Abstract
Despite drug advances for Hepatitis C virus (HCV), re-infections remain prevalent in high-risk populations. Unfortunately, the role of preexisting viral immunity and how it modulates re-infection is unclear. GBV-B infection of common marmosets is a useful model to study tissue immune responses in hepacivirus infections, and in this study we re-challenged 4 animals after clearance of primary viremia. Although only low-to-absent viremia was observed following re-challenge, GBV-B viral RNA was detectable in liver, confirming re-infection. Microscopic hepatic lesions indicated severe-to-mild lymphocyte infiltration and fibrosis in 3 out of 4 animals. Further, GBV-B-specific T cells were elevated in animals with moderate-to-severe hepatopathology, and up to 3-fold increases in myeloid dendritic and activated natural killer cells were observed after infection. Our data indicate that occult hepacivirus re-infections occur and that new liver pathology is possible even in the presence of anti-hepacivirus T cells and in the absence of high viremia.
Keywords: GBV-B, re-infections, hepatitis C virus, marmosets, liver pathology
1. Introduction
Hepatitis C infects approximately 180 million infected people worldwide causing chronic hepatitis in 80% of infected persons. While the rapidly expanding field of HCV therapeutics of approved directly acting antivirals (DAA) has a sustained virologic response rate of more than 90% in infected patients, there are still several risks and limitations to DAAs. In addition to the adverse side effects and substantial costs, there is a significant risk of frequent re-infections after successful treatment (Wandeler et al., 2015) which could lead to development of viral resistance and successive disease.
HCV re-infections occur commonly after spontaneous clearance suggesting that natural immunity could be short-lived. Studies in injection drug users have shown that previous spontaneous clearance does not reduce the incidence of new infections (Micallef et al., 2007; Osburn et al., 2010; Page et al., 2009; van de Laar et al., 2009). Peak HCV viral load was generally lower in re-infections than primary infections and clearance was also observed in 50% of participants in a shorter time duration than primary infection (Micallef et al., 2007; Osburn et al., 2010; Page et al., 2009; van de Laar et al., 2009). However, studies in humans can have limitations—most are retrospective studies and are based on heterogeneous individuals of varying age, ethnicity and injecting risk behavior. Therefore, investigations of correlates of protection in spontaneous clearance and re-infections in animal models could provide important information for vaccine development or repeat use of antivirals.
Animal models have played significant roles in identifying and understanding HCV infections in humans. Chimpanzees were the first animal model used to elucidate the natural history of HCV. In reinfected chimpanzees, rapid viral clearance was associated with proliferating CD4+ and CD8+ T cell responses after re-challenge (Bassett et al., 2001; Major et al., 2002; Nascimbeni et al., 2003; Prince et al., 2005; Zubkova et al., 2014). While chimpanzees are now rarely used in biomedical research, new world primates infected with GBV-B are commonly used as surrogate animal models for HCV (Akari et al., 2009; Beames et al., 2001; Bright et al., 2004; Jacob et al., 2004; Manickam et al., 2016). GBV-B belongs to the same family Flaviviridae and genus Hepacivirus and is closely related to HCV (Deinhardt et al., 1967; Karayiannis and McGarvey, 1995; Muerhoff et al., 1995; Tabor et al., 1980). Viremia in primary GBV-B infection clears within 2-to-3 months of infection (Bright et al., 2004; Bukh et al., 1999; Manickam et al., 2016). Several studies have shown viral immunity in primary infection, but there are only few reports of GBV-B re-infection (Bukh et al., 2008; Woollard et al., 2008). Similar to HCV re-infections, GBV-B re-infected animals also clear the virus rapidly and are associated with more robust T cell responses (Bukh et al., 2008; Woollard et al., 2008). However, the immune responses that lead to viral clearance and tissue specific immune responses in re-infection remain incompletely understood. To study the immunological processes involved in GBV-B reinfection and clearance, we re-challenged four marmosets with GBV-B and analyzed immune responses and pathology in blood and tissues within the first 4 weeks of re-infection.
2. Results
2.1. Rapid control of viremia following re-challenge
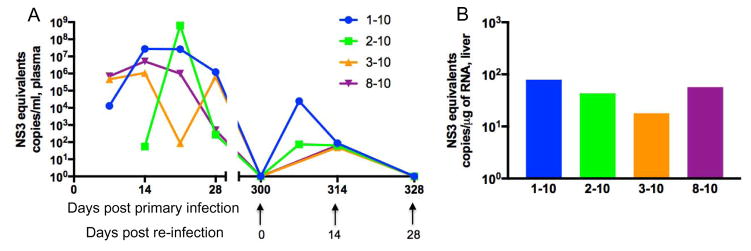
Following primary infection, all animals rapidly developed viremia with variable clearance (Fig 1) (Manickam et al., 2016). Prior to re-challenge, all animals were confirmed to be aviremic on day 300 relative to primary infection (heretofore day 0 of re-infection) (Fig 1A). Since the goal of this study was to identify viral immunity in GBV-B re-infection, we describe the immunological and pathological data only relative to re-infection. After re-infection, only transient and low level viremias were observed suggesting a more rapid control of virus replication compared to primary infection. Furthermore, only one animal, 01–10, had plasma virus loads above 100 copies/ml. Similarly, low levels of tissue virus were observed in the liver of all animals at day 28 (Fig 1B).
Figure 1. Viremia in primary infection and re-infection with GBV-B.
(A) Post-primary viral clearance animals were re-infected with GBV-B at 300 days after primary infection. Viral loads were quantified in plasma samples at serial time points following GBV-B challenge. (B) Viral loads per μg of total RNA isolated from liver at day 28-post re-infection. Standard equivalents were obtained using GBV-B NS3 plasmid as previously described (Manickam et al., 2016).
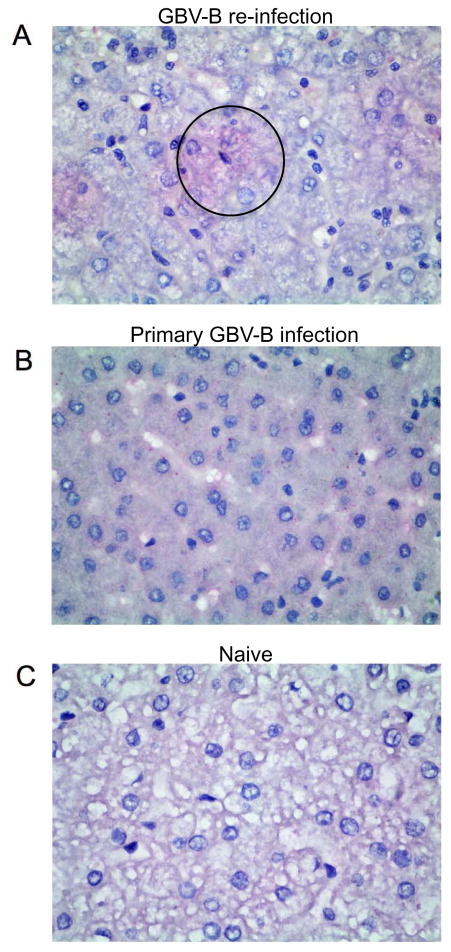
Given the low viral loads in liver detectable by standard assays, a novel in-situ hybridization approach, RNAscope, was used to detect single RNA molecules per cell in liver sections. In animal 01–10 clusters of punctate cells positive for GBV-B viral RNA were observed in the liver at day 28 following re-infection (Fig 2A). Similarly, viral RNA was detectable in 02–10 but not in 03–10 and 08–10 (data not shown). These data clearly indicate a take of infection and active replication in the liver following re-challenge concomitant with detectable viremia in plasma. Positive punctate virus RNA staining was also found in greater numbers in liver sections from marmosets acutely infected following primary GBV-B challenge as a positive control (Fig 2B), but not in livers of naïve animals as negative controls (Fig 2C).
Figure 2. In-situ hybridization of GBV-B viral RNA by RNAscope assay.
Representative photomicrographs show liver sections stained with Fast Red for GBV-B viral RNA and counterstained with Haematoxylin in (A) animal 01–10 at day 28 post re-infection (black circle indicates area containing red punctate positive signals). (B) Primary GBV-B infected animal at 14 days post infection as positive control (intracytoplasmic positive red punctate dots interspersed throughout the section) and (C) naïve animal as negative control. Magnification 600x.
2.2. Liver pathology in re-infection
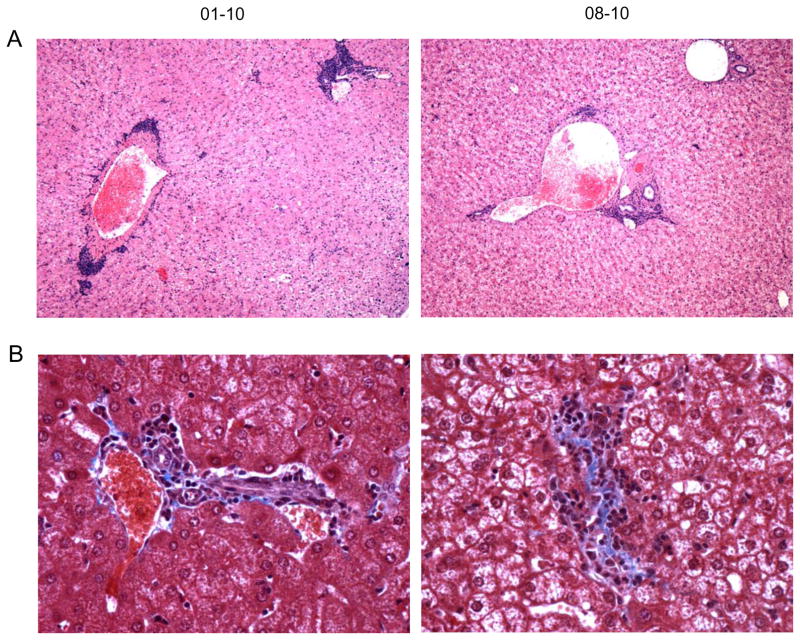
Our previous study of acute and chronic GBV-B infection suggested immune activation induces significant acute liver pathology in marmosets (Manickam et al., 2016). Strikingly, in this reinfection study, pathological manifestations were observed in the livers of re-infected animals despite only very low levels of virus replication. Hematoxylin & Eosin (H&E) stained liver sections showed multifocal moderate to severe predominantly periportal lymphocytic infiltration with random hepatocellular degeneration in all animals, with the most significant hepatitis in 01–10 and 08–10 (Table 1, Fig 3A) and mild lesions in 02–10 and 03–10 (data not shown). Additionally, moderate fibrotic changes characterized by deposition of trichome positive sinusoidal connective tissue were observed in 01–10 and at mild levels in 08–10 (Fig 3B). The animals were scored in comparison to normal animals as reported previously (Manickam et al., 2016).
Table 1.
Animal information and hepatopathological scoring of liver tissues
| Animal No. | Age (yrs) | Sex | Body weight (kg) | Hepatitis score | Fibrosis score |
|---|---|---|---|---|---|
| 01–10 | 6 | Male | 0.357 | 4 | 2 |
| 02–10 | 6 | Male | 0.334 | 2 | 0 |
| 03–10 | 6 | Male | 0.460 | 2 | 1 |
| 08–10 | 6 | Male | 0.397 | 3 | 1 |
Figure 3. Liver pathology in GBV-B re-infection.
Representative photomicrographs show (A) periportal and random lymphocytic hepatitis lesions by hematoxylin and eosin (H&E) staining (magnification, 200x) and (B) hepatic fibrosis by Trichrome staining magnification, 600x)
2.3. Elevated GBV-B-specific T cell responses in re-infected animals
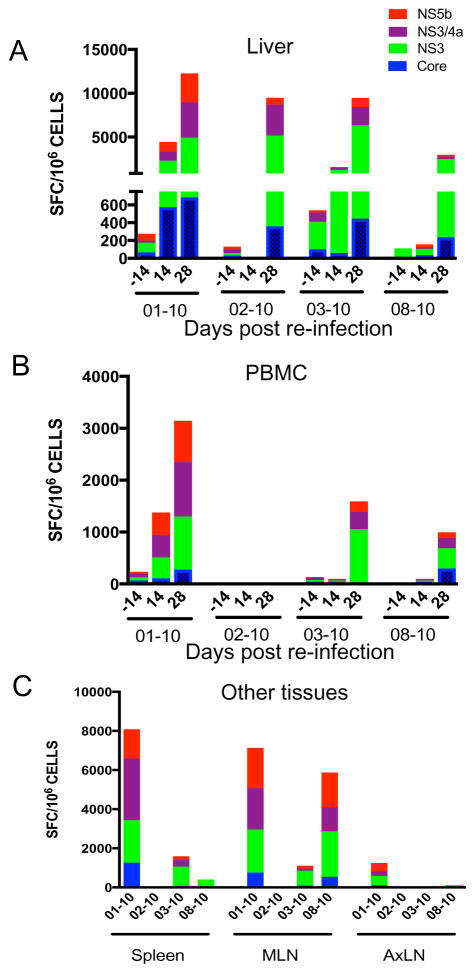
Virus-specific responses were evaluated in mononuclear cells from tissues and PBMC by ELISpot assay. Robust responses to all viral antigens (core, NS3, NS3/4a and NS5b) were observed in the liver in all four animals (Fig 4A). IFN-γ responses against GBV-B core peptides were significantly elevated at day 28 compared to day 0 (p = 0.037), but NS3 peptides were the most dominant antigens eliciting significantly elevated responses at day 28 in comparison to day 0 and day 14 (p = 0.032). However, PBMC responses were varied with increasing responses against all peptides in animal 01–10 at day 14 and day 28 while low IFN-γ responses in general were observed in the other three animals (Fig 4B). The overall percentage of IFN-γ secreting cells was lower in PBMC than in liver suggesting higher virus specific responses at the site of virus replication. In other tissues such as spleen, mesenteric lymph nodes (MLN) and axillary lymph nodes (AxLN), elevated responses were observed only in animal 01–10 which had the highest systemic and liver viral loads (Fig 4C).
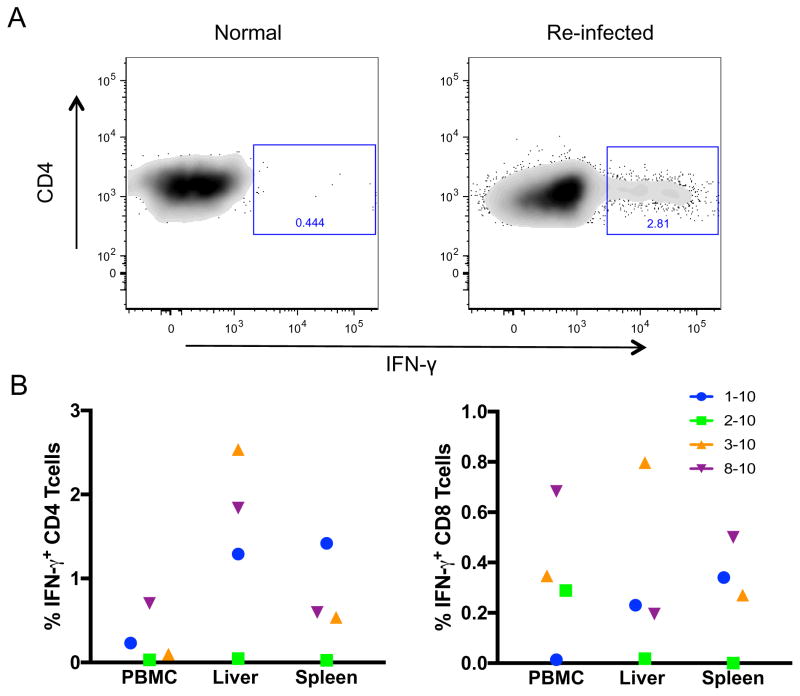
Figure 4. Elevated virus-specific T cell responses during GBV-B re-infection.
Mononuclear cells from (A) liver, (B) PBMC and (C) spleen, mesenteric lymph nodes (MLN) and axillary lymph nodes (AxLN) were stimulated with GBV-B antigens for 48 hours and analyzed for IFN-γ secreting cells by ELISpot assay.
IFN-γ secreting CD8+ T cells are known to be major effector cells that can suppress HCV replication (Frese et al., 2002; Major et al., 2004; Sung et al., 2014; Thimme et al., 2002). In order to fractionate the IFN-γ responses observed in ELISpot, we stimulated mononuclear cells with NS3 and NS3/4a pooled peptides for 6 hours and analyzed for IFN-γ secreting CD4+ and CD8+ T cells by intracellular cytokine staining (ICS). Interestingly, antigen-stimulated CD4+ T cells showed higher IFN-γ secretion than CD8+ T cells in liver (p = 0.06), spleen and PBMC (Fig 5). The highest percentage of IFN-γ+ CD4+ T cells was observed in animal 01–10 compared to near undetectable levels in 02–10. Both the ELISpot and the ICS results indicate that animal 01–10 with detectable viremia, showed the highest virus specific IFN-γ responses in re-infection among the four animals.
Figure 5. GBV-B NS3/4a-specific T cell responses in GBV-B re-infection.
Mononuclear cells were stimulated with pooled peptides (NS3 and NS3/4a) for 6 hours and stained for IFN-γ-secreting T cells and analyzed by flow cytometry. (A) Representative flow plots showing IFN-γ secreting CD4 T cells in liver. (B) Each data point represents cell percentages of IFN-γ positive CD4 and CD8 T cells in PBMC, liver and spleen at day 28 of re-infection.
2.4. Innate immune cell mobilization in circulation
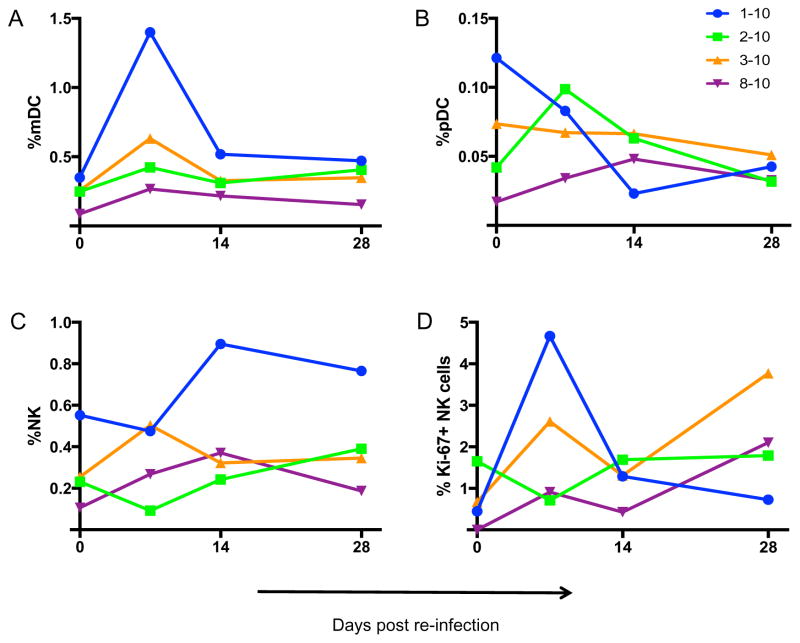
Innate immune cells can also play major roles in modulating Hepacivirus infections (Barth et al., 2011; Gonzalez et al., 2010; Kanto and Hayashi, 2007; Manickam et al., 2016; Werner et al., 2013), and innate activation is a hallmark of disease. In order to understand the role of innate immunity in hepacivirus re-infection, we phenotyped NK cells and DCs in the blood of re-challenged animals. Interestingly, up to 2-fold increase was observed in circulating myeloid dendritic cells (mDC) in 01–10 (Fig 6A) while plasmacytoid dendritic cells (pDC) were increased 2-fold in 02–10 at day 7 (Fig 6B). The percentage of mDCs were further correlated with viremia at p = 0.0004 and r = 0.89. Bulk NK cells were elevated over all time points in 01–10 but Ki-67+ NK cell populations were only transiently upregulated at day 7 post re-infection (Figure 6C&D). These data suggest that even in the absence of robust virus replication, occult re-infection is not silent and can activate systemic innate immunity.
Figure 6. Innate immune cell activation in GBV-B re-infection.
Longitudinal evaluations of innate immune cells were performed in whole blood by flow cytometry. Each data point represents cell percentages of individual animal samples stained for (A) myeloid dendritic cells (mDC), (B) plasmacytoid dendritic cells (pDC), (C) natural killer (NK) cells and (D) Ki-67 expressing NK cells.
3. Discussion
Multiple studies have shown that HCV re-infections are possible even after successful curative therapy (Corson et al., 2011; Lambers et al., 2011; Sprinzl et al., 2006). Increased recurrence rates have specifically been observed in high-risk patients including injection drug users and those individuals immunosuppressed and co-infected with HIV (Grady et al., 2013; Simmons et al., 2016). While most reports indicate a more rapid viral clearance following re-infection, the immunologic mechanisms associated are unclear, and importantly, pathologic consequences of re-infection have largely been unexplored.
In this study of GBV-B re-infection nearly one year following primary challenge, low-to-absent viremia was observed in all 4 animals when compared to primary viral loads. GBV-B infection was further confirmed by in-situ hybridization visualizing the presence of GBV-B viral RNA in liver sections. While the assay was able to detect only 2 of the 4 re-infected animals, quantitative PCR results showed viral loads in liver of all 4 animals albeit at low levels. In addition, the liver of all 4 animals exhibited virus-induced hepatitis. Our previous reports showed primary GBV-B infection could also induce lymphocytic infiltration along with fibrotic and steatotic changes even after clearance of viremia (Manickam et al., 2016; Manickam et al., 2017). In this study we observed mild (in 02–10) to severe (01–10) microscopic hepatitis and fibrotic lesions in all four re-infected animals. This indicates that hepatic damage could occur in hepacivirus re-infection even with rapid viral clearance.
Rapid control of viremia reported in GBV-B-infected animals and HCV re-infections in human and chimpanzees has been associated with virus-specific T cells (Bassett et al., 2001; Bukh et al., 2008; Major et al., 2002; Micallef et al., 2007; Nascimbeni et al., 2003; Osburn et al., 2010; Page et al., 2009; Prince et al., 2005; van de Laar et al., 2009; Woollard et al., 2008; Zubkova et al., 2014). ELISpot data from the 4 GBV-B re-infected animals indicated gradually augmented IFN-γ responses over the duration of re-infection, especially in liver. Interestingly, responses in PBMC were lower or absent in animals 02–10, 03–10 and 08–10, while animal 01–10 had the strongest responses in all tissues. A similar trend was observed in ICS assay against NS3 proteins, whereby a strong CD4+ T cell response as evidenced by elevated secretion was observed in liver at day 28 in all animals except 02–10. CD8+ T cell responses were of lesser magnitude, which could suggest that CD4+ T cells have a primary role in limiting replication following re-infection. In HCV infected patients and experimentally infected chimpanzees, weak or absent HCV specific CD4 T cell responses were reported in chronic infection, whereas strong CD4+ T cell responses have been associated with viral clearance (Gerlach et al., 1999; Semmo and Klenerman, 2007; Thimme et al., 2002). This indicates that early and vigorous CD4+ T cell responses could be involved in resolving both primary and secondary HCV infections.
In addition to T cell responses, innate immune cells such as NK cells contribute significantly to anti-hepacivirus immunity; however, they are also associated with increased pathology (Barth et al., 2011; Kanto, 2008; Manickam et al., 2016; Yamanaka et al., 2002; Zhou et al., 2014). In animal 01–10, more than 2-fold increases in levels of activated NK cells (Ki-67+ NK cells) were observed at day 7. Further, elevated mDCs at day 7 were also associated with plasma viral loads. Modest increases in NK cell and mDC populations were observed in 03–10 and 08–10, but not in 02–10. The elevated innate immune cells indicate early immune activation in re-infected animals that could contribute to overall inflammation.
Of all 4 animals, 01–10 showed the most robust T cell response in all tissues and PBMC, elevated mDCs and NK cells in circulation, low level of viremia at day 7 and, not surprisingly had the most significant liver pathology. Notably, animal 02–10 showed lower levels of immune responses in general and mild lymphocytic infiltration was observed in liver. This may indicate that pathology following re-infection is directly associated with the local immune response. Collectively, this model demonstrates that re-infection is possible following re-exposure to related hepaciviruses, and that even with undetectable or low viremia, new liver damage is likely and may even be potentiated by the local anti-virus responses. The pathogenesis of immune-mediated liver injury even in silent/resolving infections could have severe implications for multiple diseases including HBV and HCV infections.
4. Materials & Methods
4.1. Experimental animals
Four common marmosets (Callithrix jacchus) (Table 1) were housed in BSL2 biocontainment facilities at Biomere Inc., Worcester, MA, in accordance with the guidelines of the local institutional animal care and use committee, and the Department of Health and Human Services (DHHS) Guide for the Care and Use of Laboratory Animals. Animals were fed a commercial new world nonhuman primate diet along with fruits, vegetables, eggs and nuts, and ad libitum water availability.
4.2. Virus Inoculum
All animals were intravenously inoculated with 600 μl of a 1:120 dilution of an uncloned GBV-B virus stock derived at the Southwest National Primate Research Center (a kind gift from Dr. Robert Lanford (SNPRC stock) as a primary challenge. Nine months after primary infection, all four animals were re-challenged intravenously with 600ul of 1:10 dilution of a highly related uncloned GBV-B stock 9613 derived at the NEPRC (NEPRC stock) (Manickam et al., 2016).
4.3. Experimental samples
Blood and liver biopsies were collected 2 weeks before re-infection to set baseline levels and confirm the absence of viremia. Post re-infection whole blood was collected at weekly intervals and liver biopsy at 2 weeks until sacrifice at 4 weeks of re-infection. Plasma was separated from blood and was used for virus detection. At 4 weeks, the animals were euthanized and evident post mortem pathology was recorded by the attending veterinarian. In addition to blood and liver, spleen, peripheral lymph nodes and mesenteric lymph nodes were collected and further processed.
4.4. Virus Quantification
Virus quantification was performed by quantitative RT-PCR (Manickam et al., 2016). Briefly, viral RNA was extracted from cell-free plasma samples using QIAmp viral RNA mini kit (Qiagen). Liver tissues in RNAlater were processed to yield total RNA using RNeasy mini kit (Qiagen). The RNA from plasma and tissues were converted to cDNA with a high capacity reverse transcription kit (Life Technologies).
4.5. RNAscope in-situ hybridization assay
Fomalin-fixed, paraffin embedded liver sections were used for the RNAScope 2.5 assay based on the protocol developed by Advanced Cell Diagnostics (ACD, Inc). On the first day, tissue sections on slides were pretreated by baking at 60°C for 2 hours followed by deparaffinization in xylene. Antigen retrieval was done by boiling slides in RNAScope antigen retrieval reagent for 30 minutes. On the second day, slides were treated with protease for 30 min at 40°C in a humid chamber. The slides were washed in 1X RNAScope wash buffer followed by 2 hour incubation with GBV-B probes that were syntheszised by ACD Inc. Additional amplification steps were completed according to the manufacturer’s suggested protocol. Sections were finally stained with Fast Red dye and counterstained with Haematoxylin. The stained slides were dried, mounted with coverslip and observed under light microscope. Positive (Cj-PPIB) and negative (DapB) control probes provided by ACD, Inc. were used as experimental controls.
4.6. Histopathology
Liver samples were collected in formalin on the day of sacrifice. The fixed tissue samples were later paraffin embedded, sectioned and stained by Mass Histology Service, Inc. (Worcester, MA) using standard H&E and trichrome staining protocols. Liver pathology was scored as previously described (Manickam et al., 2016).
4.7. Flow cytometric staining of whole blood
Polychromatic flow cytometry to phenotype NK cells, dendritic cells and T cells were performed on whole blood using antibody combinations and protocols described earlier (Manickam et al., 2016). Briefly, the samples were incubated with antibodies for 30 minutes at RT, following which; RBCs were lysed with FACS lysing buffer (BD Biosciences) and fixed in 1% formaldehyde. Intracellular staining was performed with Fix and Perm reagents (Caltag laboratories) based on the manufacturers recommended protocol. Stained sample acquisitions were done on an LSR II (BD Biosciences), and the data was analyzed with FlowJo software (version 9.9.4, Tree Star).
4.8. Isolation of mononuclear cells from blood and tissues
Whole blood was centrifuged at 2000 rpm for 5 minutes to separate the plasma and buffy coat. The buffy coat was overlaid on Ficoll and subjected to density gradient centrifugation. The interface containing the PBMC was harvested, washed and lysed for RBCs. The cells were then counted and used for assays.
Mononuclear cells were isolated from liver, spleen and lymph nodes. Homogenized spleen and lymph nodes were washed with RPMI containing 5% FBS (R5) and then lysed for RBCs using hypotonic ammonium chloride solution. Homogenized liver sample was overlaid on 60% percoll and 30% percoll for density gradient centrifugation and the resulting interface was harvested to yield mononuclear cells.
4.9. ELISpot assay
IFN-γ responses in liver, spleen and lymph nodes against GBV-B viral peptides were assayed using anti-human IFN-γ ELISpot kit (MAb Tech). Cells were added at a concentration of 2x105 cells/well to GBV-B antigens — core (amino acids [aa] 1 to 156; 15 peptides), NS3 (aa 941 to 1250; 30 peptides), NS3/4a (aa 1241 to 1615; 37 peptides), and NS5b (aa 2275 to 2864; 58 peptides) at a concentration of 1 μg/ml in a 96 well plate. PHA at 2 μg/ml was used as positive control and no stimulation at baseline. After 40 hours of incubation at 37°C, the plates were washed and developed according to manufacturer’s instructions. Developed spots were counted by Zellnet Consulting Inc.
4.10. Intracellular cytokine staining
Mononuclear cells from liver and spleen were stimulated with 1 ug of total NS3 antigens (pool of NS3 (aa 941 to 1250; 30 peptides), NS3/4a (aa 1241 to 1615; 37 peptides) at 37°C for 6 hours. After stimulation, the cells were stained with antibodies against CD3 (clone SP34-2, BD Biosciences), CD4 (clone L-200, BD Biosciences) and CD8 (clone LT8, AbCam) followed by intracellular staining for IFN-γ (clone B27, BD Biosciences) similar to the flow cytometry staining protocol described above. Unstimulated cells and cells stimulated with PMA (0.05 ug/reaction) and Ionomycin (1 ug/reaction) served as negative and positive controls, respectively.
4.11. Statistical analysis
Statistical differences between groups and between time points was analyzed using one way-ANOVA with Tukey’s multiple comparison test and Student’s t test. Correlation between variables was evaluated by Pearson correlation test. Differences between groups or time points were considered significant when the p value was < 0.05.
Highlights.
GBV-B infection of marmosets models HCV primary and re-infections
GBV-B infection induces significant liver pathology
Hepacivirus re-infection induces liver pathology in the absence of high viremia
Hepacivirus pathology is associated with innate and adaptive immune responses
Acknowledgments
This work was supported by NIH grants R21 AI101170 and R56 AI120828 to RKR. The authors also thank the Flow Cytometry core of the CVVR (supported by Harvard Center for AIDS Research P30 AI060354) for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akari H, Iwasaki Y, Yoshida T, Iijima S. Non-human primate surrogate model of hepatitis C virus infection. Microbiology and immunology. 2009;53:53–57. doi: 10.1111/j.1348-0421.2008.00087.x. [DOI] [PubMed] [Google Scholar]
- Barth H, Rybczynska J, Patient R, Choi Y, Sapp RK, Baumert TF, Krawczynski K, Liang TJ. Both innate and adaptive immunity mediate protective immunity against hepatitis C virus infection in chimpanzees. Hepatology. 2011;54:1135–1148. doi: 10.1002/hep.24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett SE, Guerra B, Brasky K, Miskovsky E, Houghton M, Klimpel GR, Lanford RE. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;33:1479–1487. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- Beames B, Chavez D, Lanford RE. GB virus B as a model for hepatitis C virus. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2001;42:152–160. doi: 10.1093/ilar.42.2.152. [DOI] [PubMed] [Google Scholar]
- Bright H, Carroll AR, Watts PA, Fenton RJ. Development of a GB virus B marmoset model and its validation with a novel series of hepatitis C virus NS3 protease inhibitors. J Virol. 2004;78:2062–2071. doi: 10.1128/JVI.78.4.2062-2071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J, Apgar CL, Yanagi M. Toward a surrogate model for hepatitis C virus: An infectious molecular clone of the GB virus-B hepatitis agent. Virology. 1999;262:470–478. doi: 10.1006/viro.1999.9941. [DOI] [PubMed] [Google Scholar]
- Bukh J, Engle RE, Govindarajan S, Purcell RH. Immunity against the GBV-B hepatitis virus in tamarins can prevent productive infection following rechallenge and is long-lived. J Med Virol. 2008;80:87–94. doi: 10.1002/jmv.21013. [DOI] [PubMed] [Google Scholar]
- Corson S, Greenhalgh D, Palmateer N, Weir A, Hutchinson S. Risk of Hepatitis C virus reinfection following spontaneous viral clearance in injecting drug users: a systematic review. The International journal on drug policy. 2011;22:102–108. doi: 10.1016/j.drugpo.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Deinhardt F, Holmes AW, Capps RB, Popper H. Studies on the transmission of human viral hepatitis to marmoset monkeys. I. Transmission of disease, serial passages, and description of liver lesions. The Journal of experimental medicine. 1967;125:673–688. doi: 10.1084/jem.125.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese M, Schwarzle V, Barth K, Krieger N, Lohmann V, Mihm S, Haller O, Bartenschlager R. Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology. 2002;35:694–703. doi: 10.1053/jhep.2002.31770. [DOI] [PubMed] [Google Scholar]
- Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, Hoffmann R, Schirren CA, Santantonio T, Pape GR. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez VD, Landay AL, Sandberg JK. Innate immunity and chronic immune activation in HCV/HIV-1 co-infection. Clinical immunology. 2010;135:12–25. doi: 10.1016/j.clim.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Grady BP, Schinkel J, Thomas XV, Dalgard O. Hepatitis C virus reinfection following treatment among people who use drugs. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(Suppl 2):S105–110. doi: 10.1093/cid/cit301. [DOI] [PubMed] [Google Scholar]
- Jacob JR, Lin KC, Tennant BC, Mansfield KG. GB virus B infection of the common marmoset (Callithrix jacchus) and associated liver pathology. J Gen Virol. 2004;85:2525–2533. doi: 10.1099/vir.0.80036-0. [DOI] [PubMed] [Google Scholar]
- Kanto T. Virus associated innate immunity in liver. Frontiers in bioscience : a journal and virtual library. 2008;13:6183–6192. doi: 10.2741/3146. [DOI] [PubMed] [Google Scholar]
- Kanto T, Hayashi N. Innate immunity in hepatitis C virus infection: Interplay among dendritic cells, natural killer cells and natural killer T cells. Hepatology research : the official journal of the Japan Society of Hepatology. 2007;37(Suppl 3):S319–326. doi: 10.1111/j.1872-034X.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- Karayiannis P, McGarvey MJ. The GB hepatitis viruses. Journal of viral hepatitis. 1995;2:221–226. doi: 10.1111/j.1365-2893.1995.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Lambers FA, Prins M, Thomas X, Molenkamp R, Kwa D, Brinkman K, van der Meer JT, Schinkel J group Ms. Alarming incidence of hepatitis C virus re-infection after treatment of sexually acquired acute hepatitis C virus infection in HIV-infected MSM. Aids. 2011;25:F21–27. doi: 10.1097/QAD.0b013e32834bac44. [DOI] [PubMed] [Google Scholar]
- MacParland SA, Chen AY, Corkum CP, Pham TN, Michalak TI. Patient-derived hepatitis C virus inhibits CD4(+) but not CD8(+) T lymphocyte proliferation in primary T cells. Virology journal. 2015;12:93. doi: 10.1186/s12985-015-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major ME, Dahari H, Mihalik K, Puig M, Rice CM, Neumann AU, Feinstone SM. Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology. 2004;39:1709–1720. doi: 10.1002/hep.20239. [DOI] [PubMed] [Google Scholar]
- Major ME, Mihalik K, Puig M, Rehermann B, Nascimbeni M, Rice CM, Feinstone SM. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. Journal of virology. 2002;76:6586–6595. doi: 10.1128/JVI.76.13.6586-6595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manickam C, Rajakumar P, Wachtman L, Kramer JA, Martinot AJ, Varner V, Giavedoni LD, Reeves RK. Acute Liver Damage Associated with Innate Immune Activation in a Small Nonhuman Primate Model of Hepacivirus Infection. J Virol. 2016;90:9153–9162. doi: 10.1128/JVI.01051-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manickam C, Wachtman L, Martinot AJ, Giavedoni LD, Reeves RK. Metabolic Dysregulation in Hepacivirus Infection of Common Marmosets (Callithrix jacchus) PloS one. 2017;12:e0170240. doi: 10.1371/journal.pone.0170240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef JM, Macdonald V, Jauncey M, Amin J, Rawlinson W, van Beek I, Kaldor JM, White PA, Dore GJ. High incidence of hepatitis C virus reinfection within a cohort of injecting drug users. Journal of viral hepatitis. 2007;14:413–418. doi: 10.1111/j.1365-2893.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- Muerhoff AS, Leary TP, Simons JN, Pilot-Matias TJ, Dawson GJ, Erker JC, Chalmers ML, Schlauder GG, Desai SM, Mushahwar IK. Genomic organization of GB viruses A and B: two new members of the Flaviviridae associated with GB agent hepatitis. Journal of virology. 1995;69:5621–5630. doi: 10.1128/jvi.69.9.5621-5630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimbeni M, Mizukoshi E, Bosmann M, Major ME, Mihalik K, Rice CM, Feinstone SM, Rehermann B. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. Journal of virology. 2003;77:4781–4793. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Tobler L, Andrews W, Avanesyan L, Cooper S, Busch MP. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. The Journal of infectious diseases. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince AM, Brotman B, Lee DH, Pfahler W, Tricoche N, Andrus L, Shata MT. Protection against chronic hepatitis C virus infection after rechallenge with homologous, but not heterologous, genotypes in a chimpanzee model. The Journal of infectious diseases. 2005;192:1701–1709. doi: 10.1086/496889. [DOI] [PubMed] [Google Scholar]
- Semmo N, Klenerman P. CD4+ T cell responses in hepatitis C virus infection. World journal of gastroenterology : WJG. 2007;13:4831–4838. doi: 10.3748/wjg.v13.i36.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62:683–694. doi: 10.1093/cid/civ948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl MF, Otto G, Galle PR, Schuchmann M. Hepatitis C virus re-infection: new perspectives. Clinical transplantation. 2006;20(Suppl 17):117–123. doi: 10.1111/j.1399-0012.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- Sung PS, Racanelli V, Shin EC. CD8(+) T-Cell Responses in Acute Hepatitis C Virus Infection. Frontiers in immunology. 2014;5:266. doi: 10.3389/fimmu.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor E, Peterson DA, April M, Seeff LB, Gerety RJ. Transmission of human non-A, non-B hepatitis to chimpanzees following failure to transmit GB agent hepatitis. Journal of medical virology. 1980;5:103–108. doi: 10.1002/jmv.1890050202. [DOI] [PubMed] [Google Scholar]
- Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Laar TJ, Molenkamp R, van den Berg C, Schinkel J, Beld MG, Prins M, Coutinho RA, Bruisten SM. Frequent HCV reinfection and superinfection in a cohort of injecting drug users in Amsterdam. Journal of hepatology. 2009;51:667–674. doi: 10.1016/j.jhep.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Wandeler G, Dufour JF, Bruggmann P, Rauch A. Hepatitis C: a changing epidemic. Swiss medical weekly. 2015;145:w14093. doi: 10.4414/smw.2015.14093. [DOI] [PubMed] [Google Scholar]
- Werner JM, Heller T, Gordon AM, Sheets A, Sherker AH, Kessler E, Bean KS, Stevens M, Schmitt J, Rehermann B. Innate immune responses in hepatitis C virus-exposed healthcare workers who do not develop acute infection. Hepatology. 2013;58:1621–1631. doi: 10.1002/hep.26353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollard DJ, Haqshenas G, Dong X, Pratt BF, Kent SJ, Gowans EJ. Virus-specific T-cell immunity correlates with control of GB virus B infection in marmosets. J Virol. 2008;82:3054–3060. doi: 10.1128/JVI.01153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Uchida M, Doi T. Innate form of HCV core protein plays an important role in the localization and the function of HCV core protein. Biochemical and biophysical research communications. 2002;294:521–527. doi: 10.1016/S0006-291X(02)00507-7. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sun P, Lucendo-Villarin B, Angus AG, Szkolnicka D, Cameron K, Farnworth SL, Patel AH, Hay DC. Modulating innate immunity improves hepatitis C virus infection and replication in stem cell-derived hepatocytes. Stem cell reports. 2014;3:204–214. doi: 10.1016/j.stemcr.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubkova I, Duan H, Wells F, Mostowski H, Chang E, Pirollo K, Krawczynski K, Lanford R, Major M. Hepatitis C virus clearance correlates with HLA-DR expression on proliferating CD8+ T cells in immune-primed chimpanzees. Hepatology. 2014;59:803–813. doi: 10.1002/hep.26747. [DOI] [PMC free article] [PubMed] [Google Scholar]