Abstract
Nontuberculous mycobacteria (NTM) lung disease is increasing globally. Although the etiological epidemiology of NTM is different across regions, Mycobacterium avium complex (MAC) is the leading cause of NTM lung disease in most countries, including mainland Japan. Okinawa is located in the southernmost region of Japan and is the only prefecture categorized as a subtropical region in Japan, it is therefore likely the etiological epidemiology of NTM lung disease is different from mainland Japan. From 2009 to 2015, the medical records of patients, with respiratory specimens positive for NTMs, visiting or admitted to two Okinawan hospitals, were retrospectively analyzed. NTM lung disease cases were defined according to the American Thoracic Society criteria and patient epidemiology and clinical information were evaluated. Results indicate four hundred sixteen patients had bacterial cultures positive for NTM. The most common NTM was M. abscessus complex (MABC) (n = 127; 30.5%), followed by M. intracellulare (n = 85; 20.4%). NTM lung disease was diagnosed in 114 patients. Of these cases, MABC was most common (n = 41; 36.0%), followed by M. intracellulare (n = 31; 27.2%). Chronic obstructive pulmonary disease (COPD) and tracheostomy patients were more likely to develop MABC than MAC lung disease. Multivariate analysis showed a probable association between COPD and MABC lung disease. Chest computed tomography (CT) evaluation revealed bronchiectasis, nodules, and consolidation were less frequently observed in MABC patients compared with MAC patients. Our data suggests Okinawa may be one of the few places where MABC is the predominant pathogen causing NTM lung disease and our results add new insight to MABC lung disease, which is not yet well understood.
Introduction
Nontuberculous mycobacteria (NTM) are ubiquitous bacteria widely distributed in the environment. NTM can cause a variety of infectious diseases in humans and NTM-induced lung infection is increasing globally [1, 2]. A recently published article shows that the incidence of pulmonary NTM disease in Japan has increased dramatically and cases of pulmonary M. avium complex (MAC), M. kansasii, and M. abscessus complex (MABC) disease were 13.1, 0.6, and 0.5 cases per 100,000 person-years, respectively [3]. The NTM species isolated from patients with NTM lung disease are geographically diverse, nevertheless MAC is the most common species in most countries [4].
Although rapidly growing mycobacteria (RGM), represented by MABC, M. fortuitum, and M. chelonae, are not common pathogens of NTM lung disease, MABC was frequently isolated from NTM lung disease patients in South Korea and Taiwan [3–6]. A single-center study reported from Taiwan, located 500 km south of Okinawa and also categorized as a subtropical region, showed that MABC was the most common species (44.8%) followed by M. fortuitum (23.9%) isolated from NTM lung disease patients; thus, RGM accounted for approximately 70% of the caseload [6]. Because of the subtropical climate of Okinawa is different than that of mainland Japan and is more similar to Taiwan, we hypothesized that the isolation rate for certain causative species of NTM lung disease in Okinawa and mainland Japan is also different. In this study, we retrospectively investigated the causative species of NTM lung disease and the clinical features of MABC induced lung disease, in two representative hospitals from Okinawa.
Materials and methods
Data collection
Between January 2009 and December 2015, patients, with NTMs cultured from respiratory specimens, visiting the outpatient department or admitted to the ward in the Okinawa Chubu Hospital (550 hospital beds) or the University of the Ryukyu Hospital (600 hospital beds), had their medical records retrospectively reviewed. NTM lung disease cases, defined according to the American Thoracic Society (ATS) criteria [7], were identified, underwent etiological analysis by species, and had other clinical information extracted. The clinical background, symptoms, and chest computed tomography (CT) findings of MABC and MAC infected patients were also compared.
Identification of NTM
Respiratory specimens were cultured with 2% Ogawa agar and the resulting bacterial colonies were collected for species identification. The resulting suspension liquid was tested using a DNA-DNA hybridization method from a commercially available identification kit (KYOKUTO Pharmaceutical Industrial Co. Ltd., Tokyo, Japan).
Evaluation of chest CT
Chest CT was evaluated by two experienced chest physicians. Abnormal findings were categorized into; (1) bronchiectasis, (2) nodules, (3) consolidation, (4) cavity, (5) ground-glass opacity, and (6) linear scarring, according to previous reports [8, 9]. Patients could be categorized with more than one finding.
Statistical analysis
Differences among patients’ background, symptoms, and chest CT images were compared for MABC and MAC patients by Fisher’s exact test. The difference in age between these two groups was analyzed by Wilcoxon/Kruskal-Wallis test. A two-sided p-value of <0.05 was considered to be statistically significant. Previously reported risk factors [10–12] for the development of NTM lung disease and possible risk factors revealed by this study were evaluated using logistic regression. All data were analyzed with JMP ver.12 (SAS Institute Inc., North Carolina, USA).
Ethics
The Institutional Ethics Committees of the Okinawa Chubu Hospital and the University of the Ryukyus approved this study.
Results
Pathogen distribution
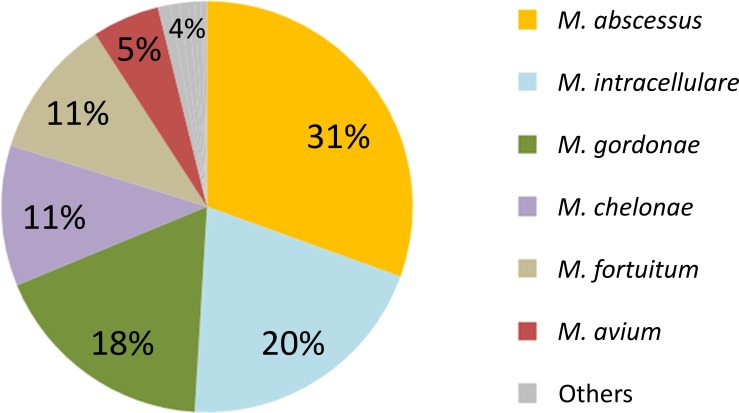
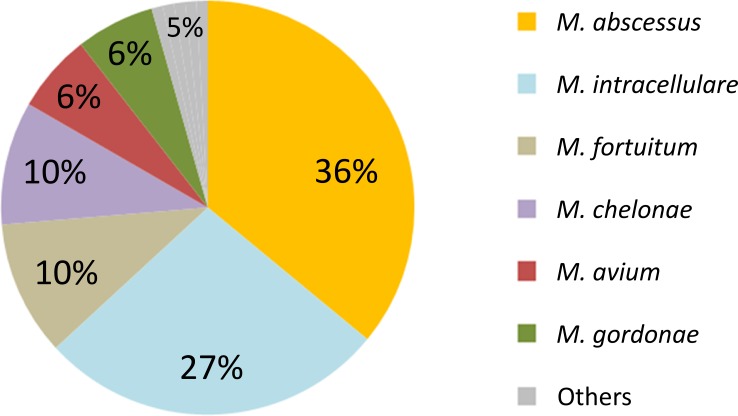
Four hundred sixteen patients were found with positive NTM culture results from respiratory specimens. As shown in Fig 1, the most frequently detected NTM was MABC (n = 127; 30.5%), followed by M. intracellulare (n = 85; 20.4%). NTM lung disease was diagnosed, according to the ATS criteria, in 114/416 patients (27%). Fig 2 shows the etiology of NTM lung disease patients. Again, the most frequently detected pathogen was MABC (n = 41; 36.0%), followed by M. intracellulare (n = 31; 27.2%).
Fig 1. NTMs cultured from respiratory samples (N = 416).
NTMs isolated from respiratory specimens were retrospectively analyzed. Others category includes: Mycobacterium kansasii (1.0%), M. szulgai (0.7%), M. xenopi (0.7%), M. peregrium (0.5%), M. scrofulaceum (0.5%), M. simiae (0.2%), and M. terrae (0.2%).
Fig 2. Epidemiology of NTM lung disease (N = 114).
NTM lung disease cases, defined according to the American Thoracic Society criteria, were extracted from NTM detected cases shown in Fig 1. Others category includes: Mycobacterium kansasii (0.9%), M. scrofulaceum (0.9%), M. szulgai (0.9%), M. terrae (0.9%), and M. xenopi (0.9%).
Characteristics of NTM lung disease patients
Clinical background and symptoms were compared for patients diagnosed with either MABC or MAC (n = 41 and 38, respectively). No statistical differences in clinical background, except chronic obstructive pulmonary disease (COPD), tracheotomy, and cerebrovascular disease, were calculated between two groups (Table 1). COPD was more frequent in MABC patients compared with MAC patients (MABC 24% vs MAC 5%, p = 0.0265). Eight tracheotomized patients were included among NTM lung disease patients and all cases were infected with RGM; six of these cases were infected with MABC (MABC 15% vs MAC 0%, p = 0.0261), the remaining two were determined to be M. chelonae. These eight instances of tracheotomy were due to amyotrophic lateral sclerosis (n = 3), disturbed consciousness after operation (n = 1), old healed tuberculosis (n = 1), right lung hypoplasia (n = 1), post-operation of hypopharyngeal cancer (n = 1), symptomatic epilepsy after cerebral infarction (n = 1). Multivariate analysis using logistic regression determined patients with COPD were significantly more likely to develop MABC (Table 2). Observed symptoms were not found to be different between the two groups (Table 3).
Table 1. Patient background.
| MABC | MAC | ||
|---|---|---|---|
| [N = 41] | [N = 38] | p value | |
| Age (median) | 74 | 78.5 | 0.0567 |
| Sex (male) | 17 (41%) | 12 (32%) | 0.4840 |
| Smoking history* | 17 (41%) | 14 (37%) | 1.0000 |
| Long-term care facility✝ | 6 (15%) | 8 (21%) | 0.5600 |
| COPD | 10 (24%) | 2 (5%) | 0.0265§ |
| Bronchial asthma | 7 (17%) | 3 (8%) | 0.3145 |
| Old healed tuberculosis | 7 (17%) | 6 (16%) | 1.0000 |
| Bronchiectasis | 6 (15%) | 4 (11%) | 0.7389 |
| Interstitial lung disease | 2 (5%) | 3 (8%) | 0.6675 |
| Thoracic surgery | 3 (7%) | 3 (8%) | 1.0000 |
| Tracheostomy | 6 (15%) | 0 | 0.0261§ |
| Ventilator | 2 (5%) | 1 (3%) | 1.0000 |
| Tracheal cannulation** | 6 (15%) | 1 (3%) | 0.1101 |
| Gastroesophageal disease | 4 (10%) | 3 (8%) | 1.0000 |
| Lung cancer | 1 (2%) | 0 | 1.0000 |
| Other solid cancer | 5 (12%) | 4 (11%) | 1.0000 |
| Hematological cancer | 1 (2%) | 0 | 1.0000 |
| Cardiovascular disease | 16 (39%) | 9 (24%) | 0.1564 |
| Chronic liver disease | 4 (10%) | 3 (8%) | 1.0000 |
| Chronic kidney disease | 7 (17%) | 6 (16%) | 1.0000 |
| Neuromuscular disease | 3 (7%) | 5 (13%) | 0.4711 |
| Cerebrovascular disease | 2 (5%) | 9 (24%) | 0.0221§ |
| Autoimmune disease | 4 (10%) | 8 (21%) | 0.2148 |
| Diabetes mellitus | 6 (15%) | 4 (11%) | 0.7389 |
| HTLV-1* | 5 (12%) | 1 (3%) | 0.1409 |
| Corticosteroid# | 9 (22%) | 8 (21%) | 1.0000 |
| Immunosuppressant | 5 (12%) | 2 (5%) | 0.4338 |
| Acid suppressant | 23 (56%) | 16 (42%) | 0.2629 |
*Only 76 and 19 patients had data included for smoking history and HTLV-1, respectively.
✝Patients admitted from long-term care facilities.
**Tracheal cannulation: includes tracheotomized patients and orally intubated patients.
#Patients receiving daily corticosteroid at any dose.
§Considered significant.
Abbreviations: MABC; Mycobacterium abscessus complex, MAC; Mycobacterium avium complex, COPD; chronic obstructive pulmonary disease, HTLV-1; human T-lymphotropic virus 1
Table 2. Multivariate analysis to determine clinical charecteristics for MABC.
| Odds ratio | 95% CI | p value | |
|---|---|---|---|
| COPD | 6.73 | 1.31–34.64 | 0.0225* |
| Tracheal cannulation** | 6.45 | 0.69–60.44 | 0.1025 |
| Bronchiectasis | 1.56 | 0.36–6.71 | 0.5474 |
| Gastroesophageal disease | 1.23 | 0.22–6.96 | 0.8157 |
| Old healed tuberculosis | 0.91 | 0.24–3.44 | 0.8854 |
*Considered significant.
**Tracheal cannulation: includes tracheotomized patients and orally intubated patients.
Abbreviations: COPD; chronic obstructive pulmonary disease, CI; confidence interval
Table 3. Symptoms.
| MABC | MAC | ||
|---|---|---|---|
| [N = 41] | [N = 38] | p value | |
| Fever (≧38°C) | 9 (22%) | 9 (24%) | 1.0000 |
| Cough | 16 (39%) | 18 (47%) | 0.5009 |
| Sputum | 31 (76%) | 27 (71%) | 0.7995 |
| Hemosputum | 4 (10%) | 5 (13%) | 0.7312 |
| Weight loss* | 2 (5%) | 0 | 0.4943 |
*Weight loss was defined as decrease of more than 5% of body weight in 6 months.
Abbreviations: MABC; Mycobacterium abscessus complex, MAC; Mycobacterium avium complex
Chest CT findings
Chest CT was performed for 40 MABC and 36 MAC patients. Bronchiectasis, nodules, and consolidation were observed less frequently in MABC patients compared with MAC patients (Table 4).
Table 4. Chest CT findings.
| MABC | MAC | ||
|---|---|---|---|
| [N = 40] | [N = 36] | p value | |
| Bronchiectasis | 21 (53%) | 29 (81%) | 0.0150* |
| Nodules | 14 (35%) | 25 (69%) | 0.0032* |
| Consolidation | 17 (43%) | 25 (69%) | 0.0224* |
| Cavity | 2 (5%) | 3 (8%) | 0.6631 |
| Ground-glass opacity | 9 (23%) | 7 (19%) | 0.7851 |
| Linear scarring | 30 (75%) | 26 (72%) | 0.8003 |
In total, 67 patients had more than one finding.
*Considered significant.
Abbreviations: MABC; Mycobacterium abscessus complex, MAC; Mycobacterium avium complex
Discussion
MABC was revealed to be the most common causative pathogen for NTM lung disease in this cohort. However, since only two hospitals were involved in the current study, our data may not precisely reveal the etiology of NTM lung disease in Okinawa Prefecture due to sampling bias. However, our data indicates the detection rate of MABC among NTM lung disease patients in Okinawa was altogether different from previous Japanese reports. Morimoto et al., reported the epidemiology of NTM in Japan based on a laboratory-based analysis [13]. Although the data for Okinawa Prefecture was not isolated, MABC was detected more frequently in the Kyushu-Okinawa region than in other, more temperate, regions of Japan. Morimoto et al., also reports that MAC was the most common pathogen to cause NTM lung disease in the Kyushu-Okinawa region [10]. This result is markedly different from our data.
For convenience, Okinawa is frequently combined with Kyushu as the "Kyushu-Okinawa region," despite Okinawa being 700 kilometers away from the Kyushu district and falling within the subtropical zone. Due to the climate change and other potential factors, Okinawa experiences unique epidemiological events for many infectious diseases, which can be completely different from those experienced on mainland Japan [14–17]. Additionally, the population of Okinawa is only one tenth the size of Kyushu's (1.4 million vs 13 million). Therefore, we can assume that Okinawa’s distinctive epidemiology, observed in our study, was diminished in the aforementioned article. Moreover, the relatively high isolation rate of MABC in our study is similar to the epidemiology seen in other Asian countries [4, 5]. In Taiwan and South Korea, the isolation rate of MABC from NTM lung disease patients is higher [6, 18–21]. Frequently, differences in epidemiology can be explained by differences in climate conditions, environmental exposure, ethnic backgrounds, and laboratory practices (i.e., culture temperatures) [5]. The climate conditions of Okinawa and Taiwan are both classified as subtropical, however climate conditions in South Korea are decidedly different. Although further research is needed, common factors among South Korea, Taiwan, and Okinawa may be able to explain this characteristic epidemiology.
Our multivariate analysis showed a possible association between COPD and MABC infection. COPD was previously reported as a risk factor for NTM lung disease, however the distinct species, associated with COPD patients, was not specified [22]. As far as we know, risk factors related to the development of MABC lung disease remain unclear. However, past studies revealed that cystic fibrosis, gastroesophageal disease, and previous mycobacterial lung disease should be considered as potential risk factors related to the development of RGM induced lung disease [23, 24]. Since RGM induced lung disease is predominantly due to MABC [25], the risk factors mentioned above should also be considered risk factors for MABC lung disease. As with cystic fibrosis and previous mycobacterial lung disease, normal lung structure is destroyed and clearance of sputum is impaired in COPD, thus it is likely that COPD patients are susceptible to MABC infection, as also described by Chan et al. [26].
Since tracheostomy was not an appropriate variable for logistic regression in this study because of no tracheotomized patient in MAC group, tracheal cannulation was evaluated by multivariate analysis. Although the relationship between MABC and tracheal cannulation was not determined to be statistically significant, it is noteworthy that all tracheotomized patients with NTM lung disease had cultures positive for RGM (MABC: 6 cases, M. chelonae: 2 cases) in our study. Although information regarding the relationship between tracheotomy and MABC is limited, Do et al., reported that MABC was frequently detected from children with tracheostomy [27]. Their study retrospectively reviewed 5-years of data on MABC infection in a pediatric pulmonary center and 16 cases infected with MABC were identified. Of these, medical records were available for 15 patients and 73% of cases (11/15) were patients with tracheostomy. Lee et al., also showed MABC was frequently identified among ventilator-dependent patients [28]. Since RGM including MABC can cause skin and soft tissue colonization and infection, we speculate that MABC infection around tracheostomy may traverse the tracheal epithelium and eventually cause lung infection.
There is limited data concerning the clinical manifestations of MABC lung disease. Griffith et al., reported that cough was the most frequent symptom (70%) in RGM patients and hemoptysis and dyspnea were also common [25]. However, there are no RGM-specific respiratory symptoms, and our results also confirm this.
Our study demonstrated that bronchiectasis, nodules, and consolidation were observed more frequently in MAC patients than in MABC patients. According to Chung et al., no significant difference was observed in the presence of small nodules, tree-in-bud pattern, and bronchiectasis between two groups [29]. However, nodule, airspace consolidation, and thin-walled cavities were observed more frequently in MAC patients. Thus, results regarding bronchiectasis and cavitation were different from our study. Although the precise reason is unknown, one possible explanation is the difference of patients’ background. There are two major radiographic features of NTM lung disease; fibrocavitary form and nodular bronchiectatic form, with the former pattern often seen in older male patients with underlying lung disease such as COPD [7, 10]. Since COPD was more frequent in MABC patients compared with MAC patients in our study, bronchiectasis and nodules may be observed less frequently in MABC group. Another explanation for the differing radiographic findings may be the multiple subspecies of MABC. It has been reported that the etiological epidemiology of three subspecies of MABC (M. abscessus, M. massiliense, and M. bolletii) varies due to geographical region. Furthermore, differences in the genotypes of M. abscessus and M. massiliense were associated with varying radiological features and abnormal shadows in the lung [30, 31]. Since the distributional pattern of MABC genotypes is assumed different between Okinawa and South Korea, it is possible that the radiological features of MABC lung disease are also different.
Our study has some limitations. As a retrospective study conducted in two hospitals, our data is sparse and potentially biased. Data from several more hospitals in Okinawa should be collected to reveal a more accurate epidemiology of NTM lung disease in Okinawa. Additionally, we did not identify MABC to the subspecies level. According to DNA sequence and drug sensitivity, MABC is divided into 3 subspecies: M. abscessus (A-type), M. massiliense (M-type), and M. bolletii (B-type) [32–35]. Because some studies have shown differences among the biological characteristics, antibiotic sensitivity, and clinical features of the three subspecies of MABC [36, 37], differentiation of three subspecies of MABC may reveal more profound results.
Nevertheless, if treated as a pilot study, our results revealed considerable differences regarding the epidemiology of NTM lung disease in Okinawa, Japan and provided valuable insight regarding the differences between the clinical characteristics of MABC and MAC lung disease. Further studies are needed to present a more accurate understanding of the epidemiology, clinical features, and risk factors of MABC lung disease.
Supporting information
[Raw data for 2nd submission.xlsx].
(XLSX)
Acknowledgments
We would like to thank Ms. Gretchen Parrott, M.P.H., for her English emendation and advice regarding statistical analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Thomson RM. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis. 2010;16(10):1576–83. Epub 2010/09/30. doi: 10.3201/eid1610.091201 ; PubMed Central PMCID: PMCPMC3294381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881–6. Epub 2012/02/09. doi: 10.1164/rccm.201111-2016OC ; PubMed Central PMCID: PMCPMC3360574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namkoong H, Kurashima A, Morimoto K, Hoshino Y, Hasegawa N, Ato M, et al. Epidemiology of Pulmonary Nontuberculous Mycobacterial Disease, Japan(1). Emerg Infect Dis. 2016;22(6):1116–7. doi: 10.3201/eid2206.151086 ; PubMed Central PMCID: PMCPMC4880076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36(1):13–34. doi: 10.1016/j.ccm.2014.10.002 ; PubMed Central PMCID: PMCPMC4332564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons S, van Ingen J, Hsueh PR, Van Hung N, Dekhuijzen PN, Boeree MJ, et al. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg Infect Dis. 2011;17(3):343–9. Epub 2011/03/12. doi: 10.3201/eid1703.100604 ; PubMed Central PMCID: PMCPMC3165997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang CC, Lin MC, Liu JW, Wang YH. Nontuberculous mycobacterial lung disease in southern Taiwan. Chang Gung Med J. 2009;32(5):499–508. . [PubMed] [Google Scholar]
- 7.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST . [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, Tanaka N, Newell JD, Degroote MA, Fulton K, Huitt G, et al. Nontuberculous mycobacterial infection: CT scan findings, genotype, and treatment responsiveness. Chest. 2005;128(6):3863–9. Epub 2005/12/16. doi: 10.1378/chest.128.6.3863 . [DOI] [PubMed] [Google Scholar]
- 9.Kim HS, Lee KS, Koh WJ, Jeon K, Lee EJ, Kang H, et al. Serial CT findings of Mycobacterium massiliense pulmonary disease compared with Mycobacterium abscessus disease after treatment with antibiotic therapy. Radiology. 2012;263(1):260–70. Epub 2012/03/01. doi: 10.1148/radiol.12111374 . [DOI] [PubMed] [Google Scholar]
- 10.Koh WJ. Nontuberculous Mycobacteria-Overview. Microbiology spectrum. 2017;5(1). Epub 2017/01/28. doi: 10.1128/microbiolspec.TNMI7-0024-2016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. The New England journal of medicine. 1989;321(13):863–8. Epub 1989/09/28. doi: 10.1056/NEJM198909283211304 . [DOI] [PubMed] [Google Scholar]
- 12.Varghese G, Shepherd R, Watt P, Bruce JH. Fatal infection with Mycobacterium fortuitum associated with oesophageal achalasia. Thorax. 1988;43(2):151–2. Epub 1988/02/01. ; PubMed Central PMCID: PMCPMC1020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morimoto K, Hasegawa N, Izumi K, Namkoong H, Uchimura K, Yoshiyama T, et al. A Laboratory-based Analysis of Nontuberculous Mycobacterial Lung Disease in Japan from 2012 to 2013. Annals of the American Thoracic Society. 2017;14(1):49–56. Epub 2016/10/28. doi: 10.1513/AnnalsATS.201607-573OC . [DOI] [PubMed] [Google Scholar]
- 14.Clark JW, Robert-Guroff M, Ikehara O, Henzan E, Blattner WA. Human T-cell leukemia-lymphoma virus type 1 and adult T-cell leukemia-lymphoma in Okinawa. Cancer research. 1985;45(6):2849–52. Epub 1985/06/01. . [PubMed] [Google Scholar]
- 15.Zaha O, Hirata T, Kinjo F, Saito A. Strongyloidiasis—progress in diagnosis and treatment. Internal medicine (Tokyo, Japan). 2000;39(9):695–700. Epub 2000/09/02. . [DOI] [PubMed] [Google Scholar]
- 16.Kamiyama K, Kinjo T, Chinen K, Iwamasa T, Uezato H, Miyagi JI, et al. Human herpesvirus 8 (HHV8) sequence variations in HHV8 related tumours in Okinawa, a subtropical island in southern Japan. Journal of clinical pathology. 2004;57(5):529–35. Epub 2004/04/29. doi: 10.1136/jcp.2003.012724 ; PubMed Central PMCID: PMCPMC1770308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakugawa H, Nakasone H, Shokita H, Nakayoshi T, Kinjo F, Saito A, et al. Seroepidemiological study of hepatitis delta virus infection in Okinawa, Japan. Journal of medical virology. 1995;45(3):312–5. Epub 1995/03/01. . [DOI] [PubMed] [Google Scholar]
- 18.Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006;129(2):341–8. doi: 10.1378/chest.129.2.341 . [DOI] [PubMed] [Google Scholar]
- 19.Shu CC, Lee CH, Hsu CL, Wang JT, Wang JY, Yu CJ, et al. Clinical characteristics and prognosis of nontuberculous mycobacterial lung disease with different radiographic patterns. Lung. 2011;189(6):467–74. Epub 2011/10/01. doi: 10.1007/s00408-011-9321-4 . [DOI] [PubMed] [Google Scholar]
- 20.Park YS, Lee CH, Lee SM, Yang SC, Yoo CG, Kim YW, et al. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2010;14(8):1069–71. Epub 2010/07/16. . [PubMed] [Google Scholar]
- 21.Lee SK, Lee EJ, Kim SK, Chang J, Jeong SH, Kang YA. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scandinavian journal of infectious diseases. 2012;44(10):733–8. Epub 2012/06/23. doi: 10.3109/00365548.2012.681695 . [DOI] [PubMed] [Google Scholar]
- 22.Andrejak C, Nielsen R, Thomsen VO, Duhaut P, Sorensen HT, Thomsen RW. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68(3):256–62. Epub 2012/07/12. doi: 10.1136/thoraxjnl-2012-201772 . [DOI] [PubMed] [Google Scholar]
- 23.Koh WJ, Lee JH, Kwon YS, Lee KS, Suh GY, Chung MP, et al. Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest. 2007;131(6):1825–30. Epub 2007/04/03. doi: 10.1378/chest.06-2280 . [DOI] [PubMed] [Google Scholar]
- 24.Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977–82. Epub 2010/05/29. doi: 10.1164/rccm.201003-0503OC . [DOI] [PubMed] [Google Scholar]
- 25.Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147(5):1271–8. doi: 10.1164/ajrccm/147.5.1271 . [DOI] [PubMed] [Google Scholar]
- 26.Chan ED, Bai X, Kartalija M, Orme IM, Ordway DJ. Host immune response to rapidly growing mycobacteria, an emerging cause of chronic lung disease. American journal of respiratory cell and molecular biology. 2010;43(4):387–93. Epub 2010/01/19. doi: 10.1165/rcmb.2009-0276TR . [DOI] [PubMed] [Google Scholar]
- 27.Do PC, Nussbaum E, Moua J, Chin T, Randhawa I. Clinical significance of respiratory isolates for Mycobacterium abscessus complex from pediatric patients. Pediatric pulmonology. 2013;48(5):470–80. Epub 2012/07/27. doi: 10.1002/ppul.22638 . [DOI] [PubMed] [Google Scholar]
- 28.Lee MR, Tsai CJ, Hu JY, Lee SW, Ko JC, Wang HC, et al. Acquisition of Mycobacterium abscessus among ventilator-dependent patients in Taiwan chronic respiratory care facilities. Future microbiology. 2016;11(4):491–500. Epub 2016/04/12. doi: 10.2217/fmb.16.6 . [DOI] [PubMed] [Google Scholar]
- 29.Chung MJ, Lee KS, Koh WJ, Lee JH, Kim TS, Kwon OJ, et al. Thin-section CT findings of nontuberculous mycobacterial pulmonary diseases: comparison between Mycobacterium avium-intracellulare complex and Mycobacterium abscessus infection. Journal of Korean medical science. 2005;20(5):777–83. Epub 2005/10/15. doi: 10.3346/jkms.2005.20.5.777 ; PubMed Central PMCID: PMCPMC2779274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh WJ, Stout JE, Yew WW. Advances in the management of pulmonary disease due to Mycobacterium abscessus complex. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2014;18(10):1141–8. Epub 2014/09/14. doi: 10.5588/ijtld.14.0134 . [DOI] [PubMed] [Google Scholar]
- 31.Shin SJ, Choi GE, Cho SN, Woo SY, Jeong BH, Jeon K, et al. Mycobacterial genotypes are associated with clinical manifestation and progression of lung disease caused by Mycobacterium abscessus and Mycobacterium massiliense. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57(1):32–9. Epub 2013/03/21. doi: 10.1093/cid/cit172 . [DOI] [PubMed] [Google Scholar]
- 32.Adekambi T, Colson P, Drancourt M. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. Journal of clinical microbiology. 2003;41(12):5699–708. Epub 2003/12/10. doi: 10.1128/JCM.41.12.5699-5708.2003 ; PubMed Central PMCID: PMCPMC308974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leao SC, Tortoli E, Euzeby JP, Garcia MJ. Proposal that Mycobacterium massiliense and Mycobacterium bolletii be united and reclassified as Mycobacterium abscessus subsp. bolletii comb. nov., designation of Mycobacterium abscessus subsp. abscessus subsp. nov. and emended description of Mycobacterium abscessus. International journal of systematic and evolutionary microbiology. 2011;61(Pt 9):2311–3. Epub 2010/11/03. doi: 10.1099/ijs.0.023770-0 . [DOI] [PubMed] [Google Scholar]
- 34.Telenti A, Marchesi F, Balz M, Bally F, Bottger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. Journal of clinical microbiology. 1993;31(2):175–8. Epub 1993/02/01. ; PubMed Central PMCID: PMCPMC262730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffith DE, Brown-Elliott BA, Benwill JL, Wallace RJ Jr. Mycobacterium abscessus. "Pleased to meet you, hope you guess my name…". Annals of the American Thoracic Society. 2015;12(3):436–9. Epub 2015/02/03. doi: 10.1513/AnnalsATS.201501-015OI . [DOI] [PubMed] [Google Scholar]
- 36.Harada T, Akiyama Y, Kurashima A, Nagai H, Tsuyuguchi K, Fujii T, et al. Clinical and microbiological differences between Mycobacterium abscessus and Mycobacterium massiliense lung diseases. Journal of clinical microbiology. 2012;50(11):3556–61. Epub 2012/08/24. doi: 10.1128/JCM.01175-12 ; PubMed Central PMCID: PMCPMC3486228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183(3):405–10. Epub 2010/09/14. doi: 10.1164/rccm.201003-0395OC . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Raw data for 2nd submission.xlsx].
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.