Abstract
The Arg64 allele of variant rs4994 (Trp64Arg) in the β3-adrenergic receptor gene has been associated with increased serum urate and risk of gout. Our objective was to investigate the relationship of rs4994 with serum urate and gout in New Zealand European, Māori and Pacific subjects. A total of 1730 clinically ascertained gout cases and 2145 controls were genotyped for rs4994 by Taqman®. Māori and Pacific subjects were subdivided into Eastern Polynesian (EP) and Western Polynesian (WP) sample sets. Publicly available genotype data from the Atherosclerosis Risk in Communities Study and the Framingham Heart Study were utilized for serum urate association analysis. Multivariate logistic and linear regression adjusted for potential confounders was carried out using R version 2.15.2. No significant association of the minor Arg64 (G) allele of rs4994 with gout was found in the combined Polynesian cohorts (OR = 0.98, P = 0.88), although there was evidence, after adjustment for renal disease, for association in both the WP (OR = 0.53, P = 0.03) and the lower Polynesian ancestry EP sample sets (OR = 1.86, P = 0.05). There was no evidence for association with gout in the European sample set (OR = 1.11, P = 0.57). However, the Arg64 allele was positively associated with urate in the WP data set (β = 0.036, P = 0.004, PCorrected = 0.032). Association of the Arg64 variant with increased urate in the WP sample set was consistent with the previous literature, although the protective effect of this variant with gout in WP was inconsistent. This association provides an etiological link between metabolic syndrome components and urate homeostasis.
Keywords: Gout, Urate, ADRB3, Genetic, Association
Introduction
Urate is the end product of endogenous and dietary purine metabolism in humans. The most important regulator of serum urate levels is reduced excretion of uric acid in the urine [1]. When supersaturation of urate is reached, mono-sodium urate (MSU) crystals are able to form within synovial fluid. Gout is the extremely painful innate immune response to these crystals. Individual variation in serum urate concentrations is partly explained by genetic factors, with 28 loci associated with serum urate in Europeans at a genome-wide level of significance [2]. The strongest genetic effects are with uric acid transporter genes, which are also associated with gout [2, 3].
Hyperuricemia and gout are closely related to metabolic diatheses like obesity, dyslipidemia, glucose intolerance and hypertension [4, 5]. Renal clearance of uric acid is inversely related to insulin resistance [6], and the evidence that gout is associated with the metabolic syndrome has led to the hypothesis that gout and hyperuricemia may also have a causal relationship with insulin resistance and obesity. This is consistent with Mendelian randomization studies that have demonstrated increased triglyceride levels and body mass index (BMI) as causal of increased urate [7, 8]. Therefore, genes influencing insulin resistance could also contribute to the development of hyperuricemia.
The beta-3 adrenergic receptor (ADRB3) is part of the adrenergic system. It is expressed mainly in adipose tissue in humans and is involved in the regulation of lipid metabolism and glucose homeostasis [9]. The Trp64Arg (rs4994) polymorphism in the first transmembrane domain of ADRB3 has been associated with BMI in meta-analysis of 44,833 individuals with Arg64 carriers having increased BMI and a stronger effect in East Asian sample sets [10]. This is consistent with reports of association of type 2 diabetes mellitus and insulin resistance with Arg64 [11, 12] and association of the Arg64 allele with increased adiposity measures, serum urate and blood pressure in older men [13].
The Trp64Arg polymorphism has previously been tested for association with hyperuricemia and gout under the hypothesis that genetic variants contributing to insulin resistance could also contribute to hyperuricemia. A combination of increased BMI and Arg64 increased the risk of developing hyperuricemia by fourfold in a postprandial diabetic group drawn from the Chinese population [14]. Rho et al. [15] and Huang et al. [16] reported association of the Arg64 allele with hyperuricemia in Korean and Chinese sample sets. A similar association was reported by Morcillo et al. [17] who reported that the Arg64 allele predicts the risk of developing hyperuricemia in a prospective study in a population from southern Spain. Wang et al. [18] reported the Arg64 allele was associated with gout in a male Chinese population.
The aim of the study reported here was to further investigate the relationship of the Trp64Arg (rs4994) variant of ADRB3 with hyperuricemia and gout in European and New Zealand Māori and Pacific Island (Polynesian) sample sets. The New Zealand Polynesian population exhibits the highest rate of gout in the world, with considerable comorbidity with type 2 diabetes and cardiovascular disease [19, 20].
Materials and methods
Study participants
All New Zealand (NZ) gout cases and controls included in this study were recruited during the years 2006–2013. Gout cases fulfilled the American Rheumatology Association criteria for gout by clinical examination [21], while controls self-reported no history of gouty arthritis. Except for the biochemical measurements and BMI, all other variables were self-reported. Written informed consent was obtained from all subjects for collection of samples and subsequent analyses. Publicly available genotype and phenotype data from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) cohorts were accessed from the ARIC and FHS studies under the project name “The Genetic Basis of Gout” and approval number 834. Table S1 reports the demographic and clinical data for all study groups.
The NZ gout case–control sample set was divided into four ancestral groups [22]: NZ European (648 cases and 877 controls), Eastern Polynesian (EP; Cook Islands and NZ Māori; 491 cases and 696 controls), Western Polynesian (WP; Samoa, Tonga, Tuvalu, Niue and Tokelau; 367 cases and 310 controls) and mixed Eastern and Western Polynesian (EP/WP; 29 cases and 70 controls). Eastern Polynesian participants were further subdivided into EPN (subjects with high EP ancestry; 334 cases and 392 controls) and EPZ (subjects with low EP ancestry; 157 cases and 311 controls) [22]. A separate Māori sample set (NPH; 195 cases and 192 controls) was also included in the study, ascertained with criteria described above. These participants were recruited in collaboration with Ngāti Porou Hauora Charitable Trust (NPHCT) from the Ngāti Porou rohe (tribal territory) located in the East Coast (Tairawhiti) region of the North Island of New Zealand. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983. The NPHCT study was approved by the Ngāti Porou Hauora Board. The Lower South Ethics Committee (OTA/99/11/098) and New Zealand Multi-region Ethics Committee (MEC/05/10/130) granted ethical approval for this study. The Northern Y Region Health Research Ethics Committee granted ethical approval for the NPHCT study (NTY07/07/074).
Control (non-gout) subjects were recruited from NZ and sourced from ARIC (http://www2.cscc.unc.edu/aric/) and FHS (Offspring and Generation 3) (http://www.framing-hamheartstudy.org/) cohorts for the purpose of evaluating the association of rs4994 with serum urate. The ARIC data set consisted of 4144 subjects and the FHS of 5109 subjects. Subjects who self-reported as taking diuretic medication, or had renal failure, gout, or had first-degree relatives with gout or were not of European ancestry were excluded.
Data collection and genotyping
The uricase oxidation method was applied to measure serum urate levels for NZ subjects using a Roche chemistry modular P/D analyzer. Data for serum urate were obtained from visit 1 (1987–1989) for ARIC and examination 1 (Offspring: 1971–1975 and Generation 3: 2002–2005) for FHS cohorts. Genotyping of NZ samples for rs4994 was done using a TaqMan® assay (C_2215549_20; Applied Biosystems, Foster City, USA) using a Lightcycler® 480 Real-Time Polymerase Chain Reaction (RT-PCR) System (Roche Applied Science, Indianapolis, IN, USA). For the FHS and ARIC cohorts, rs4994 genotype was imputed using all 1000 Genomes haplotype data (phase 1; 2012) as a reference panel using IMPUTE2 [23].
Statistical analysis
Logistic and linear regression analysis was done using statistical software R (v 2.15.2) [24] to test for an association of rs4994 (explanatory variable) with gout (binary response variable) and serum urate (continuous response variable), respectively. Any individual with missing data for any variable was excluded from the various analyses. The adjusted odds ratio (OR) and β-coefficients were obtained by including age, sex and BMI as covariates in the regression model. In additional analyses, the presence or absence of type 2 diabetes, hypertension and renal disease was included as an adjustor. Self-reported grandparental ancestry was also included as a covariate in Polynesian data sets. Adherence to Hardy–Weinberg equilibrium (HWE) was calculated using the SHEsis package (http://analysis2.bio-x.cn/myAnalysis.php) with a significant deviation from HWE if P < 0.004 (0.05 divided by 12—the number of data sets tested in Table 1). Meta-analysis was done using the meta package (http://CRAN.R-project.org/package=meta, 2014) within R using a fixed-effect model. For analyses showing heterogeneity (PHet < 0.05), the fixed-effect model was replaced with a random-effect model. A threshold of P ≤ 0.05 was used to indicate nominal statistical significance between response and explanatory variables. PCorrected was calculated by dividing the P by the number of tests performed (eight), and a PCorrected < 0.05 indicated significance.
Table 1.
Association analysis of rs4994 with gout
| AA | AG | GG | G | OR (95 % CI) (unadjusted) | Punadjusted | OR (95 % CI) (adjusted)a | Padjusted a | HWE | |
|---|---|---|---|---|---|---|---|---|---|
| EPN | |||||||||
| Cases | 266 (0.796) | 66 (0.197) | 2 (0.006) | 70 (0.105) | 0.89 (0.64–1.25) | 0.53 | 1.08 (0.69–1.68) | 0.72 | 0.33 |
| Control | 306 (0.780) | 82 (0.209) | 4 (0.011) | 90 (0.114) | 0.56 | ||||
| EPZ | |||||||||
| Cases | 122 (0.777) | 33 (0.210) | 2 (0.012) | 37 (0.117) | 1.49 (0.95–2.35) | 0.07 | 1.66 (0.91–3.08) | 0.09 | 0.89 |
| Control | 262 (0.842) | 47 (0.151) | 2 (0.006) | 51 (0.081) | 0.94 | ||||
| WP | |||||||||
| Cases | 336 (0.915) | 31 (0.084) | 0 (0.000) | 31 (0.042) | 0.62 (0.38–0.99) | 0.04 | 0.61 (0.34–1.06) | 0.08 | 0.39 |
| Control | 272 (0.877) | 34 (0.109) | 4 (0.012) | 42 (0.067) | 0.02 | ||||
| EP/WP | |||||||||
| Cases | 27 (0.931) | 2 (0.069) | 0 (0.000) | 2 (0.034) | 0.31 (0.04–1.21) | 0.13 | 0.31 (0.04–1.44) | 0.17 | 0.84 |
| Control | 57 (0.814) | 13 (0.185) | 0 (0.000) | 13 (0.092) | 0.39 | ||||
| NPH | |||||||||
| Cases | 156 (0.800) | 39 (0.200) | 0 (0.000) | 39 (0.100) | 1.07 (0.66–1.73) | 0.77 | 0.95 (0.47–1.90) | 0.89 | 0.11 |
| Control | 160 (0.833) | 28 (0.144) | 4 (0.020) | 36 (0.093) | 0.04 | ||||
| NZ European | |||||||||
| Cases | 543 (0.838) | 102 (0.157) | 3 (0.004) | 108 (0.083) | 1.00 (0.76–1.30) | 0.99 | 1.11 (0.77–1.61) | 0.57 | 0.44 |
| Control | 735 (0.838) | 138 (0.157) | 4 (0.004) | 146 (0.083) | 0.35 | ||||
| Han Chinese [18] | |||||||||
| Cases | 298 (0.793) | 104 (0.252) | 10 (0.024) | 124 (0.150) | 1.50 (1.09–2.06) | 0.01 | 1.95 (1.22–3.13) | 0.02 | 0.79 |
| Control | 248 (0.795) | 62 (0.199) | 2 (0.006) | 66 (0.106) | 0.37 | ||||
All values are adjusted against sex, age and BMI and self-reported grandparental ancestry for Polynesian data sets
Results
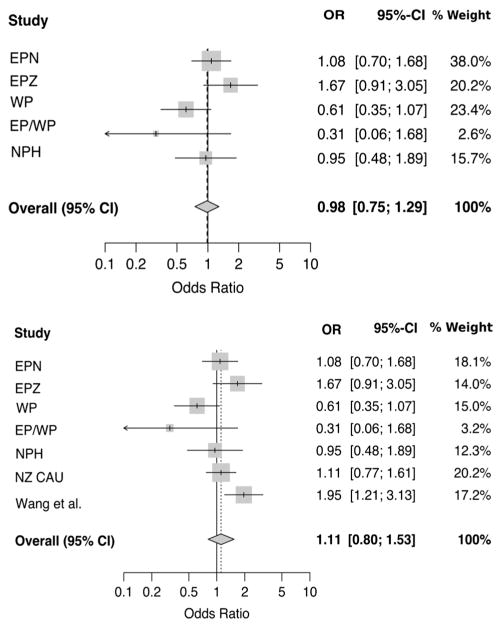
There was nominally significant association between the ADRB3 variant rs4994 and gout only in the WP sample set where the minor Arg64 (G) allele was associated with reduced risk of gout (OR = 0.62, P = 0.04) although this association became nonsignificant when adjusted for confounding variables (OR = 0.61, P = 0.08) (Table 1). A strong trend of association of the Arg64 allele toward increased risk of gout was observed in EPZ (OR = 1.49, P = 0.07) (Table 1). To increase the power of the analysis, all NZ case–control groups were combined with the Wang et al. [18] data set by meta-analysis. No evidence of association was found (Fig. 1; OR = 1.11, P = 0.52), although PHet was 0.03 which suggested some heterogeneity. Considering ancestry as a possible source of heterogeneity, Polynesian data sets only were combined in meta-analysis. Again the association was not significant (Fig. 1; OR = 0.98, P = 0.88, PHet = 0.10). Significant protective association of the rs4994 Arg64 allele was still observed in the WP sample set when adjusted for diabetes (OR = 0.57, P = 0.05), hypertension (OR = 0.56, P = 0.05) and renal dysfunction (OR = 0.53, P = 0.03), whereas there was evidence for a risk effect of Arg64 on gout in EPZ after adjustment for renal dysfunction (OR = 1.86, P = 0.05) (Table 2).
Fig. 1.
Meta-analysis of NZ Polynesian sample sets for association of rs4994 with gout (top; POR = 0.88 and PHet. = 0.10) and meta-analysis of all available sample sets for association of rs4994 with gout (bottom; POR= 0.52 and PHet.= 0.03)
Table 2.
Association analysis of rs4994 with gout adjusted for comorbidities
| Baseline adjustmenta
|
Diabetes (T2D)a
|
Hypertensiona
|
Renal dysfunctiona
|
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95 % CI) | P | OR (95 % CI) | P | OR (95 % CI) | P | OR (95 % CI) | P | |
| EPN | 1.08 (0.69–1.68) | 0.72 | 1.03 (0.66–1.62) | 0.86 | 1.08 (0.69–1.70) | 0.72 | 0.93 (0.58–1.49) | 0.77 |
| EPZ | 1.66 (0.91–3.08) | 0.09 | 1.78 (0.96–3.34) | 0.06 | 1.56 (0.80–3.05) | 0.18 | 1.86 (0.98–3.60) | 0.05 |
| WP | 0.61 (0.34–1.06) | 0.08 | 0.57 (0.32–1.01) | 0.05 | 0.56 (0.31–1.01) | 0.05 | 0.53 (0.29–0.95) | 0.03 |
| EP/WP | 0.31 (0.04–1.44) | 0.17 | 0.31 (0.04–1.45) | 0.17 | 0.35 (0.04–1.76) | 0.24 | 0.41 (0.05–2.02) | 0.31 |
| NPH | 0.95 (0.47–1.90) | 0.89 | 0.93 (0.46–1.89) | 0.85 | 0.92 (0.45–1.90) | 0.83 | 0.98 (0.48–1.98) | 0.96 |
| NZ European | 1.11 (0.77–1.61) | 0.57 | 1.12 (0.77–1.63) | 0.55 | 1.12 (0.76–1.65) | 0.56 | 1.11 (0.76–1.63) | 0.58 |
All values are adjusted against sex, age and BMI and self-reported grandparental ancestry for Polynesian data sets
Linear regression analysis was performed to test for association of rs4994 with urate in the NZ and ARIC/FHS data sets. A significant association of the Arg64 allele with urate was found in the WP sample set (β = 0.036, P = 0.004), which retained significance after correction for the eight sample sets examined (PCorrected = 0.03). This indicates that each copy of the Arg64 allele increases serum urate by 0.036 mmol/L (Table 3). To increase the power of the analysis, the various Polynesian and European (including ARIC and FHS) data sets were combined separately by meta-analysis. No significant association was found in either analysis (Arg64 allele: β = 0.01, P = 0.16 and β = 2.99 × 10−5, P = 0.98, respectively) (Fig. 2).
Table 3.
Association analysis of rs4994 with serum urate (mmol L−1)
| Unadjusted
|
Adjusteda
|
|||
|---|---|---|---|---|
| β-Coef. (95 % CI) | P | β-Coef. (95 % CI) | P | |
| EPN | 0.004 (−0.016–0.026) | 0.66 | 0.003 (−0.015–0.023) | 0.70 |
| EPZ | 0.017 (−0.018–0.054) | 0.33 | 0.011 (−0.018–0.040) | 0.45 |
| WP | 0.036 (0.009–0.063) | 0.01 | 0.036 (0.011–0.062) | 0.01 |
| EP/WP | −0.021 (−0.088–0.044) | 0.51 | −0.018 (−0.076–0.039) | 0.53 |
| NPH | −0.006 (−0.041–0.026) | 0.68 | −0.021 (−0.051–0.008) | 0.16 |
| NZ CAU | −0.025 (−0.054–0.004) | 0.09 | −0.012 (−0.037–0.012) | 0.29 |
| ARIC CAU | 0.001 (−0.003–0.006) | 0.56 | 0.001 (−0.002–0.005) | 0.49 |
| FHS CAU | 0.001 (−0.005–0.006) | 0.90 | −0.001 (−0.005–0.003) | 0.61 |
All values are adjusted against sex, age and BMI and self-reported grandparental ancestry for Polynesian data sets
Fig. 2.
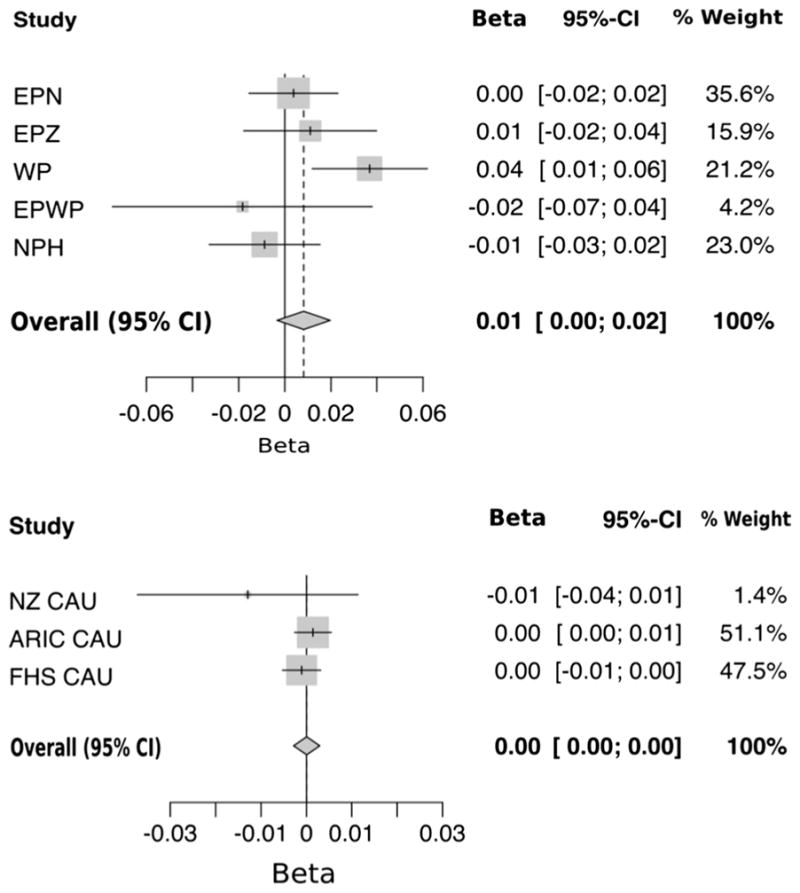
Meta-analysis of NZ Polynesian sample sets for association of rs4994 with serum urate (top; PBeta = 0.16 and PHet. = 0.08) and meta-analysis of European sample sets for association of rs4994 with serum urate (bottom; PBeta= 0.98 and PHet.= 0.41)
Discussion
Our study reports association of the Arg64 allele of the rs4994 polymorphism of ADRB3 with increased serum urate in WP individuals (β = 0.04, P = 0.004, PCorrected = 0.03). This is consistent with the previously reported findings of Morcillo et al. [17] who demonstrated that the Arg64 allele predicts the development of hyperuricemia in the population of southern Spain and with studies in Chinese and Korean sample sets that also associated the Arg64 allele with increased serum urate and risk of gout in Asian subjects [14–16, 18]. Collectively our and previous studies increase the support for a causal role of ADRB3 and the adrenergic system in urate control. We could not meta-analyze our serum urate findings with previously published studies as they were described as a secondary finding in conjunction with other metabolic conditions, or from a population subgroup, or as a binary outcome [13–15]. It is important to note that the genome-wide association study of Köttgen et al. [2] using ~140,000 individuals would not have been able to test variants in the ADRB3 region for association with urate because of the absence of any Hap-Map2 data including rs4994 that could be used for imputation (http://hapmap.ncbi.nlm.nih.gov). Using more recently available data, it should be possible to impute this region in the Kottgen et al. data using 1000 Genomes haplotype data in which the ADRB3 region is adequately covered by common variants and to test for association with urate.
The direction of association of the Arg64 allele with urate opposed that observed in gout in WP, where it was associated with reduced risk of gout. This direction of association also conflicted with the Arg64-mediated increased risk of gout reported by Wang et al. [18] and observed by us in the EPZ sample set (Table 2). Adjusting for the effect of comorbidities type 2 diabetes, hypertension and renal dysfunction did not influence the protective effect of Arg64 with gout in WP (Table 2). Acknowledging that this could be a false-positive finding, the opposing direction of association of Arg64 with gout and hyperuricemia could represent a pleiotropic effect of this allele in the WP population, perhaps having a role both in determining hyperuricemia and in the inflammatory processes leading to gout. The opposing direction to EPZ may reflect different ancestral haplotypes—we have previously observed differential effects at ABCG2 between Eastern and Western Polynesian sample sets [25]. Most importantly, however, the association of rs4994 with gout needs to be studied in larger sample sets of diverse ancestries.
The ADRB3 gene is expressed mainly in adipose tissue and encodes for the beta-3 adrenergic receptor. The activation of this receptor induces lipolysis in adipose tissue and thermogenesis in skeletal muscles. It is also responsible for the delivery of free fatty acids into the portal vein. These free fatty acids and other products of lipolysis disrupt the insulin receptor signaling pathway, thereby leading to insulin resistance [26]. The Arg64 allele of rs4994 has been reported to be associated with the development of obesity, increased BMI and insulin resistance [10, 27, 28]. Decreased activity of the Arg64 variant receptor could lead to a decline in lipolysis and increased deposition of adipose tissue. Furthermore, ADRB3 is proposed to be a part of the “leptin-sympathetic-leptin feedback loop,” whereby decreased activity of this receptor causes an increase in lep-tin secretion from the adipose tissue [29]. The observation of elevated leptin levels in hyperuricemic patients [30] is consistent with the association of Arg64 with hyperuricemia. Insulin resistance is a possible link between hyperuricemia and obesity [31, 32], which supports the hypothesis that the Arg64 allele could promote hyperuricemia in the obese.
Supplementary Material
Acknowledgments
This work was supported by the Health Research Council of New Zealand, Arthritis New Zealand, New Zealand Lottery Health and the University of Otago. The authors would like to thank Jill Drake (Canterbury District Health Board), Roddi Laurence, Chris Franklin, Meaghen House (all University of Auckland) and Gabrielle Sexton (University of Otago) for recruitment. We also thank Ria Akuhata and Nancy Aupouri of NPHCT for recruitment, coordinated by Dr. Jennie Harré Hindmarsh. The Atherosclerosis Risk in Communities and Framingham Heart study analyses (Project #834) were approved by the relevant Database of Genotype and Phenotype (dbGaP; www.ncbi.nim.nih/gov/dbgap) Data Access Committees. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01-HC-55022, R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. The authors thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant No. UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. The Framingham Heart Study and the Framingham SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University. The Framingham SHARe data used for the analyses described in this manuscript were obtained through dbGaP. This manuscript was not prepared in collaboration with investigators of the Framingham Heart Study and does not necessarily reflect the opinions or views of the Framingham Heart Study, Boston University, or the NHLBI.
Funding This study was funded by Grant 11/1075 from the Health Research Council of New Zealand.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00296-015-3370-6) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Dalbeth N, Merriman T. Crystal ball gazing: new therapeutic targets for hyperuricaemia and gout. Rheumatology. 2009;48:222–226. doi: 10.1093/rheumatology/ken460. [DOI] [PubMed] [Google Scholar]
- 2.Kottgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45:145–154. doi: 10.1038/ng.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phipps-Green A, Merriman M, Topless R, Altaf S, Montgomery G, Franklin C, et al. Twenty-eight loci that influence serum urate levels: analysis of association with gout. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205877. [DOI] [PubMed] [Google Scholar]
- 4.Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120:442–447. doi: 10.1016/j.amjmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 5.Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Care Res. 2007;57:109–115. doi: 10.1002/art.22466. [DOI] [PubMed] [Google Scholar]
- 6.Facchini F, Chen Y-DI, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266:3008–3011. [PubMed] [Google Scholar]
- 7.Lyngdoh T, Vuistiner P, Marques-Vidal P, Rousson V, Waeber G, Vollenweider P, et al. Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PLoS One. 2012;7:e39321. doi: 10.1371/journal.pone.0039321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasheed H, Hughes K, Flynn TJ, Merriman TR. Mendelian randomisation provides no evidence for a causal role of serum urate in increasing serum triglyceride levels. Circ Cardiovasc Genet. 2014;7:830–837. doi: 10.1161/CIRCGENETICS.114.000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krief S, Lönnqvist F, Raimbault S, Baude B, Van Spronsen A, Arner P, et al. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest. 1993;91:344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurokawa N, Young EH, Oka Y, Satoh H, Wareham NJ, Sandhu M, et al. The ADRB3 Trp64Arg variant and BMI: a meta-analysis of 44,833 individuals. Int J Obes. 2008;32:1240–1249. doi: 10.1038/ijo.2008.90. [DOI] [PubMed] [Google Scholar]
- 11.Gjesing A, Andersen G, Borch-Johnsen K, Jørgensen T, Hansen T, Pedersen O. Association of the β3-adrenergic receptor Trp64Arg polymorphism with common metabolic traits: studies of 7605 middle-aged white people. Mol Genet Metab. 2008;94:90–97. doi: 10.1016/j.ymgme.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Widen E, Lehto M, Kanninen T, Walston J, Shuldiner AR, Groop LC. Association of a polymorphism in the β3-adrenergic–receptor gene with features of the insulin resistance syndrome in Finns. N Engl J Med. 1995;333:348–352. doi: 10.1056/NEJM199508103330604. [DOI] [PubMed] [Google Scholar]
- 13.Strazzullo P, Iacone R, Siani A, Cappuccio FP, Russo O, Barba G, et al. Relationship of the Trp64Arg polymorphism of the beta3-adrenoceptor gene to central adiposity and high blood pressure: interaction with age. Cross-sectional and longitudinal findings of the Olivetti Prospective Heart Study. J Hypertens. 2001;19:399–406. doi: 10.1097/00004872-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Li Q, Niu T, Chen C, Xu X. Association of GYS1 and beta (3)-AR gene with postprandial hyperglycemia and serum uric acid in type 2 diabetes mellitus. Chin Med J. 2002;115:1308–1311. [PubMed] [Google Scholar]
- 15.Rho YH, Choi SJ, Lee YH, Ji JD, Song GG. The association between hyperuricemia and the Trp64Arg polymorphism of the beta-3 adrenergic receptor. Rheumatol Int. 2007;27:835–839. doi: 10.1007/s00296-006-0300-7. [DOI] [PubMed] [Google Scholar]
- 16.Huang Q, Zhang L-F, Cheng Y, Zhao Y-C, Si L, Gao Y, et al. Trp64Arg (rs4994) polymorphism of β3-adrenergic receptor gene is associated with hyperuricemia in a Chinese male population. Clin Chem Lab Med. 2013;51:1755–1760. doi: 10.1515/cclm-2012-0592. [DOI] [PubMed] [Google Scholar]
- 17.Morcillo S, Rojo-Martínez G, Martín-Núñez GM, Gómez-Zumaquero JM, García-Fuentes E, de Adana MR, et al. Trp64Arg polymorphism of the ADRB3 gene predicts hyperuricemia risk in a population from southern Spain. J Rheumatol. 2010;37:417–421. doi: 10.3899/jrheum.090637. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, Meng D, Wang J, Jia Z, Zhoub S, Liu S, et al. Positive correlation between Beta-3-Adrenergic Receptor (ADRB3) gene and gout in a Chinese male population. J Rheumatol. 2011;38:738–740. doi: 10.3899/jrheum.101037. [DOI] [PubMed] [Google Scholar]
- 19.Winnard D, Wright C, Jackson G, Gow P, Kerr A, McLachlan A, et al. Gout, diabetes and cardiovascular disease in the Aotearoa New Zealand adult population: co-prevalence and implications for clinical practice. NZ Med J. 2013;126:53–64. [PubMed] [Google Scholar]
- 20.Winnard D, Wright C, Taylor WJ, Jackson G, Te Karu L, Gow PJ, et al. National prevalence of gout derived from administrative health data in Aotearoa New Zealand. Rheumatol. 2012;51:901–909. doi: 10.1093/rheumatology/ker361. [DOI] [PubMed] [Google Scholar]
- 21.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 22.Hollis-Moffatt JE, Phipps-Green AJ, Chapman B, Jones GT, van Rij A, Gow PJ, et al. The renal urate transporter SLC17A1 locus: confirmation of association with gout. Arthritis Res Ther. 2012;14:R92. doi: 10.1186/ar3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howie B, Marchini J, Stephens M. Genotype imputation with thousands of genomes. G3: genes, genomes. Genetics. 2011;1:457–470. doi: 10.1534/g3.111.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Team RC. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: 2012. [Google Scholar]
- 25.Phipps-Green AJ, Hollis-Moffatt J, Dalbeth N, Merriman ME, Topless R, Gow PJ, et al. A strong role for the ABCG2 gene in susceptibility to gout in New Zealand Pacific Island and Caucasian, but not Māori, case and control sample sets. Hum Mol Genet. 2010;19:4813–4819. doi: 10.1093/hmg/ddq412. [DOI] [PubMed] [Google Scholar]
- 26.Savage DB, Petersen KF, Shulman GI. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension. 2005;45:828–833. doi: 10.1161/01.HYP.0000163475.04421.e4. [DOI] [PubMed] [Google Scholar]
- 27.Park HS, Shin ES, Lee JE. Genotypes and haplotypes of β2-adrenergic receptor and parameters of the metabolic syndrome in Korean adolescents. Metabolism. 2008;57:1064–1070. doi: 10.1016/j.metabol.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Zhan S, Ho SC. Meta-analysis of the association of the Trp64Arg polymorphism in the β3 adrenergic receptor with insulin resistance. Obes Res. 2005;13:1709–1719. doi: 10.1038/oby.2005.209. [DOI] [PubMed] [Google Scholar]
- 29.Mark A, Rahmouni K, Correia M, Haynes W. A leptin-sympathetic-leptin feedback loop: potential implications for regulation of arterial pressure and body fat. Acta Physiol Scand. 2003;177:345–349. doi: 10.1046/j.1365-201X.2003.01085.x. [DOI] [PubMed] [Google Scholar]
- 30.Bedir A, Topbas M, Tanyeri F, Alvur M, Arik N. Leptin might be a regulator of serum uric acid concentrations in humans. Jpn Heart J. 2003;44:527–536. doi: 10.1536/jhj.44.527. [DOI] [PubMed] [Google Scholar]
- 31.Modan M, Halkin H, Karasik A, Lusky A. Elevated serum uric acid—a facet of hyperinsulinaemia. Diabetologia. 1987;30:713–718. doi: 10.1007/BF00296994. [DOI] [PubMed] [Google Scholar]
- 32.Quiñones-Galvan A, Ferrannini E. Renal effects of insulin in man. J Nephrol. 1996;10:188–191. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.