Abstract
Development of effective novel anti-tumor treatments will require improved in vitro models that incorporate physiologic microenvironments and maintain intratumoral heterogeneity, including tumor initiating cells. Brain tumor initiating cells (BTIC) are a target for cancer therapy, because BTICs are highly tumorigenic and contribute to tumor angiogenesis, invasion, and therapeutic resistance. Current leading studies rely on BTIC isolation from patient-derived xenografts followed by propagation as neurospheres. As this process is expensive and time-consuming, we determined whether three-dimensional microtumors were an alternative in vitro method for modeling tumor growth via BITC maintenance and/or enrichment. Brain tumor cells were grown as neurospheres or as microtumors produced using the human-derived biomatrix HuBiogel™ and maintained with physiologically relevant microenvironments. BITC percentages were determined using cell surface marker expression, label retention, and neurosphere formation capacity. Our data demonstrate that expansion of brain tumor cells as hypoxic and nutrient-restricted microtumors significantly increased the percentage of both CD133+ and CFSEhigh cells. We further demonstrate that BTIC-marker positive cells isolated from microtumors maintained neurosphere formation capacity in the in vitro limiting dilution assay and tumorigenic potential in vivo. These data demonstrate that microtumors can be a useful three-dimensional biological model for the study of BTIC maintenance and targeting.
Introduction
Glioblastomas (GBMs) are the most common primary malignant brain tumor in adults and are uniformly lethal with a median life expectancy of approximately fourteen months [1]. Growing evidence demonstrates that tumors, including GBMs, are hierarchically organized and consist of a heterogeneous population of cells, in which a small subpopulation named tumor-initiating cells (TICs), or alternatively cancer stem cells, has been characterized by attributes similar to stem cells: self-renewal and differentiation [2–6]. For example, brain TICs (BTICs) form neurospheres in vitro, a functional phenotype associated with the self-renewal property of neural stem cells. BTICs have activation of stem cell related signaling pathways (such as Notch and Hedgehog) important for neural stem cell maintenance. BTICs can also asymmetrically divide to generate differentiated progeny when stimulated, as with fetal bovine serum or bone morphogenic proteins. However, BTICs are distinct from non-neoplastic stem cells in their altered genome and ability to propagate tumor growth in immunocompromised mouse models [7–14]. BTICs have been shown to be invasive and resistant to standard of care irradiation and chemotherapy. Similar results have been found with TICs isolated from other solid tumors, such as those of the colon, breast, lung, prostate, and ovary. These data suggest TICs contribute to treatment failure and disease progression, making TICs important targets for novel treatment strategies. New therapies could include inhibition of TIC growth, promotion of TIC death, or increased differentiation alone or in combination to increase radio- or chemosensitivity [15–22].
The tumor-promoting subpopulation makes an attractive opportunity for targeted drug development and therapies, but reliable methods are needed for TIC propagation. Growth and analysis of TICs encounters technical challenges due to their frequently low percentages, tendency to differentiate in culture, and functional diversity in tumors. Current TIC studies are mostly performed using neurosphere or matrix-coated monolayer culture models [23, 24]. However, TIC biomarker selection for expression and tumorigenic potential enrichment of TICs is lost with long term culture, and TICs must be continually derived from xenografts to preserve these phenotypic properties (Fig. 1) [25]. TIC proliferation, differentiation, and survival are controlled by a dynamic interaction between the tumor-stroma signals and the microenvironment including hypoxia and nutrient gradients [26–32]. Standard TIC culture systems do not fully reproduce these in vivo interactions, and a new methodology is needed for TIC expansion while preserving biomarker profiles and phenotypic properties both in vitro and in vivo. Indeed, there is a movement to provide more physiologically relevant tumor models in the form of three-dimensional (3D) tissue culture. One matrix that provides an advance over standard Engelbreth-Holm-Swarm (EHS) murine sarcoma based matrices is the Vivo Bioscience HuBiogel™ matrix. HuBiogel™ is a natural extracellular matrix obtained from human amnion that is growth factor free with high percentages of collagen and laminin (Table 1). We therefore sought to determine whether HuBiogel™ could promote TIC maintenance in microtumors with physiologically relevant growth conditions (Fig. 1). With this model, we are able to imbed, grow, and expand TICs in a reliable and relevant manner, allowing them to maintain TIC surface biomarkers and phenotypic properties while providing a growth factorinert, human extracellular matrix (ECM) to best mimic the in vivo environment.
Figure 1. Model for Microtumor Generation.
To better emulate primary tumor multicellular growth and biology ex vivo for cost-effective and accurate clinical endpoint drug screening, Vivo Biosciences developed a novel Microtumor 3D matrix. Microtumors can be generated from patient-derived xenografts (PDX), including glioblastoma (GBM), the most common primary brain tumor in adults. We tested the hypothesis that BTICs could be maintained in Microtumors and enriched in physiologically relevant 3D microenvironments. The standard method for TIC propagation in comparison to HuBiogel™ Microtumors is shown.
Table 1. Characteristics of HuBiogel™.
| Derivation | Source is Pooled, Pathogen-free Human Amnions Derived under Good Laboratory Practice and Quality Controlled Conditions |
| Extracellular Matrix Components | Natural Human Extracellular Matrix |
| Collagen-I (38%) | |
| Laminin (22%) | |
| Collagen-IV (20%) | |
| Collagen-III (7%) | |
| Enactin & Heparan Sulfate Proteoglycan (<3%) | |
| Growth Factor Free | Undetectable Epidermal Growth Factor |
| Undetectable Fibroblast Growth Factor | |
| Undetectable Transforming Growth Factor | |
| Undetectable Vascular Endothelial Growth Factor | |
| Undetectable Insulin-like Growth Factor | |
| Physical State | Viscous at 4°C and forms gel at 37°C |
| Benefits Over Engelbreth-Holm-Swarm Derived Gels | Natural Human Extracellular Matrix Scaffold Rather than Mouse |
| Tumor-derived Basement Membrane Mix |
Materials and Methods
Cell Lines and Media
All animal studies were carried out in accordance with the policies set by the UAB Institutional Animal Care and Use Committee and performed according to their guidelines. The GBM patient derived xenograft (PDX) D456 used for this study was obtained from Dr. Darell Bigner (Duke University). Propagation of PDX was implemented in athymic nu/nu mice. Complete Neurobasal® medium (NBM), used for PDX cell culture, was made with Neurobasal®-A medium (Gibco, Cat: 10888-022), 2% B-27 Supplement (Gibco, Cat: 12587-010), 10 ng/ml FGF-B (Invitrogen, Cat: PHG0264), 10 ng/ml EGF (Invitrogen, HuBiogel™ is a natural growth factor free extracellular matrix derived from human amnions that can be used to generate Microtumors. Cat: PHG0314), 1% L-Glutamine (Corning, Cat: 25-005-CI), 1% Fungizone (Corning, Cat: 30-003-CF), and 0.1% Gentamicin (Gibco, Cat: 15750-060). For low glucose conditions (0.45 g/L glucose), complete NBM was combined with complete NBM minus glucose, using the same additives as above, including 25 mg/L sodium pyruvate (Corning, Cat: 25-000-CI).
Patient-Derived Xenograft Harvesting and Dissociation
Subcutaneous xenografts were dissociated to single cells using the Worthington Biochemical Papain Dissociation System according to the manufacturer's instructions. Cells were seeded into a non-treated tissue culture flasks and allowed to form neurospheres for 3–4 days post-dissociation to initially select for BTICs.
CFSE Labeling
Neurospheres generated from PDX were dissociated into single cells using Accutase® (Corning, Cat: 25-058-CI) to prepare for CFSE labeling. 5 mM stock solution carboxyfluorescein succinimidyl ester (CFSE) was prepared from lyophilized CellTrace™ CFSE dye and 18 μl DMSO (ThermoFisher Scientific, Kit Cat: C34554). For 5 μM working concentration, 1 μl of CFSE stock solution was added per 1 ml PBS. Harvested single cells were centrifuged (150×g, 8 minutes, room temperature), and supernate was removed. Cells were resuspended with 10 ml working concentration and incubated at 37°C for 20 minutes, protected from light. 50 ml of NBM was added to the cells, and cells were incubated for 5 minutes to remove free dye. Cells were pelleted (300×g, 5 minutes, room temperature), and supernate was aspirated. Cells were resuspended in complete NBM for microtumor production or neurosphere formation, as a comparative control/standard culture condition.
Microtumor Production and Maintenance
Unstained or CFSE-stained single cells were counted with Trypan blue via the TC10™ automated cell counter (Bio-Rad Laboratories, Inc.) and precisely mixed with patented HuBiogel™ human biomatrix (US Patent: 7727750, 2010; Table 1) at a proprietary ratio (Vivo Biosciences, Inc.). Then, through a proprietary process, microtumors were generated from the mixture (Day 0) [33]. Each microtumor contained approximately 10,000 cells in10 μL in volume and 2 mm in diameter. After production, these free-floating microtumors were placed in 6-well tissue culture plates in complete NBM medium with approximately 20 microtumors per well. Cells were incubated at 37° C in 5% CO2. Remaining cells were seeded in a non-treated tissue culture flask and cultured in complete NBM and standard conditions for control neurospheres. On Day 1, 24 hours post-microtumor production, all microtumors were cultured under control (4.5 g/L glucose, 20% O2), or physiologically relevant conditions (0.45 g/L glucose, 2% O2). On Day 7, microtumors and neurospheres were collected for dissociation using Accutase or the Miltenyi Human Tumor Dissociation kit as per the manufacturer's instructions when maintenance of cell surface markers was required.
Flow Cytometry/FACS
Cells were labeled with CD133-APC (Miltenyi) and exposed to Sytox Blue (viability dye, Thermo Fisher) according to the manufacturer's instructions and live CD133 cells determined via flow cytometry. Unlabeled cells as well as those incubated with IgG-APC were used as controls. Cells labeled with CFSE at the time of microtumor formation were exposed to Sytox Blue and live CFSEhigh cells determined via flow cytometry. Unlabeled cells as well as those immediately labeled with CFSE were used as controls.
In vitro limiting dilution neurosphere assay
In a 96-well plate, 200 ml of sterile PBS was added to each outside well to control for evaporation, and 200 μl of NBM was added to each inner well. After microtumors were dissociated and cells were sorted, single cells were plated in limiting dilution down to 1 cell in 50 μl of NBM, accordingly: row B2-B11, 1 cell per well; row C2-C11, 5 cells per well; row D2-D11, 10 cells per well; row E2-E11, 25 cells per well; row F2-F11, 50 cells per well; and row G2-G11, 100 cells per well. Wells were monitored for neurospheres at 14 days. The number of wells with spheres was noted, and this information, along with the total number of wells and cells plated, was used to complete Extreme Limiting Dilution Analysis (ELDA; http://bioinf.wehi.edu.au/software/elda/).
In vivo Tumorigenic Assay
Immediately after isolation via flow cytometry, 1,000 CFSEhigh-sorted cells from dissociated microtumors cultured in low glucose and hypoxia were injected into the brains of immunocompromised mice (n =4) as in our prior reports. Mice were monitored for the development of neurologic signs indicating the presence of a brain tumor (lethargy, paralysis, seizure), at which time the brain was harvested, formalin fixed-paraffin embedded, and subsequently stained with hematoxylin and eosin.
Results
Maintenance of BTIC Markers in Microtumors and Enrichment with Micro-environmental Modeling
Many methods have been used to enrich for BTICs, including flow cytometry for expression of neural stem cell surface proteins and dye retention. One of the first and most well characterized cell surface BTIC markers is the neural stem cell marker CD133/prominin-1 [8, 9, 26, 34]. One limitation of CD133 as a BTIC marker is that tumor and cell dissociation methods used prior to flow cytometry must ensure maintenance of the cell surface protein (Fig. 2A).We therefore confirmed that the Miltenyi Tumor Dissociation kit did allow immediate sorting for CD133 post dissociation, although there may be a decrease in the percentage of positive cells when enzyme dissociated neurospheres are compared to neurospheres dissociated via pipetting alone (Supplemental Fig. 1).
Figure 2. BTIC Marker Assessment in the Microtumor Model.
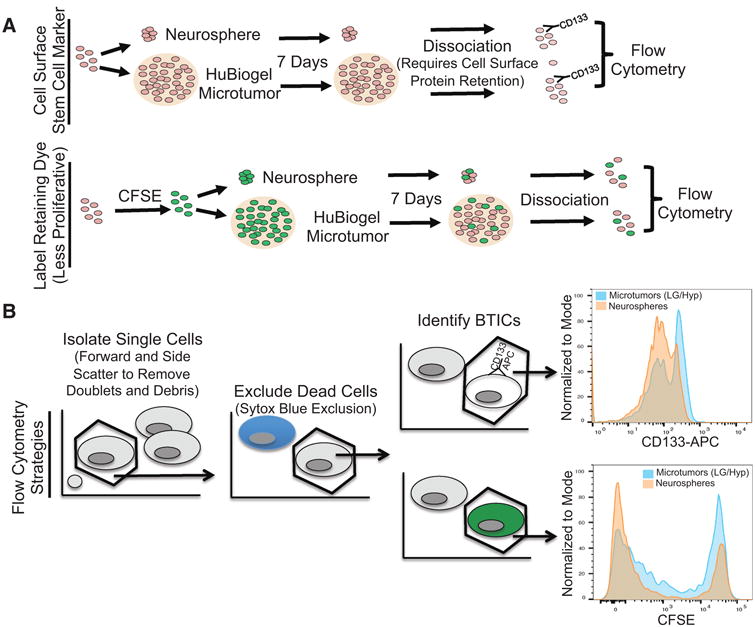
(A) Model of methods for evaluation of BTIC markers in microtumors. (B) Microtumor propagation increased BTIC markers in comparison to neurospheres. Human GBM cells isolated from a xenograft were passaged for seven days as neurospheres in standard conditions or as microtumors in low glucose and hypoxia. Histograms of normalization to mode demonstrate the percent maximum for the cell surface stem cell marker CD133 and label retaining cells as demonstrated with CFSE.
Recent data suggest that CD133 may change during the cell cycle [35] and there may be both a quiescent/slow proliferating BTIC subset as well as a more rapidly proliferating tumor progenitor cell fraction [10]. CFSE is one label retaining dye that has been successfully used to identify TICs from several cancers, including GBM [36–38]. CFSE readily crosses the plasma membrane into cells where it is converted to a fluorescent form by esterases and becomes cross-linked to proteins. Since CFSE is divided equally between daughter cells, cell division can be followed as a consecutive halving of the fluorescence intensity through divisions, making it possible to isolate label retaining (quiescent BTICs) and non-retaining populations. As CFSE is intracellular, a larger number of effective dissociation methods that can be immediately followed by flow cytometry are available (Fig. 2B). For example, we obtained similar results with the use of either Accutase or the Miltenyi Tumor Dissociation kit for microtumor dissociation (data not shown). Based on these data, we used CD133 and the label retaining dye CFSE to evaluate percentages of BTICs.
Due to the large number of TICs required for testing drug panels, there is a need for a system that propagates TICs, including the quiescent/slow proliferating fraction, in bulk. Neurospheres generated from xenograft-derived cells grown in serum-free media are a gold standard for BTIC growth and enrichment, but these models typically lack physiologic cell-microenvironmental interactions. We therefore sought to determine how the 3D HuBiogel™ microtumor model compared to the standard neurosphere model with regard to surface marker expression and CFSE retention. We believed that HuBiogel-promoted cell-ECM interactions and complex information exchange in the context of endogenous oxygen and glucose levels could be important for regulating proliferation and promoting malignancy [39, 40]. Indeed, our data demonstrated that microtumors in physiologic microenvironments are significantly enriched in BTICs in comparison to neurospheres. The percentage of CD133C cells or CFSEC cells, as indicated by the farthest right peaks in the graphs (representative images Fig. 2A, 2B; Supplemental Fig. 1), was increased under the microtumor culture conditions. CFSE was easily observed in microtumors and microtumors could be frozen and thawed with high viability (Fig. 3A). Our data suggest the potential of this method for high thru-put screening in future experiments.
Figure 3. Functional Assessment of BTICs Isolated from Microtumors.

(A) Images of microtumors made from CFSE labeled cells on Day 1 and Day 7. CalceinAM labeled microtumors 7 days after thaw from liquid nitrogen storage for 28 days, demonstrate successful storage and recovery. (B) Microtumor propagated BTICs maintain neurosphere formation capacity. Human GBM cells isolated from a xenograft were maintained as microtumors for a total of 7 days in low glucose and hypoxia. Microtumors were dissociated and cells immediately plated in limiting dilution for the neurosphere formation assay. (C-F) Microtumor propagated BTICs maintain tumorigenic potential in an orthotopic model. Human GBM cells isolated from a xenograft were maintained as microtumors for a total of 7 days in low glucose and hypoxia. Microtumors were dissociated, sorted via flow cytometry for CFSE expression, and 1000 CFSEhigh cells were immediately injected post-sorting into the brains of immunocompromised mice. (C) Kaplan Meier survival curve. (D) Even gross evaluation revealed the brains displayed highly hemorrhagic regions (indicated with arrows) and morphologic displacements characteristic of the presence of GBM. (E, F) Hematoxylin and eosin staining confirmed the presence of highly vascular and multifocal brain tumors.
Microtumors Maintain Cells with Functional Characteristics of BTICs
With the knowledge that the 3D microtumor model successfully maintained cells with BTIC markers, we sought to confirm that the microtumor-isolated BTICs also retained the required functional characteristics of this cellular subset. TICs lack specific or universally applicable cell surface markers for identification and isolation: analysis and characterization of TICs can remain challenging [41]. The most widely accepted methods therefore rely on detection of specific functional cellular features, useful for exploring unidentified TIC subpopulations without the need to rely on markers that may not be applicable. The sphere formation assay is a phenotypic method of determining stemness and has been successfully used to isolate, expand, and calculate the frequency of tumor initiating cells in cancers of the brain, breast, pancreas, bone, central nervous system, colon, and cervix [42–51]. When GBM cells were grown and propagated as microtumors, cells isolated from microtumors did maintain the capacity to form neurospheres. Figure 3B shows the log fraction of non-responding samples (wells without neurospheres) with the number of cells plated per 96 wells. If the line were vertical (black line), the data would indicate that every cell plated was a BTIC as each was sufficient to form a neurosphere. In contrast, if the line were horizontal (grey line), the data would indicate that no cell plated was a BTIC because no amount of cells was sufficient to form a neurosphere. We found that approximately 1 in 7 cells in the microtumor model with physiologic oxygen and glucose were BTICs according to this in vitro limiting dilution assay. We next sought to confirm that microtumor propagated cells were tumorigenic in vivo. We determined that low numbers of CFSEhigh cells isolated from microtumors and subsequently injected into the right lobe of immunocompromised mouse brain produced highly vascular brain tumors (Fig. 3C–F). Both functional assessments confirm BTIC maintenance in microtumors. Together, our data confirm the utility of the 3D HuBiogel™ physiologically relevant microtumor model for GBM cell propagation and BTIC maintenance.
Discussion
TICs are a subset of neoplastic cells that are highly invasive, metastatic, and resistant to standard therapies. TICs contribute to treatment failure and disease progression, making them important targets for treatment and an attractive opportunity for drug development. However, growth and analysis of TICs in a high through-put system presents major technical challenges, and reliable methods are needed for TIC propagation directly from patient specimens. The presented data demonstrate that the micro-tumor model with physiologic microenvironments not only maintains but also enriches BTICs in the context of GBM in comparison to standard neurosphere culture. Our findings are in agreement with other recent studies indicating the potential for 3D models to propagate TICs. A model-engineered 3Dinert hydrogel matrix increased neurosphere formation and expression of TIC markers in comparison to the traditional neurosphere [52]. BTICs were also enriched as determined by increased CD133C expression in a chitosan-alginate scaffold [53].
Our data suggest the physiologically-relevant HuBiogel™ microtumor model provides opportunities for TIC-targeted drug screening in a novel, 3D manner without the limitations of current 3D modeling e.g. spheroids and animal-derived Matrigel or synthetic hydrogel scaffolds. While useful, Matrigel contains intrinsic growth factors, and not all cell lines form stable spheroids [54, 55]. To combat these issues, the HuBiogel™-based microtumor culture system supports long-term growth and function of multiple cell types. As HuBiogel™ is extracted from human amnions and contains no growth factors (Table 1), the cells cultured within the natural human matrix maintain their phenotype. With financial and time costs for growth as patient-derived xenografts in mice followed by propagation as neurospheres, there is a need for a solution that solves both issues. We believe that using the microtumor model, it could be possible to accomplish direct generation of patient-derived tumor lines in a cost effective manner without animal usage (Fig. 4). Microtumors can be frozen and recovered, suggesting the potential for cell stocks to be maintained longer term. However, the microtumor models have not been characterized with the same level of genomic/transcriptomic or therapeutic response profiling already known for PDX models: the ability of the microtumor model to recapitulate the patient specimen over long term passages is unknown. Early passages of PDX models are also available, ensuring similar cell cultures and xenograft tissues can be available to multiple labs over extended periods of time: this resource is not yet available for microtumor models.
Figure 4. Microtumors as a Xenograft Alternative.
As traditional patient-derived xenograft propagation has both time and fiscal expenses, there is a need for more resource-conscious models. Microtumors provide opportunities for the generation of patient derived tumor lines in a cost effective manner with minimal animal usage and for BTIC targeted drug screening.
Most current anti-cancer treatments target rapidly proliferating cells, often through DNA damage and subsequent death of replicating cells. To develop novel treatments, one must take into consideration the unique molecular and cellular biology of the more quiescent tumor cell subset and provide the appropriate in vitro conditions. This slow proliferating tumor cell subset is likely to be one component of the BTIC fraction. While CFSE-retaining cells enriched for tumorigenic potential can be identified in neurospheres [36], current cell culture methods, including neurosphere propagation, still do not optimally model a less proliferative state. Our data suggest that 3D microtumors utilizing human extracellular matrices may better maintain a less proliferative tumor cell subset, making the model more ideal for the identification of treatments targeting cancer through novel mechanisms not previously exploited. While inhibition of self-renewal and promotion of differentiation are strategies of interest, development of drugs that release cells from dormancy to improve chemo- or radiosensitivity could be considered. Alternatively, the metabolic state or mitochondrial function of the less proliferative BTIC fraction could be targeted. The HuBiogel™ microtumors therefore offer opportunities not only for CFSE-based drug screening, but also for improved understanding of the mechanisms driving a quiescent BTIC state.
Although we have focused on the use of HuBiogel™ microtumors for BTIC maintenance, our results have the potential to be applicable to TICs from many different tumor types. The human extracellular matrices present in HuBiogel™ are found in many solid tumors from which TICs can be isolated. Furthermore, the microtumors could be modified to be more tissue specific to support TICs isolated from different tumors. For BTIC propagation for example, addition of hyaluronic acid could better model the brain extracellular matrix and may prove beneficial. Further refinement of the tissue microenvironments, as through modeling of both ischemia and reperfusion that occurs during tissue remodeling and tumor growth, could also generate a more advanced model. However, the most significant advance would be through incorporation of non-neoplastic tissue including endothelial and immune cells to recapitulate cancer as an organ system. GBM PDXs have the benefit of in vivo interactions between human tumor cells and mouse microenvironmental cells including those of the vasculature. Some immune-tumor cell interactions can also be observed, although this depends on the nature of the immunocompromised mouse models. Better in vitro modeling of tumor and vessel and/ or immune cell interactions in vitro with HuBiogel™ microtumors would be a significant advance for cancer research. We believe 3D microtumors can be one way for patient derived cancer models to continue to advance in order for personalized medicine approaches to bebased on screens other than sequencing alone.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health SBIR grant (PI: Raj Singh, HHSN26120150066C). Additional support was provided by 1F31CA200085 (Nathaniel Boyd), P30AI027767 (Center for AIDS Research Flow Cytometry Core), and P20CA151129 (Neurosurgery Brain Tumor Bank), and R21NS096531 (ABH). Additional support for the Hjelmeland laboratory was provided by the UAB Brain Tumor SPORE Career Development Award, a pilot award from the UAB-HudsonAlpha Center for Genomic Medicine, and startup funds from the University of Alabama at Birmingham.
Footnotes
Conflict of Interests: Ashley Gilbert and Raj Singh are employees of Vivo Biosciences, Inc., recently acquired by LifeNet Health (Virginia Beach, Virginia).
Authors' Contributions: Ashley N. Gilbert, HuBiogel microtumor generation, designed experiments, generation and analysis of data, manuscript preparation. Kiera Walker, generated and analyzed data. Anh N. Tran, generated and analyzed data. Nathaniel H. Boyd, analyzed data. G. Yancey Gillespie, provided reagents; reviewed manuscript. Raj K. Singh, provided reagents, designed experiments, final manuscript approval, Anita Hjelmeland, designed experiments, generation and analysis of data, manuscript preparation, and final manuscript approval.
References
- 1.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 4.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Ann Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 5.Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sinica. 2013;34:732–40. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan JP, Minna JD, Shay JW. Evidence for self-renewing lung cancer stem cells and their implications in tumor initiation, progression, and targeted therapy. Cancer Met Rev. 2010;29:61–72. doi: 10.1007/s10555-010-9216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien CA, Kreso A, Jamieson CH. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16:3113–20. doi: 10.1158/1078-0432.CCR-09-2824. [DOI] [PubMed] [Google Scholar]
- 8.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 9.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu B, Zhang D, Tao J, Tie X, Wu A, Wang Y. Human brain glioma stem cells are more invasive than their differentiated progeny cells in vitro. J Clin Neurosci. 2012;19:130–4. doi: 10.1016/j.jocn.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Cheng L, Wu Q, Guryanova OA, Huang Z, Huang Q, Rich JN, et al. Elevated invasive potential of glioblastoma stem cells. Bioch Biophy Res Commun. 2011;406:643–8. doi: 10.1016/j.bbrc.2011.02.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johannessen TC, Wang J, Skaftnesmo KO, Sakariassen PO, Enger PO, Petersen K, et al. Highly infiltrative brain tumours show reduced chemosensitivity associated with a stem cell-like phenotype. Neuropathol Appl Neurol. 2009;35:380–93. doi: 10.1111/j.1365-2990.2008.01008.x. [DOI] [PubMed] [Google Scholar]
- 14.Ye F, Zhang Y, Liu Y, Yamada K, Tso JL, Menjivar JC, et al. Protective properties of radio-chemoresistant glioblastoma stem cell clones are associated with metabolic adaptation to reduced glucose dependence. PLoS One. 2013;8:e80397. doi: 10.1371/journal.pone.0080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 16.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–5. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 18.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 20.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154–9. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 22.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–80. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Patru C, Romao L, Varlet P, Coulombel L, Raponi E, Cadusseau J, et al. CD133, CD15/SSEA-1, CD34 or side populations do not resume tumor-initiating properties of long-term cultured cancer stem cells from human malignant glio-neuronal tumors. BMC Cancer. 2010;10:66. doi: 10.1186/1471-2407-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 27.Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB, Rich JN. Hypoxia inducible factors in cancer stem cells. Br J Cancer. 2010;102:789–95. doi: 10.1038/sj.bjc.6605551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–84. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–13. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flavahan WA, Wu Q, Hitomi M, Rahim N, Kim Y, Sloan AE, et al. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat Neurosci. 2013;16:1373–82. [Google Scholar]
- 31.Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–59. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 32.Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol. 2010;177:1491–502. doi: 10.2353/ajpath.2010.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert AN, Shevin RS, Anderson JC, Langford CP, Eustace N, Gillespie GY, et al. Generation of Microtumors Using 3D Human Biogel Culture System and Patient-derived Glioblastoma Cells for Kinomic Profiling and Drug Response Testing. JoVE. 2016 doi: 10.3791/54026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 35.Barrantes-Freer A, Renovanz M, Eich M, Braukmann A, Sprang B, Spirin P, et al. CD133 Expression Is Not Synonymous to Immunoreactivity for AC133 and Fluctuates throughout the Cell Cycle in Glioma Stem-Like Cells. PLoS One. 2015;10:e0130519. doi: 10.1371/journal.pone.0130519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deleyrolle LP, Harding A, Cato K, Siebzehnrubl FA, Rahman M, Azari H, et al. Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain: J Neurology. 2011;134:1331–43. doi: 10.1093/brain/awr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–25. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 38.Holyoake TL, Jiang X, Jorgensen HG, Graham S, Alcorn MJ, Laird C, et al. Primitive quiescent leukemic cells from patients with chronic myeloid leukemia spontaneously initiate factor-independent growth in vitro in association with up-regulation of expression of interleukin-3. Blood. 2001;97:720–8. doi: 10.1182/blood.v97.3.720. [DOI] [PubMed] [Google Scholar]
- 39.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, et al. Engineering tumors with 3D scaffolds. Nat Med. 2007;4:855–60. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 40.Rao W, Zhao S, Yu J, Lu X, Zynger DL, He X. Enhanced enrichment of prostate cancer stem-like cells with miniaturized 3D culture in liquid core-hydrogel shell microcapsules. Biomaterials. 2014;35:7762–73. doi: 10.1016/j.biomaterials.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stuelten CH, Mertins SD, Busch JI, Gowens M, Scudiero DA, Burkett MW, et al. Complex display of putative tumor stem cell markers in the NCI60 tumor cell line panel. Stem Cells. 2010;28:649–60. doi: 10.1002/stem.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimshaw MJ, Cooper L, Papazisis K, Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, et al. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10:R52. doi: 10.1186/bcr2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation andinvitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 45.Lonardo E, Cioffi M, Sancho P, Crusz S, Heeschen C. Studying pancreatic cancer stem cell characteristics for developing new treatment strategies. JoVE. 2015:e52801. doi: 10.3791/52801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martins-Neves SR, Lopes AO, do Carmo A, Paiva AA, Simoes PC, Abrunhosa AJ, et al. Therapeutic implications of an enriched cancer stem-like cell population in a human osteosarcoma cell line. BMC Cancer. 2012;12:139. doi: 10.1186/1471-2407-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azari H, Louis SA, Sharififar S, Vedam-Mai V, Reynolds BA. Neural-colony forming cell assay: an assay to discriminate bona fide neural stem cells from neural progenitor cells. JoVE. 2011 doi: 10.3791/2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 49.Pacey L, Stead S, Gleave J, Tomczyk K, Doering L. Neural Stem Cell Culture: Neurosphere generation, microscopical analysis and cryopreservation. 2006 [Google Scholar]
- 50.Wang S, Kanojia D, Lo PK, Chandrashekaran V, Duan X, Berger FG, et al. Enrichment and selective targeting of cancer stem cells in colorectal cancer cell lines. Hum Genet Embry. 2012 [Google Scholar]
- 51.Wang L, Guo H, Lin C, Yang L, Wang X. Enrichment and characterization of cancer stemlike cells from a cervical cancer cell line. Mole Med Reports. 2014;9:2117–23. doi: 10.3892/mmr.2014.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang X, Sarvestani SK, Moeinzadeh S, He X, Jabbari E. Three-dimensional-engineered matrix to study cancer stem cells and tumorsphere formation: effect of matrix modulus. Tissue Engineering Part A. 2013;19:669–84. doi: 10.1089/ten.tea.2012.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kievit FM, Florczyk SJ, Leung MC, Wang K, Wu JD, Silber JR, et al. Proliferation and enrichment of CD133(C) glioblastoma cancer stem cells on 3D chitosan-alginate scaffolds. Biomaterials. 2014;35:9137–43. doi: 10.1016/j.biomaterials.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- 55.Fridman R, Benton G, Aranoutova I, Kleinman HK, Bonfil RD. Increased initiation and growth of tumor cell lines, cancer stem cells and biopsy material in mice using basement membrane matrix protein (Cultrex or Matrigel) co-injection. Nat Protocol. 2012;7:1138–44. doi: 10.1038/nprot.2012.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




