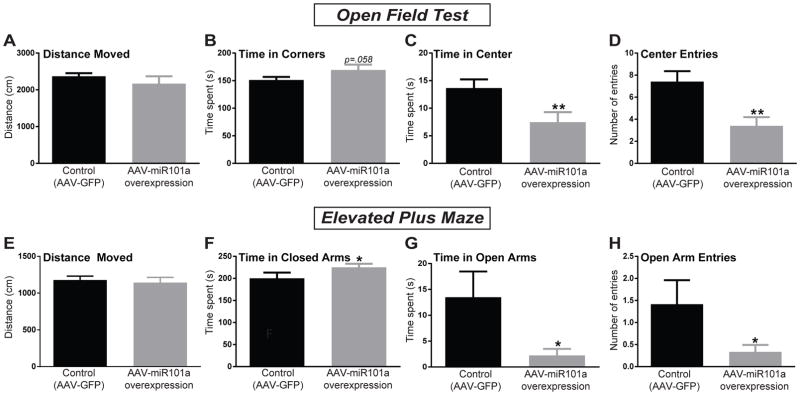
A separate cohort of HR rats received intra-amygdala injections of either AAV-miR-101a (to overexpress miR-101a-3p) or AAV-GFP (control) and were tested on two rodent tests of anxiety, the OFT and EPM. On the OFT, there was no difference in total distance moved between the two groups (Fig. 2A) indicating that the viruses did not differentially affect locomotion. HR rats injected with the miR-101a-overexpressing virus displayed increased time spent in the corners of the OFT (p=0.058), a reduction in time spent in the center (p<0.01), and fewer center entries (p<0.01; Fig. 2B–D) compared to the control group. On the EPM, there was also no group difference in total distance moved (Fig. 2E). AAV-miR-101a-injected HRs spent more time in the closed arms (p<0.05), less time in the open arms (p<0.05), and had fewer open arm entries (p<0.05; Fig. 2F–H) compared to AAV-GFP injected rats. These results indicate that overexpressing miR-101a in HR rats’ amygdala led to increased anxiety-like behavior across both the OFT and EPM.
Figure 2. Viral-mediated miR-101a over-expression in the HR amygdala increased anxiety-like behavior on the Open Field Test (OFT) and the Elevated Plus Maze (EPM).
(a) There was no difference in total distance moved between HR rats injected with AAV-miR101a and control rats injected with AAV-GFP. (b) miR-101a overexpression lead HRs to spend more time in corners of the OFT (p=0.06), (c) spend less time in the center (p<0.01), and (d) make fewer center entries (p<0.01) compared to the control group. (e) There were no group differences in overall activity in the EPM. (f) miR-101a overexpression lead HRs to spend more time in the closed arms (p<0.05), (g) spend less time in the open arms (p<0.05), and (h) show fewer open arm entries (p<0.05) compared to the AAV-GFP control animals. *p<0.05, **p<0.01
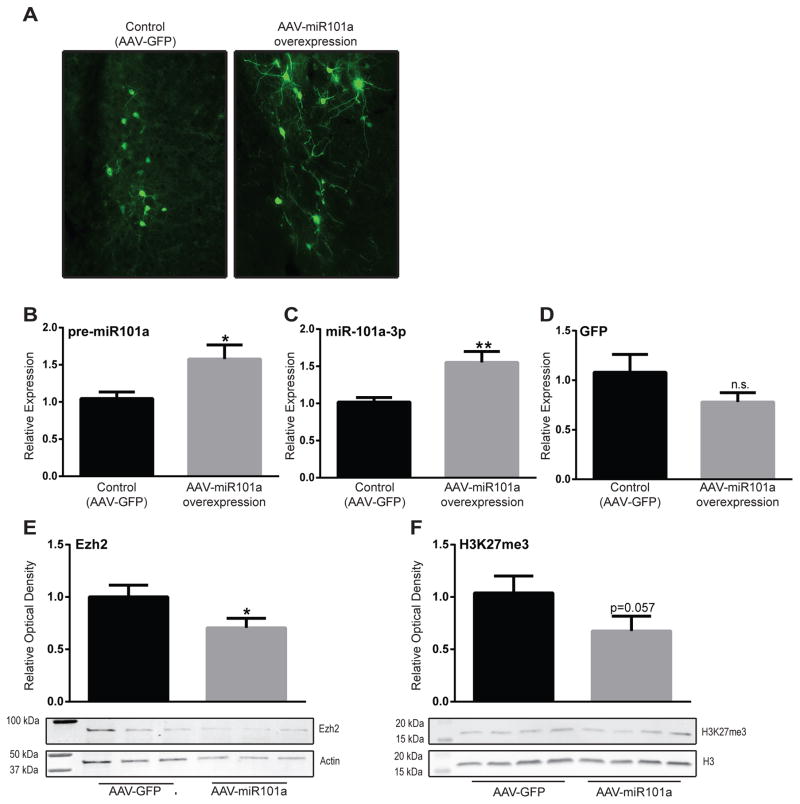
Viral expression was confirmed through visualization of GFP for both the AAV-GFP and the AAV-miR-101a viruses (Fig. 3A). Using qRT-PCR, we confirmed that HRs injected with the AAV-miR-101a virus had greater expression of pre-miR101a (p<0.05) and mature miR-101a-3p (p<0.01) compared to controls. There was no GFP expression differences between the two experimental groups (Fig. 3B–D). We also showed that miR-101a over-expression caused a reduction in Ezh2 protein levels (p<0.05; Fig. 3E) and reduced H3K27me3 levels (p=0.057; Fig. 3F), which is consistent with miR-101a’s known role in repressing Ezh2 expression and functional output.
Figure 3. Viral-mediated miR-101a-3p over-expression lead to reduced Ezh2 and H3K27me3 levels in the HR amygdala.
(a) Viral expression of AAV-miR101a and AAV-GFP. (b) HR rats that received intra-amygdalar injections of AAV-miR101a showed increased expression of pre-miR101a (p<0.05) and (c) mature miR-101a-3p (p<0.01) compared to animals injected with AAV-GFP. (d) There were no differences in GFP mRNA between AAV-miR101a and AAV-GFP animals. (e) Viral-mediated miR-101a over-expression caused a reduction in Ezh2 protein expression (p<0.05) and (f) H3K27me3 (p=0.057) in the amygdala of HR rats. *p<0.05, **p<0.01
Knockdown of Ezh2 increases HR rats’ anxiety-like behavior
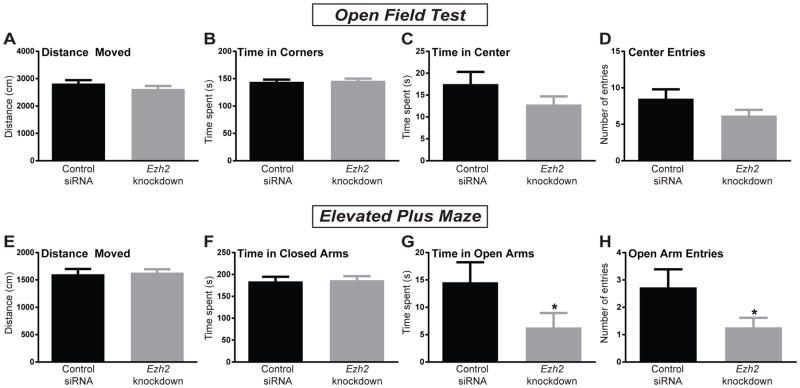
In order to determine if the anxiogenic effects of miR-101a overexpression in the HR amygdala are mediated through repression of Ezh2 by miR-101a-3p, another group of HR rats received intra-amygdalar injections of either an Ezh2 targeting siRNA or a control siRNA. Ezh2 knockdown did not affect behavior in the OFT, with no group differences in distance moved, time in corners, time in center, or center entries into the OF (Fig. 4A–D). On the EPM there were no differences in total distance moved or time in the closed arms (Fig. 4E–F). However, animals that received the Ezh2 siRNA spent less time in the open arms (p<0.05) and had fewer open arm entries (p<0.05; Fig. 4G–H). Overall these data showed that Ezh2 knockdown in the amygdala of HR rats had a weaker anxiogenic effect compared to miR-101a overexpression as it increased anxiety-like behavior on the EPM, but no the OFT.
Figure 4. siRNA knockdown of Ezh2 in the HR rat amygdala increased anxiety-like behavior in the EPM.
(a) Injection of short-interfering (siRNA) targeting Ezh2 into the amygdala of HR rats did not alter the total distance moved, (b) time spent in corners, (c) time spent in the center, or (d) the number of center entries in the Open Field Test (OFT) compared to rats injected with control siRNA. (e) Ezh2 knockdown did not alter total distanced moved or (f) time spent in the closed arms on the elevated plus maze (EPM) compared to control rats. (g) Ezh2 siRNA did reduce HR rats’ time spent in the open arms (p<0.05) and (h) the number of open arm entries (p<0.05) compared to control siRNA-treated animals. *p<0.05
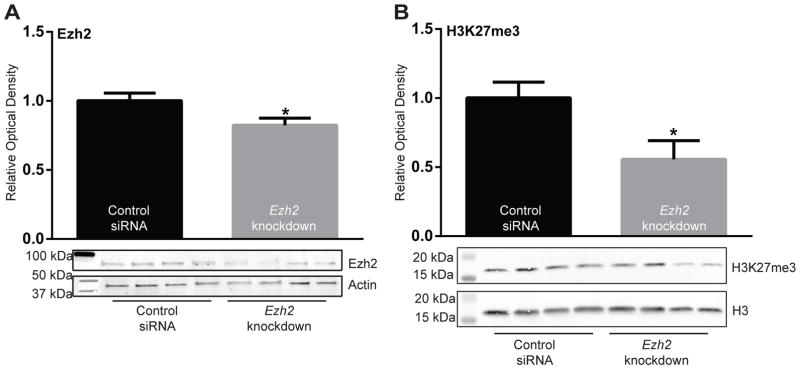
Animals were sacrificed 24 h after EPM testing and knockdown of Ezh2 in the amygdala was confirmed (p<0.05; Fig. 5A). We also showed that Ezh2 knockdown caused a reduction in total H3K27me3 in the amygdala (p<0.05; Fig. 5B)
Figure 5. Injection of siRNA targeting Ezh2 in the amygdala reduced Ezh2 protein levels and its functional output, H3K27me3.
(a) Animals injected with siRNA targeting Ezh2 displayed reduced expression of Ezh2 protein (p<0.05) and (b) reduced Ezh2 output, trimethylation of histone 3 at lysine 27 (H327me3) levels (p<0.05) compared to animals injected with control siRNA. *p<0.05
DISCUSSION
The HR/LR rat lines provide a useful tool for studying genetic and molecular factors that contribute to anxiety-like behavior. Selective breeding for high vs low locomotor response to novelty produced the behaviorally distinct HR and LR lines, which differ in a number of behavioral domains, including trait anxiety. LR rats display greater anxiety-like behavior compared to HRs on several behavior tests, including the elevated plus maze, open field test, and light dark box (Stead et al., 2006; Clinton et al., 2014; Cohen et al., 2015; Glover et al., 2015), and these differences are observable as early as the 25th day of life (Clinton et al., 2011). Since the HR/LR behavioral phenotypes are highly heritable and apparent early in life, our research group has sought to identify genetic and epigenetic factors that shape their behavior. We recently used next-generation sequencing to identify numerous miRNA and mRNA expression differences in the amygdala of HR/LR rats (Cohen et al., 2016). The miRNA miR-101a-3p emerged as an abundant miRNA in the amygdala that was expressed at significantly higher levels in LR versus HR rats. Our current study confirmed that HRs exhibit lower miR-101a levels in the amygdala compared to LRs as well as greater levels of Ezh2 and H3K27 (Ezh2’s functional output); this is consistent with the notion that LRs’ elevated miR-101a-3p levels impinge on PRC2/Ezh2 function and hinder H3K27me3 (relative to HRs). We therefore tested the hypothesis that increasing miR-101a-3p in the HR amygdala would increase their typically low levels of anxiety-like behavior. We found that viral-mediated miR-101a-3p overexpression in the HR amygdala increased their anxiety-like behavior on the EPM and OFT. Overexpressing miR-101a-3p also reduced levels of its target, the histone methyltransferase Ezh2, and H3K27me3. Using an siRNA approach, we found that direct knockdown of Ezh2 also caused an increase in HRs’ anxiety-like behavior, but to a lesser degree than miR-101a-3p over-expression.
Polycomb group proteins (like the PRC2) play an important role in gene silencing through post-translational modification of histone tails. miR-101a-3p is known to negatively regulate PRC2 by inhibiting translation of multiple PRC2 components including embryonic ectoderm development (Eed), suppressor of zeste (Suz12), and Ezh2, the histone methyltransferase that silences gene expression via tri-methylation H3K27 (Varambally et al., 2008; Cao et al., 2010). PRC2/Ezh2 function is required for a number of developmental processes (Chakrabarty et al., 2007; Marchesi et al., 2014), including maintaining embryonic and adult stem cells, likely due to its role in repressing developmental transcription factor expression (Pietersen and van Lohuizen, 2008). Given their roles in stem cell maintenance, miR-101a and Ezh2 have been widely studied in the context of cancer. For instance, miR-101a expression decreases and Ezh2 expression increases during progression of prostate cancer (Varambally et al., 2008) and bladder cancer (Friedman et al., 2009). The Ezh2 inhibitor tazemetostat is currently undergoing clinical trials to test safety and efficacy in treating several different cancers (Kurmasheva et al., 2016).
In the brain, Ezh2 has been shown to play a role in neural stem cell differentiation. Ezh2 is expressed at high levels in neural stem cells and its expression decreases during differentiation into neurons and astrocytes, but not oligodendrocytes (Sher et al., 2008); Ezh2 over-expression in astrocytes promotes de-differentiation towards a neural stem cell-like state (Sher et al., 2011). Conditional Ezh2 knockout in neural stem cells results in mice with impaired spatial learning and memory and contextual fear learning and memory as well as decreased progenitor cell proliferation (Zhang et al., 2014). While Ezh2 clearly regulates a number of key neurodevelopmental processes (Di Meglio et al., 2013; Neo et al., 2014), it continues to serve important functions in mature neurons, particularly in regards to cellular plasticity (Zhang et al., 2014). Morphine exposure causes reduced expression of Bdnf in the ventral tegmental area by recruiting Ezh2 and increasing H3K27me3 at the Bdnf promotor, and Ezh2 over-expression combined with morphine exposure increased animals conditioned place preference to the drug (Koo et al., 2015). In the cerebellum, Ezh2 restricts dendrite morphogenesis through H3K27me3-mediated repression of Bdnf (Qi et al., 2014). Other work shows that Ezh2 expression is sensitive to external stimuli, such as stress (Hunter et al., 2009; Xu et al., 2012) and neural activity, which transiently increases Ezh2 and H3K27me levels (Reynolds et al., 2015; Palomer et al., 2016). Thus, while Ezh2 has traditionally been considered in the context of cell development and differentiation, recent evidence clearly shows numerous important roles in the adult brain, particularly in the ability of neurons to respond to environmental signals.
Selectively-bred HR rats, which are known to display low levels of anxiety-like behavior, exhibit reduced miR-101a-3p expression together with increased Ezh2 and H3K27me3 levels in the amygdala compared to LRs that characteristically display high levels of behavioral inhibition and anxiety-like behavior. Since Ezh2 maintains expression to varying degrees in mature neurons, astrocytes, and oligodendrocytes, it is unclear from our current data which cell type(s) may be contributing to the observed HR/LR differences. In our viral manipulation experiment, miR-101a-3p over-expression was limited to neurons through the use of AAV vectors, which selectively transduct neurons but not glia (Mason et al., 2010; Doherty et al., 2011), and the Ezh2 knockdown experiment using Accell siRNA technology likely predominantly affected neurons since this tool was previously shown to selectively transduce mature neurons but not glia (Nakajima et al., 2012). Consequently, the observed anxiogenic effects caused by miR-101a over-expression and Ezh2 suppression are likely the result of alterations in neural function. Future work will determine if knockdown or inhibition of miR-101a-3p and/or over-expression of Ezh2 in the amygdala can produce anxiolytic effects (either in high anxiety-prone LRs or normative rates). We will also continue to investigate the genetic and epigenetic differences between HR and LR rats that may contribute to their disparate stress reactive and behavioral phenotypes. Chromatin immunoprecipitation studies will determine differences in genomic binding locations of Ezh2 and H3K27me3 between HRs and LRs. These locations could be compared to changes in Ezh2 and H3K27me3 sites that occur following miR-101a-3p overexpression and/or Ezh2 knockdown. Ultimately such analyses could potentially identify downstream genes and pathways that are responsible for the miR-101a-3p and Ezh2 driven changes in anxiety-like behavior.
In conclusion, viral mediated miR-101a-3p over-expression in the amygdala of low-anxiety HR rats increases their anxiety-like behavior and reduces Ezh2 and H3K27me3 levels. Direct Ezh2 knockdown also increases anxiety-like behavior, but not to the same extent as miR-101a-3p over-expression. Thus, increasing the expression of miR-101a-3p in the amygdala produces an anxiogenic effect that is at least partially mediated via the repression of Ezh2 and its functional output, H3K27me3. Overall, these studies point to a novel molecular mechanism that may contribute to individual differences in anxiety-like behavior. They also add to the growing body of evidence supporting a role for mechanisms once considered to be exclusive to development and differentiation, such as miRNA and epigenetic regulation, in the adult brain and in underlying behavioral phenomena.
SIGNIFICANCE STATEMENT.
We identify the microRNA miR-101a-3p and its gene target enhancer of zeste homolog 2 (Ezh2) in the amygdala as novel molecular factors that shape anxiety-like behavior. Selectively-bred rats that display low trait anxiety show reduced miR-101a-3p and increased Ezh2 expression in the amygdala compared to rats that display high trait anxiety. Viral-mediated miR-101a-3p over-expression in the amygdala increased anxiety-like behavior in low trait-anxiety rats and reduced levels of Ezh2. Knockdown of Ezh2 with short-interfering RNA also increased anxiety-like behavior, but to a lesser extent than miR-101a-3p overexpression. We show that amygdalar expression of miR-101a-3p contributes to anxiety-like behavior and that this effect is at least partially mediated via repression of Ezh2.
Acknowledgments
This study was funded by R01MH105447-01 (SMC), R01MH097909 (FDL), NIGMS MSTP 5T32GM008361 and NINDS 5T32NS061788 (which supported JLC and WMW). Viral constructs were produced by the UAB Neuroscience NINDS Protein Interactions Core C. (P30 NS047466)
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest
References
- Andolina D, Di Segni M, Bisicchia E, D’Alessandro F, Cestari V, Ventura A, Concepcion C, Puglisi-Allegra S, Ventura R. Effects of lack of microRNA-34 on the neural circuitry underlying the stress response and anxiety. Neuropharmacology. 2016;107:305–316. doi: 10.1016/j.neuropharm.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P, Voinnet O. Revisiting the principles of microRNA target recognition and mode of action. Nature reviews Molecular cell biology. 2009;10:141–148. doi: 10.1038/nrm2619. [DOI] [PubMed] [Google Scholar]
- Cao P, Deng Z, Wan M, Huang W, Cramer SD, Xu J, Lei M, Sui G. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1alpha/HIF-1beta. Molecular cancer. 2010;9:108. doi: 10.1186/1476-4598-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey SK. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci U S A. 2007;104:15144–15149. doi: 10.1073/pnas.0705917104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Watson SJ, Akil H. High novelty-seeking rats are resilient to negative physiological effects of the early life stress. Stress. 2014;17:97–107. doi: 10.3109/10253890.2013.850670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Stead JD, Miller S, Watson SJ, Akil H. Developmental underpinnings of differences in rodent novelty-seeking and emotional reactivity. The European journal of neuroscience. 2011;34:994–1005. doi: 10.1111/j.1460-9568.2011.07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JL, Glover ME, Pugh PC, Fant AD, Simmons RK, Akil H, Kerman IA, Clinton SM. Maternal Style Selectively Shapes Amygdalar Development and Social Behavior in Rats Genetically Prone to High Anxiety. Developmental neuroscience. 2015;37:203–214. doi: 10.1159/000374108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JL, Ata AE, Jackson NL, Rahn EJ, Ramaker RC, Cooper S, Kerman IA, Clinton SM. Differential stress induced c-Fos expression and identification of region-specific miRNA-mRNA networks in the dorsal raphe and amygdala of high-responder/low-responder rats. Behavioural brain research. 2016 doi: 10.1016/j.bbr.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio T, Kratochwil CF, Vilain N, Loche A, Vitobello A, Yonehara K, Hrycaj SM, Roska B, Peters AH, Eichmann A, Wellik D, Ducret S, Rijli FM. Ezh2 orchestrates topographic migration and connectivity of mouse precerebellar neurons. Science. 2013;339:204–207. doi: 10.1126/science.1229326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty FC, Schaack JB, Sladek CD. Comparison of the efficacy of four viral vectors for transducing hypothalamic magnocellular neurosecretory neurons in the rat supraoptic nucleus. Journal of neuroscience methods. 2011;197:238–248. doi: 10.1016/j.jneumeth.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Liang G, Liu CC, Wolff EM, Tsai YC, Ye W, Zhou X, Jones PA. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer research. 2009;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- Glover ME, Pugh PC, Jackson NL, Cohen JL, Fant AD, Akil H, Clinton SM. Early-life exposure to the SSRI paroxetine exacerbates depression-like behavior in anxiety/depression-prone rats. Neuroscience. 2015;284:775–797. doi: 10.1016/j.neuroscience.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauberg ME, Roussos P, Grove J, Borglum AD, Mattheisen M Schizophrenia Working Group of the Psychiatric Genomics C. Analyzing the Role of MicroRNAs in Schizophrenia in the Context of Common Genetic Risk Variants. JAMA psychiatry. 2016;73:369–377. doi: 10.1001/jamapsychiatry.2015.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommers L, et al. MicroRNA hsa-miR-4717-5p regulates RGS2 and may be a risk factor for anxiety-related traits. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2015;168B:296–306. doi: 10.1002/ajmg.b.32312. [DOI] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci U S A. 2009;106:20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Kim SN, Liu X, Zhang H, Zhang C, Seo JS, Kim Y, Sun T. miR-17-92 Cluster Regulates Adult Hippocampal Neurogenesis, Anxiety, and Depression. Cell reports. 2016;16:1653–1663. doi: 10.1016/j.celrep.2016.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman IA, Shabrang C, Taylor L, Akil H, Watson SJ. Relationship of presympathetic-premotor neurons to the serotonergic transmitter system in the rat brainstem. The Journal of comparative neurology. 2006;499:882–896. doi: 10.1002/cne.21129. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychological medicine. 2008;38:365–374. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, et al. Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nature neuroscience. 2015;18:415–422. doi: 10.1038/nn.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurmasheva RT, Sammons M, Favours E, Wu J, Kurmashev D, Cosmopoulos K, Keilhack H, Klaus CR, Houghton PJ, Smith MA. Initial testing (stage 1) of tazemetostat (EPZ-6438), a novel EZH2 inhibitor, by the Pediatric Preclinical Testing Program. Pediatric blood & cancer. 2016 doi: 10.1002/pbc.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi I, Bagella L. Role of enhancer of zeste homolog 2 polycomb protein and its significance in tumor progression and cell differentiation. In: Radzioch D, editor. Chromatin Remodelling. InTech; 2013. pp. 119–152. [Google Scholar]
- Marchesi I, Giordano A, Bagella L. Roles of enhancer of zeste homolog 2: from skeletal muscle differentiation to rhabdomyosarcoma carcinogenesis. Cell Cycle. 2014;13:516–527. doi: 10.4161/cc.27921. [DOI] [PubMed] [Google Scholar]
- Mason MR, Ehlert EM, Eggers R, Pool CW, Hermening S, Huseinovic A, Timmermans E, Blits B, Verhaagen J. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Molecular therapy: the journal of the American Society of Gene Therapy. 2010;18:715–724. doi: 10.1038/mt.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathonnet G, Fabian MR, Svitkin YV, Parsyan A, Huck L, Murata T, Biffo S, Merrick WC, Darzynkiewicz E, Pillai RS, Filipowicz W, Duchaine TF, Sonenberg N. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science. 2007;317:1764–1767. doi: 10.1126/science.1146067. [DOI] [PubMed] [Google Scholar]
- McCoy CR, Golf SR, Melendez-Ferro M, Perez-Costas E, Glover ME, Jackson NL, Stringfellow SA, Pugh PC, Fant AD, Clinton SM. Altered metabolic activity in the developing brain of rats predisposed to high versus low depression-like behavior. Neuroscience. 2016;324:469–484. doi: 10.1016/j.neuroscience.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, Kaufer D. Changes in brain MicroRNAs contribute to cholinergic stress reactions. Journal of molecular neuroscience: MN. 2010;40:47–55. doi: 10.1007/s12031-009-9252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muinos-Gimeno M, Espinosa-Parrilla Y, Guidi M, Kagerbauer B, Sipila T, Maron E, Pettai K, Kananen L, Navines R, Martin-Santos R, Gratacos M, Metspalu A, Hovatta I, Estivill X. Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biological psychiatry. 2011;69:526–533. doi: 10.1016/j.biopsych.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Kubo T, Semi Y, Itakura M, Kuwamura M, Izawa T, Azuma YT, Takeuchi T. A rapid, targeted, neuron-selective, in vivo knockdown following a single intracerebroventricular injection of a novel chemically modified siRNA in the adult rat brain. Journal of biotechnology. 2012;157:326–333. doi: 10.1016/j.jbiotec.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Nam H, Kerman IA. A2 noradrenergic neurons regulate forced swim test immobility. Physiology & behavior. 2016;165:339–349. doi: 10.1016/j.physbeh.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Neo WH, Yap K, Lee SH, Looi LS, Khandelia P, Neo SX, Makeyev EV, Su IH. MicroRNA miR-124 controls the choice between neuronal and astrocyte differentiation by fine-tuning Ezh2 expression. J Biol Chem. 2014;289:20788–20801. doi: 10.1074/jbc.M113.525493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RM, Dinan TG, Cryan JF. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Molecular psychiatry. 2012;17:359–376. doi: 10.1038/mp.2011.162. [DOI] [PubMed] [Google Scholar]
- Palomer E, Carretero J, Benvegnu S, Dotti CG, Martin MG. Neuronal activity controls Bdnf expression via Polycomb de-repression and CREB/CBP/JMJD3 activation in mature neurons. Nature communications. 2016;7:11081. doi: 10.1038/ncomms11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen AM, van Lohuizen M. Stem cell regulation by polycomb repressors: postponing commitment. Current opinion in cell biology. 2008;20:201–207. doi: 10.1016/j.ceb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Qi C, Liu S, Qin R, Zhang Y, Wang G, Shang Y, Wang Y, Liang J. Coordinated regulation of dendrite arborization by epigenetic factors CDYL and EZH2. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:4494–4508. doi: 10.1523/JNEUROSCI.3647-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JP, Miller-Delaney SF, Jimenez-Mateos EM, Sano T, McKiernan RC, Simon RP, Henshall DC. Transcriptional Response of Polycomb Group Genes to Status Epilepticus in Mice is Modified by Prior Exposure to Epileptic Preconditioning. Front Neurol. 2015;6:46. doi: 10.3389/fneur.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi A, Vincenti S, De Vito F, Bozzoni I, Oliverio A, Presutti C, Fragapane P, Mele A. Stress induces region specific alterations in microRNAs expression in mice. Behavioural brain research. 2010;208:265–269. doi: 10.1016/j.bbr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Sher F, Boddeke E, Copray S. Ezh2 expression in astrocytes induces their dedifferentiation toward neural stem cells. Cellular reprogramming. 2011;13:1–6. doi: 10.1089/cell.2010.0052. [DOI] [PubMed] [Google Scholar]
- Sher F, Rossler R, Brouwer N, Balasubramaniyan V, Boddeke E, Copray S. Differentiation of neural stem cells into oligodendrocytes: involvement of the polycomb group protein Ezh2. Stem cells. 2008;26:2875–2883. doi: 10.1634/stemcells.2008-0121. [DOI] [PubMed] [Google Scholar]
- Shin KJ, Wall EA, Zavzavadjian JR, Santat LA, Liu J, Hwang JI, Rebres R, Roach T, Seaman W, Simon MI, Fraser ID. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13759–13764. doi: 10.1073/pnas.0606179103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Lugli G, Rizavi HS, Zhang H, Torvik VI, Pandey GN, Davis JM, Dwivedi Y. MicroRNA expression in rat brain exposed to repeated inescapable shock: differential alterations in learned helplessness vs. non-learned helplessness. The international journal of neuropsychopharmacology. 2011;14:1315–1325. doi: 10.1017/S1461145710001628. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behavior genetics. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, Laxman B, Cao X, Jing X, Ramnarayanan K, Brenner JC, Yu J, Kim JH, Han B, Tan P, Kumar-Sinha C, Lonigro RJ, Palanisamy N, Maher CA, Chinnaiyan AM. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Chen Y, Zhang H, Chen Y, Shen X, Shi C, Liu Y, Yuan W. Integrated microRNA-mRNA analyses reveal OPLL specific microRNA regulatory network using high-throughput sequencing. Scientific reports. 2016;6:21580. doi: 10.1038/srep21580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Denbow CJ, Meiri N, Denbow DM. Fasting of 3-day-old chicks leads to changes in histone H3 methylation status. Physiol Behav. 2012;105:276–282. doi: 10.1016/j.physbeh.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ji F, Liu Y, Lei X, Li H, Ji G, Yuan Z, Jiao J. Ezh2 regulates adult hippocampal neurogenesis and memory. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:5184–5199. doi: 10.1523/JNEUROSCI.4129-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]