Abstract
To document the trajectory of motor and vocal behaviors in real and developmental time, researchers observed infants at each of 4 biweekly naturalistic play sessions over the transition to crawling. An exhaustive and mutually exclusive coding scheme documented every vocalization and posture. Odds ratios of the likelihood of a given posture-vocalization dyad revealed that vocalization and crawling were significantly unlikely to co-occur at the session marking the onset of crawling. Infants’ allocation of attention over the transition to crawling prompted behavioral trade-offs. During mastery of a novel skill, infants had difficulty allocating attention to multiple tasks, but with experience a decrease in attentional load for the new skill allowed performance of simultaneous behaviors in other domains to occur.
The acquisition of new motor skills and behaviors over the first year or so of life generates new contexts in which infants have opportunities to practice skills relevant for language acquisition (Iverson, 2010). For example, learning to sit upright provides infants with greater lung capacity, enabling new possibilities for vocalization (Yingling, 1981). At the onset of babbling, vocalizations were likely to occur coupled with rhythmic rattle shaking as infants practice the rhythmically organized actions that underlie both rhythmic arm shaking and babbling (Ejiri and Masataka, 2001). Moreover, having sufficient manual control over objects to bring them to the mouth for exploration facilitated infants’ exploration of vocalization of consonants (Fagan & Iverson, 2007). Between the ages of 6 and 9 months, infants commonly vocalize while mouthing objects. These vocalizations contain more consonants and more variety than vocalizations not involving mouthed objects. The multimodal feedback that infants receive during object mouthing prompts exploration of the consonants formed, which may in turn facilitate the development of speech.
Complementing the account that motor development facilitates change in infant vocalization is the idea that cognition and action compete for attentional resources. Attentional resources have been conceptualized as the effort children need to muster to express a behavior, as well as learn new behaviors (Bloom & Tinker, 2001). The more complex or novel the behavior, the greater the resources required to perform it. On this account, performing newly acquired skills requires an allocation of attentional resources at the expense of resources available to carry out other tasks (e.g., Boudreau & Bushnell, 2000; Kahneman, 1973; Surkar, Edelbrock, Stergiou, Berger, & Harbourne, 2015). For infants, whose first year is veritably defined by unremitting encounters with novel tasks, contexts, and events, this account predicts trade-offs in performance as attention is diverted to task mastery. For example, as infants acquire their first vocabulary words, their emotional expressiveness during naturalistic play decreases (Bloom & Tinker, 2001). Moreover, infants with Spinal Muscular Atrophy demonstrated better language skills compared to typically-developing infants because they diverted resources that would otherwise have been devoted to motor development to the acquisition of language (Sieratzki & Woll, 2001). In addition, attentional resources are required to maintain postural control (Maylor & Lavie, 1998), such that infants and young children have difficulty during new motor skill acquisition or while performing complex motor tasks with simultaneous cognitive processing such as planning or inhibiting (e.g., Berger, 2010; Boudreau & Bushnell, 2000; Carrico, 2013).
In an early naturalistic investigation of this phenomenon, one infant was observed weekly from 3 weeks to 41 weeks of age, over the acquisition of several early motor milestones, such as lifting the head and balancing on hands and knees (Tipps, Mira, & Cairns, 1981). The authors observed increased variability and decreased frequency in speech sounds during the onset of angular forearm support and head lifts, which suggest possible motor-vocalization trade-offs. Despite this pattern of results, the authors concluded that evidence for motor-language trade-offs remained “anecdotal” (p. 323). The contradiction between the reported findings and the authors’ own conclusions, as well as the fact that this was a case study of a single infant, may explain why the formal study of trade-offs between motor and language development did not gain much traction at the time.
The facilitation and trade-offs perspectives do not necessarily contradict one another, as there is nothing in the current accounts about the timing of the relation between motor and language acquisition. In fact, the facilitation account tends to focus on developmental time—motor development at one point facilitating language development at another—whereas the trade-offs account tends to focus on real time. Perhaps initially, as novel skills challenge and require greater attention, there may be a disruption in performance. Ultimately, the acquisition of new motor skills may create opportunities for language, but not necessarily immediately or concurrent with onset.
Aims of the Study
The primary aim of this study was to document the timing and trajectory of motor and language development via infants’ naturalistic, spontaneous behaviors. To do this we followed infants over the transition to crawling to document whether the frequency of concurrent postures and vocalizations changed as a function of expertise in that posture. If the pattern of cognition-action trade-offs previously observed in both naturalistic and experimental goal-directed tasks also held true for infants’ naturally occurring vocalizations and motor behaviors, we would expect to observe vocalization during crawling less often than during more experienced postures. We would also expect the likelihood of vocalization during crawling to increase as crawling experience increased and the skill became less taxing. However, if early vocalization and motor skills tap “multiple pools” of attentional resources, rather than “a central, unitary pool” (Park, Kim & Chun, 2007, p. 1063), then we would not expect to see any interference as newly acquired skills are added to infants’ repertoires.
Our second aim was to examine the occurrence of early motor and language skills relative to one another on multiple time scales. First, we documented this relation longitudinally over several weeks. Biweekly observations (i.e., every 2 weeks) allowed us to hone in on possible changes in this relation over the transition to crawling as infants’ motor expertise changed from week to week. Second, we microcoded frame-by-frame onsets of all motor and vocalization events as they unfolded in real time. Developmental studies frequently characterize what children know at particular points in development, providing a series of snapshots that help to illuminate children’s development. Widely spaced sampling intervals can only reveal reference points along the path of developmental change and are likely to miss critical details in that path (Adolph & Robinson, 2011). We aimed to meticulously discriminate periods of stability and change on the order of seconds and minutes, rather than the traditional weeks, months, and years. By observing infants during naturalistic play, we captured “in the moment” interactions among motor and vocal behaviors.
Method
This data set was collected as part of a larger longitudinal study examining vocal and motor development in the first two years of life. In the original study, 30 infants were observed for a 45-minute naturalistic play session in their own homes, bi-weekly from ages 2 to 19 months. Sessions were videotaped for later data coding. To enhance the audio component of the recordings, infants wore a small wireless microphone clipped to a cloth vest worn over their clothing during the session.
Participants
For 23 infants from the original data set (12 female), we selected four sessions corresponding to key time points across the transition to crawling, independent of age: 2 weeks pre-crawling, crawling onset, 2 weeks post-crawling, 4 weeks post-crawling, for a total of 80 naturalistic observation sessions. Mean age at session 1 was 7.94 months (SD = 1.49 months). Infants from the original data set were excluded if they could not contribute useable data to all 4 sessions. Missing sessions were due to lost video or if infants were constrained for 85% or more of the session (e.g., by being placed in an infant seat or other piece of furniture; see below). Families were recruited from a small Midwestern city and a large mid-Atlantic city through published birth announcements and word of mouth. Eligible families were contacted by letter and follow-up phone call. Informed consent was obtained from parents upon enrollment. All infant participants were from full-term, uncomplicated pregnancies with normal deliveries; had 5-min neonatal Apgar scores within the normal range (9 or better; Apgar, 1953); and came from monolingual, English-speaking homes.
Data Coding
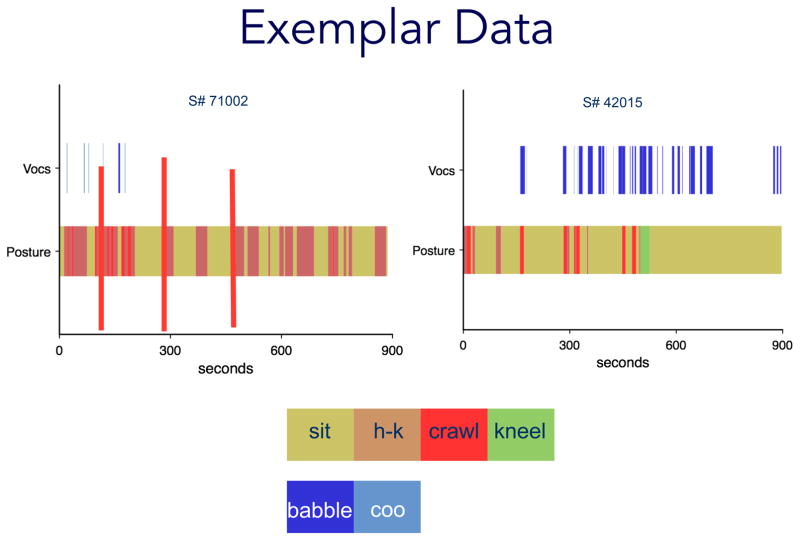
Data from each observation were coded from digital video using Datavyu (datavyu.org), a computerized coding system that records durations and frequencies of behavior. We microcoded 15 consecutive minutes from each 45-minute session. We used the first 15 minutes of the session unless the infant was not on the floor for any of that time. When that happened, we simply advanced the video until the infant was placed on the floor and commenced coding from there. If a session did not have a full 15 minutes of floor time, we coded as much of the session as possible. Using an exhaustive coding scheme, we identified the onset (first frame) and offset (last frame) of every vocalization and posture. Codes within each variable were mutually exclusive. Durations for each behavior were calculated by subtracting the onset frame from the offset frame. Over the 22.89 hours of coded video, we observed 11 postures (e.g., sitting, crawling, pulling-to-stand) and 6 types of vocalizations (e.g., babbling, cooing, crying). Operational definitions of posture and vocalization variables are provided in Table 1.
Table 1.
| Operational Definitions |
|---|
| Posture |
|
|
| balance supported |
| prone - belly resting on floor |
| supine - back resting on floor |
| supported sit - sitting position with postural support from hands, pillow, etc. |
| independent balance controlled |
| sit - sitting without hands, holds position for 10 secs |
| hands and knees - belly lifted off floor, stationary |
| kneel - stationary on knees |
| supported stand - stands upright keeping balance holding furniture, caregiver, etc. |
| crawl - pulls body forward to achieve independent locomotion |
| pull to stand - independently uses furniture, wall, caregiver, etc. to get from floor to standing |
| independent stand - stands upright without assistance, bears own weight |
| steps - take at least 2 independent steps without assistance |
| Vocalization |
|
|
| cooing - uttering only vowel sounds |
| babbling - repeated bouts of consonant sounds |
| spontaneous emotion-related (from Green & Potegal, 2011) |
| fuss - negative emotion, short, flat melody, quiet |
| cry - negative emotion, loud, up and down melody, breath may be interrupted |
| laugh - positive emotion |
| shriek - shrill, loud, short |
To get timed event sequential data, data were coded in two passes. The first pass captured posture and continuously scored the full 15 minutes, as infants were always in a posture. The second pass captured vocalizations, which occurred intermittently throughout the session. Each change in posture and/or change in vocalization was considered a new event. The context in which behavior occurred was also categorized using exhaustive and mutually exclusive codes (i.e., on the floor, in high chair). We examined the timing of postural and vocal behaviors relative to one another within a session, as well as to crawling status between sessions. By marking video frames to identify behavioral onsets and offsets, and by doing so in separate passes for posture and vocalization, we objectively classified concurrent events by identifying the frames where posture and vocalization behaviors overlapped.
A primary coder coded all variables from video. To test interrater reliability, a second coder independently coded 20% of all events. Events chosen for reliability coding were selected at random, except to ensure that each participant contributed some data from each session to the reliability sub-sample. For categorical measures, the reliability coder agreed on 100% of context codes, 93% of language codes, and 96% of motor codes (κs > .88, p values < .01). Correlation coefficients for measures of duration ranged from r = .91 to r = .94 (p values < .01). Discrepancies between coders were resolved through discussion.
Results
For the purposes of this study, all analyses refer to the 15-minute segment of coded video for each infant at each session. Data were included in analyses only when infants were free to move. Babies were considered constrained if they were held by their mother or placed in posture-supporting baby equipment like an exersaucer or doorway jumper. Constrained time accounted for just 16.5% of the total available video and ranged from 0–84% of any individual infant’s session. Most infants were free to move most of the time: in 55 of 92 sessions infants were free to move 100% of the time; in only 2 sessions were infants constrained more than 50% of the session (both at session 1). For purposes of our research question, we only included babbling and cooing as vocal behaviors in our analyses. Perlocutionary acts, such as emotional vocalizations (e.g., laughing, crying), were excluded from analyses because there is no indication that infants used these sounds intentionally (Bates, Camaioni, & Volterra, 1975). Using these criteria, 5028 motor and vocal events were eligible for analysis.
Posture
Transitions between postures
We had no a priori expectations about the amount of time infants would spend transitioning between postures. To assess whether transition between postures needed to be its own category, we pilot-coded video from 2 infants at each of the 4 sessions (8 infants total). The mean proportion of time infants spent transitioning from one posture to another, when the duration of all transitions were summed together, was less than one minute (4.5%) of the 15 minute segment (range = 2%–8%; SD = .02). Confirming what has been observed previously with adults, infants’ transition movements were typically indistinct from and overlapping with the postures bracketing the transition (Aminian, Robert, Buchser, Rutschmann, Hayoz, & Depairon, 1999; Mathie, Celler, Lovell, Coster, 2004). The majority of transitions were very brief, fluid movements. Occasionally, longer transitions would occur when infants would get distracted as they moved from one posture to another (e.g., noticing the video camera or a toy and slowing down their movement to examine it). Thus, we chose not to create a unique category for transitioning between postures, and instead operationally defined the offset of the preceding posture/onset of the following posture as the frame marking the end of the transition.
Stationary postures
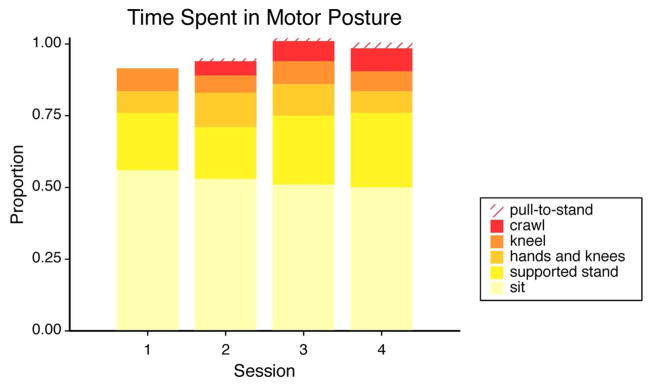
Figure 1 shows the mean proportion of time that infants spent in balanced-controlled postures at each session. Infants sat independently for approximately 50% of the observation, with little variation from session to session. Repeated measures ANOVAs of the proportions of time spent in each posture at each session revealed no change in the stationary postures of sitting and kneeling.
Figure 1.
Pre-locomotor and locomotor postures
In contrast, significant main effects of Session were observed for pre-locomotor and locomotor postures. For crawling, F(1, 22) = 27.48, p < .01, ηp2 = .56, the main effect was driven by significant differences between session 1, when infants did not crawl, and all other sessions (all p’s < .01), as well as by a significant increase between session 2 and session 4, t(22) = 2.87, p < .01.
Main effects of Session were also observed for pulling to stand, F(1, 22) = 8.14, p < .01, ηp2 = .27; this was driven by differences between sessions 1 (when infants did not pull to stand) and 3, 1 and 4, and 2 and 4 (all p’s ≤ .05); for being on hands and knees, F(1, 22) = 4.76, p < .05, ηp2 = .19, which was driven by differences between sessions 1 and 2, and between sessions 1 and 3 (p’s ≤ .05); and for supported standing, F(1, 22) = 6.99, p < .02, ηp2 = .24, which was driven by differences between sessions 1 and 4 and sessions 2 and 4. In addition, one infant took a few independent steps (between 2 and 10) at each session, but it was so rare that we did not include it in any analyses.
Vocalizations
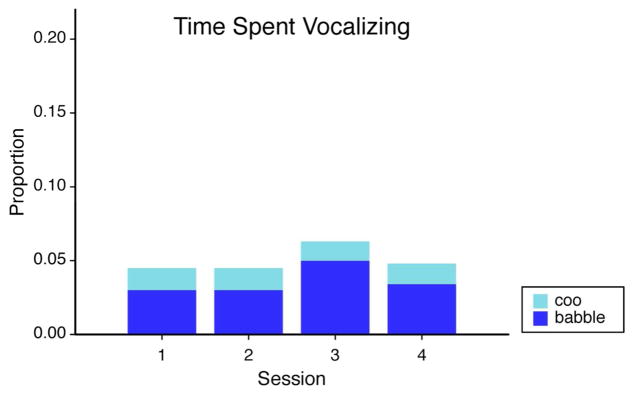
There were 1206 separate instances of vocalization included in the analyses. Total vocalization time summed over a given session for individual infants ranged from just 1.27 seconds to 3.23 minutes. A 4 (session) x 2 (vocalization type) repeated measures ANOVA revealed a main effect for vocalization type, F(1, 22) = 7.68, p < .02, ηp2 = .26. Planned comparisons revealed that infants babbled more than they cooed at sessions 3 and 4, p = .02 and p < .01, respectively (see Figure 2).
Figure 2.
Proportion of session spent in each unconstrained posture requiring balance control. Balance-controlled postures were emphasized to address the aim of examining potentially attentionally taxing behaviors. Proportions do not sum to 1 if infants spent any portion of the session constrained, prone, or supine.
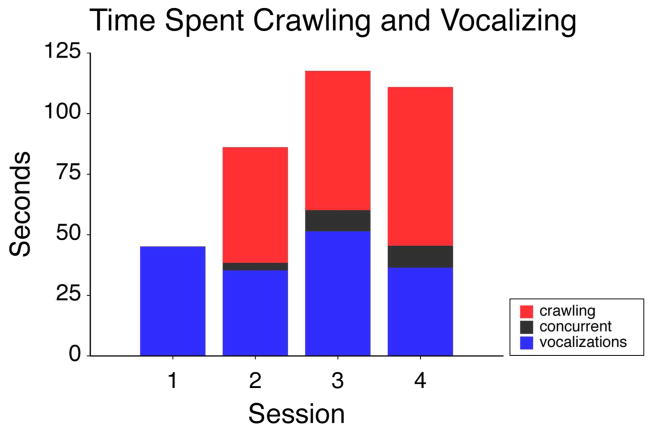
Co-Occurrences Between Crawling and Vocal Behavior Over the Transition to Crawling
To address our primary aims of understanding when and whether co-occurrences between motor and vocal behavior took place, we identified at each session the amount of time that infants spent crawling, the amount of time spent vocalizing, and when those behaviors overlapped (Figure 3). Following procedures described in Bakeman and Quera (2011; see also Bornstein, Putnick, Cote, Haynes, & Suwalsky, 2015), we created 2 × 2 contingency tables for each posture/vocalization dyad of interest at each session. For example, contingency tables for crawling and vocalization tallied the number of times (a) infants vocalized while crawling (b) vocalized while not crawling (c) crawled without vocalizing, and (d) neither vocalized, nor crawled. This process was repeated for each target posture. Odds ratios (OR) were calculated as OR = [(a/b)/(c/d)] for each session as the likelihood that a given posture-vocalization dyad would co-occur. ORs are descriptive measures of effect size (Bornstein, et al., 2015), so in this study ORs greater than 1 indicated that contingencies were more likely to occur than not.
Figure 3.
Proportion of session infants spent cooing (light blue) or babbling (dark blue). Height of the bars reflects total proportion of time spent vocalizing.
We could not run repeated measures analyses across sessions without losing data from infants who had a session where they did not perform the given motor behavior. Instead, to retain as many participants as possible, we conducted planned comparisons for the effects of session on the likelihood of concurrent given and target behaviors.
To address our primary question of whether vocal behaviors significantly co-occurred with motor behavior, one sample t-tests of whether ORs differed significantly from 1 were performed for each session. Vocalizations at sessions 2 and 3 (crawling onset and 2 weeks of crawling experience) were significantly less likely to be concurrent with crawling, whereas vocalization at session 4 (4 weeks crawling experience) was equally likely to occur while infants were crawling as when they were not crawling (see Table 2, Row 1). To examine whether concurrent performance changed as a function of expertise, we compared the odds ratio for the crawling/vocalization dyad across the 3 sessions (session 1 was excluded as it was the pre-crawling session). Paired samples t-tests on OR revealed that all sessions were significantly different from all other sessions: infants were significantly less likely to vocalize while crawling in session 2 than in sessions 3 and 4, t(8) = 2.26, p =.05 and t(12) = 2.78, p =.02, respectively, and significantly less likely to vocalize while crawling during session 3 than 4, t(15) = 2.51, p < .03.
Table 2.
Mean Odds Ratio at each session
| Mean OR Value
|
||||
|---|---|---|---|---|
| Session | 1 | 2 | 3 | 4 |
| Crawling | NA | ***0.13 (n=13) | **0.54 (n=16) | 1.33 (n=22) |
| Sitting | 1.24 (n=15) | 1.87 (n=18) | 1.21 (n=17) | 1.92 (n=22) |
| Standing with support | .54 (n=8) | 1.26 (n=12) | **2.14 (n=12) | 1.62 (n=20) |
| Pulling to stand | NA | **.10 (n=3) | ***.05 (n=6) | .45 (n=10) |
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001
Concurrence Between Other Postures and Vocal Behavior
To test the hypothesis that more experienced postures require less effort and would free up attentional resources for other demands, and because sitting was the “default” posture that infants spent the most time in at all sessions, we calculated the odds ratio for the sitting/vocalization dyad across the 4 sessions. Unlike with crawling, infants were equally likely to vocalize as not while sitting at all 4 sessions. Planned paired samples t-tests revealed no significant differences between any of the sessions for OR (see Table 2, Row 2).
We also calculated contingency tables for other postures that are typically acquired later in the first year. Unlike crawling, we did not have onset dates, but we did want to examine the contingency patterns for other postures that may have been in flux at the time of observation. We calculated the odds ratios for the standing with support/vocalization dyad and for the pulling-to-stand/vocalization dyad across the 4 sessions. As shown in Table 2 (Row 3), few infants were standing with support at session 1, so the likelihood that infants would not vocalize while standing with support at that session did not reach statistical significance. By session 3, however, infants were more likely to vocalize than not while standing with support.
No infants pulled to a stand in session 1 and very few did so in session 2 (see Table 2, Row 4). By session 3, those infants who pulled to a stand were significantly unlikely to vocalize while doing so. However, by session 4 infants were equally likely to vocalize as not while pulling to a stand.
Discussion
This study examined the frequency of behaviors in motor and language domains, as well as changes in both over real and developmental time. We chose observation points surrounding the onset of crawling and found that infants were less likely to vocalize simultaneously while crawling when they were novice crawlers, but after a month of crawling experience they were equally like to vocalize while crawling as when not crawling. Furthermore, although we did not intentionally time our observations to capture the onset of pulling-to-stand, we also found a similar pattern for this milestone—infants were less likely to vocalize simultaneously while pulling-to-stand at the first session where they performed that skill, but equally like to vocalize at the next session while doing so.
Our documentation of the timing and developmental trajectory of concurrent postural and vocal behaviors reflects the organization and development of attention during infancy. Using skill acquisition as a model for understanding resource allocation, we observed that infants’ allocation of attention over the transition to crawling prompted behavioral trade-offs. During mastery of a novel skill, infants had difficulty allocating attention to multiple tasks, but with experience, a decrease in attentional load for the new skill allowed performance of simultaneous behaviors in other domains to occur (Warburton, Wilson, Lynch, & Cuykendall, 2013).
Postures that depend on balance abilities require significant attention to maintain control (Woollacott & Shumway-Cook, 2002). In elderly, balance-impaired adults, for example, falls frequently occur when they are walking and simultaneously performing a secondary task, such as talking (Shumway-Cook & Woollacott, 2000). Researchers posit that falls are not due to impairments to balance alone, but to decrements in the ability to allocate attention to balance while also carrying out other tasks. Likewise, infants acquiring multiple new skills simultaneously may have to strategize how to effectively allocate attention during mastery and execution. Rather than sacrificing performance in either domain, they may suspend one behavior while carrying out the other.
Several recent studies have demonstrated positive relations between motor and language development (He, Walle & Campos, 2015; Oudgenoeg-Paz, Volman & Leseman, 2012; Walle & Campos, 2014), which at first glance may seem to contradict our current findings. How can motor behaviors compete with vocalization for attentional resources, while also facilitating language development? One explanation is that these relations are operating on different time scales. The acquisition of motor milestones supports language development as new postures and skills create new opportunities (Alcock & Krawczyk, 2010; Iverson, 2010; LeBarton & Iverson, 2013; Yingling, 1981). The onset of crawling or walking at one time point triggers a cascade of later experiences that shape opportunities for vocalization, exploration of new contexts, and social interaction. However, when language comprehension and motor abilities of 21 month olds were observed, only oral motor control was associated with language production and no relation was found between language comprehension and measures of motor control. In contrast, our findings dealt with motor and vocalization behaviors as they unfolded “in the moment” in real time and as they emerged in development. Emergent behaviors elicit temporary instabilities that ultimately resolve as infants become more skilled in the new posture (Smith & Thelen, 2003).
Conclusion
Examining the relation between the developmental trajectories of motor and language milestones using dense sampling (Siegler, 2006), yields new insights into our understanding of the interaction between these developmental domains. Once skills are mastered, they may indeed facilitate one another, but in the short term the acquisition of one may put the development of the other on hiatus. As infants allocate limited attention to mastering newly acquired developmental milestones, they may find it difficult to simultaneously perform other novel behavior. Thus, trade-offs in the acquisition of developmental milestones on multiple time scales as infants concentrate on one effortful behavior at a time may help to explain the natural variability in timing and order of milestone acquisition (Adolph, Berger & Leo, 2011; Berger, Theuring, & Adolph, 2007).
Figure 4.
The amount of time at each session infants spent crawling (red), vocalizing (blue), or both concurrently (black).
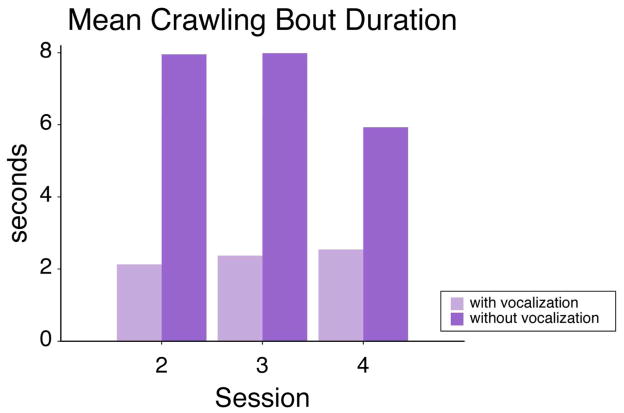
Figure 5.
Mean duration of crawling bouts that contained instances of simultaneous vocalization (light purple) and that were absent of any vocalization (dark purple).
Contributor Information
Sarah E. Berger, Department of Psychology, College of Staten Island and the Graduate Center of the City University of New York
Marian Cunsolo, Department of Psychology, College of Staten Island, City University of New York.
Mariam Ali, Department of Psychology, College of Staten Island, City University of New York.
Jana M. Iverson, Department of Psychology, University of Pittsburgh
References
- Adolph KE, Berger SE, Leo AJ. Developmental continuity? Crawling, cruising, and walking. Developmental Science. 2011;14(2):306–318. doi: 10.1111/j.1467-7687.2010.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Robinson SR. Sampling development. Journal of Cognition and Development. 2011;12(4):411–423. doi: 10.1080/15248372.2011.608190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcock KJ, Krawczyk K. Individual differences in language development: Relationship with motor skill at 21 months. Developmental Science. 2010;13(5):677–691. doi: 10.1111/j.1467-7687.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- Aminian K, Robert Ph, Buchser EE, Rutschmann B, Hayoz D, Depairon M. Physical activity monitoring based on accelerometry: Validation and comparison with video observation. Medical & Biological Engineering & Computing. 1999;37:304–308. doi: 10.1007/BF02513304. [DOI] [PubMed] [Google Scholar]
- Apgar V. A proposal for a new method of evaluation of the newborn. Classic Papers in Critical Care. 1953;32(449):97. [Google Scholar]
- Bakeman R, Quera V. Sequential analysis and observational methods for the behavioral sciences. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- Bates E, Camaioni L, Volterra V. The acquisition of performatives prior to speech. Merrill-Palmer Quarterly of Behavior and Development. 1975;21(3):205–226. [Google Scholar]
- Berger SE. Locomotor expertise predicts infants’ perseverative errors. Developmental Psychology. 2010;46(2):326–336. doi: 10.1037/a0018285. [DOI] [PubMed] [Google Scholar]
- Berger SE, Theuring C, Adolph KE. How and when infants learn to climb stairs. Infant Behavior and Development. 2007;30(1):36–49. doi: 10.1016/j.infbeh.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Bloom L, Tinker E. The intentionality model and language acquisition: Engagement, effort, and the essential tension in development. Monographs of the Society for Research in Child Development. 2001;66(4 Serial No. 267) [PubMed] [Google Scholar]
- Bornstein MH, Putnick DL, Cote LR, Haynes OM, Suwalsky JT. Mother-infant contingent vocalizations in 11 countries. Psychological Science. 2015;26(8):1272–1284. doi: 10.1177/0956797615586796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau JP, Bushnell EW. Spilling thoughts: Configuring attentional resources in infants’ goal-directed actions. Infant Behavior and Development. 2000;23(3):543–566. [Google Scholar]
- Datavyu Team. Databrary Project. New York University; 2014. Datavyu: A Video Coding Tool. URL http://datavyu.org. [Google Scholar]
- Ejiri K, Masataka N. Co-occurences of preverbal vocal behavior and motor action in early infancy. Developmental Science. 2001;4(1):40–48. [Google Scholar]
- Fagan MK, Iverson JM. The influence of mouthing on infant vocalization. Infancy. 2007;11(2):191–202. doi: 10.1111/j.1532-7078.2007.tb00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Walle EA, Campos JJ. A cross-national investigation of the relationship between infant walking and language development. Infancy. 2015;20(3):283–305. [Google Scholar]
- Iverson JM. Developing language in a developing body: The relationship between motor development and language development. Journal of Child Language. 2010;37(02):229–261. doi: 10.1017/S0305000909990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Attention and effort. Upper Saddle River, NJ: Prentice Hall; 1973. [Google Scholar]
- LeBarton ES, Iverson JM. Fine motor skill predicts expressive language in infant siblings of children with autism. Developmental Science. 2013;16(6):815. doi: 10.1111/desc.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie MJ, Celler BG, Lovell NH, Coster ACF. Classification of basic daily movements using a triaxial accelerometer. Medical & Biological Engineering & Computing. 2004;42:679–687. doi: 10.1007/BF02347551. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Lavie N. The influence of perceptual load on age differences in selective attention. Psychology and Aging. 1998;13(4):563. doi: 10.1037//0882-7974.13.4.563. [DOI] [PubMed] [Google Scholar]
- Oudgenoeg-Paz O, Volman MC, Leseman PP. Attainment of sitting and walking predicts development of productive vocabulary between ages 16 and 28 months. Infant Behavior & Development. 2012;35(4):733. doi: 10.1016/j.infbeh.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Park S, Kim MS, Chun MM. Concurrent working memory load can facilitate selective attention: Evidence for specialized load. Journal of Experimental Psychology: Human Perception and Performance. 2007;33:1062–1075. doi: 10.1037/0096-1523.33.5.1062. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M. Attentional demands and postural control: The effect of sensory context. Journal of Gerontology. 2000;55A:M10–M16. doi: 10.1093/gerona/55.1.m10. [DOI] [PubMed] [Google Scholar]
- Siegler RS. Microgenetic analyses of learning. In: Damon W, Lerner R, Kuhn D, Siegler RS, editors. Handbook of child psychology: Vol. 2: Cognition, Perception and Language. 6. New York: John Wiley & Sons; 2006. pp. 464–510. [Google Scholar]
- Sieratzki JS, Woll B. Toddling into language: Precocious language development in motor-impaired children with spinal muscular atrophy. Lingua. 2002;112(6):423. [Google Scholar]
- Smith LB, Thelen E. Development as a dynamic system. Trend in Cognitive Sciences. 2003;7:343–348. doi: 10.1016/s1364-6613(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Surkar SM, Edelbrock C, Stergiou N, Berger S, Harbourne R. Sitting postural control affects the development of focused attention in children with cerebral palsy. Pediatric Physical Therapy. 2015;27(1):16–22. doi: 10.1097/PEP.0000000000000097. [DOI] [PubMed] [Google Scholar]
- Tipps ST, Mira MP, Cairns GF. Concurrent tracking of infant motor and speech development. Genetic Psychology Monographs. 1981;104:303–324. [PubMed] [Google Scholar]
- Walle EA, Campos JJ. Infant language development is related to the acquisition of walking. Developmental Psychology. 2014;50(2):336. doi: 10.1037/a0033238. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Wilson M, Lynch M, Cuykendall S. The cognitive benefits of movement reduction: Evidence from dance marking. Psychological Science. 2013;24(9):1732–1739. doi: 10.1177/0956797613478824. [DOI] [PubMed] [Google Scholar]
- Brauer SG, Woollacott M, Shumway-Cook A. The influence of a concurrent cognitive task on the compensatory stepping response to a perturbation in balance-impaired and healthy elders. Gait & Posture. 2002;15(1):83. doi: 10.1016/s0966-6362(01)00163-1. [DOI] [PubMed] [Google Scholar]
- Yingling J. Unpublished doctoral dissertation. University of Denver; 1981. Temporal features of infant speech: A description of babbling patterns circumscribed by postural achievement. [Google Scholar]