Abstract
Multiple neurodegenerative disorders typically result from irrevocable damage and improper functioning of specialized neuronal cells or populations of neuronal cells. These disorders have the potential to contribute to an already overburdened health care system unless the progression of neurodegeneration can be altered. Progress in understanding neurodegenerative cell biology has been hampered by a lack of predictive and, some would claim, relevant cellular models. Additionally, the research needed to develop new drugs and determine methods for repair or replacement of damaged neurons is severely hampered by the lack of an adequate in vitro human neuron cell-based model. In this context, pluripotent stem cells and neural progenitors and their properties—including unlimited proliferation, plasticity to generate other cell types, and a readily available source of cells—pose an excellent alternative to ex vivo primary cultures or established immortalized cell lines in contributing to our understanding of neurodegenerative cell biology and our ability to analyze the therapeutic or cytotoxic effects of chemicals, drugs, and xenobiotics. Many questions that define the underlying “genesis” of the neuronal death in these disorders also remain unanswered, with evidence suggesting a key role for mitochondrial dysfunction. The assessment of stem cells, neural progenitors, and engineered adult cells can provide useful insights into neuronal development and neurodegenerative processes. Finally, the potential for a combination of cell- and gene-based therapeutics for neurodegenerative disorders is also discussed.
Keywords: pluripotent stem cells, neural progenitors, mitochondria, gene therapy, cellular differentiation
I. STEM CELLS AND NEURONAL DEVELOPMENT
I.A. Basics of Neuronal Development
During early embryogenesis and prior to gastrulation, signals originating from extra-embryonic and visceral endoderm govern the induction of the three germ layers (ectoderm, endoderm, and mesoderm).1 After the formation of the three germ layers, initial signals from the mesoderm induce early neuroectoderm formation that forms the neural plate, which folds and closes itself to form the neural tube. The neural tube gives rise to the essential elements in the central nervous system (CNS): neurons, astrocytes, and oligodendrocytes. These early specification events are coordinated by multiple components of four signaling pathways that play a major role during mammalian neurogenesis. Coordination between the fibroblast growth factor-2 (FGF2), bone morphogenetic protein (BMP), Wnt-β-catenin, and Notch pathways is crucial for the initiation of neurogenesis.
In neural specification during early embryonic development, FGF signaling may induce an early neural state while also stabilizing neural identity by serving as an antagonist to BMP signaling.2 Based on available information on patterning cues, FGF signaling may enrich survival and proliferation of the early forebrain neural precursor cell types. BMPs are members of the transforming growth factor-alpha (TGFα) superfamily and play multiple roles in CNS and peripheral nervous system development. Loss of BMP signaling is implicated in neural induction from the ectoderm at or before gastrulation,3 with expression of BMP antagonists such as noggin, chordin, and follistatin involved in dorsoventral patterning of the spinal cord.4,5 Thus, early in development, BMPs mainly specify forebrain cells, and their inhibition before or during gastrulation causes neural induction. Retinoic acid plays important roles in many aspects of neural development, including neuronal differentiation and patterning of the neural plate and neural tube in the early embryos.6 Retinoic acid is specifically implicated in organizing the posterior hindbrain and anterior spinal cord. In the dorsoventral axis of the neural tube, retinoic acid works synergistically with other molecules such as sonic hedgehog (SHH), FGF, and BMP to determine the fates of sensory neurons, interneurons, and motor neurons. The molecular mechanisms by which the different signaling pathways converge on the expression of early neural genes are still poorly understand and are the subject of numerous ongoing studies. One study has shown that Wnt and FGF signaling cooperate independent of BMP signaling to activate Sox2 expression, which is particularly important in the specification of neuroectoderm.7 Several members of the SoxB transcription factor family are expressed throughout the neural plate and are important for the maintenance of a neuroepithelial character, as their expression in embryonic stem cells (ESCs) is correlated with neuroectodermal lineage commitment.8,9
During in vivo development, formation of the neural plate and eventually the neural tube occurs when the human embryo is approximately 3 weeks old. Human ESCs (hESCs) are in vitro equivalents of the inner cell mass of a 5- to 6-d-old blastocyst-stage embryo, and can be propagated indefinitely in culture. 10–12 A striking feature during early neural differentiation of hESCs is the formation of neural rosettes,13 which are considered to be an early intermediate composed of radially organized columnar neuroepithelial cells resembling the stage of neurulation.14,15 Initial studies involving neural differentiation of hESCs suggest that neural rosettes represent abortive neural tube structures expressing a broad range of neural precursor markers including Nestin, NCAM, Pax6, Sox1, and Sox2.14,16–18 One critical feature of the in vitro formation of neural rosettes is the close temporal link with in vivo neural patterning potential.19–21 As neural rosettes continue to propagate in vitro, they acquire features that mimic the processes occurring during neurulation and neural tube growth. Upon further differentiation, neural rosette cells gradually give rise to differentiated cells such as neurons, migrating radially away from the neural rosette structure and a set of more restricted progenitors. Further studies need to address the proliferation and differentiation potential of neural rosette cell populations at different stages of in vitro differentiation.
It is widely acknowledged that mitochondria play an important role in metabolism, calcium signaling, apoptosis, and differentiation, with their key function being the generation of ATP as the cell’s major source of energy.22 Undifferentiated stem cells have small, immature mitochondria located around the nucleus, low oxygen consumption, and express high levels of glycolytic enzymes. However, as stem cells lose their pluripotent capabilities and commit to specific cell fates, the expression of mitochondrial DNA (mtDNA) transcription and replication factors is up-regulated with a concomitant increase in mitochondria and mtDNA copy number.23 Specifically, mitochondria play a critical role in neuronal development and synaptic formation through their specific role in supporting ATP production based on requirements by different neuronal processes.24 During differentiation, the number of mitochondria increases per cell, with increases in the nerve terminal leading to an increase in synaptic activity. In the sections that follow, we will focus our discussions on neural stem cells that contribute to differentiation and regeneration in the adult and pluripotent stem cells that contribute to early neuronal development.
I.B. Neural Stem Cells
Extensive studies over the past few decades have classified neural stem cells (NSCs) as self-renewing, multipotent progenitors residing in the adult nervous system. In the adult brain, NSCs are primarily located in the subventricular zone of the lateral ventricle and the subgranular zone of the hippocampal dentate gyrus.25–28 It is widely acknowledged that primary adult subventricular zone NSCs divide slowly in vivo, and are progenitors that retain bromodeoxyuridine in the long-term. They tend to exhibit several common features of subventricular zone radial glia-like astrocytes and ventricular ependymal cells, including morphological characteristics and expression of glial fibrillary acidic protein (GFAP) and the CD133 glycoprotein. A subset of these cells have also been classified as positive for expression of progenitor markers including LeX, CD133, GFAP, and Nestin, while they are negative for differentiated cell markers CD24, O4, NeuN, and S100β.27
The identification of trophic and mitogenic actions of growth factors, in particular components of FGF and epidermal growth factor (EGF) family proteins, have immensely contributed to the culture and maintenance of a variety of neural cells in vitro.29,30 Seminal studies in the 1990s demonstrated that neural cells derived from the adult rodent brain were capable of self-replicating and giving rise to both neurons and glia in culture.31,32 Further studies revealed the presence of NSCs with similar properties as adult subventricular zone NSCs in many other brain regions of mammals.26,33–37 Research into the development of defined culture and propagation conditions for adult NSCs have contributed to numerous in vitro studies on their functionality and differentiation potential. Most of the adult NSCs can be expanded continually in suspension culture as free-floating cell clusters called “neurospheres,” which exhibit the self-renewal and multilineage potential of the starting NSC population.33,38 In vitro, expanded adult NSCs appear to regain some glial characteristics when cultured as neurospheres,39 and may exhibit the same diversity as those found in vivo.40 The core difference between adult NSCs and lineage-restricted progenitors is that adult NSCs are capable of serial neurosphere formation while maintaining multipotentiality at the clonal level.41 However, neurosphere data need to be interpreted with caution, as they are typically composed of heterogeneous cell types including many differentiated cells, leading to the conclusion that neither primary nor secondary neurosphere formation is a conclusive assay of adult NSC identity.15
In addition to being propagated as neurospheres, adult subgranular zone-derived NSCs can also be expanded as a monolayer for a prolonged period of time.34,42 In the presence of the FGF-2 trophic factor, they can be clonally derived, maintain the ability to remain undifferentiated and proliferate, and differentiate into neurons and glia both in vitro and after transplantation.34,35,43–47 However, the predominant neuron types generated from adult NSCs expanded either as neurospheres or in FGF-2-expanded monolayer cultures are GABA-ergic and glutamatergic neurons, indicating their limited differentiation potential into many functional neuronal subtypes.15 The challenge still remains in the isolation and consistent propagation of adult NSCs in vitro; however, once this challenge is overcome, their capacity to self-renew and generate multiple neural lineages, including neurons, astrocytes, and oligodendrocytes, can be better defined. In vitro-cultured adult NSCs thus provide a useful system for analysis of their self-renewal and differentiation capabilities, while also providing opportunities for developing therapeutic strategies.
I.C. Pluripotent Stem Cells
hESCs, which are derived from the inner cell mass of a blastocyst-stage embryo, have the dual properties of prolonged self-renewal and the ability to differentiate into multiple cell types that constitute the human body.10–12 The pluripotent nature of these cells—that is, their ability to differentiate into three different germ layers (ectoderm, endoderm, and mesoderm) and eventually all somatic cell types—has opened avenues for potential cell-based therapies, drug-discovery platforms, and unique in vitro model systems for the study of early human development. The core questions that are being addressed by researchers in the stem cell community relate to the molecular mechanisms that drive self-renewal and directed differentiation into specialized cell types. Spontaneous differentiation of hESCs occurs either in prolonged cultures or in the absence of supporting feeder layers, leading to populations consisting of multiple cell types from the three different germ layers. The formation of embryoid bodies, three-dimensional hESC aggregates in suspension or in an environment that does not support adhesion, has been used as a more controlled method to induce differentiation.48 Heterogeneous subpopulations that are enriched for a specialized cell type are then often isolated and replated, with differentiation induced by the addition of growth factors to the medium.49 Thus, directed differentiation into neural subtypes involves: a) exposure of hESCs to growth factors that mimic natural pathways in neuronal development, b) co-culture with suitable cytokine-secreting feeders, or c) replating enriched populations derived from embryoid bodies. Cell types that have been derived through the use of embryoid bodies include many that have been largely random in their phenotype and organization. Thus, there is a need for directed differentiation strategies for converting hESCs to highly specific neural derivatives with increased reproducibility and capacity for scale-up.
Given their pluripotent capabilities, hESCs can generate neural cells, including functional neurons, glial cells, and oligodendrocytes, under appropriate culture conditions.17,21,50–53 To promote neural differentiation, hESCs have been co-cultured in the presence of stromal cells to generate dopaminergic neurons in culture.19,54 Additionally, many researchers have used retinoic acid in medium formulations to induce neural differentiation of hESCs,18,50,53,55–59 while others have used the BMP-antagonist noggin on adherent hESC cultures, causing up-regulation of the neural transcription factors Pax6, Sox2, and Nestin.55,60–62 Based on the numerous studies that have demonstrated the neural differentiation potential of pluripotent stem cells, these cells provide unique opportunities for understanding the mechanisms related to neural development while also presenting avenues for cell-based therapies.
II. NEURODEGENERATION AND GENOME DYSFUNCTION
II.A. Examples of Neurodegenerative Disorders
Parkinson’s disease (PD) is the most prevalent neurodegenerative movement disorder of adults, with the principal affected region being the substantia nigra of the mesencephalon. In PD patients, degeneration of dopaminergic neurons in the substantia nigra causes dysregulation of motor coordination. Most PD (approximately 90%) occurs sporadically without any obvious autosomal inheritance patterns. Over the last decade, mutations in several apparently unrelated genes have been described that are associated with recessively or dominantly inherited PD. These genetic PD variants produce clinical and pathological phenotypes that are similar or identical to those of sporadic PD through as yet unclear mechanisms. However, these genetic forms of PD teach us that it is possible to disrupt disparate pathways that, when combined with brain aging, produce a common neurodegeneration. Mitochondrial mechanisms for cell death have assumed prominence for several of these genetic PD variants, implicating mitochondrial dysfunction as a potential pathogenic process linking some autosomal forms of PD to sporadic PD.
Another common neurodegenerative disease is Alzheimer’s disease (AD), which is the most common form of dementia and is defined as an irreversible, progressive brain disorder that slowly destroys memory and cognitive skills. Early symptoms of this disorder can be mistaken for age-related stress. Another early symptom is memory loss and difficulty in remembering recent events or incidents. As the disease advances, other symptoms appear, such as mood swings, aggression, long-term memory loss, and confusion, ultimately leading to loss of bodily functions and death.63
Ischemic stroke is most frequently caused by the rupture of an atherosclerosis plaque, and is characterized by the loss of neurons in vascular regions of the brain. It often affects several functionally distinct areas and different neuronal subtypes. The symptoms of this disorder are highly variable and are mostly associated with sensorimotor deficits and less cognitive impairments. Multiple sclerosis (MS) is a degenerative disease characterized by the destruction of myelin sheets produced by oligodendrocytes, resulting in the impairment of neuronal connections throughout the CNS, and the ultimate loss of oligodendrocytes and neurons. Amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease) is characterized by a selective loss of motor neurons in the spinal cord and subsequently in the cerebral cortex. Huntington’s disease (HD) is a genetically inheritable disease caused by defects in the Huntington protein, leading to neurotoxicity and neurodegeneration, which are most pronounced in the striatum.
II.B. Genome Dysfunction in Neurodegeneration
Inherited mutations causing familial forms of neurodegenerative diseases have provided important insights into the molecular networks implicated in disease pathogenesis, with a number of underlying molecular causes found to be common in most of these disorders. Among these common underlying causes are the accumulation of aberrant or misfolded proteins, mitochondrial injury, failure of synapse, oxidative stress, ubiquitin-proteosome system dysfunction, abnormal metal homeostasis, and axonal or dendritic transport failure. Understanding of PD has improved in the last decade with the identification of multiple gene mutations that may reveal a lot about its pathogenesis.64,65 To date, 11 genes have been mapped by linkage studies, and six of them have been identified: SNCA (synuclein), UCH-L1 (ubiquitin hydrolase like-1), PRKN (parkin), LRRK 2, PINK 1, and DJ-1.66 Because many of these genes are associated with the formation of Lewy bodies (protein inclusions), including those involved in proteolysis, it has been hypothesized that the pathogenesis of PD relies on a defective ubiquitin-proteosome system. Given that ample evidence is emerging about the role of these genes in the common sporadic form of PD, future studies should focus on the cellular pathways impacted in relevant cell-based models.
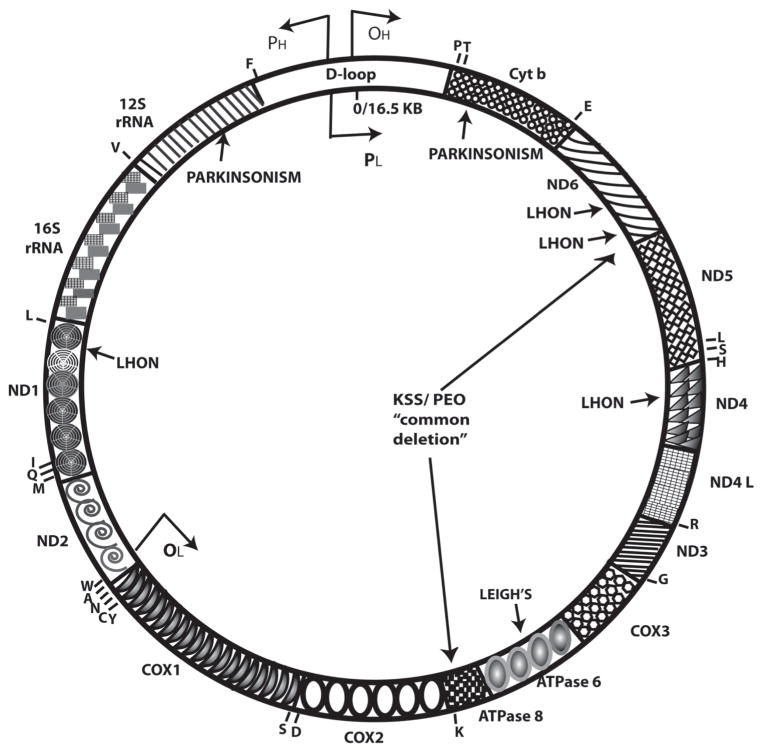
In addition to dysfunctions in the nuclear genome being correlated with neurodegenerative disorders, there is ample evidence to suggest that insults to the mitochondrial genome are an equally important contributor.24 Unlike nuclear DNA, mtDNA lacks introns, has an inefficient repair mechanism, and mutates more rapidly, giving rise to a high probability of affected genes leading to neuronal dysfunction. Most of the diseases are a group of progressive disorders and are defined by a lack of bioenergy owing to defects in the OXPHOS system.67,68 Examples of some of the rare diseases with mutations in the mtDNA and defective OXPHOS systems (Fig. 1), are Kearns-Sayers syndrome (KSS),69 mitochondrial encephalopathy with lactis acidosis and stroke like episodes (MELAS),70 mitochondrial encephalopathy with ragged red fibers (MERRF),71 Leber’s hereditary optic neuropathy (LHON),72,73 and maternally inherited Leigh’s syndrome (MILS).74 Furthermore, mitochondrial functions are impaired in PD,75 ALS,76,77 and Down’s syndrome,78 demonstrating the importance of mitochondria for neurological functioning in development and in adulthood.
FIGURE 1.
Map of the human mitochondrial genome (about 16 kb) with several diseases that impact the nervous system marked on the genome. (ND = NADH dehydrogenase; COX = cytochrome oxidase; Cytb = cytochrome oxidoreductase). A 5-kb common deletion is associated with PD, KSS, PEO (progressive external ophthalmoplegia), and other aging-related disorders. Individual letters on the genome relate to different amino acids.
Mutant mtDNA can be generated by large deletions (reported in KSS syndrome), duplications or point mutations (reported for MELAS, LHON, KSS), or depletion in mtDNA copy number due to mutations in the nuclear-encoded replication machinery.79,80 Given that mtDNA is maternally inherited, offspring tend to inherit the mtDNA mutation; however, the clinical manifestation of mtDNA-related diseases is highly variable and dependent on the nature and amount of mtDNA mutation within the same family. Tissues with high oxidative energy metabolism requirements, such as the brain, have relatively low thresholds and are particularly vulnerable to mtDNA mutations. Disorders are characterized by the presence of wild-type and mutant mtDNA within cells and tissues in either homoplasmic (containing 100% identical copies of mtDNA) or heteroplasmic (variable mixtures of mtDNA copies) proportions.81,82 The cause of homoplasmy or heteroplasmy is currently unknown and is an important subject for future scrutiny. A detailed understanding of how a certain percentage of mutant mtDNA molecules manifests in a diseased phenotype in appropriate cell-based models is vital for understanding the cause of mitochondrial-associated neurodegenerative disorders and their treatment.
II.C. Bioenergetics in Neuronal Differentiation and Neurodegeneration
Given that mitochondria are the energy powerhouses of the cell, deficits in bioenergetics are increasingly recognized to be of pathological importance in neurodegenerative disorders. Studies have shown that neuronal differentiation is highly dependent on the complex I subunits of the electron-transport chain.83 Subsequent studies have demonstrated that defects in the mt genes encoding the complex I subunits results in defective respiration, decreased ATP production, and incomplete differentiation of ESCs into functional neurons.84 During the transfer of electrons through the subunits of the electron-transport chain, the reduced O2 would now be more accessible for oxidation by leaking electrons. Other studies have shown that mtDNA is particularly susceptible to such oxidative damage at complex I and III because it is not protected by histones and has a lower ability to correct mistakes during mtDNA replication.84,85 Studies in aged and PD individuals have shown that substantia nigra neurons containing high levels of defective mtDNA also display impaired mt respiration and increased oxidative stress.86–88 Very compelling evidence for mt dysfunction and reduced bioenergy, such as ATP, has also been demonstrated in mesostriatal neurons of putamen and midbrain in living patients with PD.89 Other studies have also shown that hippocampal neurons from AD brains have increased cytochrome oxidase (the last enzyme in the respiratory electron-transport chain) deficiency compared with normal and aged individuals.90 Together, these findings support the significance of complex I activity and its requirement for ESC differentiation into functional neurons for ATP production and for maintenance of normal bioenergy levels in neuronal systems.
III. MODELS FOR NEURONAL DEVELOPMENT AND NEURODEGENERATION
III.A. Cybrid Models for Neurodegeneration
“Cybrid” (cytoplasmic hybrid) cell lines have been created by fusing platelets from mtDNA from PD or disease-free volunteers with host mtDNA-free human neuroblastomas. This is usually followed by metabolic selection to yield cybrid cell lines with uniform genetic backgrounds, resulting in cell-based models that mimic the disease.91,92 PD and AD cybrid cell lines have been successfully used to explore the contribution of mitochondrial dysfunction and mtDNA gene mutations to PD and/or AD pathogenesis.93–96 Cybrids have also served the purpose of providing a window into early stages of pathogenesis that is typically not available from pathology samples. However, major limitations that exist with this model include the use of tumor cells as host cells that undoubtedly influence their cell cycle progression and mitochondrial biogenesis. Further, cybrids are made from mesodermal sources of mtDNA such as platelets that may not accurately reflect the genome inside non-mitotic neural cells.
III.B. Neural Progenitors Derived from Pluripotent Stem Cells
To take full advantage of hPSCs as a source for neuronal therapies or for the development of appropriate cell-based models that mimic neuronal development, it is important to direct its differentiation towards a neural progenitor that can uniformly produce cell types that constitute the nervous system. In vitro differentiation of hESCs to neural lineage is characterized by the formation of neural rosettes, which is considered an equivalent of the transverse section of a neural tube formed during early development. Under specific culture conditions, neural rosettes are observed 2 weeks after the induction of hESC differentiation, and seem to indicate that in vivo spatial and temporal developmental events are grossly mimicked during the in vitro formation of neural rosettes.14–16 Stepwise procedures have been developed for the generation of uniform populations of neural progenitors from hPSCs (Fig. 2) based on use of FGF2, retinoic acid, and media supplements such as N2 and B27.16–18,50,58 The significance of these studies that have contributed to generation of homogeneous neural progenitor populations is based on the capability of these cells to renew indefinitely in culture, remain genomically stable, and provide the unique capability of serving as an unlimited lineage-restricted cell source for replacement therapy and drug-screening applications.13,16,17 These methodologies also avoid use of the embryoid body approach that results in spontaneous differentiation, and utilize an adherent monolayer approach that provides more control of the differentiation and propagation conditions. Further studies that aim to promote symmetric divisions of undifferentiated neural progenitors in vitro should focus on systems that mimic the in vivo niche by providing an appropriate stem cell micro-environment based on a synergy of biochemical and biophysical cues.97,98
FIGURE 2.
(A) Phase-contrast image of neural progenitors derived from human embryonic stem cells. Neural rosette morphology is maintained and neural progenitors continue to exhibit high nuclear-cytoplasmic ratio, representative of actively dividing cells. (B) Immunocytochemical staining indicates uniform expression of nestin, a marker for neural progenitor cells in their undifferentiated state.
III.C. Induced Pluripotent Stem Cells
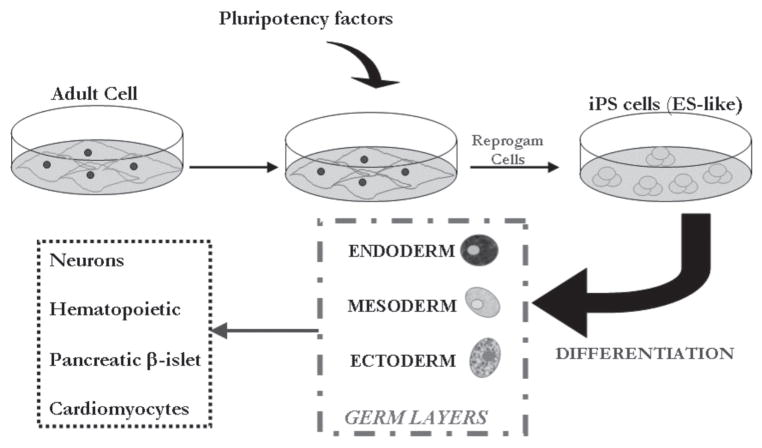
The development of induced pluripotent stem cells based on reprogramming of adult cells have created significant opportunities generationtion of unique in vitro models for studying mechanisms of pathogenesis of different neurodegenerative disorders. Initial studies in the mouse, which were later replicated in the human system, demonstrated that transduction of candidate pluripotency-related genes (Oct3/4, Sox2, c-Myc, Klf4, Nanog, and Lin28) contributed to the generation of “ESC-like” induced pluripotent stem cells99–103 (Fig. 3). The excitement behind the generation of induced pluripotent stem cells relates to the fact that they exhibited all of the defined hallmarks of pluripotency, including prolonged self-renewal and multilineage differentiation potential (Fig. 4). Following these studies, many researchers are focusing their efforts on generating customized induced pluripotent stem lines from patients with various neurodegenerative diseases such as spinal muscular atrophy (SMA), ALS, and PD.104,105
FIGURE 3.
Reprogamming of adult cells using pluripotency-associated factors generates induced pluripotent stem cells that can then be differentiated into multiple cell types for customized regenerative biomedical therapies.
FIGURE 4.
(A) Phase-contrast image of a human induced pluripotent stem cell colony propagated in direct co-culture on mouse embryonic fibroblast feeders. Colony exhibits compact morphology with individual cells within the colony, exhibiting high nuclear-cytoplasmic ratio, representative of actively dividing cells. (B) Neural rosettes in histological sections of embryoid bodies derived from induced pluripotent stem cells demonstrate differentiation potential.
SMA is an autosomal recessive genetic disorder caused by mutations in the SMN1 (survival motor neuron 1)gene that significantly reduce the level of SMN protein expression,106 resulting in the selective degeneration of lower α-motor neurons.107 Another form of the SMN1 gene is the SMN2 gene, which is almost identical to SMN1 but results in only 10% of the full-length protein being produced. It has been shown that patients with multiple copies of SMN2 produce more full-length protein and have a less severe phenotype.108 Prior models used to understand SMA pathogenesis have relied on nematodes, fruit flies, and mice, which produced invaluable data concerning the genetic cause of SMA, mechanisms of motor neuron death, and potential drug therapies.109 Among several limitations has been the absence of the SMN2 gene in these model systems, requiring complicated knockout and overexpression strategies. Additionally, some therapies required the activation of the SMN2 gene as a potential modifier of the disease, thereby requiring a human cell-based assay system. Although fibroblast samples from SMA patients were readily available for in vitro studies, they did not exhibit the same vulnerability as motor neurons, and the processing and functioning of the SMN protein was significantly different in the two cell types.110 The generation of induced pluripotent stem cells from a SMA-type 1 patient fibroblast and subsequent induction to neural tissue and motor neurons, while maintaining the absence of SMN1 expression correlated with the disease phenotype of selective motor neuron death, is proving to be the ideal in vitro model system for SMA research.105 These customized induced pluripotent stem cells are now expected to permit drug screening that may increase expression of the SMN protein and the added advantage of using neural tissue and motor neurons obtained from the patient’s reprogrammed induced pluripotent stem cells to perform combinatorial drug screening in a more relevant system.
Another disorder that has been directly impacted by advances in induced pluripotent stem research is ALS. Recent studies have demonstrated the potential for retrovirally transducing fibroblasts ALS patients with pluripotency factors (c-Myc, Klf4, Sox2, and Oct4) and generating induced pluripotent stem cells with active cell cycle profiles, strong alkaline phosphatase activity, and positive expression of multiple ESC-associated antigens (SSEA-3, SSEA-4, TRA1-60, and NANOG).104 Additional results confirmed the differentiation into motor neurons, the loss of which is associated with ALS. This study has shown that induced pluripotent stem cells have the ability to respond appropriately to developmentally relevant patterning signals and can be used to generate a potentially limitless supply of the cells affected in ALS or other diseases.
Recent studies have also provided opportunities for the development of induced pluripotent stem-based models and therapies that target PD.111–113 In one study, reprogramming of fibroblasts PD patients and subsequent differentiation into dopaminergic neurons was achieved based on use of Cre-recombinase excisable viruses, making them more amenable for future therapeutic applications.112 Another study demonstrated that dopaminergic neurons generated from mouse fibroblast-derived induced pluripotent stem cells rescued behavioral dysfunction in a rodent model of PD, highlighting the potential of induced pluripotent stem cells as being good candidate cell sources for the treatment of PD. The generation of induced pluripotent stem cells thus make the generation of in vitro disease models from the affected individuals themselves possible, which would greatly enhance our understanding of the mechanisms underlying different neurodegenerative diseases and neuronal development.
IV. THERAPEUTIC APPROACHES
IV.A. Cell-based Therapies
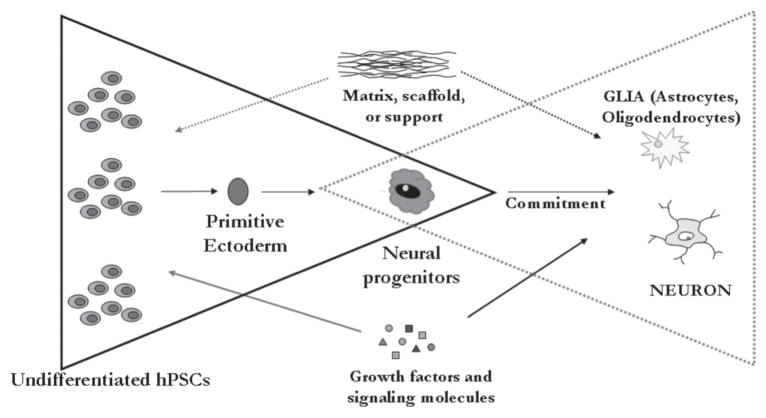
Cell-replacement therapy is emerging as an important component of tissue engineering and regenerative medicine and is primarily targeted at the treatment of various disorders that involve the loss of a specialized cell type (Fig. 5). The ability to restore function to the nervous system based on the replacement of cells lost to trauma, disease, or developmental defects is one of the most exciting concepts in the future treatment of neurodegenerative disorders. Given that current interventions involving drugs or surgeries have contributed to limited therapeutic outcomes, cell-based therapies merit serious consideration. It is possible that cell-based therapies might not be useful for the treatment of many disorders that affect the nervous system, and are most likely to work in cases in which lost neurons have relatively nonspecific and whose function does not depend on complex and precise networks.
FIGURE 5.
Cell-based therapy requires the development of directed differentiation strategies to generate neural progenitors from undifferentiated hPSCs based on a combination of biochemical (signaling molecules, growth factors) and biophysical (matrix, scaffold) cues. Subsequent commitment to neurons and glia aid in the generation of specialized functional cell types for the treatment of disorders of the nervous system.
Most research involving cell-based therapy for neurodegenerative disorders has focused on PD, primarily because the end result revolves around replacing only one type of neuron that releases dopamine. Direct implantation of stem cell-derived dopaminergic neurons into the substantia nigra presents a very attractive option and one that could allow the original restoration of the nigro-striatal pathway (Fig. 6). Initial clinical studies in PD patients after intrastriatal transplantation of human fetal mesencephalic tissue, which is rich in postmitotic dopaminergic neurons, have shown significant reduction in PD-related motor deficits, increased release of dopamine, and synaptic integration of dopaminergic neurons.114–117 However, the impractical use of human fetal mesencephalic tissue presents the necessity to generate neural tissue in vitro for developing cell therapies for CNS disorders.118 Many research groups have now focused their efforts at differentiating hESCs toward dopaminergic neurons.19,20,119–122 Several studies based on the implantation of hESC-derived dopaminergic neurons into the striatum of animal models of PD have also reported clinical outcomes, thus proving the potential of this approach.19,123–125 Despite these promising results, obtaining functional in vivo data after transplantation in animal models of PD is an important step before clinical trials using hESC-derived dopaminergic neurons can be initiated.126
FIGURE 6.

Schematic of cell-based therapy for PD and expected outcomes that can contribute to functional recovery.
Another promising target for cell-based therapy is stroke. Although the ultimate goal of replacing dead cells with new healthy ones may seem remote due to the complexity of the neuronal architecture, currently available data from animal models of stroke are promising. hESC-derived neuronal cells transplanted into ischemic-damaged areas were able to survive, integrate, and promote functional recovery.127–129 Functional outcomes in these studies were demonstrated through a number of mechanisms, including: formation of new neurons and synaptic connections with the host cells, increased neovascularization, and increased native axonal reorganization (sprouting and dendritic branching).
Recent progress in generating motor neurons from hESCs provides opportunities for potential treatment of motor neuropathies, including ALS, SMA, and several major viral infections that affect the nervous system.130–132 HD is another promising candidate disease for cell-based therapies, with experiments in animal models demonstrating the potential of transplanted fetal striatal neurons in restoring synthesis of the GABA neurotransmitter and reformation of neuronal connections.133,134 Cell-replacement therapy for treatment of MS should aim at the implantation of in vitro-generated oligodendrocytes to reconstitute damaged myelin sheets around axons. Recent studies have demonstrated the ability to generate oligodendrocytes from hESCs, followed by the ability to remyelinate axons in vivo, with very minimal functional recovery.135–139 Another promising cell-based therapy approach for the treatment of MS involves re-setting the patient’s immune system to limit the destruction of the myelin sheaths. The procedure of autologous non-myeloablative hemopoietic stem cell transplantation was used to deliver intense immune suppression, and it was demonstrated in a clinical trial that 17 out of 23 MS patients showed enhancement by at least one point on the standard disability scale, while none of the patients deteriorated.140
Although most studies have succeeded in providing relevant cell sources and experimental models for a number of different neurodegenerative diseases, there are still important issues that need to be resolved before any potential applications of these promising therapies can be investigated in human clinical trials. The mechanisms that maintain both repair capabilities and long-term functional integration of any cell type upon transplantation are still not clear. The homing capacity of cells, reconstruction of the three-dimensional brain architecture, adequate numbers of functioning cells, and route of administration are issues that also need to be addressed before cell-based therapies can be incorporated in human clinical trials and treatments for different neurodegenerative disorders.
IV.B. Gene-based Therapies
Treatments for neurodegenerative disorders caused by mtDNA mutations are inadequate. Given that abundant deleted mtDNAs and reduced mtDNA gene levels are correlated with many neural disorders, an approach to restore bioenergetic capacity in vivo based on mtDNA manipulation warrants attention. The recent development of a protein-based mtDNA transfection (protofection), which utilizes an engineered human mitochondrial transcription factor A (TFAM) protein, provides opportunities to access the mitochondria for therapeutic interventions.141–143 The components of the engineered protein are based on the naturally occurring, non-immunogenic human proteins TFAM and mitochondrial localization sequence/signal (MLS), to target mitochondria. Studies conducted with sporadic PD cybrid cells have demonstrated the potential for this technology to restore respiration, increase mtDNA levels and gene expression, and increase levels of proteins associated with the electron transport chain.144 Additional studies with cybrid cells carrying the LHON mutation have demonstrated the potential of TFAM to increase respiration and levels of respiratory proteins.141 These promising outcomes appear to result from stimulation of mitochondrial biogenesis, and present opportunities for the development of this mitochondrial gene therapy approach for therapeutic applications in human neurodegenerative disorders.
IV. CONCLUSIONS
Stem cells from different sources are emerging as exciting areas of research, not only in terms of cell-based models for the study of neuronal development, but also in potential therapeutic strategies for the treatment of severe neurodegenerative disorders (Table 1). However, many basic issues in vitro, in vivo, and in their use in clinical trials need to resolved before they can be used in a safe and efficacious manner for therapeutic applications. Although success has been achieved in the generation of neural progenitors from hPSCs,14–17 further studies need to be conducted on the culture medium and matrix components that will drive differentiation into a range of cell types and tissues required for replacement therapies of nervous system disorders. It is important for future studies to take advantage of knowledge from embryogenesis, where hESC and progenitors undergo a temporal developmental program involving a complex multidimensional microenvironment composed of signaling molecules, growth factors, mitochondrial respiration, extracellular matrix interactions, and physical forces. A better understanding of these variables and mimicking them in vitro will contribute immensely to the development of functional cell types for use in transplantation.97 Another important research question that needs to be addressed is functional integration of the different cell types with existing neural networks in repairing damaged circuitry. Studies that have demonstrated functional integration in animal models are highly encouraging; however, understanding the mechanisms that control neuronal networks demand further experimentation. Additionally, extensive electrophysiological and behavioral studies need to be conducted in preclinical large-animal models prior to use in human clinical trials and in therapies. Other issues that need to be addressed include genomic stability and teratoma formation from hPSCs.145 This issue might require a combination of cell- and gene-based strategies based on the incorporation of suicidal genes into undifferentiated cells that will eliminate their presence in progenitor populations to be used in transplantation. Further, bioprocessing paradigms that involve development of humanized components are crucial in eliminating the risk of contamination with pathological agents capable of transmitting disease to patients.97
TABLE 1.
List of Neurodegenerative Diseases That Can Be Impacted by Cell-and/or Gene-Based Therapies
| Disease | Underlying Cause | Potential Treatment Strategy |
|---|---|---|
| PD | Death of dopaminergic neurons | Transplantation of dopaminergic neurons derived from ESC/iPS cells |
| Stroke | Neuronal death | Cell replacement with fully functional neurons derived from ESC/iPS cells |
| ALS | Loss of motor neurons | Cell therapy based on motor neurons derived from ESC/iPS cells |
| SMA | Spinal motor neuron death | Drug screening based on in vitro models of NPs/iPS cells for SMA; generation of spinal motor neurons from iPS cells |
| HD | Death of neurons in the striatum | Cell replacement with striatal neurons derived from iPS cells |
| AD | Loss of neurons and synapses throughout the brain | Drug screening based on in vitro models of AD; gene and cell therapy: transplantation of cells that overexpress NGF |
| MS | Immune system attacking myelin sheaths | Cell therapy based on transplantation of oligodendrocytes derived from ESC/iPS cells; autologous stem cell transplantation to repopulate the immune system |
iPS, induced pluripotent stem
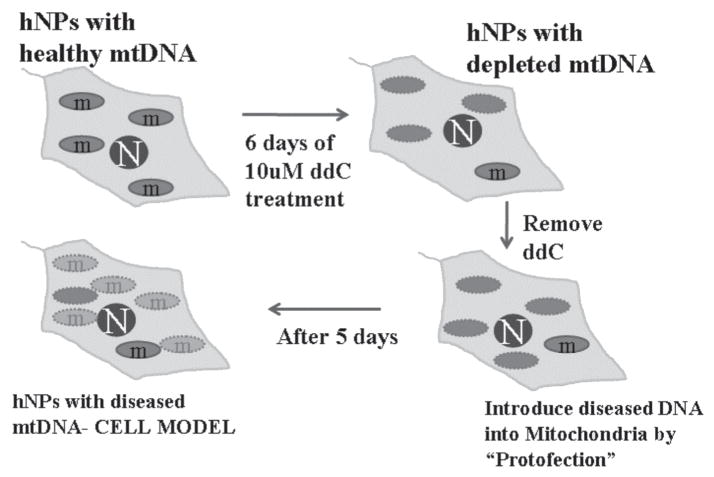
To aid in the development of therapies, it is equally important to take advantage of in vitro model systems that are relevant to the understanding of human development and differentiation. Although cybrids have been useful in demonstrating that mtDNA is a significant pathogenic factor in driving neurodegeneration in AD and PD, they have relied exclusively on human neural tumor cells for their creation.95 Given that tumor cells are more glycolytic than primary cells, carry mutations, and easily revert to de-differentiated cells, it is important to consider cell lines that are efficacious and embryo and the developing brain. The use of neural progenitors that are genomically stable can thus contribute to the generation of models that can be differentiated into neural, astrocytic, and oligodendroglial lineages.16 Further, in combination with protofection technology,141 possibilities exist for the development of novel group of cell lines in neural progenitors or hPSCs that have had their nascent mtDNA removed and disease-causing mutant mtDNA introduced into them (Fig. 7). The creation of these cell models will provide novel insights into how these separate neuroepithelial lineages are affected by mutant mtDNAs and insights into human neurodegenerative disease cell biology, and will serve as ideal neuroprotective drug-development platforms.
FIGURE 7.
Schematic of the development of in vitro-based neural progenitor models that will mimic specific disease phenotypes by manipulation of the mitochondrial genome. Treatment of the human neural progenitors with dideoxycytidine (ddC) depletes host mtDNA, after which protofection can be used to introduce diseased mtDNA for the creation of cell-based models. The approach also forms the basis for potential gene-based therapy in vivo.
Without a doubt, induced pluripotent stem cells are emerging as exciting cell sources for the study of neuronal development, for the generation of unique cell-based models, and for therapeutic applications. Despite their enormous potential, many obstacles need to be resolved before these cells can be fully used by the clinical or the pharmaceutical community. Although induced pluripotent stem cells can overcome two important obstacles associated with the use of hESCs—immune rejection following transplantation and ethical concerns regarding the use of human embryos—they still share the major obstacle of teratoma formation with hESCs. Major efforts should focus on the induction of differentiation of hPSCs with the absence of undifferentiated cells that can result in the formation of teratomas. Additional efforts need to be focused on increasing the efficiency of reprogramming and reduction in aberrant reprogramming that may inhibit differentiation.
In conclusion, stem cells are not only exciting sources for neuroscience research, but are also components of therapeutic strategies for the treatment of neurodegenerative disorders. However, further research into the control of proliferation and differentiation from an in vitro perspective, and survival, regeneration, and functional recovery from an in vivo perspective, are needed. Given the complexities of the nervous system and the disorders associated with it, care should be taken in evaluating progress and in determining the safety and efficacy of therapies in preclinical trials before their application in human trials and clinical use. In this regard, the development of appropriate human cell-based models, whose cell biology closely mimics that occurring in the human brain, sets the stage for significantly enriching translational opportunities for therapy development.
ABBREVIATIONS
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- BMP
bone morphogenetic protein
- CNS
central nervous system
- EB
embryoid body
- EGF
epidermal growth factor
- ESC
embryonic stem cell
- FGF
fibroblast growth factor
- GFAP
glial fibrillary acidic protein
- HD
Huntington’s disease
- hESC
human embryonic stem cell
- iPS
induced pluripotent stem
- KSS
Kearns-Sayers syndrome
- LHON
Leber’s hereditary optic neuropathy
- LIF
leukemia inhibitory factor
- MELAS
mitochondrial encephalopathy with lactis acidosis and stroke-like episodes
- MERRF
mitochondrial encephalopathy with ragged red fibers
- MILS
maternally inherited Leigh’s syndrome
- MLS
mitochondrial localization sequence/signal
- MS
multiple sclerosis
- mtDNA
mitochondrial DNA
- NP
neural progenitor
- NR
neural rosette
- NSC
neural stem cell
- PD
Parkinson’s disease
- PNS
peripheral nervous system
- RA
retinoic acid
- SHH
sonic hedgehog
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
- TFAM
human mitochondrial transcription factor A
- TGF
transforming growth factor
References
- 1.Rossant J. Lineage development and polar asymmetries in the peri-implantation mouse blastocyst. Semin Cell Dev Biol. 2004 Oct;15(5):573–81. doi: 10.1016/j.semcdb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Du ZW, Zhang SC. Neural differentiation from embryonic stem cells: which way? Stem Cells Dev. 2004 Aug;13(4):372–81. doi: 10.1089/scd.2004.13.372. [DOI] [PubMed] [Google Scholar]
- 3.Harland R. Neural induction. Curr Opin Genet Dev. 2000 Aug;10(4):357–62. doi: 10.1016/s0959-437x(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 4.Graham A, Francis-West P, Brickell P, Lumsden A. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature. 1994 Dec 15;372(6507):684–6. doi: 10.1038/372684a0. [DOI] [PubMed] [Google Scholar]
- 5.Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997 Jul;124(14):2709–18. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- 6.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007 Oct;8(10):755–65. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- 7.Takemoto T, Uchikawa M, Kamachi Y, Kondoh H. Convergence of Wnt and FGF signals in the genesis of posterior neural plate through activation of the Sox2 enhancer N-1. Development. 2006 Jan;133(2):297–306. doi: 10.1242/dev.02196. [DOI] [PubMed] [Google Scholar]
- 8.Pevny L, Placzek M. SOX genes and neural progenitor identity. Curr Opin Neurobiol. 2005 Feb;15(1):7–13. doi: 10.1016/j.conb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Zhao S, Nichols J, Smith AG, Li M. SoxB transcription factors specify neuroectodermal lineage choice in ES cells. Mol Cell Neurosci. 2004 Nov;27(3):332–42. doi: 10.1016/j.mcn.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Mitalipova M, Calhoun J, Shin S, Wininger D, Schulz T, Noggle S, Venable A, Lyons I, Robins A, Stice S. Human embryonic stem cell lines derived from discarded embryos. Stem Cells. 2003;21(5):521–6. doi: 10.1634/stemcells.21-5-521. [DOI] [PubMed] [Google Scholar]
- 11.Reubinoff BE, Pera MF, Fong C-Y, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotech. 2000;18(4):399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 12.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 13.Dhara SK, Stice SL. Neural differentiation of human embryonic stem cells. J Cell Biochem. 2008 Oct 15;105(3):633–40. doi: 10.1002/jcb.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008 Jan 15;22(2):152–65. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkabetz Y, Studer L. Human ESC-derived neural rosettes and neural stem cell progression. Cold Spring Harb Symp Quant Biol. 2008;73:377–87. doi: 10.1101/sqb.2008.73.052. [DOI] [PubMed] [Google Scholar]
- 16.Dhara SK, Hasneen K, Machacek DW, Boyd NL, Rao RR, Stice SL. Human neural progenitor cells derived from embryonic stem cells in feeder-free cultures. Differentiation. 2008 May;76(5):454–64. doi: 10.1111/j.1432-0436.2007.00256.x. [DOI] [PubMed] [Google Scholar]
- 17.Shin S, Mitalipova M, Noggle S, Tibbitts D, Venable A, Rao R, Stice SL. Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells. 2006 Jan;24(1):125–38. doi: 10.1634/stemcells.2004-0150. [DOI] [PubMed] [Google Scholar]
- 18.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001 Dec;19(12):1129–33. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 19.Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004 Aug 24;101(34):12543–8. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005 Jun-Jul;23(6):781–90. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SC. Neural subtype specification from embryonic stem cells. Brain Pathol. 2006 Apr;16(2):132–42. doi: 10.1111/j.1750-3639.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crimi M, Rigolio R. The mitochondrial genome, a growing interest inside an organelle. Int J Nanomedicine. 2008;3(1):51–7. doi: 10.2147/ijn.s2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker GC, Acsadi G, Brenner CA. Mitochondria: determinants of stem cell fate? Stem Cells Dev. 2009 Jul-Aug;18(6):803–6. doi: 10.1089/scd.2009.1806.edi. [DOI] [PubMed] [Google Scholar]
- 24.Sekine S, Miura M, Chihara T. Organelles in developing neurons: essential regulators of neuronal morphogenesis and function. Int J Dev Biol. 2009;53(1):19–27. doi: 10.1387/ijdb.082618ss. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004 Mar 4;41(5):683–6. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 26.Gage FH. Mammalian neural stem cells. Science. 2000 Feb 25;287(5457):1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 27.Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009 Jun;19(6):672–82. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005 Oct;15(5):514–20. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Morrison RS, Kornblum HI, Leslie FM, Bradshaw RA. Trophic stimulation of cultured neurons from neonatal rat brain by epidermal growth factor. Science. 1987 Oct 2;238(4823):72–5. doi: 10.1126/science.3498986. [DOI] [PubMed] [Google Scholar]
- 30.Nurcombe V, Ford MD, Wildschut JA, Bartlett PF. Developmental regulation of neural response to FGF-1 and FGF-2 by heparan sulfate proteoglycan. Science. 1993 Apr 2;260(5104):103–6. doi: 10.1126/science.7682010. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992 Mar 27;255(5052):1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996 Apr 10;175(1):1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 33.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994 Nov;13(5):1071–82. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 34.Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995 Oct;6(5):474–86. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 35.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8(6):389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 36.Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996 Dec 1;16(23):7599–609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996 Sep;19(9):387–93. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 38.Bonaguidi MA, Peng CY, McGuire T, Falciglia G, Gobeske KT, Czeisler C, Kessler JA. Noggin expands neural stem cells in the adult hippocampus. J Neurosci. 2008 Sep 10;28(37):9194–204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dromard C, Bartolami S, Deleyrolle L, Takebayashi H, Ripoll C, Simonneau L, Prome S, Puech S, Tran VB, Duperray C, Valmier J, Privat A, Hugnot JP. NG2 and Olig2 expression provides evidence for phenotypic deregulation of cultured central nervous system and peripheral nervous system neural precursor cells. Stem Cells. 2007 Feb;25(2):340–53. doi: 10.1634/stemcells.2005-0556. [DOI] [PubMed] [Google Scholar]
- 40.Brazel CY, Limke TL, Osborne JK, Miura T, Cai J, Pevny L, Rao MS. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell. 2005 Aug;4(4):197–207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 41.Louis SA, Rietze RL, Deleyrolle L, Wagey RE, Thomas TE, Eaves AC, Reynolds BA. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells. 2008 Apr;26(4):988–96. doi: 10.1634/stemcells.2007-0867. [DOI] [PubMed] [Google Scholar]
- 42.Davis AA, Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994 Nov 17;372(6503):263–6. doi: 10.1038/372263a0. [DOI] [PubMed] [Google Scholar]
- 43.Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11879–83. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002 May 2;417(6884):39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 45.Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002 May;5(5):438–45. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 46.Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996 Oct 17;383(6601):624–7. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- 47.Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S, Ying QL, Cattaneo E, Smith A. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005 Sep;3(9):e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1441–5. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11307–12. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001 Dec;172(2):383–97. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 51.Hong S, Kang UJ, Isacson O, Kim KS. Neural precursors derived from human embryonic stem cells maintain long-term proliferation without losing the potential to differentiate into all three neural lineages, including dopaminergic neurons. J Neurochem. 2008 Jan;104(2):316–24. doi: 10.1111/j.1471-4159.2007.04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005 May 11;25(19):4694–705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schulz TC, Palmarini GM, Noggle SA, Weiler DA, Mitalipova MM, Condie BG. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci. 2003 Oct 22;4:27. doi: 10.1186/1471-2202-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vazin T, Chen J, Lee CT, Amable R, Freed WJ. Assessment of stromal-derived inducing activity in the generation of dopaminergic neurons from human embryonic stem cells. Stem Cells. 2008 Jun;26(6):1517–25. doi: 10.1634/stemcells.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baharvand H, Mehrjardi NZ, Hatami M, Kiani S, Rao M, Haghighi MM. Neural differentiation from human embryonic stem cells in a defined adherent culture condition. Int J Dev Biol. 2007;51(5):371–8. doi: 10.1387/ijdb.72280hb. [DOI] [PubMed] [Google Scholar]
- 56.Erceg S, Lainez S, Ronaghi M, Stojkovic P, Perez-Arago MA, Moreno-Manzano V, Moreno-Palanques R, Planells-Cases R, Stojkovic M. Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PLoS One. 2008;3(5):e2122. doi: 10.1371/journal.pone.0002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A. 2003 Oct 28;100(22):12741–6. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotech. 2001;19(12):1134–40. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 59.Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, Goldstein RS, Benvenisty N. Induced neuronal differentiation of human embryonic stem cells. Brain Res. 2001 Sep 21;913(2):201–5. doi: 10.1016/s0006-8993(01)02776-7. [DOI] [PubMed] [Google Scholar]
- 60.Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005 Oct;23(9):1234–41. doi: 10.1634/stemcells.2005-0110. [DOI] [PubMed] [Google Scholar]
- 61.Itsykson P, Ilouz N, Turetsky T, Goldstein RS, Pera MF, Fishbein I, Segal M, Reubinoff BE. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci. 2005 Sep;30(1):24–36. doi: 10.1016/j.mcn.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van Oostwaard D, Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004 Mar 1;117(Pt 7):1269–80. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- 63.Tabert MH, Liu X, Doty RL, Serby M, Zamora D, Pelton GH, Marder K, Albers MW, Stern Y, Devanand DP. A 10-item smell identification scale related to risk for Alzheimer’s disease. Ann Neurol. 2005 Jul;58(1):155–60. doi: 10.1002/ana.20533. [DOI] [PubMed] [Google Scholar]
- 64.Gasser T. Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev Mol Med. 2009;11:e22. doi: 10.1017/S1462399409001148. [DOI] [PubMed] [Google Scholar]
- 65.Gasser T. Genomic and proteomic biomarkers for Parkinson disease. Neurology. 2009 Feb 17;72(7 Suppl):S27–31. doi: 10.1212/WNL.0b013e318198e054. [DOI] [PubMed] [Google Scholar]
- 66.Davie CA. A review of Parkinson’s disease. Br Med Bull. 2008;86:109–27. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- 67.Zeviani M. Mitochondrial disorders. Suppl Clin Neurophysiol. 2004;57:304–12. [PubMed] [Google Scholar]
- 68.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999 Mar 5;283(5407):1482–8. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 69.Bosbach S, Kornblum C, Schroder R, Wagner M. Executive and visuospatial deficits in patients with chronic progressive external ophthalmoplegia and Kearns-Sayre syndrome. Brain. 2003 May;126(Pt 5):1231–40. doi: 10.1093/brain/awg101. [DOI] [PubMed] [Google Scholar]
- 70.Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–3. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 71.Shoffner JM, Lott MT, Lezza AM, Seibel P, Ballinger SW, Wallace DC. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–7. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- 72.Huoponen K, Vilkki J, Aula P, Nikoskelainen EK, Savontaus ML. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am J Hum Genet. 1991 Jun;48(6):1147–53. [PMC free article] [PubMed] [Google Scholar]
- 73.Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, 2nd, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–30. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 74.Kirby DM, McFarland R, Ohtake A, Dunning C, Ryan MT, Wilson C, Ketteridge D, Turnbull DM, Thorburn DR, Taylor RW. Mutations of the mitochondrial ND1 gene as a cause of MELAS. J Med Genet. 2004 Oct;41(10):784–9. doi: 10.1136/jmg.2004.020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4078–83. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu J, Lillo C, Jonsson PA, Vande Velde C, Ward CM, Miller TM, Subramaniam JR, Rothstein JD, Marklund S, Andersen PM, Brannstrom T, Gredal O, Wong PC, Williams DS, Cleveland DW. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004 Jul 8;43(1):5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 77.Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, Brown RH., Jr Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004 Jul 8;43(1):19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 78.Chang KT, Min KT. Drosophila melanogaster homolog of Down syndrome critical region 1 is critical for mitochondrial function. Nat Neurosci. 2005 Nov;8(11):1577–85. doi: 10.1038/nn1564. [DOI] [PubMed] [Google Scholar]
- 79.Schapira AH. Mitochondrial disease. Lancet. 2006 Jul 1;368(9529):70–82. doi: 10.1016/S0140-6736(06)68970-8. [DOI] [PubMed] [Google Scholar]
- 80.Jacobs LJ, de Coo IF, Nijland JG, Galjaard RJ, Los FJ, Schoonderwoerd K, Niermeijer MF, Geraedts JP, Scholte HR, Smeets HJ. Transmission and prenatal diagnosis of the T9176C mitochondrial DNA mutation. Mol Hum Reprod. 2005 Mar;11(3):223–8. doi: 10.1093/molehr/gah152. [DOI] [PubMed] [Google Scholar]
- 81.Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–9. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- 82.Lightowlers RN, Chinnery PF, Turnbull DM, Howell N. Mammalian mitochondrial genetics: heredity, heteroplasmy and disease. Trends Genet. 1997 Nov;13(11):450–5. doi: 10.1016/s0168-9525(97)01266-3. [DOI] [PubMed] [Google Scholar]
- 83.Papa S, Petruzzella V, Scacco S, Vergari R, Panelli D, Tamborra R, Corsi P, Picciariello M, Lambo R, Bertini E, Santorelli FM. Respiratory complex I in brain development and genetic disease. Neurochem Res. 2004 Mar;29(3):547–60. doi: 10.1023/b:nere.0000014825.42365.16. [DOI] [PubMed] [Google Scholar]
- 84.Kirby DM, Rennie KJ, Smulders-Srinivasan TK, Acin-Perez R, Whittington M, Enriquez JA, Trevelyan AJ, Turnbull DM, Lightowlers RN. Transmitochondrial embryonic stem cells containing pathogenic mtDNA mutations are compromised in neuronal differentiation. Cell Prolif. 2009 Aug;42(4):413–24. doi: 10.1111/j.1365-2184.2009.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atamna H, Frey WH., 2nd Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer’s disease. Mitochondrion. 2007 Sep;7(5):297–310. doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 86.Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006 May;38(5):518–20. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- 87.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006 May;38(5):515–7. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 88.Parker WD, Jr, Parks JK, Swerdlow RH. Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res. 2008 Jan 16;1189:215–8. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hattingen E, Magerkurth J, Pilatus U, Mozer A, Seifried C, Steinmetz H, Zanella F, Hilker R. Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain. 2009 Dec;132(Pt 12):3285–97. doi: 10.1093/brain/awp293. [DOI] [PubMed] [Google Scholar]
- 90.Parker WD, Jr, Parks J, Filley CM, Kleinschmidt-DeMasters BK. Electron transport chain defects in Alzheimer’s disease brain. Neurology. 1994 Jun;44(6):1090–6. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- 91.Ghosh SS, Swerdlow RH, Miller SW, Sheeman B, Parker WD, Jr, Davis RE. Use of cytoplasmic hybrid cell lines for elucidating the role of mitochondrial dysfunction in Alzheimer’s disease and Parkinson’s disease. Ann N Y Acad Sci. 1999;893:176–91. doi: 10.1111/j.1749-6632.1999.tb07825.x. [DOI] [PubMed] [Google Scholar]
- 92.Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Jr, Davis RE, Parker WD., Jr Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol. 1996 Oct;40(4):663–71. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 93.Borland MK, Mohanakumar KP, Rubinstein JD, Keeney PM, Xie J, Capaldi R, Dunham LD, Trimmer PA, Bennett JP., Jr Relationships among molecular genetic and respiratory properties of Parkinson’s disease cybrid cells show similarities to Parkinson’s brain tissues. Biochim Biophys Acta. 2009 Jan;1792(1):68–74. doi: 10.1016/j.bbadis.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, Krebs CT, Bennett JC, Parks JK, Swerdlow RH, Parker WD, Jr, Bennett JP., Jr Alzheimer’s disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol. 2000 Aug;48(2):148–55. [PubMed] [Google Scholar]
- 95.Trimmer PA, Bennett JP., Jr The cybrid model of sporadic Parkinson’s disease. Exp Neurol. 2009 Aug;218(2):320–5. doi: 10.1016/j.expneurol.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trimmer PA, Swerdlow RH, Parks JK, Keeney P, Bennett JP, Jr, Miller SW, Davis RE, Parker WD., Jr Abnormal mitochondrial morphology in sporadic Parkinson’s and Alzheimer’s disease cybrid cell lines. Exp Neurol. 2000 Mar;162(1):37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- 97.Abraham S, Eroshenko N, Rao RR. Role of bioinspired polymers in determination of pluripotent stem cell fate. Regen Med. 2009 Jul;4(4):561–78. doi: 10.2217/rme.09.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol. 2005 Dec;17(6):648–57. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 99.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008 Jan;26(1):101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 101.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 102.Yamanaka S. Molecular mechanisms underlying pluripotency of embryonic stem cells. Seikagaku. 2006 Jan;78(1):27–33. [PubMed] [Google Scholar]
- 103.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007 Dec 21;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 104.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008 Aug 29;321(5893):1218–21. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 105.Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009 Jan 15;457(7227):277–80. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, Le Paslier D, Frézal J, Cohen D, Weissenbach J, Munnich A, Melki J. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995 Jan 13;80(1):155–65. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 107.Crawford TO, Pardo CA. The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis. 1996 Apr;3(2):97–110. doi: 10.1006/nbdi.1996.0010. [DOI] [PubMed] [Google Scholar]
- 108.Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, Dreyfuss G, Melki J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997 Jul;16(3):265–9. doi: 10.1038/ng0797-265. [DOI] [PubMed] [Google Scholar]
- 109.Schmid A, DiDonato CJ. Animal models of spinal muscular atrophy. J Child Neurol. 2007 Aug;22(8):1004–12. doi: 10.1177/0883073807305667. [DOI] [PubMed] [Google Scholar]
- 110.Wilson PG, Cherry JJ, Schwamberger S, Adams AM, Zhou J, Shin S, Stice SL. An SMA project report: neural cell-based assays derived from human embryonic stem cells. Stem Cells Dev. 2007 Dec;16(6):1027–41. doi: 10.1089/scd.2007.0061. [DOI] [PubMed] [Google Scholar]
- 111.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008 Sep 5;134(5):877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O, Jaenisch R. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009 Mar 6;136(5):964–77. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A. 2008 Apr 15;105(15):5856–61. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L, Dagher A, Isacson O. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005 Jul;128(Pt 7):1498–510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kordower JH, Freeman TB, Snow BJ, Vingerhoets FJ, Mufson EJ, Sanberg PR, Hauser RA, Smith DA, Nauert GM, Perl DP, Olanow CW. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N Engl J Med. 1995 Apr 27;332(17):1118–24. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 116.Piccini P, Brooks DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, Brundin P, Hagell P, Rehncrona S, Widner H, Lindvall O. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat Neurosci. 1999 Dec;2(12):1137–40. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 117.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001 Mar 8;344(10):710–9. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 118.Lindvall O, Bjorklund A. Cell therapy in Parkinson’s disease. NeuroRx. 2004 Oct;1(4):382–93. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schulz TC, Noggle SA, Palmarini GM, Weiler DA, Lyons IG, Pensa KA, Meedeniya AC, Davidson BP, Lambert NA, Condie BG. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22(7):1218–38. doi: 10.1634/stemcells.2004-0114. [DOI] [PubMed] [Google Scholar]
- 120.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006 Nov;12(11):1259–68. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 121.Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2344–9. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH, Leem JW, Oh SK, Choi YM, Hwang DY, Chang JW, Kim DW. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2008 Mar 4;105(9):3392–7. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005 Jan;115(1):102–9. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang D, Zhang ZJ, Oldenburg M, Ayala M, Zhang SC. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008 Jan;26(1):55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chiba S, Lee YM, Zhou W, Freed CR. Noggin enhances dopamine neuron production from human embryonic stem cells and improves behavioral outcome after transplantation into Parkinsonian rats. Stem Cells. 2008 Nov;26(11):2810–20. doi: 10.1634/stemcells.2008-0085. [DOI] [PubMed] [Google Scholar]
- 126.Srivastava AS, Malhotra R, Sharp J, Berggren T. Potentials of ES cell therapy in neurodegenerative diseases. Curr Pharm Des. 2008;14(36):3873–9. doi: 10.2174/138161208786898617. [DOI] [PubMed] [Google Scholar]
- 127.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Cerebral ischemia and CNS transplantation: differential effects of grafted fetal rat striatal cells and human neurons derived from a clonal cell line. Neuroreport. 1998 Nov 16;9(16):3703–9. doi: 10.1097/00001756-199811160-00025. [DOI] [PubMed] [Google Scholar]
- 128.Muir JK, Raghupathi R, Saatman KE, Wilson CA, Lee VM, Trojanowski JQ, Philips MF, McIntosh TK. Terminally differentiated human neurons survive and integrate following transplantation into the traumatically injured rat brain. J Neurotrauma. 1999 May;16(5):403–14. doi: 10.1089/neu.1999.16.403. [DOI] [PubMed] [Google Scholar]
- 129.Buhnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, Reymann KG, Dihne M. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006 Dec;129(Pt 12):3238–48. doi: 10.1093/brain/awl261. [DOI] [PubMed] [Google Scholar]
- 130.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005 Feb;23(2):215–21. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 131.Shin S, Dalton S, Stice SL. Human motor neuron differentiation from human embryonic stem cells. Stem Cells Dev. 2005 Jun;14(3):266–9. doi: 10.1089/scd.2005.14.266. [DOI] [PubMed] [Google Scholar]
- 132.Singh Roy N, Nakano T, Xuing L, Kang J, Nedergaard M, Goldman SA. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp Neurol. 2005 Dec;196(2):224–34. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 133.Garbuzova-Davis S, Willing AE, Milliken M, Saporta S, Zigova T, Cahill DW, Sanberg PR. Positive effect of transplantation of hNT neurons (NTera 2/D1 cell-line) in a model of familial amyotrophic lateral sclerosis. Exp Neurol. 2002 Apr;174(2):169–80. doi: 10.1006/exnr.2002.7860. [DOI] [PubMed] [Google Scholar]
- 134.Kendall AL, Rayment FD, Torres EM, Baker HF, Ridley RM, Dunnett SB. Functional integration of striatal allografts in a primate model of Huntington’s disease. Nat Med. 1998 Jun;4(6):727–9. doi: 10.1038/nm0698-727. [DOI] [PubMed] [Google Scholar]
- 135.Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. 2000 May 23;97(11):6126–31. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kang SM, Cho MS, Seo H, Yoon CJ, Oh SK, Choi YM, Kim DW. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007 Feb;25(2):419–24. doi: 10.1634/stemcells.2005-0482. [DOI] [PubMed] [Google Scholar]
- 137.Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999 Jul 30;285(5428):754–6. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 138.Glaser T, Perez-Bouza A, Klein K, Brustle O. Generation of purified oligodendrocyte progenitors from embryonic stem cells. Faseb J. 2005 Jan;19(1):112–4. doi: 10.1096/fj.04-1931fje. [DOI] [PubMed] [Google Scholar]
- 139.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005 Feb;49(3):385–96. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 140.Burt RK, Loh Y, Cohen B, Stefoski D, Balabanov R, Katsamakis G, Oyama Y, Russell EJ, Stern J, Muraro P, Rose J, Testori A, Bucha J, Jovanovic B, Milanetti F, Storek J, Voltarelli JC, Burns WH. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. 2009 Mar;8(3):244–53. doi: 10.1016/S1474-4422(09)70017-1. [DOI] [PubMed] [Google Scholar]
- 141.Iyer S, Thomas RR, Portell FR, Dunham LD, Quigley CK, Bennett JP., Jr Recombinant mitochondrial transcription factor A with N-terminal mitochondrial transduction domain increases respiration and mitochondrial gene expression. Mitochondrion. 2009 Jun;9(3):196–203. doi: 10.1016/j.mito.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khan SM, Bennett JP., Jr Development of mitochondrial gene replacement therapy. J Bioenerg Biomembr. 2004 Aug;36(4):387–93. doi: 10.1023/B:JOBB.0000041773.20072.9e. [DOI] [PubMed] [Google Scholar]
- 143.Khan SM, Smigrodzki RM, Swerdlow RH. Cell and animal models of mtDNA biology: progress and prospects. Am J Physiol Cell Physiol. 2007 Feb;292(2):C658–69. doi: 10.1152/ajpcell.00224.2006. [DOI] [PubMed] [Google Scholar]
- 144.Keeney PM, Quigley CK, Dunham LD, Papageorge CM, Iyer S, Thomas RR, Schwarz KM, Trimmer PA, Khan SM, Portell FR, Bergquist KE, Bennett JP., Jr Mitochondrial gene therapy augments mitochondrial physiology in a Parkinson’s disease cell model. Hum Gene Ther. 2009 Aug;20(8):897–907. doi: 10.1089/hum.2009.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mitalipova MM, Rao RR, Hoyer DM, Johnson JA, Meisner LF, Jones KL, Dalton S, Stice SL. Preserving the genetic integrity of human embryonic stem cells. Nat Biotechnol. 2005 Jan;23(1):19–20. doi: 10.1038/nbt0105-19. [DOI] [PubMed] [Google Scholar]