Abstract
The development of new agents to target HBV cccDNA is urgently needed because of the limitations of current available drugs for treatment of hepatitis B. By using a cell-based assay in which the production of HBeAg is in a cccDNA-dependent manner, we screened a compound library derived from Chinese herbal remedies for inhibitors against HBV cccDNA. Three hydrolyzable tannins, specifically punicalagin, punicalin and geraniin, emerged as novel anti-HBV agents. These compounds significantly reduced the production of secreted HBeAg and cccDNA in a dose-dependent manner in our assay, without dramatic alteration of viral DNA replication. Furthermore, punicalagin did not affect precore/core promoter activity, pgRNA transcription, core protein expression, or HBsAg secretion. By employing the cell-based cccDNA accumulation and stability assay, we found that these tannins significantly inhibited the establishment of cccDNA and modestly facilitated the degradation of preexisting cccDNA. Collectively, our results suggest that hydrolyzable tannins inhibit HBV cccDNA production via a dual mechanism through preventing the formation of cccDNA and promoting cccDNA decay, although the latter effect is rather minor. These hydrolyzable tannins may serve as lead compounds for the development of new agents to cure HBV infection.
Keywords: HBV, Antiviral, cccDNA, Hydrolyzable tannins
1. Introduction
Hepatitis B virus (HBV) infection remains a major global health problem with an estimated 240 million chronically infected people worldwide (Grimm et al., 2011; Zeisel et al., 2015). Every year approximately 1 million people die of severe liver diseases caused by chronic hepatitis B (CHB) infection, such as liver failure, cirrhosis and hepatocellular carcinoma (HCC) (Ganem and Prince, 2004; Grimm et al., 2011; Hoofnagle et al., 2007). HBV is a small double-stranded DNA virus belonging to the family of Hepadnaviridae. After an endocytotic process, the viral nucleocapsid is released into cytoplasm and the 3.2 kb relaxed circular partially double-stranded DNA (rcDNA) is transferred to nucleus where the rcDNA is repaired to form covalently closed circular DNA (cccDNA) by host functions (Guo and Guo, 2015; Summers et al., 1975; Tuttleman et al., 1986). This episomal cccDNA serves as the transcription template for production of 3.5 kb pregenomic RNA (pgRNA) and four other viral mRNAs under the control of their own promoters, specifically 3.5 kb precore mRNA, 2.4 kb and 2.1 kb surface mRNA, and 0.7 kb X mRNA. The 3.5 kb precore mRNA contains all the ORFs of viral proteins but only translates precore protein, which is further processed and secreted as e antigen (HBeAg). The pgRNA is a multifunctional transcript which encodes the viral polymerase and core protein, and it serves as the template of HBV genome DNA synthesis as well. Following the binding of viral polymerase to pgRNA, the complex is packaged into nucleocapsid, inside of which the pgRNA is converted to rcDNA by polymerase. The mature nucleocapsid can either acquire the viral envelope and secrete as virion or redirect the rcDNA into nucleus to refill the cccDNA pool (Ganem and Varmus, 1987; Seeger and Mason, 2000; Tuttleman et al., 1986; Wu et al., 1990). Therefore, cccDNA plays a pivotal role in HBV life cycle and its elimination is critical for a cure of hepatitis B.
Although vaccination programs against HBV infection have shown a high efficiency in blocking vertical transmission and protect 90% health people from infection with HBV, an 100% effective antiviral treatment has not been available for patients with CHB yet (Raney et al., 2003; Shepard et al., 2006; Thermet et al., 2003). There are currently two major classes of drugs approved for the treatment of CHB in Europe and the US, namely interferons (IFN-α and pegylated IFN-α) and nucleotide analogues (lamivudine, adefovir, entecavir, telbivudine, and tenofovir) (Zoulim and Durantel, 2015). Each agent has individual advantages and drawbacks. Interferon is costly, poorly tolerated, and only responsive in a small fraction of CHB patients. Nucleotide analogues can effectively inhibit HBV DNA synthesis, but drug resistance emerges after long-term treatment. More importantly, cccDNA persists even after years of antiviral therapy and results in rapid reactivation of viral replication upon withdrawal of treatment (Khanbabaee and van Ree, 2001; Litwin et al., 2005; Locarnini, 2005; Moraleda et al., 1997; Zoulim, 2005). In recent years, several non-nucleotide substances with novel antiviral targets have been evaluated in preclinical studies or clinical trials for their anti-HBV activities. For example, Petersen et al. showed that the acylated PreS1-derived peptides could prevent HBV infection of immunodeficient uPA mice repopulated with primary human hepatocytes (Petersen et al., 2008). AT-61 and AT-130, the phenyl-propenamide derivatives, interfere with the packing of pgRNA into core particles (Delaney et al., 2002; Deres et al., 2003; King et al., 1998). Heteroaryldihydropyrimidines (HAPs) inhibit HBV capsid formation through altering the kinetics of capsid assembly and/or promoting the degradation of capsid protein (Deres et al., 2003; Stray et al., 2005). However, a complete eradication of viral cccDNA from the nuclei of infected hepatocytes cannot be achieved by any of the aforementioned drugs and compounds. Thus, the development of new drugs preventing the formation of cccDNA, or ideally, eliminating established cccDNA, in the infected hepatocytes, is of special clinical interest (Levrero et al., 2009; Zoulim and Locarnini, 2009).
In a previous attempt to find small molecules that can inhibit HBV cccDNA accumulation, Cai et al. screened a compound library by using HepDE19 cell system which inducibly expresses cccDNA-dependent HBeAg as a surrogate marker for cccDNA, and identified two structurally related compounds that act as cccDNA formation inhibitors through blocking rcDNA deproteinization (removal of the covalently attached polymerase from rcDNA), a presumably mandatory step in the conversion of rcDNA to cccDNA (Cai et al., 2012). In this report, we utilized a cell line HepG2.117 for anti-HBV cccDNA screening. With the similar cloning strategy for the transgene in HepAD38 cell line and its upgraded version HepDE19 cells (Cai et al., 2012; Zhou et al., 2006), HBV production in HepG2.117 cell is also under the control of a tetracycline-responsive promoter, and the authentic HBV precore mRNA can only be transcribed from cccDNA but not the transgene, giving rise to cccDNA-dependent HBeAg production (Sun and Nassal, 2006; Zhou et al., 2006). Through screening of compounds derived from Chinese herbal remedies for their activity against HBeAg production in HepG2.117 cells, three hydrolyzable tannins (Punicalagin, Punicalin and Geraniin) were identified as antiviral hits. Punicalagin, punicalin and geraniin inhibited the production of HBeAg but not HBsAg in HepG2.117 cells, along with a significant reduction of cccDNA without dramatic alteration of viral DNA replication. Further study revealed that tannins inhibited cccDNA accumulation in HepDES19 and HepG2.117 cells primarily through preventing cccDNA establishment, and partly through promoting cccDNA degradation. These results suggested that hydrolyzable tannins represent a novel class of natural product compounds that hold promise for development of cccDNA eliminators to cure hepatitis B.
2. Methods and materials
2.1. Cell lines and cell culture
HepG2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 10% FCS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in 5% carbon dioxide atmosphere. HepG2.2.15 is a HepG2-based stable cell line that carries two integrated tandem head-to-tail dimers of HBV genome and a G418-resistant gene (Sells et al., 1987). HepG2.2.15 cell were cultured in the same way as HepG2 cells with the addition of 500 μg/mL G418. HepG2.117, a gift from Dr. Dian-Xing Sun (University Hospital Freiburg, Germany), is an inducible HBV-replicating cell line that expresses HBV pgRNA under the control of a tetracycline responsive CMV promoter (Sun and Nassal, 2006). HepDES19 is a sibling cell line of HepDE19 cells with artificial gene mutations to block the expression of HBV envelope proteins, it produces more cccDNA than other HBV stable cell lines and thus was often used in direct cccDNA measurement by Southern blot (Cai et al., 2012; Guo et al., 2007a). HepG2.117 and HepDES19 cells were cultured in the same way as HepG2.2.15 cells with additional 80 μg/mL hygromycin and 1.5 μg/mL doxycycline (Dox) (for HepG2.117 cells) or additional 1 μg/mL tetracycline (Tet) (for HepDES19 cells). When needed, Dox or Tet was withdrawn to induce HBV pgRNA transcription.
2.2. Natural product library screening
The natural product library, which contains a large amount of compounds (purity ≥ 98%) from Chinese traditional herbs, was obtained from Sichuan Weikeqi Biological Technology Co., Ltd. We selected 400 compounds from the natural product library to evaluate their anti-HBV activities according to the reported antiviral functions of those Chinese traditional herbs. HepG2.117 cells were plated in 96-well plates at a density of 8 × 103 cells/well in the presence of Dox. Twenty-four hours after seeding, Dox was removed and cells were treated with natural product compounds at concentration of 3.125 μM, 6.25 μM, 12.5 μM, 25 μM, 50 μM and 100 μM, and 10 μM of lamivudine (NIH AIDS Research and Reference Reagent Program, Rockville, MD, USA. purity ≥98%) was included as positive control, FBS and DMSO were normalized to 5% and 1% in all the mock and treatment groups, respectively. The drug-containing media were replaced every three days. The culture media were harvested at day 6 post treatment and subjected to HBeAg and HBsAg enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions (Kehua, Shanghai). Compounds that reduced HBeAg more than 50% but did not reduce HBsAg were considered as hits.
2.3. Cell viability assay
The effect of testing compounds on cell viability was measured by using the CellTiter-Glo reagent (Promega) according to the manufacturer’s instructions.
2.4. Analysis of HBV RNA
Total cellular RNA was extracted with Trizol reagent (Invitrogen). HBV RNA Northern blot was performed according to previously published (Cai et al., 2012).
To determine viral mRNA levels by quantitative real-time PCR, 1 μg of total cellular RNA were treated with RQ1 RNase-Free DNase (Promega) and reverse transcribed using M-MLV Reverse Transcriptase (Promega) and oligo dT (Promega) following the manufacturer’s instructions. The cDNAs of HBV total RNA, precore mRNA and pgRNA were quantified by real-time PCR. PCRs were performed using SYBR Green PCR Master Mix and the primer pairs with the following program: initial denaturation at 95 °C for 5 min, followed by 37 cycles of amplification at 95 °C for 15 s and annealing/extension at 56 °C for 45 s.
The specific primers for precore mRNA were as follows: forward, 5′-TCTGCGCACCAGCACCATG-3′ (nt 1800–1818); reverse, 5′-TGCCTCGTCGTCTAACAA-3′ (nt 2362–2345). The forward primer for pgRNA amplification was 5′-TCGGGAAGCCTTAGAGTC-3′ (nt 2016–2033), the reverse primer was 5′-TGCCTCGTCGTCTAACAA-3′ (nt 2362–2345) (He et al., 2011). The primers for total HBV RNA were 5′-CCGTCTGTCCTTCTCATCT-3′ (nt 1551–1570) and 5′-GACCAATT-TATGCCTACAGCCTC-3′ (nt 1801–1779). Primers used for GAPDH mRNA amplification were as previously reported, as 5′-GAAGGT-GAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTC-3′ (Zhong et al., 2005).
2.5. Detection of HBV core promoter activity by dual luciferase reporter assay
HepG2 and Huh7 cells were transiently cotransfected with plasmid pHBVCP-Luc reporter, which was constructed by inserting HBV core promoter before the firefly luciferase gene in the pGL3-basic vector, and the reporter plasmid pRL-TK as an internal control with FuGENE-HD reagent according to the manufacturer’s instructions (Roche Applied Science, Manheim, Germany) (He et al., 2011). Twenty-four hours post-transfection, cells were treated with compounds for three days with fresh media changed every day. HBV core promoter activity was determined by measuring luciferase activity using the Dual Luciferase Reporter Assay System (Promega).
2.6. Western blot assay
Cells were treated with different concentrations of compounds for six days, washed with cold PBS, and lysed with radio immunoprecipitation assay (RIPA) lysis buffer supplemented with complete mini protease inhibitor cocktail (Roche) at 4 °C for 1 h. Cell lysates were subjected to 15% SDS-PAGE and transferred to poly-vinylidene difluoride (PVDF) membranes. After incubation with rabbit anti-HBcAg (Dako) at 4 °C overnight, each blot was probed with horseradish peroxidase-conjugated secondary antibody. Immunoreactive signals were detected with an enhanced chemiluminescence substrate (Thermo) using an AlphaEaseH FC Imaging System (Alpha Innotech Corporation).
2.7. Extraction and detection of HBV DNA
HepG2.117 cells were lysed with lysis buffer containing 10 mM Tris–HCl (pH 7.5), 10 mM EDTA and 0.7% SDS, 150 mM NaCl. After 30 min incubation at room temperature, samples were divided into two parts, one for total DNA purification and the other for cccDNA extraction. Briefly, the total DNA containing lysate was treated with 0.4 mg/mL Proteinase K for 4 h at 58 °C followed by phenol/chloroform extraction and ethanol precipitation. For cccDNA extraction, the lysate was added 0.25 vol 2.5 M KCL and incubated at room temperature for 30 min, then clarified protein-DNA-complexes by centrifugation at 12,000g for 10 min (Werle-Lapostolle et al., 2004; Wu et al., 1990). The supernatant containing cccDNA was extracted twice with phenol/chloroform and once with chloroform. DNA was precipitated with ethanol overnight at −20 °C and dissolved in ddH2O. The cccDNA samples were heated to 85 °C to denature the non-cccDNA into single strand DNA and then treated with plasmid-safe ATP-dependent DNase (PSAD) (preferentially digest double or single stranded DNA over nicked circular dsDNA) to remove the non-cccDNA molecules. Then cccDNA was purified with PCR/DNA Purification Kit (Beyotime, China). DNA samples were subjected to real-time PCR using SYBR GREEN Realtime PCR Master Mix (TOYOBO). To quantify total intracellular HBV DNA (core DNA and cccDNA), primers corresponding to HBV S ORF were introduced (Liu et al., 2007). CccDNA selective primers NCCC1 5′-CTCCCCGTCTGTGCCTTCT -3′ plus CCCAS2 5′-GCCCCAAAGCCACC-CAAG -3′ were used for cccDNA amplification (Werle-Lapostolle et al., 2004). The quantification was normalized to the GAPDH DNA copies. Mitochondrial DNA was analyzed as an internal reference for normalization purpose for cccDNA quantification in the cccDNA decay kinetics assay. Primers for Mitochondrial DNA quantification were 5′-CCCCACAAACCCCATTACTAAACCCA -3′ plus 5′-TTTCATCATGCGGAGATGTTGGATGG -3′.
The extraction and Southern blot analysis of HBV core DNA and cccDNA from HepDES19 cells were performed as previously described (Cai et al., 2013; Guo et al., 2007a). Quantitative real-time PCR detection of core DNA and cccDNA from HepDES19 cells was performed with the FastStart Essential DNA Probes Master (Roche), using a 20 μl reaction mixture. The primers and probe used for core DNA detection were forward primer: 5′-CCGTCTGTGCCTTCTCATCTG -3′, reverse primer: 5′-AGTCCAA-GAGTYCTCTTATGYAAGACCTT -3′ and probe: 5′-FAM-CCGTGTGCACTTCGCTTCACCTCTGC -TAMRA-3′. The PCR reaction contains 0.8 μM of primers and 0.2 μM of probe and the thermal cycling conditions are as follow: 10 min at 95 °C, 45 cycles of 15 s at 95 °C and 30 s at 64 °C. The primers and probe used for cccDNA qPCR were forward primer 5′-GTCTGTGCCTTCTCATCTGC-3′, reverse Primer: 5′-AGTAACTCCACAGTAGCTCCAAATT-3′, and probe 5′-FAM-TTCAAGCCTCCAAGCTGTGCCTTGGGTGGC-TAMRA-3′. The amplification setting included 0.9 μM primers and 0.2 μM probe, annealing, and extension at 61 °C for 50 cycles.
2.8. Statistical analysis
Statistical analysis was performed by using a two-tailed student’s t-test by SPSS software. Results were presented as mean value ± SD with p-value.
3. Results
3.1. Identification of hydrolyzable tannins as novel anti-HBV agents
Thus far, HepDE19 cell line remains the only reported cell system which was specially designed to express HBeAg as a cccDNA reporter and has been successfully applied in high throughput screening of small molecule compound libraries for cccDNA inhibitors (Cai et al., 2012). The principle of cccDNA-dependent HBeAg expression in HepDE19 cells is built upon a transgene cloning strategy that prevents precore (the precursor of HBeAg) expression from the integrated HBV genome, but allows precore expression from cccDNA template once the separated ORF of pre-core on both termini of pgRNA is rejoined after reverse transcription and cccDNA formation (Cai et al., 2012; Zhou et al., 2006). In this regard, HepG2.117 cell line carries a HBV pgRNA expression cassette under the control of tetracycline inducible promoter, starting from a nucleotide right behind the start codon of precore ORF (Sun and Nassal, 2006). Theoretically, HepG2.117 cells should not make precore/HBeAg from transgene, but the pgRNA transcribed from transgene will initiate DNA replication and the pre-core ORF will be restored on the cccDNA, giving rise to the cccDNA-dependent HBeAg production. To test this, the kinetics of HBeAg secretion and cccDNA production upon doxycycline removal were monitored. As expected, there is a good correlation between the extracellular levels of HBeAg and the amounts of intracellular cccDNA (Fig. S1). Therefore, the secretion of HBeAg can serve as a cccDNA surrogate marker in HepG2.117 cells, and we thus measured HBeAg to evaluate the antiviral activities of the compounds against HBV cccDNA.
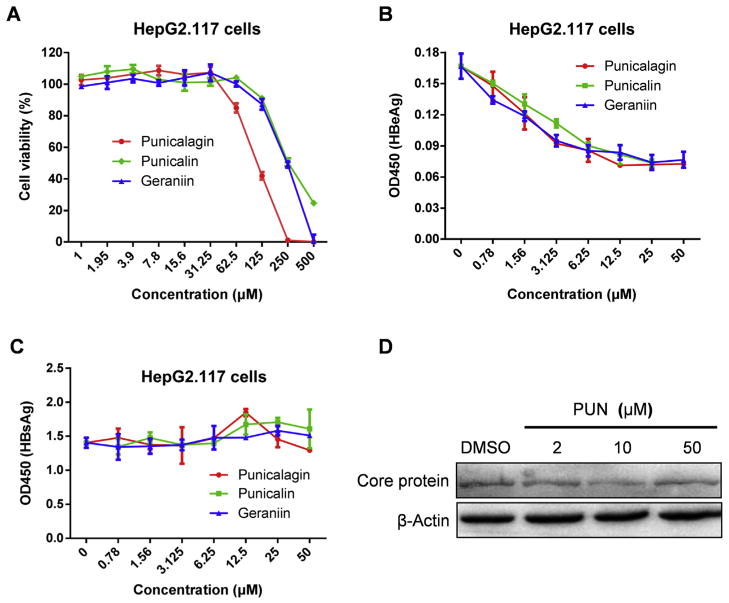
Among 400 compounds isolated from traditional Chinese medicinal herbs, three structurally related compounds, specifically punicalagin, punicalin and geraniin, emerged as confirmed hits from the screening. Punicalagin, punicalin and geraniin are hy-drolyzable tannins as they possess structures that generally consist of gallic or ellagic acid esters conjugated to a sugar moiety (Khanbabaee and van Ree, 2001) (Fig. 1). All the three tannins exhibited dose-dependent reduction of supernatant HBeAg level in HepG2.117 cells but had no effect on the secretion of HBsAg at non-cytotoxic doses (Fig. 2A, B and 2C). Next, we chose punicalagin as a representative compound to test its effect on core protein expression by Western Blot. As shown in Fig. 2D, punicalagin did not affect the expression of core protein, which was predominantly expressed from the HBV transgene in HepG2.117 cells. These results demonstrated that hydrolyzable tannins displayed a specific inhibition of HBeAg production in HepG2.117 cells, inferring a possible antiviral effect of tannins on cccDNA synthesis, stability, or transcription.
Fig. 1.
Chemical structures of punicalagin, punicalin and geraniin.
Fig. 2. Identification of punicalagin, punicalin and geraniin as novel anti-HBV agents.
After the induction of HBV replication, HepG2.117 cells were treated with various indicated concentrations of compounds for six days. (A) Cell viability was measured to assess the compound cytotoxicity. HBeAg (B) and HBsAg (C) in the culture supernatants were determined by ELISA. Error bars indicate standard deviation of three independent experiments. (D) Intracellular HBV core protein was analyzed by Western blot. β-Actin served as loading control.
3.2. Punicalagin treatment reduced HBV precore mRNA level in HepG2.117 cells
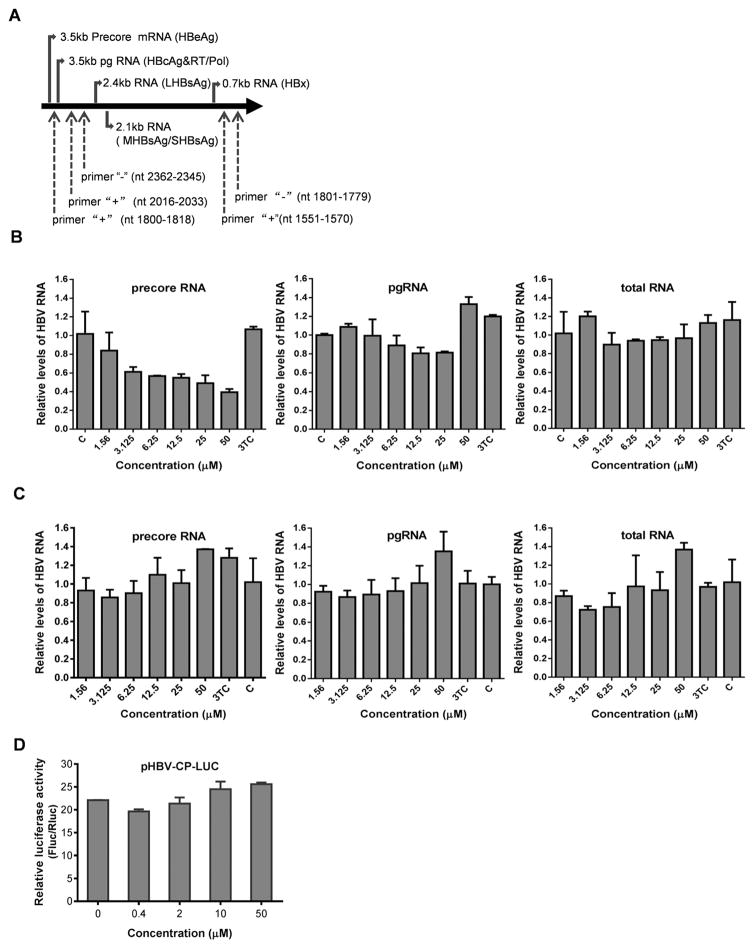
Since the authentic precore mRNA is only transcribed from cccDNA template in HepG2.117 cells, but other HBV RNAs, such as pgRNA, should be predominantly synthesized by using transgene as template, precore mRNA, pgRNA and total HBV RNA were examined to evaluate the antiviral specificity of punicalagin on HBV RNA production. Because precore mRNA is only 35 nt longer than pgRNA at the 5′ terminus, in order to distinguish precore mRNA from pgRNA in PCR assay, we used a specific forward primer (nt 1800–1818) in the 5′-end 35 nt region of precore RNA, which is absent in pgRNA, and the reverse primer (nt 2362–2345) is present in both precore mRNA and pgRNA, to amplify precore mRNA (He et al., 2011). Another pair of primers that amplifies a fragment of core ORF was used to detect both pgRNA and precore mRNA. Since all HBV mRNAs contain the same sequence of X mRNA, a pair of primers flanking the X ORF region was utilized to amplify HBV total RNA (Fig. 3A). As shown in Fig. 3B, the treatment of punicalagin significantly reduced the levels of precore mRNA in HepG2.117 cells, but had no impact on the levels of pgRNA and total mRNAs, suggesting that punicalagin treatment may reduce the accumulation of cccDNA or block cccDNA transcription. It is worth to note that, although either the reduction of cccDNA copy number or inhibition of cccDNA transcription will result in the loss of cccDNA-derived HBV pgRNA and total RNA levels, such contribution from cccDNA has been shown to be minor compared to that from transgene in HBV stable cell lines (Cai et al., 2012; Chou et al., 2005; Guo et al., 2007b), thus the levels of pgRNA and total HBV RNA remained unchanged under 3TC or punicalagin treatment (Fig. 3B).
Fig. 3. Punicalagin downregulates levels of precore mRNA in HepG2.117 cells, but has no effect on core promoter activity.
(A) Schematic illustration of HBV RNAs and the PCR primer positions. The primers for precore mRNA detection are primer “+” (nt 1800–1818) and primer “−” (nt 2362–2345). Primer “+” (nt 2016–2033) and primer “−” (nt 2362–2345) were used for pgRNA and precore mRNA amplification. A pair of primers flanking the X ORF region, primer “+” (nt 1551–1570) and primer “−” (nt 1801–1779), were used to amplify HBV total RNA. (B) After induction of HBV replication for three days in the absence of Dox, HepG2.117 cells were treated with various concentrations of punicalagin and lamividine (3TC, 1 μM) for an additional three days and harvested for total RNA extraction. Viral RNAs were detected by real-time PCR. GAPDH was analyzed as an internal reference for normalization purpose. C, untreated control. (C) HepG2.2.15 cells were treated with drugs for 3 days and harvested for total RNA extraction. Viral RNAs were detected by real-time PCR. (D) Twenty four hours after cotransfection with pHBVCP-Luc and pRL-TK, HepG2 cells were treated with different concentrations of punicalagin for 3 days and lysed for dual luciferase reporter analysis.
Next, we tested punicalagin in HepG2.2.15 cells, in which all the viral RNAs are constitutively and predominately transcribed from the integrated HBV genome. As shown in Fig. 3C, 3TC or punicalagin treatment did not down-regulate precore mRNA, pgRNA or HBV total mRNAs at all in HepG2.2.15 cells, suggesting that punicalagin specifically reduces the level of precore mRNA with cccDNA being the exclusive transcription template.
3.3. Punicalagin has no impact on the precore/core promoter activity
As shown above, precore mRNA was down-regulated by punicalagin in HepG2.117 cells but not in HepG2.2.15 cells. Considering precore mRNA can be transcribed under the control of viral pre-core/core promoter from cccDNA template in HepG2.117 cells, or from both integrated viral DNA (major) and cccDNA (minor) templates in HepG2.2.15 cells, it is possible that punicalagin may inhibit HBV basal core promoter in an episomal DNA template. To test this possibility, we examined the effect of punicalagin on precore/core promoter activity by using the reporter plasmid pHBVCP-Luc. The results showed that punicalagin did not suppress the transcriptional activity of HBV core promoter in HepG2 cells (Fig. 3D) or in Huh7 cells (data not shown) at non-cytotoxic doses.
3.4. Punicalagin inhibits the accumulation of cccDNA in HepG2.117 cells
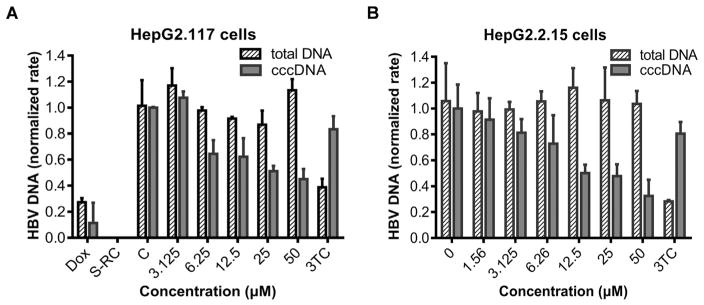
The above data demonstrated that punicalagin specifically down-regulated the production of cccDNA-dependent precore mRNA and HBeAg without affecting precore/core promoter activity, we thus speculated that the observed antiviral effect of punicalagin might be a consequence of the decreased level of HBV cccDNA upon treatment. To evaluate the effect of punicalagin on cccDNA, HepG2.117 cells were treated with or without punicalagin from the third day after the removal of doxycycline and lasted for 3 days. Then total DNA and cccDNA were extracted and subjected to real- time PCR quantification. As shown in Fig. 4A, punicalagin markedly reduced the levels of cccDNA in a dose-dependent manner, but only slightly reduced the amount of total HBV DNA at higher concentrations. DNA from HepG2.117 cells treated with doxycycline (Dox) and core DNA in the supernatant of HepG2.117 cells without doxycycline were included as negative controls for cccDNA qPCR. It has been reported that HBV DNA and HBeAg significantly accumulated from the third day after Dox induction in HepG2.117 cells (Sun and Nassal, 2006). Thus, we reasoned that the observed weak effect of 3TC on levels of cccDNA (Fig. 4A) and precore RNA (Fig. 3B) in HepG2.117 cells after 3-day Dox induction might be due to an established cccDNA pool before treatment or a fast dynamics of rcDNA to cccDNA conversion. To further confirm the inhibitory effect of punicalagin on the accumulation of HBV cccDNA, HepG2.2.15 cell line was treated with punicalagin and a similar result was observed in this cell line, though it had an obvious effect until the treatment was extended to 6 days (Fig. 4B). Taken together, these results demonstrated that punicalagin has a direct down-regulative effect on the accumulation of cccDNA.
Fig. 4. Punicalagin treatment reduces cccDNA level in both HepG2.117 and HepG2.2.15 cells.
Cells were treated with punicalagin at different concentrations for the indicated time. Cells were collected and DNAs were extracted and analyzed by specific real-time qPCR. GAPDH was used for normalization. DNA level in the untreated control was arbitrarily designated as 1. Error bars indicate standard deviation of three independent experiments. (A) After induction of HBV replication for three days, HepG2.117 cells were treated with various concentrations of punicalagin and lamividine (3TC, 1 μM) in the absence of Dox for an additional three days. Intracellular total HBV DNA and cccDNA were detected. C, untreated control; Dox, DNA from the cells treated with Dox, used as negative control for cccDNA qPCR to assess the contamination of the integrated HBV DNA; S-RC, supernatant DNA (2.0 × 105 copies/ml) from HepG2.117 cells cultured in the absence of Dox was treated by PSAD and used as negative control for cccDNA qPCR to assess the contamination of RC DNA. (B) HepG2.2.15 cells were treated with drugs for six days and the effect of punicalagin on intracellular HBV total DNA and cccDNA was determined. Data were expressed as mean ± S.D. (n = 3).
3.5. Hydrolyzable tannins directly inhibit cccDNA production in HepDES19 cells
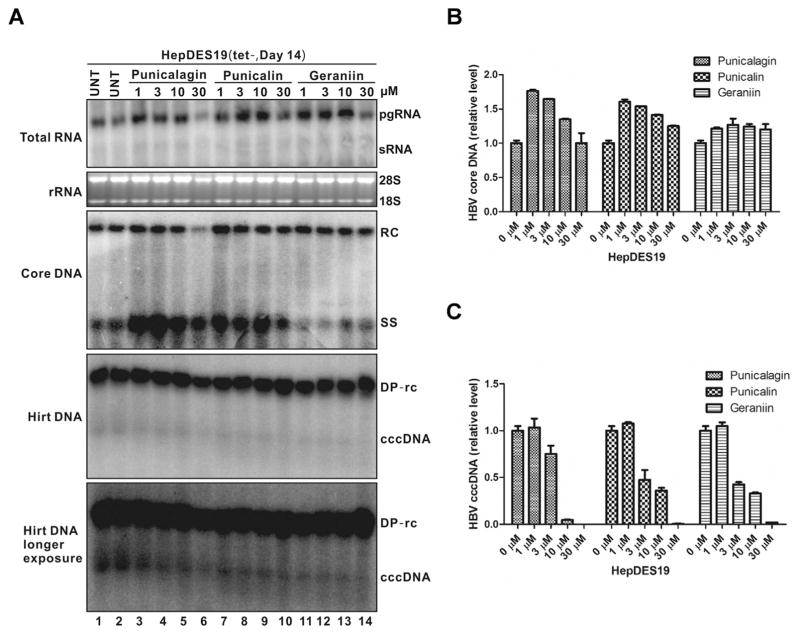
To further confirm the inhibitory effects of tannins on cccDNA accumulation, HepDES19 cells were treated with punicalagin, punicalin and geraniin immediately after withdrawal of tetracycline. HepDES19 supports the replication of an envelope-deficient HBV genome in a tetracycline-inducible manner. After induction, the cells exhibit a higher ratio of cccDNA to rcDNA than cells that contain wild-type HBV genome, likely due to the loss of negative regulations of viral envelope proteins on cccDNA amplification (Guo et al., 2007a; Lentz and Loeb, 2011). As shown in Fig. 5, except that punicalagin exhibited cytotoxicity at 30 μM after 2-week treatment, all three tannins resulted in a dose-dependent reduction of cccDNA in HepEDS19 cells without dramatic alterations of the steady state levels of viral RNA, core DNA, and deproteinized rcDNA (DP-rcDNA), which is consistent with the observations in HepG2.117 and HepG2.2.15 cells. Furthermore, quantitative PCR analyses demonstrated the similar results with DNA Southern blot. All these results clearly suggest that hydrolyzable tannins inhibit HBV cccDNA accumulation at a post DNA replication step(s).
Fig. 5. Hydrolyzable tannins directly inhibit cccDNA production in HepDES19 cells.
HepDES19 cells were untreated (UNT) or treated with punicalagin, punicalin or geraniin at the indicated concentrations upon withdrawal of tetracycline. The treatment was repeated every two days and cells were harvested at day 14 post treatment, viral RNA (top panel), core DNA (middle panel), and Hirt DNAs (DP-rcDNA and cccDNA) (bottom two panels, the blot with longer exposure was used to highlight cccDNA bands) were extracted and analyzed by Northern blotting and Southern blotting, respectively (A). HBV cytoplasmic core DNA (B) and cccDNA (C) were also quantified by qPCR. The quantitative data was normalized to untreated control and expressed as mean ± SD (n = 3).
3.6. Tannins modestly promote the decay of cccDNA
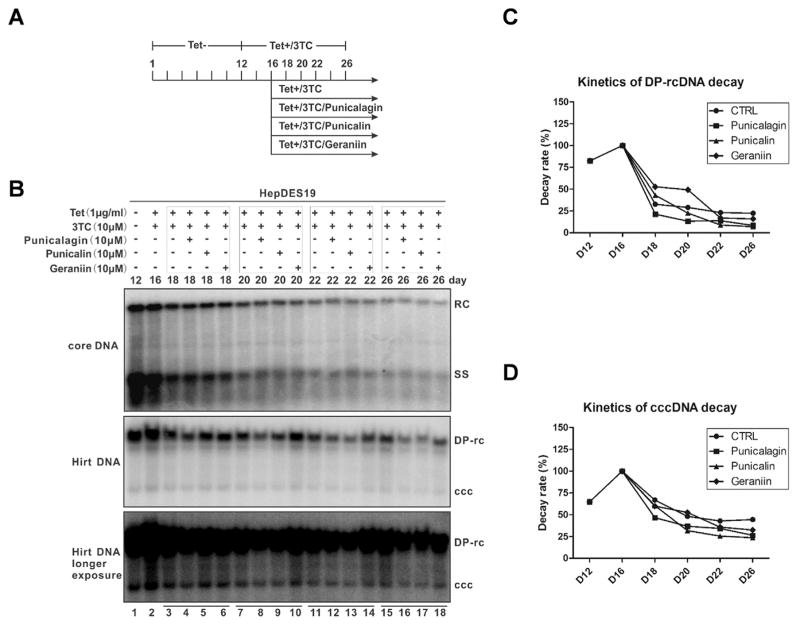
In stably transfected HBV cell cultures, the maintenance of cccDNA pool is relying on the intracellular rcDNA to cccDNA amplification pathway and the longevity of cccDNA (Guo and Guo, 2015). To elucidate the antiviral mechanism by which HBV cccDNA was affected by tannins, we made use of the inducible HepDES19 cells to assess the stability of cccDNA under compound treatment. As shown in Fig. 6A and B, HBV core DNA, DP-rcDNA and cccDNA accumulated at day 12 after the removal of tetracycline, then the de novo synthesis of cccDNA was inhibited by treating the cells with tetracycline and 3TC to shut down the transgene-based pgRNA transcription and viral DNA replication, respectively. Four days later, the decay kinetics of existing core DNA, DP-rcDNA, and cccDNA were determined with or without tannins treatment in the continuous presence of tetracycline and 3TC. The results revealed the following observations: 1) all three types of HBV DNA species degraded gradually over time, cccDNA was more stable than core DNA and DP-rcDNA (Fig. 6B–D); 2) tannins did not alter the decay kinetics of cytoplasmic core DNA (Fig. 6B, upper panel); 3) among these three tannins, punicalagin and punicalin modestly but clearly promoted the degradation of DP-rcDNA and cccDNA, but geraniin had little effect on the stability of either DNA molecules (Fig. 6B–D). In order to quantitatively measure the tannin-mediated cccDNA decay and to rule out the possible cell line specific effect, HepG2.117 cells were tested with three tannins for the cccDNA decay kinetics, a similar result was observed in this cell line (Fig. S2). However, comparing the antiviral effect of tannins on the accumulation of cccDNA to its stability (Fig. 5 vs. Fig. 6; Fig. 4 vs. Fig. S2), we speculate that the acceleration of cccDNA decay plays less important role than preventing cccDNA formation in the observed inhibition of cccDNA accumulation by tannins, although a possible stronger effect of tannins on cccDNA stability in the early cccDNA establishing phase could not be completely ruled out. Nevertheless, our data suggest that hydrolyzable tannins inhibit HBV cccDNA through a dual mode of action, by blocking cccDNA formation and promoting cccDNA degradation, though the latter effect is rather minor.
Fig. 6. The effects of tannins on the decay kinetics of HBV DP-rcDNA and cccDNA in HepDES19 cells.
(A) Schematic illustration of experimental procedures: HepDES19 cells were cultured in 6-well plate in the presence of tetracycline until the cells reached confluent state, then tetracycline was removed from the culture medium to induce HBV replication and cccDNA formation. After 12 days, tetracycline and 3TC (10 μM) were added back to the culture medium to shut off viral pgRNA transcription from the integrated HBV genome and prevent viral DNA replication. Four days later, one set of cells were cultured with medium containing tetracycline and 3TC and another three sets of cells were treated with the tannins in the presence of tetracycline and 3TC for the indicated period of time. (B) Cells were harvested at the indicated time points, HBV core DNA and Hirt DNA were analyzed by Southern blot. The relative intensities of viral DP-rcDNA and cccDNA signals in each sample were expressed as percentages of the signals from the sample at day 16 (lane 2 in panel B) and were plotted in graphs C and D, respectively.
4. Discussion
Continuous development of new agents to treat HBV infections is urgently needed because of the limitation and side effects of current available drugs, and antiviral substances that are able to eradicate cccDNA from HBV infected hepatocytes are highly warranted (Guo and Guo, 2015; Zoulim and Durantel, 2015). Traditional Chinese medicine (TCM) is used extensively for the treatment of CHB in China. Thousands of different herbs have been used in numerous TCM formulations (mixture of different herbs) for the treatment of CHB. Many TCMs and related active compounds have been reported to have promising and potent anti-HBV activities. These compounds including Phyllanthus, Salvia miltiorrhiza, Rheum palmatum L., Radix Astragali, oxymatrine, artemisinin and artesunate, and wogonin (Cui et al., 2010). A meta-analysis of randomized, controlled, clinical trials (RCTs) of TCM for treatment of CHB with either TCM alone or in combination with interferon or lamivudine revealed that: (i) TCM had a greater beneficial effect than IFN and slightly better effect than lamivudine on normalization of serum ALT; (ii) TCM had a similar beneficial effect when compared with IFN or lamivudine for CHB on antiviral activity as evidenced by the loss of serum HBeAg and HBV DNA; (iii) TCM enhanced IFN and lamivudine antiviral activities and improvements of liver function (Zhang et al., 2010). Thus, TCM can serve as a resource for identification of direct anti-HBV drug.
To identify new drug candidates against HBV cccDNA, we screened 400 compounds isolated from traditional Chinese medicinal herbs via a cell-based assay using HBeAg as cccDNA reporter, and three hydrolyzable tannins (punicalagin, punicalin and geraniin) were found to possess potent inhibition activity on HBeAg secretion and cccDNA establishment. Interestingly, hydrolyzable tannins have been reported to exhibit antiviral activities against viral adsorption to the host cell membrane (for HSV and HIV), as well as HIV reverse transcriptase (Buzzini et al., 2008; Serrano et al., 2009). Haidari et al. found that punicalagin blocked the replication of the influenza virus RNA, inhibited agglutination of chicken red blood cells by the virus and had virucidal effects. In addition, punicalagin synergized the anti-influenza activity of oseltamivir (Haidari et al., 2009). Punicalagin and geraniin have been reported to inhibit EV71 infection both in vitro and in vivo (Yang et al., 2012a,b). These observations, together with our findings, suggest that tannins possess a broad antiviral spectrum.
In our attempt to characterize the antiviral target(s) of tannins in HBV life cycle, we found that tannins down regulated the level of cccDNA in HBV stable cell lines without inhibiting viral RNA transcription and DNA replication, indicating that tannins directly target cccDNA metabolism. Further analyses demonstrated a potential dual mode of action of tannins against cccDNA homeostasis. First, tannins, especially punicalagin and punicalin, were able to modestly promote the degradation of preexisting cccDNA and DP-rcDNA, the latter has been suggested to be a precursor for cccDNA (Gao and Hu, 2007; Guo et al., 2007a, 2010). However, the degree of cccDNA reduction through degradation could not account for the total reduction of cccDNA under tannin treatment (comparing Fig. 6 to Fig. 5), not to mention that geraniin had little effect on the stability of both DNA molecules (Fig. 6). In addition, the degradation of DP-rcDNA during active virus replication, if any, was negligible (Fig. 5A). Thus, we speculated that tannins, especially geraniin, possess a major antiviral activity against cccDNA formation. Nevertheless, since the assays of tannin’s antiviral effect on cccDNA accumulation and stability were performed under different experimental conditions, such as the timing and duration of treatment, cell density and vitality, and the presence of other drugs, a possible stronger inhibitory effect of tannins on cccDNA stability during the early phase of cccDNA establishment cannot be completely ruled out.
It is not unprecedented that tannins possess more than one antiviral activity against HBV. Hydrolyzable tannins are known to be hydrolyzed into smaller polyphenols, such as gallic or ellagic acids, it is therefore conceivable that the inhibition of cccDNA formation and stability by tannin is attributed to different hydrolysis products and/or other metabolites. Gallic or ellagic acids have been shown to inhibit multiple steps in HBV life cycle, including virus entry (Huang et al., 2014), DNA replication (He et al., 2011; Zhong et al., 2015), and HBeAg secretion (Shin et al., 2005). However, the direct effect of hydrolyzable tannins on cccDNA metabolism has not been studied. In the present study, we, for the first time, identified and characterized the bivalent antiviral activities of tannins against cccDNA. Comparing these three tannins in the current study, punicalagin and punicalin are more structurally closely related and both tannins could be hydrolyzed into an identical ellagic acid-containing fragment, while the hydrolysis of geraniin will produce gallic acid instead, which may explain the observed similar antiviral activity between punicalagin and punicalin and the less inhibitory effect of geraniin on cccDNA stability. What’s more, hydrolysis of punicalagin will generate an additional C–C bond connected dimeric gallic acids. Therefore, it is of interest to test each individual tannin metabolites to distinguish their effects on cccDNA in the future study.
Despite of the key role of cccDNA in HBV infection, thus far little is known about cccDNA formation and maintenance. Comparing the structure of cccDNA to that of rcDNA, the conversion of rcDNA to cccDNA requires: (i) removal of viral polymerase covalently attached to the 5′ end of the negative DNA strand; (ii) removal of the 5′–capped short RNA primer on plus-strand DNA; (iii) the completion of viral plus-strand DNA; (iv) the removal of one copy of the short terminal redundancy from the minus-strand DNA; (v) the ligation of two viral strands (Cai et al., 2012; Guo et al., 2007a; Guo and Guo, 2015; Levrero et al., 2009). The only known viral proteins to regulate cccDNA formation from mature rcDNA are the viral envelope proteins which were shown to suppress cccDNA amplification by a negative-feedback mechanism (Lentz and Loeb, 2011; Summers et al., 1990). It is widely accepted that host DNA repair machinery is involved in cccDNA formation, this notion is supported by evidence showing that the host non-homologues end joining (NHEJ) pathway is required for conversion of duck hepatitis B virus (DHBV) double stranded linear DNA into cccDNA (Guo et al., 2012). However, host DNA repair factors that participate in authentic cccDNA formation from rcDNA have not been identified. As discussed above, cccDNA formation requires the removal of viral polymerase covalently attached to rcDNA, this deproteinization reaction generates a DP-rcDNA as functional precursor for cccDNA and may serve as an antiviral target. Recently, two structurally related disubstituted sulfonamides, CCC-0975 and CCC-0346, have been identified as cccDDNA formation inhibitors through high throughput compound screening, which interfere primarily with rcDNA conversion into cccDNA by reducing the cccDNA putative precursor DP-rcDNA. In the present study, punicalagin, punicalin and geraniin inhibited cccDNA formation not by suppressing the production of DP-rcDNA (Fig. 5A), which imply that the hydrolyzable tannins impair cccDNA biosynthesis through a novel mechanism. Regarding the stability of cccDNA, it is known that cccDNA exists in nucleus as a nucleosome-decorated minichromosome, however, critical questions, such as, whether or not nuclear DP-rcDNA is a minichromosome, how does cccDNA minichromosome maintain its stability, whether cccDNA unpacks its chromatin architecture during mitosis, and whether cccDNA survives cell division, remain unanswered. Interestingly, we found that punicalagin and punicalin exhibited activity to promote cccDNA and DP-rcDNA degradation, albeit at a low potency, indicating that the chemical approach to eliminate nuclear non-host episomal viral DNA is feasible. More importantly, eradication of cccDNA from infected hepatocytes is an undisputed holy grail for a definite cure of hepatitis B. To our best knowledge, punicalagin and punicalin are the only chemical compounds that have been reported to reduce the stability of preexisting cccDNA in cell cultures, albeit the potency is rather modest. On the other hand, further study of the mechanism of action of hydrolyzable tannins, including identification of their exact antiviral targets, will reciprocally lead to a better understanding of the mechanisms of cccDNA formation and maintenance.
Supplementary Material
Acknowledgments
We thank Dr. Dian-Xing Sun (Bethune International Peace Hospital, Shijiazhuang, China) for providing HepG2.117 cells, and Dr. Yanming Du (Baruch S. Blumberg Institute) for helpful discussion on the chemistry of hydrolyzable tannins. This work was supported by The Important Hubei Science and Technology Innovation Plan 2015ACA062 (to Xulin Chen) and NIH grants R01AI094474 and R01AI110762 (to Haitao Guo).
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.antiviral.2016.08.026.
References
- Buzzini P, Arapitsas P, Goretti M, Branda E, Turchetti B, Pinelli P, Ieri F, Romani A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev Med Chem. 2008;8:1179–1187. doi: 10.2174/138955708786140990. [DOI] [PubMed] [Google Scholar]
- Cai D, Mills C, Yu W, Yan R, Aldrich CE, Saputelli JR, Mason WS, Xu X, Guo JT, Block TM, Cuconati A, Guo H. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents Chemother. 2012;56:4277–4288. doi: 10.1128/AAC.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Nie H, Yan R, Guo JT, Block TM, Guo H. A southern blot assay for detection of hepatitis B virus covalently closed circular DNA from cell cultures. Methods Mol Biol. 2013;1030:151–161. doi: 10.1007/978-1-62703-484-5_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YC, Jeng KS, Chen ML, Liu HH, Liu TL, Chen YL, Liu YC, Hu CP, Chang C. Evaluation of transcriptional efficiency of hepatitis B virus covalently closed circular DNA by reverse transcription-PCR combined with the restriction enzyme digestion method. J Virol. 2005;79:1813–1823. doi: 10.1128/JVI.79.3.1813-1823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Wang Y, Kokudo N, Fang D, Tang W. Traditional Chinese medicine and related active compounds against hepatitis B virus infection. Biosci Trends. 2010;4:39–47. [PubMed] [Google Scholar]
- Delaney WEt, Edwards R, Colledge D, Shaw T, Furman P, Painter G, Locarnini S. Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro. Antimicrob Agents Chemother. 2002;46:3057–3060. doi: 10.1128/AAC.46.9.3057-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deres K, Schroder CH, Paessens A, Goldmann S, Hacker HJ, Weber O, Kramer T, Niewohner U, Pleiss U, Stoltefuss J, Graef E, Koletzki D, Masantschek RN, Reimann A, Jaeger R, Gross R, Beckermann B, Schlemmer KH, Haebich D, Rubsamen-Waigmann H. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science. 2003;299:893–896. doi: 10.1126/science.1077215. [DOI] [PubMed] [Google Scholar]
- Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- Ganem D, Varmus HE. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Gao W, Hu J. Formation of hepatitis B virus covalently closed circular DNA: removal of genome-linked protein. J Virol. 2007;81:6164–6174. doi: 10.1128/JVI.02721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Thimme R, Blum HE. HBV life cycle and novel drug targets. Hepatol Int. 2011;5:644–653. doi: 10.1007/s12072-011-9261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Jiang D, Zhou T, Cuconati A, Block TM, Guo JT. Characterization of the intracellular deproteinized relaxed circular DNA of hepatitis B virus: an intermediate of covalently closed circular DNA formation. J Virol. 2007a;81:12472–12484. doi: 10.1128/JVI.01123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Mao R, Block TM, Guo JT. Production and function of the cytoplasmic deproteinized relaxed circular DNA of hepadnaviruses. J Virol. 2010;84:387–396. doi: 10.1128/JVI.01921-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Xu C, Zhou T, Block TM, Guo JT. Characterization of the host factors required for hepadnavirus covalently closed circular (ccc) DNA formation. PLoS One. 2012;7:e43270. doi: 10.1371/journal.pone.0043270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Zhou T, Jiang D, Cuconati A, Xiao GH, Block TM, Guo JT. Regulation of hepatitis B virus replication by the phosphatidylinositol 3-kinase-akt signal transduction pathway. J Virol. 2007b;81:10072–10080. doi: 10.1128/JVI.00541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JT, Guo H. Metabolism and function of hepatitis B virus cccDNA: implications for the development of cccDNA-targeting antiviral therapeutics. Antivir Res. 2015;122:91–100. doi: 10.1016/j.antiviral.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidari M, Ali M, Ward Casscells S, 3rd, Madjid M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine. 2009;16:1127–1136. doi: 10.1016/j.phymed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- He W, Li LX, Liao QJ, Liu CL, Chen XL. Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication - inducible cell line. World J Gastroenterol. 2011;17:1507–1514. doi: 10.3748/wjg.v17.i11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–1075. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- Huang HC, Tao MH, Hung TM, Chen JC, Lin ZJ, Huang C. (-)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antivir Res. 2014;111:100–111. doi: 10.1016/j.antiviral.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Khanbabaee K, van Ree T. Tannins: classification and definition. Nat Product Rep. 2001;18:641–649. doi: 10.1039/b101061l. [DOI] [PubMed] [Google Scholar]
- King RW, Ladner SK, Miller TJ, Zaifert K, Perni RB, Conway SC, Otto MJ. Inhibition of human hepatitis B virus replication by AT-61, a phenyl-propenamide derivative, alone and in combination with (-)beta-L-2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1998;42:3179–3186. doi: 10.1128/aac.42.12.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz TB, Loeb DD. Roles of the envelope proteins in the amplification of covalently closed circular DNA and completion of synthesis of the plus-strand DNA in hepatitis B virus. J Virol. 2011;85:11916–11927. doi: 10.1128/JVI.05373-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009;51:581–592. doi: 10.1016/j.jhep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Litwin S, Toll E, Jilbert AR, Mason WS. The competing roles of virus replication and hepatocyte death rates in the emergence of drug-resistant mutants: theoretical considerations. J Clin Virol. 2005;34(Suppl 1):S96–S107. doi: 10.1016/s1386-6532(05)80018-6. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hussain M, Wong S, Fung SK, Yim HJ, Lok AS. A genotype-independent real-time PCR assay for quantification of hepatitis B virus DNA. J Clin Microbiol. 2007;45:553–558. doi: 10.1128/JCM.00709-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locarnini S. Molecular virology and the development of resistant mutants: implications for therapy. Semin Liver Dis. 2005;25(Suppl 1):9–19. doi: 10.1055/s-2005-915645. [DOI] [PubMed] [Google Scholar]
- Moraleda G, Saputelli J, Aldrich CE, Averett D, Condreay L, Mason WS. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J Virol. 1997;71:9392–9399. doi: 10.1128/jvi.71.12.9392-9399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J, Dandri M, Mier W, Lutgehetmann M, Volz T, von Weizsacker F, Haberkorn U, Fischer L, Pollok JM, Erbes B, Seitz S, Urban S. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26:335–341. doi: 10.1038/nbt1389. [DOI] [PubMed] [Google Scholar]
- Raney AK, Hamatake RK, Hong Z. Agents in clinical development for the treatment of chronic hepatitis B. Expert Opin Investig Drugs. 2003;12:1281–1295. doi: 10.1517/13543784.12.8.1281. [DOI] [PubMed] [Google Scholar]
- Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A. 1987;84:1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano J, Puupponen-Pimia R, Dauer A, Aura AM, Saura-Calixto F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009;53(Suppl 2):S310–S329. doi: 10.1002/mnfr.200900039. [DOI] [PubMed] [Google Scholar]
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112–125. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- Shin MS, Kang EH, Lee YI. A flavonoid from medicinal plants blocks hepatitis B virus-e antigen secretion in HBV-infected hepatocytes. Antivir Res. 2005;67:163–168. doi: 10.1016/j.antiviral.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Stray SJ, Bourne CR, Punna S, Lewis WG, Finn MG, Zlotnick A. A heteroaryldihydropyrimidine activates and can misdirect hepatitis B virus capsid assembly. Proc Natl Acad Sci U S A. 2005;102:8138–8143. doi: 10.1073/pnas.0409732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J, O’Connell A, Millman I. Genome of hepatitis B virus: restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc Natl Acad Sci U S A. 1975;72:4597–4601. doi: 10.1073/pnas.72.11.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J, Smith PM, Horwich AL. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990;64:2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Nassal M. Stable HepG2- and Huh7-based human hepatoma cell lines for efficient regulated expression of infectious hepatitis B virus. J Hepatol. 2006;45:636–645. doi: 10.1016/j.jhep.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Thermet A, Rollier C, Zoulim F, Trepo C, Cova L. Progress in DNA vaccine for prophylaxis and therapy of hepatitis B. Vaccine. 2003;21:659–662. doi: 10.1016/s0264-410x(02)00575-3. [DOI] [PubMed] [Google Scholar]
- Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney WEt, Xiong S, Brosgart CL, Chen SS, Gibbs CS, Zoulim F. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Wu TT, Coates L, Aldrich CE, Summers J, Mason WS. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology. 1990;175:255–261. doi: 10.1016/0042-6822(90)90206-7. [DOI] [PubMed] [Google Scholar]
- Yang Y, Xiu J, Zhang L, Qin C, Liu J. Antiviral activity of punicalagin toward human enterovirus 71 in vitro and in vivo. Phytomedicine. 2012a;20:67–70. doi: 10.1016/j.phymed.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhang L, Fan X, Qin C, Liu J. Antiviral effect of geraniin on human enterovirus 71 in vitro and in vivo. Bioorg Med Chem Lett. 2012b;22:2209–2211. doi: 10.1016/j.bmcl.2012.01.102. [DOI] [PubMed] [Google Scholar]
- Zeisel MB, Lucifora J, Mason WS, Sureau C, Beck J, Levrero M, Kann M, Knolle PA, Benkirane M, Durantel D, Michel ML, Autran B, Cosset FL, Strick-Marchand H, Trepo C, Kao JH, Carrat F, Lacombe K, Schinazi RF, Barre-Sinoussi F, Delfraissy JF, Zoulim F. Towards an HBV cure: state-of-the-art and unresolved questions–report of the ANRS workshop on HBV cure. Gut. 2015;64:1314–1326. doi: 10.1136/gutjnl-2014-308943. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang G, Hou W, Li P, Dulin A, Bonkovsky HL. Contemporary clinical research of traditional Chinese medicines for chronic hepatitis B in China: an analytical review. Hepatology. 2010;51:690–698. doi: 10.1002/hep.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Hu J, Shu W, Gao B, Xiong S. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015;6:e1770. doi: 10.1038/cddis.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Guo H, Guo JT, Cuconati A, Mehta A, Block TM. Hepatitis B virus e antigen production is dependent upon covalently closed circular (ccc) DNA in HepAD38 cell cultures and may serve as a cccDNA surrogate in antiviral screening assays. Antivir Res. 2006;72:116–124. doi: 10.1016/j.antiviral.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Zoulim F. Combination of nucleoside analogues in the treatment of chronic hepatitis B virus infection: lesson from experimental models. J Antimicrob Chemother. 2005;55:608–611. doi: 10.1093/jac/dki095. [DOI] [PubMed] [Google Scholar]
- Zoulim F, Durantel D. Antiviral therapies and prospects for a cure of chronic hepatitis B. Cold Spring Harb Perspect Med. 2015:5. doi: 10.1101/cshperspect.a021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009;137:1593–1608. e1591–1592. doi: 10.1053/j.gastro.2009.08.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.