INTRODUCTION
Planning and transfer of a new technology platform developed in an academic setting to a start-up company for medical diagnostic product development may appear daunting and costly in terms of complexity, time and resources. In this review we outline the key steps taken and lessons learned when a technology platform developed in an academic setting was transferred to a start-up company for medical diagnostic product development in the interest of elucidating development toolkits for academic groups and small start-up companies starting on the path to commercialization and regulatory approval.
ENABLING THE TRANSLATIONAL POTENTIAL OF A CIRCULATING TUMOR CELL DETECTION PLATFORM
Among the new diagnostic technologies that have appeared in recent years are those for analysis of non-invasive fluid biopsies for clinical use, including those for assessment of circulating tumor cells (CTCs) (1). CTCs are intact tumor-derived cells that extravasate into the bloodstream and travel through the circulatory system; some may be messengers of information between tumor sites, form secondary metastases, self-seed, or remain in the circulatory system until clearance. Although the potential value of CTC analysis in cancer diagnostics lies in the relative ease of frequent acquisition of simple blood draws rather than requiring invasive tumor biopsies, detection and characterization of CTCs is technically challenging due to their low numbers (10−8/mL) in the peripheral blood of cancer patients, requiring specialized instrumentation not typically available in a clinical chemistry laboratory. The need for development and validation of individual assays as well as development, validation and approval of specialized instrumentation drives the need for commercial entities to select promising approaches and work with investors to advance them through the development process. In the interest of spurring the deployment of these assays, a number of groups have proposed performance criteria that should be demonstrated to validate these complex, potentially multi-analyte platforms, particularly in the context of a FDA qualified biomarker platform (2). However, the pace of commercialization of CTC platforms has remained slow, despite the fact that there are over 40 CTC technologies identified in the literature, the CellSearch® system (Veridex LLC, Raritan, New Jersey), introduced in 2004, is the only commercial instrument for CTC analysis that has received 510(k) clearance; it is approved to enumerate CTCs as a prognostic marker in a limited number of settings (3, 4). The CellSearch® platform methodology isolates and counts cells based on enrichment and enumeration of epithelial cells.
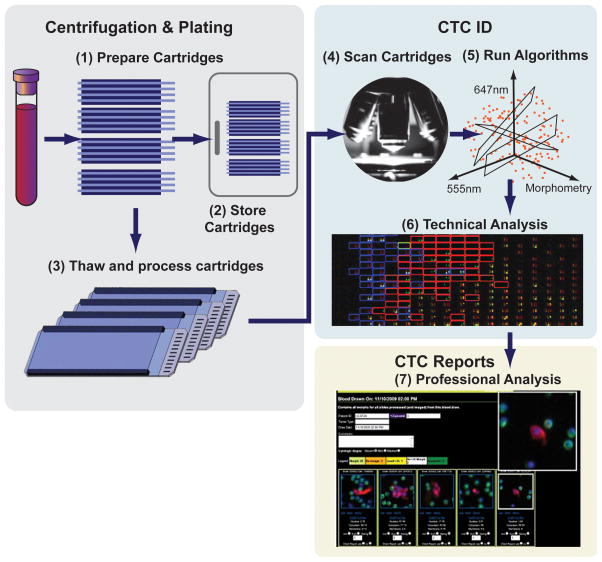
The No-Cell-Left-Behind platform and its High Definition CTC (HD-CTC) assay, developed at the Scripps Physics Oncology Center (Kuhn laboratory at The Scripps Research Institute [TSRI], San Diego, California) is one of a number of second-generation assays under development. The HD-CTC system is an ex vivo platform employing a non-enrichment technique (independent of EpCAM expression) for morphogenic, protein and ultimately genomic analysis of rare cell populations in peripheral blood (5, 6). As diagrammed in Figure 1, nucleated cells are plated on custom microscope slides and frozen until use for single cell characterization by fluorescent immunolabeling and imaging at a later time. A number of slides are generated from each patient sample; thus, the multiple samples can be differentially imaged and analyzed for different assay purposes.
Figure 1. The HD-CTC platform sample preparation and measurement.
(1) Whole blood samples are collected in commercially available tubes, RBCs are lysed and the nucleated cells are plated on custom microscope slides (≤ 12 slides/patient sample). (2) The slides are stored at −80°C until further use. (3) Slides are thawed and the cells on the slides are permeabilized and stained with the nuclear DNA counterstain 4′,6-diamidino-2-phenylindole (DAPI), as well as fluorescently labeled antibodies to cytokeratin (CK) and leukocyte-common antigen (CD45). (4) The slides are scanned on a customized microscope platform and (5) analyzed using a proprietary algorithm that employs a large number of cellular parameters to identify candidate CTCs. (7) A sample report with candidate HD-CTCs identified as nucleated, morphologically distinct, CD45– (negative) and CK+ (positive) cells with white blood cells (CD45+ ) serving as internal standards for sample/slide preparation.
The TSRI group realized the new HD-CTC platform had the potential to be developed as a “fluid phase biopsy” for detection and monitoring of disease progress and therapy (6–8). One of their first steps in exploring the potential for commercialization after the initial invention and patent application was to work with the TSRI internal office of technology development. These technology transfer experts exist in many academic centers to promote and assist successful out-licensing and spin-offs as is the responsibility of institutions taking federal grants per the Bayh-Dole Act of 1980 (9); they have the legal and commercialization knowledge required to match commercial sector interests to the technology (10). As a result, Kuhn and TSRI initiated steps to exclusively license the technology for commercial development to a newly created start-up company, Epic Sciences, (La Jolla, California) to focus on the development of the system as a medical diagnostic platform. In addition, a collaboration was initiated with CCS Associates relatively early in the start-up phase to assess the platform and define what would be needed to effectively develop and commercialize the platform, including obtaining regulatory approval. The collaboration resulted in a set of recommendations for Epic Sciences that would enable the company to move the platform from the feasibility stage into initial clinical trials. The recommendations spanned the steps from generation of a business development plan and a regulatory strategy to analytical validation studies taking into consideration the discussion around the “evidence” needed for introduction of a diagnostic test into clinical practice (11), and the evolution in the US Food and Drug Administration’s (FDA) approach to regulation of diagnostic tests (12). In addition, the group planned for a mock audit of Epic Sciences to be performed before clinical validation to assess how successfully the company had followed the recommended path towards commercialization. The key points from the initial set of recommendations and the subsequent mock audit of Epic Sciences, as well as lessons learned from this process are described below. While financial/capital resources and IP will not be discussed here, other publications may be consulted for insight (13, 14). Recommendations for the academic laboratory included focus on further research use only (RUO) exploration of the technology under standard operating procedures to provide scientifically robust and reproducible data as well as pursuit of new technological innovations to further extend the platform.
DEVELOPMENT STEPS
High Level Business and Regulatory Strategy
It is important to define the first intended use of the platform and establish the business and regulatory strategy very early in the development process. Intended use refers to the specific purpose of the test—i.e., what is being measured and why. Maintaining focus on this starting early in assay development will help ensure the new test will be fit for purpose and appropriately staged. Here, it is important to understand any pre-existing test methods for an analyte and to be clear as to how the new test would be an improvement over current technology (e.g., in terms of efficiency, accuracy, cost) and would add real value to clinical practice, ultimately benefiting establishment of clinical utility (15, 16). It is helpful to consult with potential customers, end users, and funders while defining the intended use, as well as periodically throughout development to assure that the strategy remains sound (14, 15).
The route to market or the setting in which the assay will be implemented should be considered along with the regulatory implications e.g., as a RUO platform, or LDT or as a FDA approved IVD, since there are differences in the validation, documentation and certifications required for each scenario (see Table 1). The regulatory oversight of clinical assay platforms in the US is conducted by both the FDA and the Center for Medicare and Medicaid Services (CMS) in a complementary fashion. CMS provides oversight of laboratory testing services performed on human samples for quality through Clinical Laboratory Improvement Amendments (CLIA) certification, accreditation, and inspections, as well as compliance related functions. CLIA certification is required for any clinical laboratory, including those analyzing Phase 2 and 3 clinical trial patient samples. The FDA Center for Devices and Radiological Health (CDRH), Office of In Vitro Diagnostics and Radiological Health (OIR) regulates tests or assay platforms, historically IVDs, with the approval path dependent on the setting and intended use of the product. For more information, see the guidance given on the FDA’s website (17, 18), as well as other publications (19).
Table 1.
Examples of Regulatory Implications of Routes to Market for Medical Devices
| FDA Test Designation | Staging of Assay/ Intended Use | Regulatory Implications |
|---|---|---|
| RUO 1 |
|
|
| IVD |
|
|
| LDT |
|
|
| IVDMIA |
|
|
Abbreviations: CLIA, Clinical Laboratory Improvement Amendments; IVD, in vitro diagnostic; IVDMIA, IVD Multivariate Index Assay; LDT, laboratory developed test; RUO, research use only. Adapted from (12, 18, 26, 34–37)
The FDA is reevaluating regulatory oversight of LDTs, working with Center for Medicare and Medicaid Services to establish efficient, collaborative joint oversight mechanisms
RUO tests can be used as exploratory (e.g., biomarker) endpoints in clinical trials, but the resultant data cannot be used in clinical decision making. The goal of this approach is to provide a space where assays can be developed (as RUO) in the research laboratory and tested in a CLIA/College of American Pathologists (CAP) approved laboratory running LDTs on patient samples with retrospective analysis for their potential prospective use. Data obtained from a reimbursable (billable) CLIA assay can then be collected in a highly controlled environment of the CLIA laboratory with the goal of generating the evidence necessary for FDA approval as a marketable IVD. However, there are times when these distinctions are not as simple as it would seem. For example, under certain circumstances results from an RUO assay can be presented in a tumor board and may inform the discussion but must never cross the boundary of being the critical decision making data point.
As the routes to market are mapped out for implementation of an LDT or an FDA-approved IVD, it is also worth considering working with the FDA and business partners on a staged or non-traditional approach to market entry. For example, Epic Sciences needed to both meet more short term business needs while retaining its long term goal to provide companion diagnostics through Pre-Market Approvals (PMAs). That development path started with the company conducting contract research in a RUO mode for biopharmaceutical companies using Good Laboratory Practice (GLP), while working towards establishment of a CLIA-certified CAP-approved laboratory and assays for performing in-house patient sample analysis and before consideration of IVD approval via the FDA.
Getting the System under Design Control, Implementation of Quality Systems, and GLP Compliant Laboratory Practices
Transferring a new technology from an academic laboratory to a start-up company for medical diagnostic product development involves a shift in approach for those who switch from the academic environment into the start-up environment including implementation of documented systems and design control (20). With the ultimate goal of impacting patient care through innovative technology approaches, a mindset of reproducibility and transferability is required for the academic scientists involved in these projects. It’s also important to educate the academic researchers on how to protect IP to avoid any premature disclosure of the invention prior to patent protection. This team brought the local technology transfer officers into the academic laboratory to educate all team members on responsibilities in accordance with the Bayh-Dole act as well as the appropriate conduct of research in the context of both filed intellectual property (IP) and subsequently during the time period of an existing license to a commercial entity. Disclosure procedures and appropriate management processes for then existing conflicts of interest (COI) were put in place early on to ensure public disclosure and awareness for all personnel at all times.
The steps needed to meet the requirements for demonstrating assay system performance for regulatory review include establishing an organizational structure and infrastructure for implementing GLP and “quality systems” to support platform optimization activities. A document control system with standard operating procedures (SOPs) and system design documents with quality and management review, and mechanisms for control of quality records and laboratory notebooks or records should be implemented. The system documentation required for design control includes proprietary in-house computer systems and programs, developed according to system specifications and requirements documents, and change control procedures for making platform upgrades. Ultimately the hardware (HW) and software (SW) have to be under complete design control before performance validation, which has to be version specific. Documentation should include inventory (including material management), maintenance records, disaster recovery plans, data back-up procedures, data handling, and employee training procedures. For information on GLP for nonclinical laboratory studies see (21).
The effort needed to bring an organization under design control can be daunting, the business strategy noted above that was undertaken by Epic Sciences resulted in their ability to meet the long term goal of implementing quality control systems in accordance with the requirements for CLIA certification, while at the same time pursuing technology optimization and near term business goals by running RUO tests. This approach allowed Epic Sciences to lay the groundwork for their long term regulatory and commercial goals, while still advancing the necessary research and development programs both internally and with external partners.
Assay/ System Optimization and Verification
Development of the assay platform involves optimization of the steps and components consistent with clinical sample analysis and commercialization in a laboratory setting. At this stage assay development is directed at ultimately fine-tuning and locking down assay parameters and variables (e.g., specificity, reproducibility, dynamic range, lower and upper limits of quantitation (LLOQ, ULOQ), intra- and inter-assay precision), in addition to typical assay development checks such as sample matrix effects, optimized incubation times, temperature effects, resolving method-specific performance issues, and determining the ultimate data analysis and reporting procedures.
In this phase it is also important to focus on pre-analytic factors, limiting sources of variability and degradation due to sample acquisition, storage and preparation. Pre-analytic optimization can include working out issues with sample procurement, shipping and tracking systems, transit times, storage conditions and length of storage. Similarly, this is the time to work on procurement and optimization of commercial grade and reliable sources of critical assay materials, reagents, and equipment, as well as to establish redundancies in back-up systems.
Once the assay and system are optimized it is helpful to plan for performance verification measurements, conducted in a laboratory setting before the actual assay and system “lock-down” for later validation measurements. Note that systems verification confirms that HW and SW development output meets input requirements.
Analytical Validation
Analytical validation is a regulatory requirement for CLIA certification and for FDA-approval of IVDs (Table 1). It is part of a quality management system directed at checking that the system meets specifications and that it fulfills its intended purpose, i.e., will consistently lead to the expected results in the laboratory setting. Analytical or performance validation testing includes in-house qualification of assay procedures, systems and equipment used in the process once the platform development is complete. This is typically accomplished through a series of tests according to a pre-approved Validation Plan. For example, the tests demonstrate assay sensitivity, specificity, etc., as described in the pre-analytic, analytic and post-analytic components in Table 2. Systems validation confirms that requirements implemented through HW and SW development are traceable to the system specifications and requirements and can be consistently fulfilled once development is complete. Systems validation tests cover data acquisition, data analysis, data reporting, and computer systems (workstations and servers). A validation report is compiled with the results for review and sign-off by quality assurance and management representatives.
Table 2.
Clinical Development Audit Checklist1
| Business Development Plan |
|
| Regulatory Strategy |
|
| GLP Compliance |
|
| Assay System |
|
| Analytical Validation |
|
Publication of validation results can be considered as it provides a mechanism to increase confidence in the assay platform for target users, especially when this involves third-party validation by experts in the field. The Kuhn laboratory has published numerous studies using the platform with initial performance verification data for the RUO system, including comparison to CTC detection in patient samples using the Cell Search® system (see for example, refs. (8, 22)). The first publication included demonstration of linearity of detection of SKBR3 (breast cell line) cells spiked into normal blood from approximately 10 to 300 cells/slide; detection of >5 CTCs/ml in patients with metastatic breast, prostate and pancreatic cancer patients relative to none detected in blood from normal patients; and inter-analyst and inter-experiment precision (R2= 0.979 for analysis of nine patient samples between two analysts). Epic Sciences has also published two manuscripts, the first describing analytical validation results with the Epic Sciences CTC platform, and the second a demonstration of detection of CTCs from frozen PBMCs (23, 24). The first reports on analytical performance of CTC enumeration including accuracy (% recovery of nucleated cells), assay linearity or reportable range, specificity, and intra- and inter-assay precision. The studies were conducted using healthy donor blood samples spiked with cells from a cancer cell line, COLO-205. Clinical feasibility was demonstrated through CTC detection from metastatic castrate-resistant prostate cancer patients. In the second report Epic Sciences characterized the detection of CTCs from archived frozen PBMC samples in comparison to that from the company’s standard protocol for preparation of CTCs from fresh samples, opening up the possibility of retrospective analysis of frozen samples.
CLIA Certification
As noted, both laboratories offering IVDs for clinical use as LDTs and those using FDA approved devices are required to obtain CLIA certification (see Table 1 and ref. (25)). Obtaining certification involves inspections and proficiency testing through CMS, CAP, state agencies or other designated third parties. (26). Keep in mind that the analytical validation review for CLIA certification of a laboratory is meaningful only to that laboratory, as it is limited to the conditions of that specific laboratory environment, staff, equipment, and patient population for routine biennial surveys. Conversely, the FDA reviews the analytical validation results before the assay is used on patient samples for devices submitted to the agency for approval. In addition, the FDA review assesses the test system on safety and effectiveness and may uncover errors in test design or other problems with the system that may not be detected through the CLIA laboratory certification review. Given this, CLIA inspections can also include examination of whether the laboratory is following GLP requirements, and they cover sample and data management. Table 2 provides a list of elements that may be reviewed. The inspectors may follow sample processing from receipt in the laboratory through result reporting, requesting a laboratory tour, including one of computer systems. Thus, early planning for meeting inspection guidelines is helpful as the documentation and processes can be established over time as they are being developed.
Possible Further Steps, Clinical Validation, FDA approval and Clinical Utility
After achieving CLIA certification, Epic Sciences has announced plans to pursue FDA 510(k) clearance of the platform. A 510(k) premarket submission is required when introducing a device into commercial distribution for the first time. This submission documents that the device to be marketed is at least as safe and effective and “substantially equivalent,’ to a legally marketed Class I or II device. “Substantial equivalence” can refer to intended use, design, performance, labeling, standards, and other characteristics of the test. If a medical device is classified as a Class III device by the FDA a PMA of the entire assay platform is required. The documentation and review for a PMA is much more detailed and stringent than for a 510(k) and can take much longer to prepare.
Clinical validation is required for FDA-approval of IVDs and demonstration of some level of clinical validation is also recommended for non-FDA-approved LDTs. Clinical validation demonstrates that the test platform generates analytically valid results in a clinical setting, generating an association between the test result and a clinical outcome related to the condition. This step follows analytical validation and is accomplished through analysis of patient samples, usually from clinical studies, often performed with the new assay platform in parallel with a comparable (gold standard) or reference method. An appropriate clinical validation plan is developed that accounts for the setting, i.e., the disease settings, patient populations, and assay indication as defined by the intended use. When using samples obtained in a clinical trial, the clinical protocol design is incorporated into the validation plan. The clinical protocol should also include the scientific rationale for the study, statement of the procedures to be used on the subjects, summary of the data analysis methods with a complete and detailed statistical analysis plan, accounting for statistically appropriate sample size. Clinical evaluation of devices not cleared for marketing should be performed under Good Clinical Practice (GCP) (27). It is also useful to plan the clinical studies to facilitate later demonstration of clinical utility, described below. Thus, it is key in the early planning stages to begin to identify samples to support the intended use of the assay and to form partnerships to obtain well-annotated samples from on-going, new or completed trials or sample repositories. There are a number of approaches to clinical study design, dependent on the intended use of the product, if a biomarker or not (19, 28, 29). In addition, when a test device is used in clinical studies to collect trial safety and effectiveness data to support a PMA submission an IDE exemption may also be required by the FDA. If the results are masked and not used in clinical decision making an IDE is not required; thus it can be useful to consult with the FDA in a pre-IDE meeting about whether an IDE will be required by the agency for the clinical trials (for more details, see reference (30)). Additional details around a 510(k) application before the planned market launch, including documentation, can be found on the FDA website, (31, 32). Keep in mind the FDA does not perform 510(k) pre-clearance facility inspections, but the manufacturer should be prepared for a FDA quality system inspection (21 CFR 820) at any point after the clearance.
Demonstration of clinical utility is not required for FDA approval, but recommended for non-FDA-approved LDTs and FDA-approved IVDs, as it is useful for the long term acceptance of a test, including physician adoption and payer reimbursement decisions. Clinical utility refers to evidence that the new test results in improved measurable clinical outcomes for patients and added value to patient management decision-making compared with current management without the test. Planning for performing the work to demonstrate clinical utility after approvals and commercialization can appear daunting, especially to relatively small medical device companies. Thus, it pays to integrate plans for demonstration of clinical utility into the development planning process early (16). For example, carefully design clinical trials to show that the assay meets the intended use early and assess the impact on physician behavior before commercialization. As noted earlier, it is helpful to generate publications in peer-reviewed journals to add to a chain of evidence and to include plans for supporting infrastructure into the product development plan, take into consideration the practicality of performing the assay in clinical practice, anticipate costs for reimbursement decisions, and perform cost-effectiveness studies.
LESSONS LEARNED
Approximately two years after the initial recommendations were developed, Epic Sciences reported that the company had made progress in bringing their systems under the quality control needed to conduct early clinical projects in an observational (RUO) capacity and were ready for a mock audit. The mock audit of Epic Sciences was performed with the goals of documenting how successfully the Kuhn Laboratory at TSRI had transferred the HD-CTC assay to Epic Sciences, while continuing to perform CTC analysis in the academic setting, assess if and how successfully Epic Sciences had followed the recommended path towards commercialization as a clinical assay to date, and assist the company in finding loopholes that would need to be addressed as they prepared to apply for CLIA certification. The audit included a review of the company’s success in formulating and implementing a business strategy, facility tour, overview of the sample chain of custody, processing and storage, and assay and data analysis procedures. In addition Epic Sciences’ progress in bringing the systems into regulatory compliance, including implementation of SOPs and documents to be completed, equipment controls, disaster recovery plan, material management procedures and training. The company noted they were developing an analytical validation plan; thus validation results were not reviewed. The conclusions of the mock audits were that the company had made progress in implementing GLP and other quality systems consistent with the original recommendations, but still had some additional work to do in implementing these systems, including documentation, and assay validation studies. After the mock audit, Epic Sciences continued to complete analytical validation and apply for CLIA certification, and in fact announced in January 2015 that the laboratory had achieved that goal.
In the transfer of the HD-CTC platform from the Kuhn laboratory at TSRI to Epic Sciences, from the point of view of the platform developers and company leadership at the time, some of the critical elements of success included the timing of the spin-off, making use of the university technology transfer office to facilitate and expedite the spin-off process and having the necessary intellectual leadership team throughout with a focus on the ultimate company goal while keeping quality in mind. The start-up found that it was important to build an organizational structure that paralleled the development and commercialization steps, and maintain focus on the required documentation, regulatory framework, and processes such as GLP compliant laboratory practices. The group also found that breaking down the process to launch into incremental steps helps planning and implementation, while periodic assessments facilitates beneficial decision making. The company made use of consultants to supplement the company staff as needed and these independent assessments provided useful input.
From both the company and academics’ perspectives, it is also helpful to formalize the working relationship and firewalls between the academic laboratory and commercial start-up. Academic technologies are often not ready for “licensing across a firewall,” and success requires active and constructive collaboration from all parties, as also noted recently by others (20). This in turn requires well established mechanisms for the management of the conflict of interest (COI). It is helpful if the academic laboratories understand the processes required for development and validation, including the applicable regulatory requirements, so they can help facilitate the prototype design process (e.g., documenting the initial systems hardware and software development).
From the Kuhn laboratory’s perspective, maintaining a respectful, constructive relationship with the spin-off company resulted in seeing their ideas and concepts impact patient care, as well as providing the academic staff with practical knowledge of quality procedures, useful for future enterprises. They gained a greater understanding of how technology development and maturation in an academic setting would benefit the transfer process as well as “best (quality) practices” that are implementable in the experimental setting. All along a clearly defined conflict of interest management plan was used to ensure integrity of both the academic and commercial enterprise. The academic laboratory worked to identify a path that enabled them to produce highly reproducible and reliable data, while accounting for process developments, yet not setup to run the full GLP or CLIA/CAP processes. The group also found that obtaining grants for R&D in an academic setting in parallel to start-up funds can help spread risk in discovery and development phases. The academic laboratory has continued to conduct numerous investigations of CTCs with both academic and commercial collaborators to measure CTCs in a number of studies of patients with metastatic disease incorporating genomics and proteomics for downstream analysis (33).
DISCUSSION
For a company, early definition of the business strategy to plan for the first intended use of the platform, as well as planning a regulatory strategy and organizational structure required to match the commercialization strategy with regulatory requirements is key. Given this overall framework, critical steps to follow even during system and assay optimization are implementation of GLP compliant laboratory practices, documenting the assay and system hardware and software structure and development, and bringing these under “design control.” These are followed by verification and validation studies of the assay and system performance, as well as carefully planned clinical trials to support clinical validation, FDA review and clinical utility and to plan ahead to lay additional groundwork for demonstration of clinical utility for assay adoption and payer reimbursement. Appropriate planning to implement these processes early in the assay development lifecycle will facilitate the move from the feasibility stage of development to commercialization. It is also important to maintain a balance of keeping the focus on developing these systems while optimizing the assay system and meeting short term business goals. Maintaining and appropriate and defined relationship between the initial inventors and the commercial developers promotes efficient development.
While relative novices in commercial platform development, Dr. Kuhn, TSRI and Epic Sciences started early to determine the first indicated use, business and regulatory framework and kept focused on the long term goals while working through the incremental steps to achieve these goals. Epic Sciences proceeded to eventually publish analytical validation data for the platform, and has obtained CLIA certification, and are looking towards a 510(k) filing.
STUDY HIGHLIGHTS.
The key steps taken and lessons learned when a technology platform developed in an academic setting was transferred to a start-up company for medical diagnostic product development include an early focus on the intended use of the platform, incorporating the appropriate business and regulatory strategy, and implementation of the organizational structure in parallel to development and commercialization steps. Maintenance of a constructive relationship with the academic laboratory can also provide downstream rewards for both the public and private institutions.
Acknowledgments
We would like to thank Brenda Gumbs Petty (CCS Associates, Inc.), Liz Lison (Advocea LLC), and Scott Forrest (TSRI Technology Transfer Office) for their contributions.
Footnotes
Author contributions: SMK, PK, and CCS contributed to the writing of the manuscript. PK and AK developed the platform technology. SMK and CCS contributed to the development recommendations and audit report with GTB. GTB and KT performed the mock audit. All authors contributed to lessons learned.
Conflict of Interest/Disclosures: PK is a shareholder in and advisor to Epic Sciences, which holds the exclusive commercialization license to the HD-CTC technology from The Scripps Research Institute. This work was partially funded by grant U54 CA143906 from the National Cancer Institute of the National Institutes of Health (NIH).
References
- 1.Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med. 2014;7:1–11. doi: 10.15252/emmm.201303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkinson DR, et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med. 2012;10:138. doi: 10.1186/1479-5876-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andree KC, van Dalum G, Terstappen LW. Challenges in circulating tumor cell detection by the CellSearch system. Mol Oncol. 2016;10:395–407. doi: 10.1016/j.molonc.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allard WJ, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh HB, et al. High speed detection of circulating tumor cells. Biosens Bioelectron. 2006;21:1893–9. doi: 10.1016/j.bios.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Marrinucci D, et al. Cytomorphology of circulating colorectal tumor cells:a small case series. J Oncol. 2010;2010:861341. doi: 10.1155/2010/861341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrinucci D, et al. Case study of the morphologic variation of circulating tumor cells. Hum Pathol. 2007;38:514–9. doi: 10.1016/j.humpath.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Marrinucci D, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol. 2012;9:016003. doi: 10.1088/1478-3975/9/1/016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markel H. Patents, profits, and the American people--the Bayh-Dole Act of 1980. N Engl J Med. 2013;369:794–6. doi: 10.1056/NEJMp1306553. [DOI] [PubMed] [Google Scholar]
- 10.Wapner J. Technology transfer: The leap to industry. Nature. 2016;533:S13–S5. doi: 10.1038/533S13a. [DOI] [PubMed] [Google Scholar]
- 11.Woodcock J. Assessing the clinical utility of diagnostics used in drug therapy. Clin Pharmacol Ther. 2010;88:765–73. doi: 10.1038/clpt.2010.230. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs) Draft Guidance. 2014 Oct 3; http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM416685.pdf. (2014)
- 13.Kleinbeck K, Anderson E, Ogle M, Burmania J, Kao WJ. The new (challenging) role of academia in biomaterial translational research and medical device development. Biointerphases. 2012;7:12. doi: 10.1007/s13758-011-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledford H. Biotech boot camp. U.S. funding agencies are turning to a Silicon Valley entrepreneur to focus fledgling biomedical companies on success — even when that means making a scientific course correction. Nature. 2015;519:402–5. doi: 10.1038/519402a. [DOI] [PubMed] [Google Scholar]
- 15.Anagostou A, Liotta LA. Small business development for molecular diagnostics. Methods Mol Biol. 2012;823:421–37. doi: 10.1007/978-1-60327-216-2_28. [DOI] [PubMed] [Google Scholar]
- 16.Parkinson DR, et al. Evidence of clinical utility: An unmet need in molecular diagnostics for patients with cancer. Clin Cancer Res. 2014;20:1428–44. doi: 10.1158/1078-0432.CCR-13-2961. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration. Device Advice: Comprehensive Regulatory Assistance. 2015 Nov 25; Available: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/default.htm. (2015)
- 18.U.S. Food and Drug Administration. Discussion paper on laboratory developed tests (LDTs) 2017 Jan 13; http://www.fda.gov/downloads/medicaldevices/productsandmedicalprocedures/invitrodiagnostics/laboratorydevelopedtests/ucm536965.pdf. (2017)
- 19.Tezak Z, Kondratovich MV, Mansfield E. US FDA and personalized medicine: In vitro diagnostic regulatory perspective. Personalized Medicine. 2010;7:517–30. doi: 10.2217/pme.10.53. [DOI] [PubMed] [Google Scholar]
- 20.Zappe H. Innovation: Bridging the market gap. Nature. 2013;501:483–5. doi: 10.1038/501483a. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration. Bioresearch Monitoring. 2015 Jul 31; Available: http://www.fda.gov/ICECI/EnforcementActions/BioresearchMonitoring. (2015)
- 22.Nieva J, et al. High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: A longitudinal analysis. Phys Biol. 2012;9:016004. doi: 10.1088/1478-3975/9/1/016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner SL, et al. Analytical validation and capabilities of the Epic CTC Platform: Enrichment-free circulating tumour cell detection and characterization. J Circ Biomark. 2015;4:3. doi: 10.5772/60725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu D, et al. Detection and characterization of circulating tumour cells from frozen peripheral blood mononuclear cells. J Circ Biomark. 2015;4:4. doi: 10.5772/60745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Medicare & Medicaid Services. Clinical Laboratory Improvement Amendments (CLIA) 2015 Aug 10; Available: https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html?redirect=/clia. (2015)
- 26.U.S. Food and Drug Administration. Administrative Procedures for CLIA Categorization. Guidance for Industry and Food and Drug Administration Staff. 2014 Mar 12; Available: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm070889.pdf. (2014)
- 27.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) E6 Good Clinical Practice: Consolidated Guidance. ICH; Apr, 1996. Guidance for Industry. Available: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm073122.pdf. (1996) [Google Scholar]
- 28.U.S. Food and Drug Administration. Design Considerations for Pivotal Clinical Investigations for Medical Devices - Guidance for Industry, Clinical Investigators Institutional Review Boards and Food and Drug Administration Staff. Nov 7, 2013. [Google Scholar]
- 29.Freidlin B, McShane LM, Korn EL. Randomized clinical trials with biomarkers: Design issues. J Natl Cancer Inst. 2010;102:152–60. doi: 10.1093/jnci/djp477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Food and Drug Administration. Device Advice: Investigational Device Exemption (IDE) 2015 Sep 04; Available: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/InvestigationalDeviceExemptionIDE/default.htm. (2015)
- 31.U.S. Food and Drug Administration. Premarket Notification 510(k) 2015 Sep 16; Available: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketNotification510k/default.htm. (2015)
- 32.U.S. Food and Drug Administration. Guidance for Industry and FDA Staff. Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices. 2005 May 11; http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm089593.pdf. (2005)
- 33.Dago AE, et al. Rapid phenotypic and genomic change in response to therapeutic pressure in prostate cancer inferred by high content analysis of single circulating tumor cells. PLoS ONE. 2014;9:e101777. doi: 10.1371/journal.pone.0101777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U. S. Food and Drug Administration. Guidance for Industry and FDA Staff. Commercially Distributed Analyte Specific Reagents (ASRs): Frequently Asked Questions. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm071269.pdf. (2007)
- 35.U. S. Food and Drug Administration. Overview of IVD regulation. 2015 Page Last Updated: 03/19/2015. Available: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/IVDRegulatoryAssistance/ucm123682.htm.
- 36.U. S. Food and Drug Administration. In Vitro Companion Diagnostic Devices. Guidance for Industry and Food and Drug Administration Staff. 2014 Aug 6; Available: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM262327.pdf. (2014)
- 37.U. S. Food and Drug Administration. Draft Guidance for Industry, Clinical Laboratories, and FDA Staff. In Vitro Diagnostic Multivariate Index Assays. 2007 Jul 26; Available: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm071455.pdf. (2007)
- 38.U. S. Food and Drug Administration. General Principles of Software Validation; Final Guidance for Industry and FDA Staff. 2002 Jan 11; Available: http://www.fda.gov/RegulatoryInformation/Guidances/ucm085281.htm. (2002)
- 39.U. S. Food and Drug Administration. Guidance for Industry, FDA Reviewers and Compliance on Off-the-Shelf Software Use in Medical Devices. 1999 Available: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm073779.htm.