Abstract
Objective
To describe the characteristics of patients who undergo total artificial heart support and to explore the ethical aspects of its withdrawal.
Patients and Methods
We retrospectively reviewed the medical records of all adult recipients of total artificial heart at our institution from the program’s inception in 2007 to June 30, 2015. Management of other life-sustaining therapies, approach to end-of-life decision making, engagement of ethics and palliative care consultation, and causes of death were analyzed.
Results
Of 47 total artificial heart recipients, 14 patients or their surrogates (30%) requested withdrawal of total artificial heart support. No request was denied by treatment teams. All 14 patients were supported with at least 1 other life-sustaining therapy. Only 1 patient was able to participate in decision making.
Conclusion
It is widely held to be ethically permissible to withdraw a life-sustaining treatment when the treatment no longer meets the patient’s health care–related goals (ie, the burdens outweigh the benefits). Our data suggest that some patients, surrogates, physicians, and other care providers believe that this principle extends to the withdrawal of total artificial heart support.
Keywords: cardiovascular disease, medical ethics, quality of life
Introduction
Historical Perspective
Replacing an ailing heart with a man-made organ has been the dream of scientists since the time of Leonardo da Vinci and the Renaissance.1 In 1964, Dr Michael DeBakey and colleagues appealed to President Lyndon B. Johnson to establish a total artificial heart (TAH) program, which was ultimately allocated approximately $160 million in federal funding.2 This ambitious endeavor was to parallel President Kennedy’s space program, with the objective of developing a fully functional TAH before the lunar landing.1,3 In April 1969, a mere 3 months before Neil Armstrong alighted on the moon, Dr Denton Cooley implanted the first human TAH, which supported a man for 64 hours until he received a heart transplant.1
In 1982, Dr William DeVries implanted the first TAH, intended as destination therapy, at the University of Utah.2 Justifiably, this pioneering case received prominent and exuberant international news coverage, with reporters packing the hospital auditorium. A fact receiving little publicity was that the surgeons had given the patient, Dr Barney Clark, a dentist, a “key” to the compressor of his TAH.4 Dr Willem Kolff, founder of University of Utah’s artificial heart program, explained, “if the man suffers and feels it isn’t worth it anymore, he has a key that he can apply. … I think it is entirely legitimate that this man whose life has been extended should have the right to cut it off if he doesn’t want it, if life ceases to be enjoyable …”5 Dr Clark survived 112 days, never leaving the hospital and never using his key, eventually dying “with peace and dignity” from circulatory collapse and secondary multiorgan system failure.6
Medical Indications and the State of the Art
Approximately 400,000 patients die of heart failure in the United States annually.7 Only 2,000 to 2,500 hearts are transplanted annually, a number that has remained roughly stable for 20 years.1 Mechanical circulatory support devices such as TAHs and ventricular assist devices (VADs) have the potential to address, in part, the mismatch between the demand for and availability of hearts for transplant.
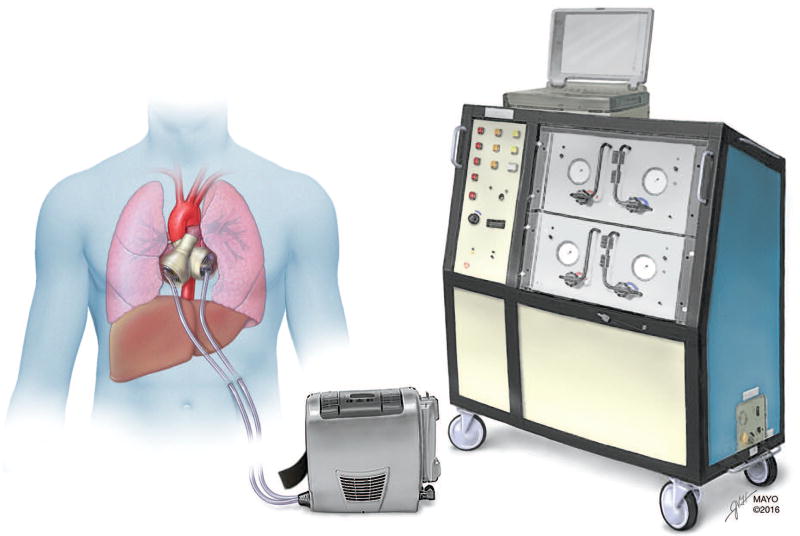
TAH implantation requires excision of the native heart, leaving atrial remnants to which cuffs can be secured (Figure). Vascular grafts are sutured to the pulmonary artery and aorta. A pneumatically driven diaphragm directs blood through 2 artificial ventricles in a pulsatile fashion, with 4 mechanical valves ensuring unidirectional flow. Percutaneous drive lines tether hospitalized patients to a washing machine–sized air compressor terminal, also known as “big blue”.8 A portable alternative weighing approximately 6 kg (Freedom driver; SynCardia Systems, Inc) allows patients to walk and even to leave the hospital.
Figure.
Total Artificial Heart and Driver Consoles. (Used with permission of Mayo Foundation for Medical Education and Research.)
Indications for TAH implantation include severe biventricular heart failure, intractable arrhythmia, septal defects or myocardial aneurysms, previous valve prosthesis or congenital heart disease, infiltrative or restrictive cardiomyopathy, cardiac malignancies, and cardiac graft failure (heart transplant recipients awaiting second allograft).1,8 The TAH was implanted in 4 patients in the 1980s as a permanent therapy, or “destination therapy,” before US Food and Drug Administration (FDA) approval for this application of the TAH was withdrawn.9 Since the 1990s, TAH has been approved only as a bridge to transplant, although in 2015 the FDA approved a clinical trial with TAH as destination therapy.9,10
More than 1,300 SynCardia temporary TAHs have been implanted, with 80% successfully bridging the patient to transplant. One-year survival with the device is 70%,11 with the longest successful bridge to transplant being more than 3.5 years.12 The annual number of TAH implantations remains in the double digits; the Interagency Registry for Mechanically Assisted Circulatory Support recorded 278 SynCardia temporary TAH implantations between 2008 and 2014.13
Ethical Q uestions Raised by the TAH
Though the TAH touches the lives of a relative few, many ethical considerations accompany its use. A patient undergoing TAH implantation consents and submits to a lethal alteration in his or her anatomy, whereby the native heart is explanted and replaced with a mechanical device. Infection, hemorrhage, thrombosis, and multiorgan failure may occur in patients with a TAH, all of which would preclude heart transplant. One-fifth of TAH recipients do not survive to transplant,11 yet their experience is seldom described in the medical literature. Several questions related to TAH use remain to be answered, such as under what circumstance is withdrawal of TAH support—leading to certain, near-instantaneous death—ethically and legally permissible? And when a patient’s circulation is entirely reliant on implanted mechanical support, is the patient required to continue TAH support even if the treatment becomes more burdensome than beneficial?
The aim of this study was to describe the characteristics of patients who underwent TAH implantation at our institution and from whom TAH support was withdrawn. We also aimed to evaluate the ethical and legal permissibility of withdrawing this life-sustaining therapy (LST) from patients who no longer desire it.
Patients and Methods
We searched our electronic patient database for the records of all patients who have undergone TAH implantation at Mayo Clinic in Scottsdale, Arizona, and Rochester, Minnesota, since the inception of the TAH program in 2007, through June 30, 2015. We reviewed the records of all patients identified to further identify those who did not receive heart transplant and who requested, or their surrogate decision-makers requested, withdrawal of TAH support. Permission to perform a retrospective review of the patients’ medical records was granted by the Mayo Clinic Institutional Review Board.
Results
During the study period, 47 patients (32 in Arizona, 15 in Minnesota) had a TAH implanted. Of these, 26 were alive at the time of data collection; of the 21 patients who died, 7 died after heart transplant. The remaining 14 did not receive heart transplant, and withdrawal of TAH support was requested. These 14 patients make up the study group; their clinical features are shown in Table 1.
Table 1.
Clinical Features of 14 Study Patients
| Pt/Sex/Age, y |
LVEF, % |
Cardiac Devices |
RRT | Nutritional Support |
Mechanical Ventilation |
Cause of Death |
TAH Duration, d |
Other Life- Sustaining Therapies |
|---|---|---|---|---|---|---|---|---|
| 1/M/64 | 20–29 | IABP, PM | Yes | Yes | Yes | MOF/sepsis | 231 | 3 |
| 2/M/61 | N/A (VAD) | IABP, VAD | Yes | Yes | Yes | MOF | 186 | 3 |
| 3/M/58 | 10–19 | AICD, IABP | Yes | Yes | No (NPPV) | MOF/sepsis | 247 | 3 |
| 4/M/62 | 20–29 | PM | Yes | Yes | Yes | MOF/sepsis | 98 | 3 |
| 5/M/64 | 10–19 | AICD, prior VA ECMO | Yes | Yes | Yes | MOF | 46 | 3 |
| 6/M/54 | 10–19 | Prior VA ECMO, VV ECMO (post TAH) | Yes | Yes | Yes | MOF | 1 | 2 |
| 7/M/67 | 10–19 | IABP | No | Yes | Yes | CVA/MOF | 7 | 3 |
| 8/M/60 | <10 | IABP | Yes | Yes | Yes | MOF | 8 | 3 |
| 9/M/31 | 30–39 | None | Yes | Yes | Yes | MOF | 21 | 3 |
| 10/M/45 | 20–29 | IABP | Yes | No | No | MOF | 308 | 1 |
| 11/M/61 | N/A (VAD) | VAD | Yes | Yes | Yes | MOF | 11 | 3 |
| 12/F/65 | 50–59 | VA ECMO | Yes | Yes | Yes | MOF | 10 | 3 |
| 13/M/38 | 10–19 | None | Yes | Yes | Yes | MOF/pulmonary hemorrhage | 14 | 3 |
| 14/M/55 | <10 | VA ECMO | Yes | No | No | MOF | 158 | 2 |
Abbreviations: AICD, automatic implantable cardioverter-defibrillator; CVA, cerebrovascular accident; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction; MOF, multiple organ failure; N/A, not applicable; NPPV, noninvasive positive-pressure ventilation; PM, pacemaker; Pt, patient; RRT, renal replacement therapy; TAH, total artificial heart; VA, venoarterial; VAD, ventricular assist device; VV, venovenous.
The median age was 60.5 (range, 31–67) years, and 13 patients were men. All TAH implants were intended as bridge to transplant. The median ejection fraction range before implantation was between 10% and 19% (ejection fraction estimates in the cohort ranged from <10% to >50%). The highest ejection fraction was seen in a patient with acute ventricular septal defect (case 12). Before TAH implantation, 11 patients had other cardiac devices, such as implantable cardioverter-defibrillator (ICD), pacemaker, VAD, and intra-aortic balloon pump, and 4 were supported with extracorporeal membrane oxygenation. Patients lived with TAH support for a median of 33.5 (range, 1–308) days. All 14 patients were supported with at least 1 other LST, in addition to TAH, until just before death, including mechanical ventilation, noninvasive positive pressure ventilation, renal replacement therapy (RRT), or artificial nutrition and hydration; 1 patient had 1 additional LST, 2 had 2, and 11 were receiving 3 (RRT, ventilatory support, and nutritional support). The most common cause of death was multiorgan failure (n=12). All 14 patients progressed to clinical death within minutes after withdrawal of TAH support. Thirteen patients died in the hospital, and 1 died in an inpatient hospice facility.
Only 1 patient had decisional capacity when the decision to withdraw TAH support was reached (Table 2). For the other 13 patients, surrogate decision-makers requested withdrawal or cessation of all LSTs. All requests to withdraw TAH support were carried out by the medical teams. Six patients had completed an advance directive, 2 of which were written after TAH implantation, although no directive specifically mentioned the TAH. All decisions to withdraw LST were reached after a care conference or series of care conferences. Palliative medicine consultation was requested in 7 cases. Chaplains participated in all cases. Only 1 ethics consultation was requested.
Table 2.
End-of-Life Factors for 14 Study Patients
| Pt | Decisional Capacity |
AD | AD Before TAH |
Surrogate Decision- Maker(s) |
Ethics Consultation |
Palliative Care Involvement |
Chaplain Involvement |
Site/Year of Death |
|---|---|---|---|---|---|---|---|---|
| 1 | No | No | … | Spouse | Yes | Yes | Yes | ICU/2014 |
| 2 | No | Yes | No | Spouse | No | Yes | Yes | ICU/2014 |
| 3 | No | Yes | Yes | Spouse | No | Yes | Yes | ICU/2015 |
| 4 | No | No | … | Family | No | Yes | Yes | ICU/2015 |
| 5 | No | No | … | Family | No | Yes | Yes | ICU/2015 |
| 6 | No | No | … | Family | No | No | Yes | ICU/2010 |
| 7 | No | Yes | Yes | Spouse | No | No | Yes | ICU/2008 |
| 8 | No | Yes | Yes | Spouse | No | No | Yes | ICU/2009 |
| 9 | No | Yes | Yes | Parents | No | No | Yes | ICU/2011 |
| 10 | No | No | … | Father | No | Yes | Yes | Hospital/2012 |
| 11 | No | No | … | Family | No | No | Yes | ICU/2012 |
| 12 | No | No | … | Family | No | No | Yes | ICU/2012 |
| 13 | No | No | … | Spouse | No | No | Yes | Hospital/2013 |
| 14 | Yes | Yes | No | Spouse | No | Yes | Yes | Inpatient hospice/2013 |
Abbreviations: AD, advance directive; ICU, intensive care unit; Pt, patient; TAH, total artificial heart.
Cases
Several cases illustrate the medical and psychosocial considerations that affected the decisions to withdraw TAH support. Case numbers correspond with those in the tables.
Case 4
A 62-year-old man with arrhythmogenic nonischemic cardiomyopathy underwent TAH implantation for acute-on-chronic biventricular heart failure. Postoperatively, the patient’s hepatic and renal dysfunction persisted, and recurrent bloodstream infections developed. A life-threatening abdominal hemorrhage necessitated arterial embolization and resuscitation with blood products. In the final days of the patient’s life, septic shock and respiratory distress developed requiring endotracheal intubation and mechanical ventilation. The palliative care team led a conference with all care providers and the patient’s wife. Given his grave condition, he was transitioned to comfort-directed care. He had no advance directive, but in the last week of life he voiced that he was “done fighting.” Sedation was administered, and support was withdrawn by the attending cardiologist, first with cessation of dialysis and vasopressors; then, the TAH rate was gradually decreased and the patient was extubated. He had been supported with TAH for 98 days.
Case 9
A 31-year-old man with complex congenital heart disease (transposition of the great vessels, pulmonary atresia, and ventricular septal defect) underwent ICD removal followed by TAH implantation. Postoperatively, the patient had multiple complications including mediastinal hemorrhage, ventilatory failure, and renal failure requiring RRT. A multidisciplinary care conference was held with the power of attorney (mother) and other family members, during which his poor prognosis was discussed. A family member expressed uncertainty to a hospital chaplain about the morality of withdrawing TAH support and wondered about lightening the patient’s sedation to allow for his participation in decision making. The chaplain offered supportive listening and counseled him on substituted judgment. Shortly thereafter, the patient’s condition deteriorated further, he was found to have pneumoperitoneum, and he underwent exploratory laparotomy, which revealed infarction and necrosis of the liver and spleen. With ongoing clinical decline, his parents requested withdrawal of the TAH after 21 days of support. The patient died minutes after the programmed TAH heart was stopped.
Discussion
Patient Experience
We found that, in patients who underwent TAH implantation, requests to withdraw TAH support were not uncommon. Of the 47 patients who received this LST during our study period, 14 (30%) eventually underwent withdrawal of TAH support. Notably, all 14 of these patients were supported with at least 1 other LST such as mechanical ventilation, noninvasive positive pressure ventilation, RRT, and artificial nutrition and hydration, and 11 were receiving 3 other LSTs at the end of life. Catastrophic central nervous system injury, hemorrhage, sepsis, and thrombosis were common among decedents, with multiple organ failure as the most commonly listed cause of death (13 patients).
Nearly all of the patients (13 of 14) from whom TAH support was withdrawn were unable to participate in end-of-life decision making. Although only 6 had completed advance directives expressing care preferences and designating a durable power of attorney for health care, care providers carried out all of the requests to withdraw LST. These data suggest that clinical practice at our institution follows the widely held ethical principle permitting withdrawal of treatments, including LSTs, that are perceived by patients or their surrogates as nonbeneficial or burdensome. Decisions to withdraw TAH support were universally reached when the patient’s condition was moribund and it had become evident that the patient would never stabilize to the point of receiving a heart transplant. This circumstance, in which a patient is sustained by mechanical circulatory support, but has no hope of transplant or recovery, has been termed “destination nowhere.”14
Of note, the topics discussed during family conferences, and descriptions of end-of-life processes and procedures (ie, around the time of TAH withdrawal), were inconsistently documented in our patients’ medical records. When details were available, an attending physician specializing in palliative medicine or heart failure administered sedation for anticipatory symptom management and supervised withdrawal of therapies, including discontinuation of vasopressors and inotropes, extubation, and decreasing the programmed heart rate of the TAH to approximately 40 beats/min or stopped it entirely. Whereas LSTs were sequentially withdrawn in some patients, in other cases therapies were simultaneously stopped. This heterogeneity of approach reflects the lack of best-practice guidelines for withdrawing TAH support and perhaps also varying provider practices. An individualized plan for withdrawing LST and palliating terminal symptoms should be reached for each patient. Eliciting patient or surrogate preference as to when the TAH is “turned off” (before or after clinical death) is an important element in anticipatory planning for end-of-life care.15 Patients may meet clinical criteria for death, including absence of corneal and pupillary reflexes and cessation of respiration while circulation is still maintained. If the TAH is turned off after clinical death is evident, the formal death examination and pronouncement may be performed within minutes.
Ethical Analysis
Clinical medical ethics commonly involves decisions concerning withdrawing or withholding LSTs. Legal and ethical analyses coalesce around the patient’s or surrogate’s right to refuse, or request the withdrawal of, LST, as an expression of that individual’s right to bodily integrity. As a rule, physicians ought to comply with these requests if the therapy has become ineffective or if the patient or surrogate judges that continuing therapy (such as mechanical ventilation, artificial nutrition and hydration, dialysis, or pacemaker support) constitutes an unacceptable burden that is no longer consistent with his or her health care–related values, goals, or preferences.16,17 Under appropriate circumstances, carrying out requests to withdraw LST is legally permissible.18 The right to withdraw LST is codified in nearly every US state’s legislation and also by the judicial system, including the US Supreme Court,19 even if death of the patient follows. This right extends to incapacitated patients, who exercise it through advance directives and surrogate decision-makers. Moreover, a patient has the right to refuse a treatment to which he or she previously consented.20–22 We address distinct considerations for withdrawing TAH support in the following paragraphs.
Mechanical circulatory support (eg, VAD, TAH) offers patients with advanced heart failure the hope of a longer life and improved symptom control. Some would question whether specific features of mechanical circulatory support place it in a category distinct from other LSTs. TAH support is long-term, continuous (as distinct from episodic and unpredictable discharges from an ICD), and constitutive (assuming a function the body is no longer able to perform independently).23 However, it does not follow that any of these properties makes TAHs ethically distinguishable from other long-term, continuous, and constitutive therapies (such as mechanical ventilation) for which permissibility of withdrawing support is well established.16,24 To the extent that VAD support comes closest in its clinical characteristics to TAH, it is noteworthy that ethicists have already argued that it is ethically permissible to withdraw VAD support.25,26
The location of the artificial heart within the thorax, flanked by the lungs, in a pocket previously occupied by the patient’s native heart, does not distinguish it from other forms of LST. There is no morally decisive boundary between withholding defibrillation delivered transcutaneously and withholding defibrillation from a device tucked beneath the skin.17,23 That the TAH sits deep within the body does not constitute an ethically substantive difference.
Some may believe that the TAH is distinct from other LSTs because it represents “replacement therapy” more so than any previous technology. Replacement therapies completely assume the lost functionality of the native body part, becoming part of the patient as a unified whole individual, such as with solid organ transplant. A genuine replacement, however, does not derive power from an external source, does not rely on the control of an expert, and is responsive to the physiologic state of the person.23 Whereas a transplanted heart could be classified by these criteria as a replacement, the TAH requires an external power source, transcutaneous drive lines, and expert management to accommodate changes in the patient’s physiology.
We might ask whether withdrawing TAH support constitutes assisted suicide or euthanasia. At the core of this question is intention. Assisted suicide and euthanasia introduce a new pathology (a lethal prescription or injection) with the intention of ending life. Death after withdrawal of TAH support results from the absence of circulatory blood flow. The patient had previously consented to this lethal anatomic alteration, removal of the native heart, to enable TAH implantation, providing a bridge to transplant and a better quality of life. The removal of a vital organ and consequent reliance on LST is analogous to bilateral nephrectomy for polycystic kidney disease followed by dialysis dependence. When an LST, such as TAH or dialysis, is withdrawn, the intention is to remove an intervention perceived by the patient as nonbeneficial or burdensome. It does not follow that because death is foreseeable that the intention of the act itself was to terminate the patient’s life.27
Despite ethical and legal arguments, some clinicians may conscientiously object to carrying out a patient’s or surrogate’s request to withdraw TAH support. In these situations, the clinician should acknowledge the patient’s right to refuse unwanted treatment, inform the patient or surrogate of the objection, and facilitate timely transfer the patient’s care to another clinician.18
Conclusion
Requests to withdraw TAH support are not uncommon among patients who have received this LST, occurring in almost one-third of the recipients in this series. All patients described in this series were moribund at the time of TAH withdrawal. If the FDA were to approve TAH as destination therapy, prevalence of these difficult decisions would almost certainly increase. A patient or surrogate may forgo extraordinary measures by requesting cessation of life-prolonging treatments such as mechanical ventilation, dialysis, or TAH support. From the first human application of the artificial heart decades ago, an exit strategy was espoused if a patient found the therapy burdensome or nonbeneficial. Legal and ethical precedents have affirmed the permissibility of carrying out these requests, thereby removing a technological impediment to death. In this sense, withdrawing TAH support is not akin to assisted suicide or euthanasia and ought to be considered morally justifiable under the same conditions that would justify the withdrawal of any other LST. Preimplantation advance care planning is strongly recommended for all TAH recipients.
Acknowledgments
Financial support: Database support provided by Mayo Clinic Center for Clinical and Translational Science grant support (UL1 TR000135).
Role of the funding source: The sponsor had no involvement in the study design; collection, analysis, and interpretation of data; writing of the report; and decision to publish.
Abbreviations
- FDA
US Food and Drug Administration
- ICD
implantable cardioverter-defibrillator
- LST
life-sustaining therapy
- RRT
renal replacement therapy
- TAH
total artificial heart
- VAD
ventricular assist device
Footnotes
Conflicts of interest: None.
References
- 1.Cohn WE, Timms DL, Frazier OH. Total artificial hearts: past, present, and future. Nat Rev Cardiol. 2015;12(10):609–617. doi: 10.1038/nrcardio.2015.79. [DOI] [PubMed] [Google Scholar]
- 2.DeVries WC, Anderson JL, Joyce LD, et al. Clinical use of the total artificial heart. N Engl J Med. 1984;310(5):273–278. doi: 10.1056/NEJM198402023100501. [DOI] [PubMed] [Google Scholar]
- 3.Nose Y. Toward a totally implantable artificial heart: development status at Cleveland Clinic. In: Akutsu T, Koyanagi H, Anderson JM, et al., editors. Artificial Heart 3: proceedings of the 3rd International Symposium on Artificial Heart and Assist Devices. Tokyo, Japan. Tokyo: Springer Japan; Feb 16–17, 1990. pp. 147–165. c1991. [Google Scholar]
- 4.Rachels J. Barney Clark’s key. Hastings Cent Rep. 1983;13(2):17–19. [PubMed] [Google Scholar]
- 5.Commercial Appeal (Memphis, TN). 1982 Dec 5: pg 3. Quoted by: Rachels J. Barney Clark’s key. Hastings Cent Rep. 1983;13(2):17–19. [PubMed] [Google Scholar]
- 6.Altman LK. Barney Clark dies on 112th day with permanent artificial heart. [Accessed June 27, 2016];New York Times website. www.nytimes.com.
- 7.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics: 2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 8.Gerosa G, Scuri S, Iop L, Torregrossa G. Present and future perspectives on total artificial hearts. Ann Cardiothorac Surg. 2014;3(6):595–602. doi: 10.3978/j.issn.2225-319X.2014.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haberman C. Artificial hearts ticking along decades after Jarvik-7 debate. [Accessed June 27, 2016];New York Times website. www.nytimes.com.
- 10.SynCardia Systems Inc. SynCardia 70cc TAH-t for Destination Therapy (DT) (RA-540) [Accessed June 27, 2016];Clinical Trials website. https://clinicaltrials.gov.
- 11.Copeland JG, Smith RG, Arabia FA, et al. Cardio West Total Artificial Heart Investigators. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004;351(9):859–867. doi: 10.1056/NEJMoa040186. [DOI] [PubMed] [Google Scholar]
- 12.Torregrossa G, Morshuis M, Varghese R, et al. Results with SynCardia total artificial heart beyond 1 year. ASAIO J. 2014;60(6):626–634. doi: 10.1097/MAT.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 13.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Bramstedt KA. Destination nowhere: a potential dilemma with ventricular assist devices. ASAIO J. 2008;54(1):1–2. doi: 10.1097/MAT.0b013e3181614f18. [DOI] [PubMed] [Google Scholar]
- 15.Brush S, Budge D, Alharethi R, et al. End-of-life decision making and implementation in recipients of a destination left ventricular assist device. J Heart Lung Transplant. 2010;29(12):1337–1341. doi: 10.1016/j.healun.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Brody H, Hermer LD, Scott LD, Grumbles LL, Kutac JE, McCammon SD. Artificial nutrition and hydration: the evolution of ethics, evidence, and policy. J Gen Intern Med. 2011;26(9):1053–1058. doi: 10.1007/s11606-011-1659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller PS, Hook CC, Hayes DL. Ethical analysis of withdrawal of pacemaker or implantable cardioverter-defibrillator support at the end of life. Mayo Clin Proc. 2003;78(8):959–963. doi: 10.4065/78.8.959. [DOI] [PubMed] [Google Scholar]
- 18.Snyder L American College of Physicians Ethics, Professionalism, and Human Rights Committee. Ethics manual: sixth edition. Ann Intern Med. 2012;156(1 Pt 2):73–104. doi: 10.7326/0003-4819-156-1-201201031-00001. [DOI] [PubMed] [Google Scholar]
- 19.US Supreme Court. Cruzan v. Director, Missouri Department of Health. Wests Supreme Court Report. 1990;110:2841–2892. [PubMed] [Google Scholar]
- 20.Francis LP. Legal rights to health care at the end of life. JAMA. 1999;282(21):2079. [PubMed] [Google Scholar]
- 21.Emanuel EJ. Securing patients’ right to refuse medical care: in praise of the Cruzan decision. Am J Med. 1992;92(3):307–312. doi: 10.1016/0002-9343(92)90081-l. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrino ED. Decisions to withdraw life-sustaining treatment: a moral algorithm. JAMA. 2000;283(8):1065–1067. doi: 10.1001/jama.283.8.1065. [DOI] [PubMed] [Google Scholar]
- 23.Sulmasy DP. Within you/without you: biotechnology, ontology, and ethics. J Gen Intern Med. 2008;23(Suppl 1):69–72. doi: 10.1007/s11606-007-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce CR, Allen NG, Fahy BN, Gordon HL, Suarez EE, Bruckner BA. Challenges in deactivating a total artificial heart for a patient with capacity. Chest. 2014;145(3):625–631. doi: 10.1378/chest.13-1103. [DOI] [PubMed] [Google Scholar]
- 25.Mueller PS, Swetz KM, Freeman MR, et al. Ethical analysis of withdrawing ventricular assist device support. Mayo Clin Proc. 2010;85(9):791–797. doi: 10.4065/mcp.2010.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLean S, Dhonnchu TN, Mahon N, McQuillan R, Gordijn B, Ryan K. Left ventricular assist device withdrawal: an ethical discussion. [published online ahead of print January 7, 2013] BMJ Support Palliat Care. doi: 10.1136/bmjspcare-2012-000347. [DOI] [PubMed] [Google Scholar]
- 27.Sulmasy DP. Killing and allowing to die: another look. J Law Med Ethics. 1998;26(1):55–64. 4. doi: 10.1111/j.1748-720x.1998.tb01906.x. [DOI] [PubMed] [Google Scholar]