Abstract
Objective: Scalp acupuncture is a somatic stimulation therapy that produces prominent clinical effects when used to treat cerebral diseases. However, this acupuncture's therapeutic mechanisms have not yet been well-addressed. Scalp acupoints are innervated by the trigeminal nerve, which is coincident with the intracranial sensory afferents as well as with the meningeal vessels. In recent years, cerebrospinal fluid–contacting neurons have been found and proved to transmit allergic substances between brain the parenchyma and meninges, representing a possible network between scalp acupuncture and the brain. The aim of the current study was to observe the connections between scalp acupoints and the meninges and to establish a possible mechanism for scalp acupuncture.
Materials and Methods: Twenty-five adult Sprague-Dawley rats were used for the present study. Evans Blue dye (Sigma Chemical Co, St. Louis, MO) was injected though each rat's caudal vein after trigeminal stimulation for plasma extravasation observation. Cerebral blood flow (CBF) values of the rat's brain surface were measured at different timepoints before and after electroacupuncture (EA) on GB 15 (Toulinqi) or ST 36 (Zusanli).
Results: These preliminary studies indicated that neurogenic plasma extravasation on a rat's skin and dura mater after mechanical or electrical stimulation of the trigeminal nerves is a reliable way to show the pathologic connection between scalp acupoints and the meninges. Moreover, CBF of the rat's brain surface is increased significantly after EA stimulation at GB 15 (Toulinqi), which is located in the receptive field of the supraorbital nerve.
Conclusions: These findings suggest that the mechanism of scalp acupuncture might lie in the specific neurologic pathway that could be termed as trigeminal nerve–meninges–cerebrospinal fluid–contacting neurons–brain, which is a possible shortcut to brain functional regulation and cerebral disease treatment.
Keywords: : scalp acupuncture, mechanism, neuronal pathway, trigeminal nerve, cerebrospinal fluid–contacting neurons
Introduction
Scalp acupuncture was formed as an independent and complete acupuncture system in the early 1970s. It is based on the theory of neuroanatomy, neurophysiology, and bio-holographic principles of modern medicine. Scalp acupuncture also differs from body acupuncture, which uses the pathology of Yin–Yang, Five Elements, and meridians as its basis according to Traditional Chinese Medicine.
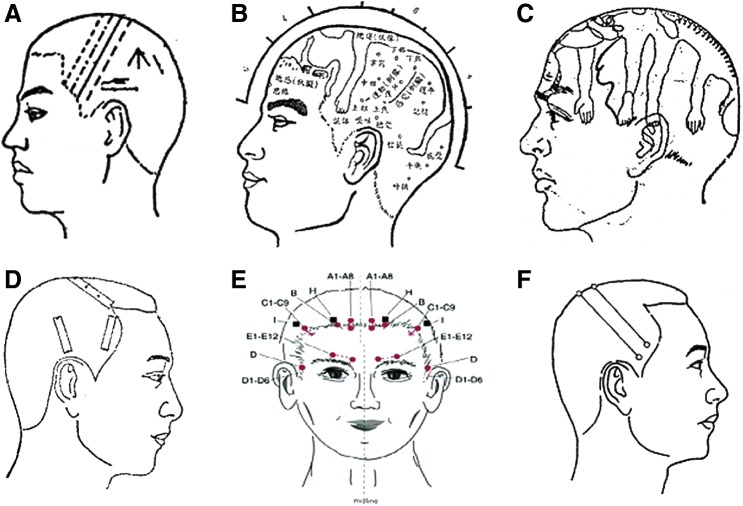
In 1971, Prof. Jiao Shunfa was the first to establish a modern acupuncture technique, combining traditional needling methods with Western medical knowledge of representative areas of the cerebral cortex.1 Jiao's scalp acupuncture stems from the nervous system concept from Western medicine. This acupuncture is used to stimulate specific scalp areas, such as the motor area, the sensory area, and the praxis area (Fig. 1A). Jiao's scalp areas, corresponding to the functional locations of the cerebral cortex, are used as the stimulated areas in scalp acupuncture for the treatment of diseases. Later, in 1976, Prof. Fang Yunpeng published another scalp acupuncture concept, which stipulated that the scalp was divided into seven zones and twenty-one acupoints2 (Fig. 1B). Other scalp acupuncture therapies that emerged included Prof. Tang Songyan's scalp acupuncture (Fig.1C) and Prof. Zhu Mingqing's scalp acupuncture (Fig. 1D). Each of them used different diagrams and locations of scalp acupoints.
FIG. 1.
Illustrations of different scalp acupuncture theories. (A) Jiao Shunfa Scalp Acupuncture, (B) Fang Yunpeng Scalp Acupuncture, (C) Tang Songyan Scalp Acupuncture, (D) Zhu Mingqing Scalp Acupuncture, (E) Yamamoto New Scalp Acupuncture (YNSA), and (F) the Standard Nomenclature of Scalp Acupuncture.
Then, in the 1960s, the Japanese physician Toshikatsu Yamamoto, MD, PhD, developed Yamamoto New Scalp Acupuncture (YNSA), which was based on somatotopic localization. YNSA involves five highly effective points, termed Basal points, and several other points named Sensory points, Brain points, Y points, and Extra points, which are all selected through abdominal or neck test zone palpation3 (Fig. 1E).
Although there are numerous differences between Japanese and Chinese scalp acupuncture therapies, there are many common aspects in clinical indications and manipulating methods. A Standard Nomenclature of Scalp Acupuncture was promulgated by the World Health Organization (WHO) in 1989, and this nomenclature has been used since that time.4
In view of the standardization of scalp acupuncture, there is no doubt regarding its wide range of applications. Scalp acupuncture therapy during clinical treatment is required to achieve a certain amount of stimulation; therefore, scalp acupuncture is different from body acupuncture, which only uses De Qi to show that stimulation is occurring. The selection of scalp acupoints relies on functional localization of the cerebral cortex, rather than traveling along the courses of meridians, as is done in body acupuncture. In addition, although scalp acupuncture is widely used in clinical practice, there are few supportive scientific studies yet published. Thus, the aim of the present research was to develop a hypothesis about the mechanism of scalp acupuncture that originates from neurophysiology and recent advances in neurobiology.
Materials and Methods
Animal Preparation
Experiments were carried out in accordance with the Guide for Care and Use of Laboratory Animals issued by the U.S. National Institutes of Health, and the Institutional Animal Care and Use Committee of the China Academy of Chinese Medical Sciences. Twenty-five adult male Sprague-Dawley rats, weighing 250 g–280 g, were used in the experiment. All rats were kept in an animal house maintained at 21°C ± 2°C, with a 12-hour light–dark cycle and with free access to food and water. All efforts were made to minimize the number of animals used and mitigate their suffering.
Plasma Extravasation Observation
The rats were anesthetized with pentobarbital (50 mg/kg i.p.). Each rat's body temperature was kept at 37°C ± 1°C, using a heating lamp controlled by a rectal thermistor probe. The fur on the right face was clipped before exposing the right mandibular nerve. In an experimental group of rats, electrical stimulation (1 mA, 10 Hz, 0.5 msec, 20 minutes) was applied to each rat's right maxillary nerve. Afterward, Evans Blue dye (EB; Sigma Chemical Co, St. Louis, MO), dissolved in sterile water (50 mg/kg), was injected through the caudal vein. Skin-color changes were observed after 2 hours, and the area of dye extravasation of skin and dura mater was photographed (Canon Inc., Ōta,Tokyo, Japan) after a craniotomy. Care was taken not to cause any injury to the skin before and during hair removal with depilatory cream.
Measurement of Cerebral Blood Flow
Each rat's cranium was removed from the bragma to the lambda to expose the dura mater and the brain surface. Once the preparation was complete, the rat's mean cerebral blood flow (CBF) was studied with full-field laser perfusion imaging (Moor FLPI,tm Moor Instruments, Devon, United Kingdom). A charge-coupled-device camera was set for low-resolution/high-speed images, including a display rate of 25 Hz, a time constant of 1.0 second, and a camera exposure time of 8.3 seconds. The contrast images were processed to produce a color-coded live-flux image (red denoted high perfusion and blue signified low perfusion). The rats in the experimental group were stimulated on GB 15 (Toulinqi) or on ST 36 (Zusanli) with an Hawato electroacupuncture (EA) apparatus (3 mA, 15 Hz; Hwato, China) for 20 minutes. GB 15 is located on the forehead straight up from the pupil of the eye and is innervated by the supraorbital nerve (the first branch of the trigeminal nerve). ST 36 is located on the anterolateral side of the hind limb near the anterior crest of the tibia below the knee, under the tibialis anterior muscle, and is innervated by the peroneal nerve. CBF values were measured at different timepoints before and after EA stimulation.
Results
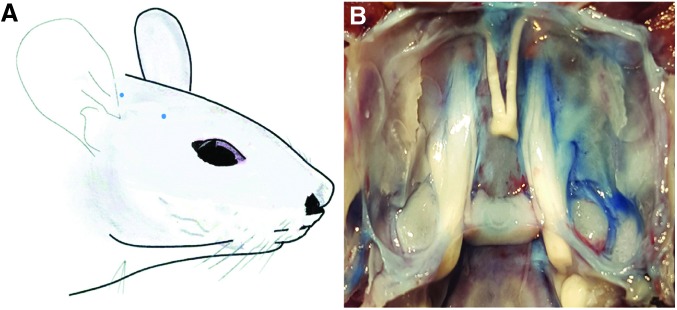
Nerve Stimulation Induced EB-Extravasated Points in Facial Skin and Dura Mater
In rats who received electrical stimulation, small blue extravasation EB dots were observed in the right orofacial skin, especially in the infraorbital area (Fig. 2A). After a craniotomy operation, the dura mater on the right side of the posterior and middle cranial fossa was stained and found to be darker than the contralateral dura mater in the experimental group (Fig. 2B), which indicated that there was plasma extravasations after nerve stimulation, while, no blue EB extravasation was observed in the skin or dura mater of rats in the control group (not shown).
FIG. 2.
After right trigeminal nerve stimulation, neurogenic plasma extravasation dots can be observed in facial skin (A) and dura mater (B) by means of Evans Blue dye injection.
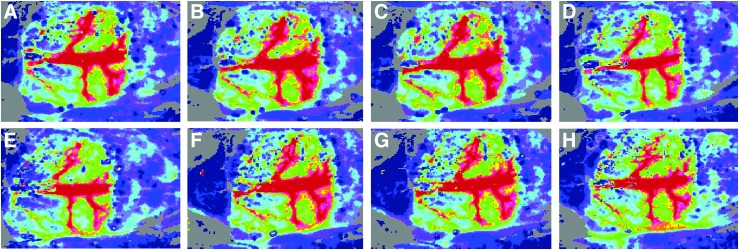
CBF Increased After EA Stimulation
During the entire observation period, CBF values of a group of rats stimulated at GB 15 (the GB 15 Group) were increased significantly after EA stimulation, compared to values before and after EA stimulation. In the GB 15 Group, 10 minutes and 20 minutes of EA stimulation led to a direct increase of each rat's sagittal sinus and superior cerebral veins (Fig. 3A–D). However, there was no obvious increase of CBF values in a group of rats stimulated at ST 36 (the ST 36 Group) during the observation period (Fig. 3E–H).
FIG. 3.
Mean cerebral blood flow (CBF) values showing the effects of scalp acupuncture (electroacupuncture; EA) and somatic acupuncture at different timepoints: (A and E), 5 minutes before EA; (B and F), 10 minutes of EA; (C and G), 20 minutes of EA; and (D and H), 5 minutes after EA. During the entire observation period, mean CBF values increased significantly after GB 15 (Toulinqi) stimulation (A–D), while ST 36 (Zusanli) stimulation produced no obvious increase of CBF values during the observation period (E–H).
Discussion
This study demonstrated that scalp acupuncture could cause greater and more consistent reflexes than somatic acupuncture. This response was related to greater inputs into the trigeminal nerve and to the special roles of the trigeminal ganglion (TG) and spinal trigeminal nucleus (STN) in scalp acupuncture modulation of meningeal function.
The trigeminal nerve is mainly responsible for facial sensation. The three branches of the trigeminal nerve also carry sensory messages from the meninges and modify the pial arteries as well.5 The primary sensory neurons are located in the TG and give rise to pseudo-unipolar trigeminal afferents, which project impulses to synapses on intra- and extracranial structures as well as to the STN. Second-order neurons in the STN ascend in the trigeminothalamic tract to send impulses to synapses on third-order thalamocortical neurons. Under migrainous pathologic circumstances, activation of the afferent fibers of the meninges can be transmitted by the TG to the STN. This sensitization of the second-order neurons in the STN induces allodynia in corresponding facial areas, including the frontal, temporal, parietal, occipital, and high cervical regions.6 Via axon reflex and dorsal-horn reflex, inputs from the facial area and meninges interact in the TG or STN; this has already been proven to occur in animal experiments and in the human body7,8 in migraine studies.
An axon reflex mechanism has been added to the explanations of the effects of acupuncture.9 Activation of unmyelinated cutaneous afferents leads to inflammatory reactions—such as increased vascular permeability and plasma extravasation—which are also considered to be neurogenic inflammations. This reaction can occur in tissues such as the derma, joints, and dura mater.10 It is commonly recognized that afferents are activated by intense local stimulation on the sensory nerves through the axon reflex. This reflex occurs between somatic and visceral organs; that is, the same axon branches dominate the body surface and viscera. The stimulation of one branch will lead to the antidromic activation of another branch of major axons.11
Although the effector action of primary afferent fibers is often attributed to axon reflexes, this action can also be initiated in the spinal cord by primary afferent depolarization large enough to trigger dorsal root reflexes (DRRs).12 DRRs can be induced by electrical stimulation of peripheral nerves.13 This antidromic activity could, in turn, result in the release of inflammatory mediators, making a positive contribution to the development of neurogenic inflammation.14
Cerebrospinal fluid (CSF)–contacting neurons (CSF-cNs) are mainly found in the thalamic paraventricular nucleus, hypothalamus, dorsal raphe nucleus, median raphe nucleus, linear nucleus, and spinal cord. Most of the CSF-cNs conduct dendritic processes into the internal CSF of the brain ventricles or the central canal. Some of these neurons are similar to chemoreceptor-type sensory cells. Presently, several lines of inquiry have indicated that CSF-cNs are involved in the transduction and regulation of encephalalgia. The distribution of neuronal nitric oxide synthase, gamma-aminobutyric acid, 5-hydroxytryptamine and its receptors, substance P, and other types of neurotropic substances in CSF-cNs have been found to be involved in pain modulation.15
In the present experiment, electrical stimulation of the trigeminal nerve was performed, followed by EB-solution injection. EB dye is visible and is able to bind to plasma proteins; therefore, EB provides a convenient way of showing areas of plasma extravasation.16 With the observation of extravasation spots of EB dye on the rats' dural mater and facial skin, it was proven that the trigeminal nerve could cause neurogenic inflammation through the axon reflex or the dorsal root reflex, thus making connections between the facial skin and the meninges. This CBF experiment offers further results, showing the different influences of scalp acupuncture and body acupuncture on brain-surface blood flew. Furthermore, the functions of CSF-cNs provide a direct connection between the brain surface and the meninges during physiologic or pathogenetic states, such as migraine.
Conclusions
Both the neurogenic plasma extravasation and microcirculation of the brain surface of the experimental animals after scalp acupoints stimulation suggest that scalp acupuncture might play a more important role in regulating functions of the meninges or cerebral parenchyma than somatic acupuncture does. On the basis of the existing evidence and this primary investigation, the authors want to explore the possibility of mediation of scalp acupuncture for cerebral diseases, with the shortcut of the axon reflex from the primary neurons in the TG and that of the DRRs from the second-order neurons in STN by morphologic, neurophysiologic, and pharmacologic approaches used with laboratory rats in the future.
Acknowledgments
This work was supported by the Natural Science Foundation of China granted to Xinyan Gao (No. 81473778) and Bing Zhu (No. 81674075), the Fundamental Research Funds for the Central Public Welfare Research Institutes (No. ZZZD16001) and the International Science & Technology Cooperation Program of China (No. 2014DFG32700).
Author Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Jiao SF. Scalp Acupuncture. Taiyuan, China: Shanxi People's Publishing House; 1982 [Google Scholar]
- 2.Wang FC. Chinese Alternative Treatment Methods—Scalp Acupuncture Therapy Beijing: People's Medical Publishing House; 2008 [Google Scholar]
- 3.Yamamoto T, Yamamoto H. Yamamoto New Scalp Acupuncture. Tokyo: Axel Springer Japan Publishing; 1998 [Google Scholar]
- 4.World Health Organization (WHO) Scientific Group on International Acupuncture Nomenclature. A Proposed Standard International Acupuncture Nomenclature: Report of a WHO Scientific Group. Geneva: WHO; 1991 [Google Scholar]
- 5.Moskowitz MA, Wei EP, Saito K, et al. Trigeminalectomy modifies pial arteriolar responses to hypertension or norepinephrine. Am J Physiol. 1998;255(1[pt2]):H1–H6 [DOI] [PubMed] [Google Scholar]
- 6.Ellrich J, Andersen OK, Messlinger K, et al. Convergence of meningeal and facial afferents onto trigeminal brainstem neurons: An electrophysiological study in rat and man. Pain. 1999;82(3):229–237 [DOI] [PubMed] [Google Scholar]
- 7.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33(1):48–56 [DOI] [PubMed] [Google Scholar]
- 8.Schwedt TJ, Larson-Prior L, Coalson RS, et al. Allodynia and descending pain modulation in migraine: A resting state functional connectivity analysis. Pain Med. 2014;15(1):154–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsson CP, Sundler F, Wallengren J. Cutaneous innervation before and after one treatment period of acupuncture. Br J Dermatol. 2006;155(5):970–976 [DOI] [PubMed] [Google Scholar]
- 10.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: Involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24(3):739–768 [DOI] [PubMed] [Google Scholar]
- 11.Cao DY, Niu HZ, Du JQ, Zhu ZL. Acupoint electrical stimulation initiates visceral neurogenic inflammation reaction via primary afferent reflex in the rat [in Chinese]. Zhen Ci Yan Jiu. 2002;27(1):45–49 [Google Scholar]
- 12.Willis WD., Jr Dorsal root potentials and dorsal root reflexes: A double-edged sword. Exp Brain Res. 1999;124(4):395–421 [DOI] [PubMed] [Google Scholar]
- 13.Toennies JF. Reflex discharge from the spinal cord over the dorsal roots. J Neurophysiol. 1938;(1):378–390 [Google Scholar]
- 14.Lobanov OV, Peng YB. Differential contribution of electrically evoked dorsal root reflexes to peripheral vasodilatation and plasma extravasation. J Neuroinflammation. 2011;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vígh B, Manzano e Silva MJ, Frank CL, et al. The system of cerebrospinal fluid-contacting neurons: Its supposed role in the nonsynaptic signal transmission of the brain. Histol Histopathol. 2004;19(2):607–628 [DOI] [PubMed] [Google Scholar]
- 16.Bharali LA, Lisney SJ. The relationship between unmyelinated afferent type and neurogenic plasma extravasation in normal and reinnervated rat skin. Neuroscience. 1992;47(3):703–712 [DOI] [PubMed] [Google Scholar]