Abstract
A subset of autoimmune diseases result from autoantibodies targeting epitopes on matrix collagen. The most extensively studied are anti-glomerular basement membrane glomerulonephritis (or its systemic counterpart Goodpasture’s disease) that destroys kidneys and lungs, and rheumatoid arthritis that leads to disabling arthritis. Autoantibodies in these disorders bind evolutionarily conserved conformational epitopes on the noncollagenous domain 1 (NC1) of the alpha3 chain of type IV [alpha3(IV)NC1] collagen in glomerular and alveolar basement membranes, and on native or citrullinated type II collagen (CII) in joint cartilage, respectively. The genetic origins of pathogenic anti-collagen B cells in these diseases is unknown, but observations from murine models raise the possibility that they overlap despite distinct in vivo immunopathologies. Monoclonal autoantibodies isolated from mice immunized with alpha3(IV)NC1 collagen or CII show a biased use of Ig light chains (LC) encoded by genes of the IGKV3 subgroup (previously Vk21 family), paired with diverse Ig heavy chains. To further explore this relationship and determine if a single murine IGKV3 LC independently predisposes to both anti-collagen responses, we generated a novel transgenic (Tg) C57BL/6 mouse that expresses a productively rearranged IGKV3-encoded LC, termed mLCV3-Tg, in conjunction with endogenously rearranged Ig heavy chains. Tg mice are also genetically deficient in endogenous kappa chains to permit tracking of the mLCV3 transgene. We show that mLCV3-Tg mice are susceptible to humoral autoimmunity against both collagen chains. Anti-alpha3(IV)NC1 collagen, but not anti-CII, mLCV3-encoded Ig are detected in serum of unmanipulated Tg mice, while Toll-like receptor ligands induce secretion of mLCV3-Tg autoantibodies of both collagen specificities from splenocytes ex vivo. This indicates developmental survival of mLCV3-Tg B cells reactive with each antigen, and is consistent with production of the two anti-collagen autoIg from distinct B cell populations. Reduced B cell numbers, low serum Ig kappa levels, low cell surface Ig kappa density, and abundant endogenous lambda chain expression suggest that subsets of IGKV3-encoded B cells are regulated in vivo by mechanisms that include deletion, anergy, and LC editing. These results support the notion that murine IGKV3 LCs contribute structural fitness to antigen binding sites that support diverse anti-collagen autoimmune responses, that these responses are regulated in vivo, and that these cells can nonetheless readily escape immune regulation.
Keywords: collagen, autoantibody, kappa light chain, IGKV3
1. Introduction
Autoimmune diseases affect an estimated 50 million Americans, causing extensive morbidity due to spontaneous immune attack on vital organs and tissues. Autoantibodies are prominent in many autoimmune diseases, mediating tissue destruction and serving as biomarkers to facilitate diagnosis, monitor disease, and inform treatment decisions. Therefore, understanding how autoantibodies are generated is key to altering disease pathogenesis and outcomes.
In an important subset of autoimmune diseases, pathogenic autoantibodies target epitopes on matrix collagen (reviewed in (Foster, 2017). Anti-glomerular basement membrane glomerulonephritis (anti-GBM GN), and its systemic counterpart, Goodpasture’s disease, rheumatoid arthritis (RA), bronchiolitis obliterans in lung allografts, and epidermolysis bullosa acquisita are mediated in part by anti-collagen Ig that bind epitopes on collagen II, IV, V, and VII respectively (Burkhardt et al., 2002; Burlingham et al., 2007; Lindh et al., 2014; Saus et al., 1988; Woodley et al., 1984). Autoreactivity to collagen V was also recently implicated in atherosclerosis (Dart et al., 2010).
Anti-GBM GN and RA are the most extensively studied diseases linked to anti-collagen reactivity. In anti-GBM GN, pathogenic anti-GBM IgG recognize conformational epitopes on the noncollagenous domain 1 (NC1) of the alpha3 chain of type IV collagen [hereafter alpha3(IV)NC1] that are expressed in capillary and alveolar basement membranes in kidneys and lungs, respectively (Borza and Hudson, 2003; Lerner et al., 1967; Saus et al., 1988). Autoantibody deposition can precipitate acute inflammation leading to kidney failure and life-threatening lung hemorrhage. In RA, a chronic autoinflammatory disease characterized by peripheral joint destruction, patients develop autoantibody responses to triple-helical fibrillar type II collagen (CII), a major component of hyaline and articular cartilage, as well as to citrullinated proteins, including citrullinated CII, and immunoglobulin (rheumatoid factor) (Burkhardt et al., 2002; Lindh et al., 2014). IgG and B cells reactive with native CII are frequently recovered from inflamed joints but not blood of RA patients, an enrichment that suggests that anti-CII IgG production predominantly occurs locally, rather than in secondary lymphoid organs (Lindh et al., 2014; Ronnelid et al., 1994; Tarkowski et al., 1989). Direct pathogenicity of both sets of anti-collagen autoantibodies is suggested by transfer experiments; patient-derived anti-alpha3(IV)NC1 IgG induce nephritis in squirrel monkeys (Lerner et al., 1967), and mouse anti-CII IgG that crossreact with CII epitopes recognized by RA patients’ IgG transfer an RA-like severe erosive polyarthritis to naïve mice (Holmdahl et al., 1990; Nandakumar and Holmdahl, 2005; Nandakumar et al., 2003; Stuart and Dixon, 1983; Terato et al., 1992). Anti-CII IgG and B cells also appear to participate in a joint-destructive amplification cycle that promotes formation and pathogenicity of their anti-citrullinated-CII counterparts (Uysal et al., 2009).
The genetic origins of pathogenic anti-collagen B cells and IgG in anti-GBM GN and RA, and their potential as therapeutic targets, remain unknown. Efforts to sequence patients’ anti-collagen IgG have been unsuccessful to date, frustrated in part by limitations of current technologies to sequence patients’ polyclonal IgG mixtures or to capture B cells that recognize conformational epitopes. However, restricted epitope specificity and shared idiotypes among anti-collagen Ig are reported for both anti-GBM GN and RA (Lindh et al., 2014; Meyers et al., 1998; Netzer et al., 1999). Thus within each disease, pathogenic Ig may arise from a limited subset of B cells bearing conformationally-related Ig receptors.
Observations from mouse models raise the possibility that these anti-collagen autoimmune diseases overlap in immune origins, despite their distinct disease manifestations, target Ag distributions, and immunopathologies. We previously reported sequence analysis of anti-alpha3(IV)NC1 collagen monoclonal autoantibodies (mAb) isolated from immunized C57BL/6 and SJL mice (Sackey et al., 2008). The anti-alpha3(IV)NC1 mAb exclusively use light chains (LC) encoded by genes of the IGKV3 subgroup (previously termed Vk21 family), paired with diverse Ig heavy chains. Preferential use of IGKV3 genes is notable; this subgroup is uncommon in the expressed repertoire of healthy adult mice, despite its being one of the larger IGKV subgroups with respect to number of functional genes (Kaushik et al., 1989). A strikingly biased use of IGKV3 genes was also observed among anti-CII IgG mAb isolated from mice with collagen-induced arthritis (CIA), a commonly used model of RA (Mo et al., 1993; Mo and Holmdahl, 1996). Up to 68% of anti-CII IgG mAb recovered from DBA/1 mice with CIA use IGKV3-encoded LCs, many of which are minimally mutated (Mo et al., 1993; Mo and Holmdahl, 1996). The IGKV3 LCs are paired with diverse VH, DH, and JH genes, and with HCDR3 of varied sequence and length. IGKV3-encoded LCs are also used by the 6 C1 epitope-specific anti-CII mAb recovered from immunized B10.Q mice bearing an anti-CII VHDHJH knock-in (Cao et al., 2011).
Biased use of minimally mutated IGKV3 genes in both anti-alpha3(IV)NC1 and anti-CII murine anti-collagen responses indicates that a germline-encoded LC conformation can predispose to multiple anti-collagen autoreactivities. This suggests an unexpected relationship between distinct anti-collagen B cell populations, and raises questions about their mechanisms of regulation in healthy animals and of activation in disease. In this regard, we previously generated an IgM autoantibody transgenic mouse expressing the IGKV3-encoded LC and IGHV1-encoded HC of a murine mAb that targets an alpha3(IV)NC1 collagen epitope recognized by IgG from patients with anti-GBM GN (Zhang et al., 2008). In this model, HC+LC Ig Tg B cells are deleted in the bone marrow unless rescued by secondary rearrangements on the endogenous light and/or heavy chain alleles. Whereas this B cell fate was attributed in large part to expression of a dominant anti-alpha3(IV)NC1 HC, the findings described above raise the possibility that the IGKV3-encoded LC plays a prominent role in determining B cell fate in this model.
To test the hypothesis that the murine IGKV3 LC independently predisposes to autoimmunity that is regulated in vivo, we generated a novel transgenic mouse that expresses the IGKV3-encoded LC in conjunction with endogenously rearranged Ig HCs, using superimposed genetic deficiency of endogenous kappa chains to facilitate monitoring of the LC Tg. We show that subsets of B cells expressing a single IGKV3 LC Tg can react with alpha3(IV)NC1 collagen or CII and survive in peripheral lymphoid organs of LC Tg mice. Anti-alpha3(IV)NC1 collagen but not anti-CII IGKV3 LC-encoded Ig are detected in serum of unmanipulated Tg mice, and Toll-like receptor (TLR) ligands induce distinct patterns of anti-collagen Tg Ig secretion from splenocytes ex vivo, consistent with origin of the two anti-collagen autoIg from distinct B cell populations. These results support the notion that murine IGKV3 LCs contribute structural fitness to the Ag binding site for generating diverse anti-collagen autoimmune responses. Nonetheless, reduced B cell numbers, low cell surface Ig kappa density, and endogenous lambda chain expression suggest that subsets of IGKV3-encoded B cells are regulated in vivo by mechanisms that include deletion, anergy, and LC editing.
2. Materials and Methods
2.1 Generation of IGKV3 LC Tg mice with endogenous kappa knockout
Isolation and characterization of a murine prototypic anti-α3(IV)NC1 mAb derived from an immunized B6 mouse and production of a DNA construct expressing the mAb IGKV3-2*01 (former Vk21C)/Jk2 kappa light chain (hereafter mLCV3-Tg) was described previously (Sackey et al., 2008; Zhang et al., 2008). C57BL/6NHsd founders bearing the mLCV3-Tg were created by the Duke University Transgenic Mouse Core Facility. Four founder lines were bred with C57BL/6J (B6) mice lacking endogenous kappa chains (kKO, Jackson Laboratory stock #002400, Bar Harbor, ME, USA) (Zou et al., 1993), to facilitate tracking of mLCV3-Tg expression in vivo and in vitro. All data presented herein are from mice bearing the mLCV3-Tg and homozygous for endogenous kappa knockout (mLCV3-Tg/kKO; mean age 8.7 months, range 1.5–18 mo), or controls including non-transgenic kKO (non-Tg/kKO, devoid of kappa) and non-transgenic kappa sufficient (k+) littermates (mean age 6.2 months, range 2–12 mo), of either sex, reared under conventional specific pathogen-free conditions. Kappa+ controls include homozygous wildtype k+/+ or heterozygous k+/− genotypes, depending on availability. The care and use of all experimental animals were in accordance with institutional guidelines, and all studies and procedures were approved by the Animal Care and Use Committee of Duke University. Blood was collected and serum stored -20C until assay. Spleens were removed into culture media for processing as described below. Bone marrow was flushed from the leg bones using sterile PBS.
2.2 Flow cytometry
Single cell suspensions of 0.5−1.0× ×106 bone marrow or red blood cell-depleted spleen cells were incubated with fluorescently labeled antibodies and fixed in 1% paraformaldehyde prior to analysis by flow cytometry. Fluorescent stains include (NIF antibody registry number, where available): anti-CD45R/B220-PE (AB_394620) and –PerCP (AB_394622) (Becton Dickinson- Pharmingen, San Jose, CA, USA), anti-lambda-FTC (AB_609767), anti-kappa-PE (AB_616864), anti-IgM-FITC, anti-IgD-FITC, and anti-CD19-PE/Cy5.5 (Southern Biotech, Birmingham, AL, USA). Stained cells were analyzed using a FACScan analyzer (Becton Dickinson), and results visualized using FlowJo (Treestar, Ashland, OR, USA). Lymphocytes were gated based on FSC and SSC properties. The surface expression level of various Ig was calculated using the mean fluorescence intensity (MFI) of the stained cells, normalized to the MFI of splenic B cells from a non-Tg k+ mouse (either fresh B6 or frozen thawed Balb/c splenocytes, depending on availability).
2.3 B cell purification and culture and splenic B cell fusion
Untouched resting B cells were isolated from splenocytes using magnetic CD43 (Ly-48) MicroBeads (Miltenyi Biotec, San Diego, CA), per the manufacturer’s protocol. Following isolation, B cells were enumerated and viability assessed by trypan dye exclusion. Whole splenocytes or purified B cells were cultured at a concentration of 1×106 cells/mL in RPMI-1640 media containing 10% HI-FBS plus 2mM L-glutamine, 100 units/mL Penicillin-Streptomycin, and 55nM beta-mercaptoethanol, with or without Toll-like receptor stimulation by either lipopolysaccharide (LPS, 50 μg/mL, L6386, Sigma, St. Louis, MO, USA) or resiquimod R848 plus CpG oligos (R848 at 2μg/mL, Sigma, and CpG Oligo ODN 1668 at 1 μM, Invivogen, San Diego, CA). Cells were plated 1 mL per well in 48-well treated plates for 8–10 days. For hybridoma production, splenocytes were harvested from 4 mLCV3-Tg/kKO mice (age 11 months), cultured overnight with resiquimod R848 plus CpG oligos as described above, then fused with murine Sp2 myeloma cells as described (Zhang et al., 2008). Each fusion was plated in 180 wells in 96-well plates, and supernatants from hybridoma lines with sustained growth after passage were screened for secreted Ig kappa (Tg) and lambda and for binding to CII and alpha3(IV)NC1 by ELISA as described below.
2.4 Ig and autoantibody quantitation
Ig concentration in serum and culture supernatants was determined by ELISA. 96-well Immunlon II HB plates (Thermo Scientific, Waltham, MA) were coated with goat-anti-mouse IgG + IgM (Boehringer Mannheim) and blocked with 3% bovine serum albumin (BSA, A7030, Sigma) prior to application of serum samples. Bound antibodies were detected using alkaline phosphatase conjugated goat-anti-mouse Ig-kappa- or Ig-lambda secondary Ig (Southern Biotech), and quantified against a standard curve of purified mouse Ig.
Autoantibodies binding alpha3(IV)NC1 or CII were also detected by ELISA: 1) Bovine purified collagen alpha3(IV)NC1 (Eurodiagnostica, Malmo, Sweden) was coated at 3 μg/mL in 6 M guanidine, using MAB3 (Eurodiagnostica, Malmo, Sweden) as positive control Ig. A sample was considered positive with vehicle-coat subtracted OD405 >0.05. 2) Purified collagen II (chicken sternal, Sigma) was coated at 5 μg/mL in tris buffered saline, using as positive control mouse IgG2a monoclonal antibody CIIC1 that binds native but not denatured triple-helical CII from multiple species (Lindh et al., 2014). The CIIC1 mAb, deposited by R. Holmdahl and K. Rubin, was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. Ig binding was detected using alkaline phosphatase-conjugated goat-anti-mouse Ig-kappa secondary Ig (Southern Biotech). Positive samples were defined as those with an OD405 > mean + 3 S.D. of non-Tg/kKO sera.
2.5 Immunohistochemistry
Sections of kidneys frozen in OCT medium were stained for mouse kappa Ig and images acquired essentially as described (direct IF) (Worni-Schudel et al., 2015), with the following modification: sections were blocked with the avidin-biotin blocking kit (Life Technologies, Frederick, MD, USA), and deposited mLCV3-Tg detected using biotin-conjugated goat-anti-mouse-Ig-kappa (Southern Biotech) and streptavidin-Alexa Fluor 488 (Life Technologies). For indirect IF, sections of kidney from NOD-scid-gamma immunodeficient mice (Jackson Laboratory) were incubated with supernatant from mLCV3-Tg/kKO mouse splenocytes cultured with R848 + CpG as described above, or with positive control mouse anti-alpha3(IV)NC1 IgG MAB3 (Eurodiagnostica), and mouse kappa detected as described above.
2.6 Statistical analysis
Data management and statistical analysis were performed using JMP 11 software (SAS, Cary, NC). P-values are via the Wilcoxon test or the Wilcoxon each-pair multiple comparison test.
3. Results
3.1 In vivo expression of the mLCV3-Tg
To determine the fate of B cells expressing a rearranged VK gene from the IGKV3 subgroup that is associated with reactivity to CII and alpha3(IV)NC1 collagen, we generated the mLCV3-Tg and established it in the B6/kKO background. Genetic elimination of endogenous kappa alleles (kKO) prevents kappa editing and permits tracking of mLCV3-Tg-encoded LCs using kappa-specific reagents. Flow cytometric analysis of splenocytes revealed three founder lines expressing abundant B cell surface kappa and detectable serum kappa, consistent with expression of the mLCV3-Tg (Fig. 1); data from the three mLCV3-Tg/kKO lines are pooled. A fourth founder line exhibited little to no kappa expression and is not discussed further.
Figure 1.
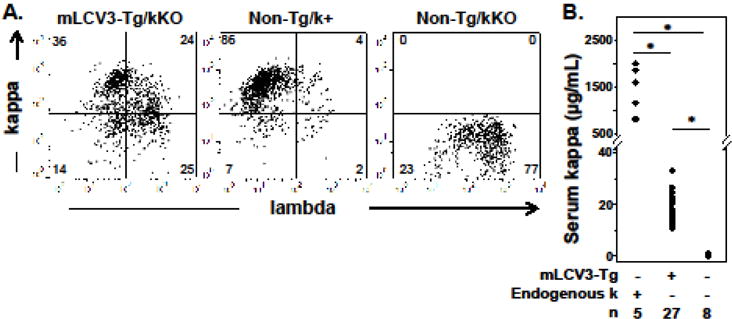
In vivo expression of the IGKV3-encoded mLCV3-Tg kappa light chain. A. Representative flow cytometry plots of splenocytes, gated on lymphocytes by forward and side scatter and on B cells using the CD19 marker. B. Serum kappa Ig levels as determined by ELISA. Endogenous k refers to genetic sufficiency at the endogenous kappa locus. * p<0.01.
The majority of spleen B cells express mLCV3-Tg on their surface (Table 1), indicating that the mLCV3-Tg can readily pair with endogenous HCs to form functional BCRs and support B cell survival and migration to the spleen. The percentage and total number of kappa+ B cells are significantly lower, however, than those observed in non-Tg/k+ mice that have a full complement of kappa V genes and LCs (Table 1): mean 66% vs 85.3% of B cells express surface kappa in mLCV3-Tg/kKO vs nonTg/k+ mice, p<0.05. There is a trend to lower total spleen B cell numbers in mLCV3-Tg/kKO mice compared to non-Tg/k+ mice (Table 1, p=0.054 for mLCV3-Tg/kKO vs non-Tg/k+). B cell and kappa+ B cell percentages in the bone marrow of mLCV3-Tg/kKO mice are significantly reduced relative to non-Tg/k+ controls (Table 1).
Table 1.
| mLCV3-Tg/kKO | non-Tg/k+ | non-Tg/kKO | |
|---|---|---|---|
| Spleen | |||
| B cells, millions | 17.1 ± 13.9# (44) |
23.1 ± 10.1 (6) |
11.6 ± 8.8 (12) |
| % kappa+, of B cells | 66.0 ± 13.1*+ (42) |
85.3 ± 8.5 (5) |
0.8 ± 0.6 (10) |
| kappa+ B cells, m illions | 12.1 ± 11.0*+ (42) |
20.4 ± 9.3 (5) |
0.1 ± 0.1 (10) |
| % lambda+, of B cells | 36.2 ± 13.8*+ (44) |
6.8 ± 3.2 (6) |
76.7 ± 8.0 (12) |
| % k/l dual positive, of B cells | 19.7 ± 11.7*+ (44) |
3.5 ± 1.6 (6) |
0.7 ± 0.6 (12) |
| Bone Marrow | |||
| % B cells, of lymphocytes | 25.9 ± 6.6* (23) |
36.7 ± 7.6 (6) |
29.0 ± 7.8 (8) |
| % kappa+ of B cells | 30.2 ± 10.6*+ (23) |
47.4 ± 4.9 (6) |
0.2 ± 0.3 (8) |
| Serum | |||
| Kappa concentration, μg/mL | 18.2 ± 5.2*+ (27) |
1497.5 ± 492.8 (5) |
0.3 ± 0.3 (8) |
| Lambda concentration, μg/mL | 573.1 ± 446.0* (27) |
35.7 ± 11.3 (5) |
581.4 ± 136.3 (8) |
p<0.05 vs non-Tg/k+
p<0.05 vs non-Tg/kKO
p=0.054 vs non-Tg/k+ and p=0.062 vs non-Tg/kKO
Almost half of the B cells in mLCV3-Tg/kKO B cells exclusively express the mLCV3-Tg, although expression of surface lambda+ LCs occurs as well (Fig. 2A). Among the B cells exclusively expressing mLCV3-Tg, there is a trend to lower surface kappa density compared to kappa-only cells in non-Tg/k+ mice that have diverse endogenous kappa repertoires (normalized MFI 0.90 vs 1.0 in non-Tg/k+ mice, p=0.0579) (Fig. 2B). Decreased surface Ig density suggests that a subset of mLCV3-Tg B cells may be anergic, due to autoreactivity generated by mLCV3-Tg pairing with a permissive endogenous HC.
Figure 2.
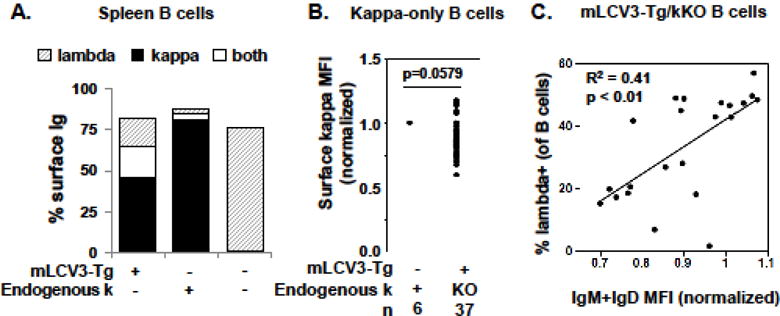
Spleen B cell surface kappa and lambda light chain expression. A. Percent of spleen B cells expressing surface kappa, lambda, or both kappa and lambda light chains is indicated. Values were determined by flow cytometry, after gating on lymphocytes and B cells. B. Surface kappa density on B cells expressing exclusively kappa light chains is shown. Expression is determined by mean fluorescence intensity (MFI), normalized to kappa expression on B cells of non-transgenic kappa-sufficient mice. C. Correlation between percent lambda-expressing B cells and B cell surface Ig density among B cells from mLCV3-Tg/kKO mice. Total Ig density is measured by flow cytometry as IgM+IgD MFI, normalized to spleen B cells from non-transgenic kappa-sufficient mice.
Approximately one-third of spleen B cells in mLCV3-Tg/kKO mice express lambda+ LCs in their BCR, half of which co-express with the mLCV3-Tg LC (Table 1, Fig. 2A). This is significantly higher than the 6.8% lambda expression found in non-Tg/k+ mice, indicating a high degree of secondary rearrangement at the lambda loci in the presence of the mLCV3-Tg. Lambda LC expression indicates receptor editing, presumably in an attempt by the B cell to reduce autoreactivity imparted by the mLCV3-Tg. Indeed, the proportion of lambda-edited B cells (Fig. 2C), and lambda MFI (not shown, p=0.02), correlate with normalized surface Ig (IgM+IgD) expression, a key marker of anergic phenotype.
Kappa+ Ig is detected in serum of all mLCV3-Tg mice (Fig. 1B). However, despite the presence of large numbers of kappa+ mLCV3-Tg B cells, serum kappa levels are significantly lower (over 80-fold) than in their non-Tg/k+ counterparts (Table 1). The vast majority of circulating Ig in mLCV3-Tg mice is lambda isotype, compared to nonTg/k+ mice that have only low levels of circulating lambda.
3.2 Anti-collagen autoantibodies in mLCV3-Tg mice
A subset of mLCV3-Tg mice spontaneously produce circulating Tg+ (kappa+) anti-alpha3(IV)NC1 autoIg (Fig. 3). Of 27 mLCV3-Tg/kKO subjects, 12 (44.4%) have detectable serum Tg+ anti-alpha3(IV)NC1 autoIg, indicating that the mLCV3-Tg LC is capable of pairing with endogenous heavy chains to generate Ig specific for alpha3(IV)NC1, and that in some cases these cells spontaneously avoid or escape regulation in vivo. The mLCV3-Tg-encoded autoIg are detected despite low levels of overall kappa+ Ig in serum of mLCV3-Tg/kKO mice (Fig. 1B). In contrast, anti-CII Tg+ Ig activity in mLCV3-Tg/kKO mouse serum is not increased above baseline (Fig. 3).
Figure 3.
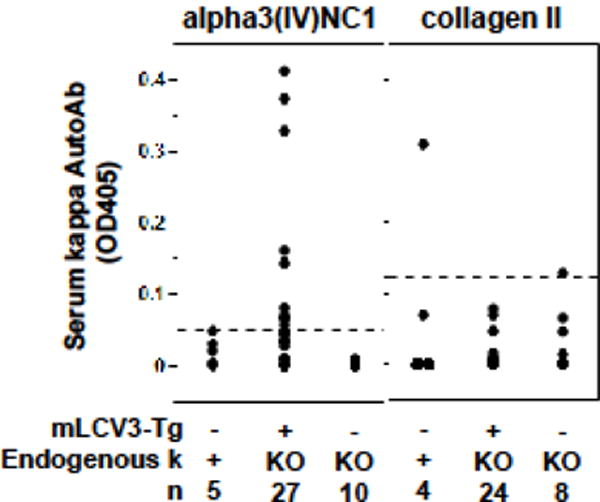
Serum mLCV3-Tg autoantibodies. Binding of serum Ig to alpha3(IV)NC1 collagen (left panel) or collagen II (right panel) was determined in ELISA. Serum was diluted 1:20 and binding detected with anti-mouse-kappa-chain Ig. The dashed line indicates the cut-off for positive binding in each assay.
Immunostaining revealed granular kappa (Tg) Ig deposits in glomeruli of an anti-alpha3(IV)NC1 seropositive, but not of a seronegative, mLCV3-Tg/kKO mouse (not shown). Sera from three seropositive mLCV3-Tg/kKO mice did not stain kidney sections in vitro; isotype analysis indicates that the vast majority of serum anti-alpha3(IV)NC1 Ig in these unmanipulated Tg mice is IgM, not IgG.
Whereas Tg+ anti-collagen Ig are not detected in supernatants of whole splenocytes or purified B cells cultured in medium alone, coculture with TLR ligands reveals the presence of anti-collagen B cells (Fig. 4). Low to modest levels of Tg+ anti-collagen Ig of both specificities are induced when either cell preparation is cultured with TLR4 agonist LPS or with a TLR7 and TLR9 agonist combination (R848 and CpG oligos) (Fig. 4). Both TLR ligand solutions significantly increased levels of kappa Ig and of both autoantibodies relative to cells from the same subjects cultured in medium alone.
Figure 4.
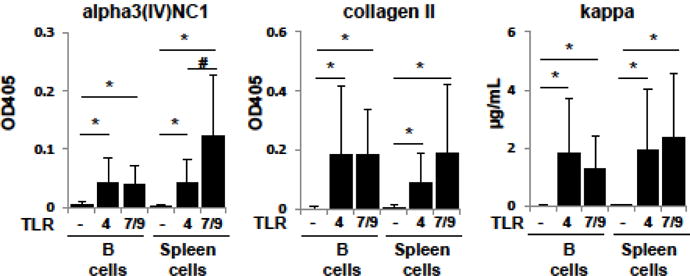
Anti-collagen mLCV3-Tg autoantibodies induced by TLR ligand stimulation. Purified B cells or whole spleen cell preparations from mLCV3-Tg/kKO mice were incubated with medium alone or with medium containing a ligand to TLR4 (LPS) or a combination of ligands that bind TLR7 and TLR9 (R848 and CpG oligos). Autoantibodies and total kappa Ig concentrations were detected in ELISA using goat-anti-mouse-kappa Ig. Shown are mean±SD, n=22–26 mice per group, * p≤0.0001, # p=0.0006.
Induced anti-alpha3(IV)NC1 kappa+ Ig responses were highest when whole splenocyte preparations were stimulated with R848+CpG (Fig. 4); autoantibody binding was significantly higher than levels in similarly stimulated purified B cell cultures (p=0.0004), despite the presence of, on average, 65% fewer B cells in the whole splenocyte wells. Anti-alpha3(IV)NC1 kappa+ Ig levels induced by R848+CpG were also significantly higher than those induced by LPS stimulation of comparable splenocyte preparations (Fig. 4). This effect on autoIg production was not observed in the anti-CII kappa+ responses from whole splenocytes stimulated with R848+CpG (p=0.42 vs LPS stimulation of comparable splenocyte cultures), suggesting that the anti-CII and anti-collagen IV antibodies arose from non-overlapping B cell subsets regulated by different mechanisms. This is consistent with the lack of correlation between mLCV3-Tg anti-alpha3(IV)NC1 and anti-CII Ig levels in supernatants of B cell cultures regardless of TLR stimulant (not shown). An autoantibody-positive cell culture supernatant produced patchy linear glomerular and tubular basement membrane staining when incubated on mouse kidney (not shown), suggesting pathogenic potential of a subset of mLCV3-Tg-encoded anti-alpha3(IV)NC1 kappa Ig.
For both anti-alpha3(IV)NC1 and anti-CII responses, Tg+ autoIg levels generated following R848 + CpG stimulation, in both B cell and whole splenocyte cultures, correlated positively with the level of mLCV3-Tg expression on the surface of kappa+ B cells, as measured by mean fluorescence intensity of kappa staining (n=14–15 group). For anti-alpha3(IV)NC1: R2 = 0.33, p=0.03 for B cell cultures, and R2 = 0.39, p=0.01 for splenocyte cultures; and, for anti-CII: R2 = 0.44, p<0.01 for B cells, R2 = 0.59, p<0.001 for splenocyte cultures.
To better assess the capacity of individual mLCV3-Tg B cells to produce autoantibodies, splenocytes from four mLCV3-Tg/kKO mice were stimulated with R848+CpG overnight prior to fusion and plating. 80 hybridoma cell lines with sustained growth, obtained from 720 plated wells (180 per spleen), were tested for Ig and binding properties by ELISA. Overall, 78/80 (97.5%) secreted Tg kappa, and 67/80 (83.8%) secreted endogenous lambda LCs. Only 1 of 79 supernatants tested contained Tg kappa Ig reactive with alpha3(IV)NC1, and none (0/79) reacted with CII.
4. Discussion
Here, we show that transgenic mice bearing a rearranged IGKV3-encoded Ig kappa light chain are susceptible to autoimmunity against both α3(IV)NC1 collagen and CII, the antigens targeted in anti-GBM GN and RA, respectively. Numerous Tg-bearing mice have detectable circulating anti-α3(IV)NC1 autoantibodies, and TLR ligand-stimulated splenic B cells produce autoantibodies to each antigen. These results support the notion that murine IGKV3 LCs contribute structural fitness to Ag binding sites that generate diverse anti-collagen autoimmune reactivity.
To our knowledge this is the first report of single Ig LC predisposing to generation of disparate anti-collagen autoreactivities. The use of a single LC to bind both alpha3(IV)NC1 and CII, and the preferred use of IGKV3 LCs in immune responses to both Ags, suggest the presence of conserved epitopes on the two collagen chains. The structural basis for this possibility remains unclear, however, because there is no obvious similarity between the known autolg epitopes on the type IV collagen non-collagenous (globular) domain and epitopes targeted on the collagenous repeated Gly-X-Y motif region of CII. Specificity may rely on a key amino acid or side chain. It is of note that all three murine anti-alpha3(IV)NC1 mAb previously derived from immunized mice, including the prototype for mLCV3 (mAb 1G6), conserve the germline IGKV3-encoded negatively charged glutamic acid (Glu, E) residue in the center of LCDR3 (Sackey et al., 2008). These mAb LCs are derived from 3 different IGKV3 genes (IGKV 3–2, 3–7, and 3–10) and differ in their LCDR3 by 4 or 5 amino acids. The IGKV3-encoded central Glu is also found in the LCDR3 of three well-characterized anti-CII IgG mAb: C1-epitope-reactive mAb CIIC1, encoded by IGKV3-5/Jk2; mAb CB20, encoded by IGKV3-4/Jk2; and J1-eptiope specific mAb M2139 encoded by IGKV3-1/Jk2 (Cao et al., 2011; Mo and Holmdahl, 1996; Uysal et al., 2009). The conserved Glu may facilitate binding to residues within the collagen epitopes, or neutralize basic residues in the HCDR3 that might otherwise veto collagen binding, analogous to the role ascribed to acidic LCDR1 aspartate residues in anti-DNA IGKV3-4 (former VK21D) LC editors (Kalinina et al., 2011; Li et al., 2001). In this regard, arginine residues are prominent among known CII B cell epitopes (Lindh et al., 2014), and a positively charged lysine and centrally located histidine are found in one of two major type IV collagen epitopes (C6, also known as EB) targeted by pathogenic Ig from Goodpasture patients (Netzer et al., 1999). Crystal structure analysis of the M2139 Fab fragment complexed with the CII J1 peptide confirms that LCDR3 is a key contributor to the epitope interface, including contact between the central LCDR3 Glu and several J1 a.a. residues (Raposo et al., 2014). Mutagenesis experiments could further explore the importance of LCDR3 Glu in Ig-collagen interactions.
It is possible that the IGKV3 LC has different functions in the two anti-collagen responses. We found evidence in the murine anti-alpha3(IV)NC1 collagen immune response of not only IGKV3 bias, but also a shared hydrophobic amino acid motif in the HC HCDR3 (Sackey et al., 2008). A shared HCDR3 motif (exceptionally long HCDR3) was also observed among human anti-a3(IV)NC1 IgM mAb derived from NOD-scid-IL2Rgamma-null mice engrafted with human hematopoietic stem cells (Foster et al., 2016). This suggests that the HCDR3 is critical for binding epitopes on alpha3(IV)NC1 collagen, following the conventional paradigm that HCDR3 is a major determinant of Ag binding. The IGKV3 bias observed in the murine anti-alpha3(IV)NC1 collagen response may reflect a permissive role for Ag binding, wherein IGKV3 LCs stabilize the Ig and do not block Ag binding. In contrast, in the murine anti-CII response, IGKV3 LCs pair with diverse HC and HCDR3 of diverse length and amino acid sequence (Mo et al., 1993; Mo and Holmdahl, 1996), suggesting that the IGKV3 LC plays a dominant role in CII binding. Although LC dominance is rare among analyzed immune responses, the importance of LC CDR residues in several anti-self or anti-microbial immune responses has been reported (Gorny et al., 2011; Liang et al., 2003; Wiehe et al., 2014; Zhuang et al., 2014). It should be noted, however, that genetic mapping in heterogeneous stock-derived mice with CIA suggests an important role for the Ig heavy chain (Igh) locus in controlling anti-CII IgG responses, including responses to the major CII epitopes (Forster et al., 2012). Nonetheless, the Igk locus modifies this influence, as shown by its mapping to production of high anti-J1 Ig titers in mice lacking the high risk Igh haplotype for this response (Raposo et al., 2014).
The relevance of these studies to human anti-GBM GN and RA necessarily awaits availability of sequence data from pathogenic IgG from patients with these diseases. There is little evidence of clinical overlap in the two diseases, but this can be explained in part by their distinct HLA associations. Anti-GBM GN is strongly associated with HLA Class II DRB1*1501 (Phelps and Rees, 1999 ), whereas RA is linked to the “shared epitope” found in a subset of HLA Class II DRB1*04 alleles (Gregersen et al., 1987). Thus immunoregulation of the two responses is distinct, independent of Ig kappa haplotype. It is not yet determined if a subset of the human population carries LC kappa genes identical to those at the murine IGKV3 locus. Query of existing databases (IMGT and GenBank) indicates that the human IGKV subgroup most related to the murine mLCV3 gene (77.7% nucleotide identity) is designated IGKV7, whose only member, IGKV7-3*01, is a pseudogene. Functional Vk alleles related to mLCV3-Tg are found among the human IGKV3 subgroup, and include human IGKV3-11 and IGKV3-20 alleles (72–73% identity to mLCV3). These alleles are also found in anti-self-Ag crossreactive neutralizing Ig responses against human cytomegalovirus and in stereotypical VH/VL combinations found in clonal B cell expansions and malignancies associated with hepatitis C virus (De Re et al., 2000; McLean et al., 2006). It is also of note that considerable evidence points to extensive germline variation at the human immunoglobulin loci (Watson and Breden, 2012), such that it is likely that many human kappa alleles and haplotypes, and their links to human diseases, have yet to be defined. Identification of specific Ig alleles recurrently recruited into anti-collagen responses would reveal a genetic risk variant and suggest a target for directed intervention in disease. Awareness of Ig gene associations could also help prevent development of therapeutic mAbs that carry risk of inadvertent autoimmune complications when administered for intervention in other human disorders.
Despite detection of anti-collagen Ig, we find evidence that B cells expressing the mLCV3-Tg are regulated in vivo. The majority of splenic B cells in Tg mice express mLCV3-Tg on their surface (noting that all kappa expression derives from the Tg due to genetic elimination of endogenous kappa alleles). Thus the mLCV3-Tg supports production of functional B cell receptors and B cell survival. However, adult Tg mice in lineages derived from 3 different mLCV3-Tg/kKO founders express only low levels of serum kappa (mLCV3-Tg) Ig. This suggests that most mLCV3-Tg-expressing B cells are not activated in vivo and do not contribute substantively to homeostatic levels of serum Ig. This could reflect immunological ignorance; however, additional observations support a role for active regulation. In multiple mLCV3-Tg mice, B cells express mean surface kappa levels that are substantially lower than that of their non-Tg/k+ counterparts. Among B cells that express exclusively mLCV3-Tg on their surface, without expression of lambda LC, there is a trend toward lower surface kappa density compared to cells from non-Tg/k+ mice with diverse endogenous kappa repertoires. This is consistent with anergy in a subset of mLCV3-Tg B cells, presumably induced by autoreactivity generated by mLCV3-Tg pairing with a permissive endogenous HC.
There is also a trend to lower total spleen B cell numbers in adult mLCV3-Tg/kKO mice compared to their non-Tg k+ counterparts. This contrasts with the near normal B cell numbers in spleens of mice homozygous for a gene-targeted replacement of a rearranged Vk8/Jk5 gene that encodes an LC derived from an anti-influenza Ig (Brady et al., 2004). In the latter case, an innocuous (non-autoreactive) LC reconstitutes a near normal B cell number by combining with multiple endogenous HCs. This does not require kappa editing, which is prevented by the homozygous Vk8/Jk5 knock-in. Our finding of low B cells numbers in mLCV3-Tg mice thus suggests that a substantial subset of mLCV3-Tg B cells are autoreactive and deleted in the absence of endogenous kappa editing, which is prevented in this model by homozygous knockout of the endogenous kappa locus. The significantly reduced frequencies of bone marrow total and kappa+ B cells are consistent with this possibility. Alternatively, it is conceivable that repertoire restriction related in part to absence of a large diverse kappa repertoire for serial LC replacement may have limited autoreactivity-driven positive selection and B cell expansion in our Tg/kKO model (Kohler et al., 2008). By comparison, the endogenous lambda locus is intact, and lambda editing is prominent among mLCV3-Tg B cells, suggesting that lambda LC revision contributes to regulation of a subset of autoreactive mLCV3-Tg B cells.
Despite evidence of regulation, some mLCV3-encoded anti-collagen B cells manage to escape tolerance mechanisms and become activated in vivo. Our demonstration of dual mLCV3-Tg and endogenous lambda editor LC expression in a substantial proportion of Tg B cells raises the possibility that allelic inclusion contributes to this phenotype. Co-expression of autoreactive and non-autoreactive LCs is well described in both Ig transgenic and wildtype mice (Casellas et al., 2007; Li et al., 2001; Liu et al., 2005). Low level or absent surface expression of the mLCV3-Tg, possibly due to receptor dilution or failure to compete successfully with the endogenous LC for HC binding, may prevent B cell negative selection, giving rise to a mature B cell that can secrete both antibodies upon activation. Such allelically included dual LC B cells have been shown to engage effectively in antigen-specific immune responses in vivo (Velez et al., 2007). Nonetheless, our findings from fusions using mLCV3-Tg/kKO splenocytes suggest that the majority of R848+CpG-stimulated mLCV3-Tg/endogenous HC dual LC-expressing Ig do not react with CII or alpha3(IV)NC1.
Whether a single mLCV3-Tg-encoded B cell can produce an Ig crossreactive with both alpha3(IV)NC1 and CII is not determined by these experiments, as B cells were assessed in bulk, not individual, culture conditions. Available evidence presented here suggests that the mLCV3-Tg encoded anti-alpha3(IV)NC1 and anti-CII Ig derive from non-overlapping, differentially regulated B cell populations; TLR7/TLR9 costimulation of splenocyte preparations enhances levels of anti-alpha3(IV)NC1, but not anti-CII, Tg Ig, compared to similar cultures stimulated with TLR4 ligand. Spontaneously produced Tg-encoded Ig specific for alpha3(IV)NC1, but not anti-CII, are detected in serum of a subset of mLCV3-Tg mice. Additionally, the prototypic murine anti-alpha3(IV)NC1 HC+LC mAb 1G6 does not bind CII (not shown). Molecular analysis of the only hybridoma, termed 66-4, isolated from a 1G6 HC+LC Tg mouse and confirmed to bind both alpha3(IV)NC1 collagen and CII revealed a productively rearranged endogenous IGKV14-111 gene (formerly Vk9B or Vk9/10), in addition to an unmutated 1G6 LC and HC (unpublished). Thus anti-CII reactivity in this cell line presumably resulted from pairing of the IGKV14 editor LC with the 1G6 HC. LC dilution may have played a role in in vivo survival of this dual-reactive B cell. A 1G6 HC-only Ig Tg model does not exist, such that the full capacity of the 1G6 HC to encode anti-CII reactivity when paired with different endogenous LC is unknown.
Our experiments do not reveal the identity or nature of the tolerizing self-antigen(s) that direct deletion, anergy, and/or editing in subsets of mLCV3-Tg-encoded B cells. We previously determined that endogenous alpha3(IV)NC1 was not the tolerogen that regulated anti-alpha3(IV)NC1 B cells in 1G6 HC+LC Tg mice on the C57BL/6 background (Zhang et al., 2008), because superimposed targeted inactivation of the Col4a3 gene failed to rescue the tolerance phenotype (Clark et al., 2011). Inactivation of CII to test its role in regulation of anti-collagen B cells is precluded by perinatal lethality of homozygous CII deficiency (Li et al., 1995). It is of note that B cells bearing the CB20 Ig VHDHJH knock-in that binds the CII C1 epitope survive, express normal surface density of Tg Ig receptors, and produce spontaneous anti-CII Ig in vivo, consistent with activation rather than deletion or anergy (Cao et al., 2011). The CB20 HC knockin is expressed on the arthritis-susceptibile MHC H-2q B10Q background, and CII immunization induces high levels of anti-CII Ig, though little clinical arthritis (Cao et al., 2011). CII immunization was not tested in the mLCV3-Tg mice described here, because mLCV3-Tg was established on the B6 strain to facilitate comparison with the 1G6 HC+LC Ig Tg, and B6 mice express the arthritis-resistant MHC H-2b haplotype.
Highlights.
IGKV3-encoded light chains are prominent among murine anti-type II and anti-type IV collagen autoantibodies
Mice bearing an IGKV3 light chain transgene develop subsets of B cells reactive with each collagen
IGKV3 subgroup genes contribute structural fitness to diverse anti-collagen responses
IGKV3-encoded B cells are regulated in vivo
Acknowledgments
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK088904 (MHF), the Swiss National Science Foundation (IMWS), the Novartis Foundation (IMWS), the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001117 (MHF), a Wellesley Career Education Internship (FMK), and the Research Service of the DVAMC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor was the NIH involved in the study design, data collection/interpretation, writing, or submission of this manuscript. We thank the Shared Resources of the Duke Cancer Institute, including the Transgenic and Knockout Mouse Shared Resource, Flow Cytometry Shared Resource, and DNA Analysis Facility, and Julie Fuller of the Duke Surgical Sciences Histopathology Core/Shared Resource Lab. We thank Melissa Weston Boor for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Borza DB, Hudson BG. Molecular characterization of the target antigens of anti-glomerular basement membrane antibody disease. Springer Semin Immunopathol. 2003;24:345–361. doi: 10.1007/s00281-002-0103-1. [DOI] [PubMed] [Google Scholar]
- Brady GF, Congdon KL, Clark AG, Sackey FN, Rudolph EH, Radic MZ, Foster MH. Kappa editing rescues autoreactive B cells destined for deletion in mice transgenic for a dual specific anti-laminin Ig. J Immunol. 2004;172:5313–5321. doi: 10.4049/jimmunol.172.9.5313. [DOI] [PubMed] [Google Scholar]
- Burkhardt H, Koller T, Engstrom A, Nandakumar KS, Turnay J, Kraetsch HG, Kalden JR, Holmdahl R. Epitope-specific recognition of type II collagen by rheumatoid arthritis antibodies is shared with recognition by antibodies that are arthritogenic in collagen-induced arthritis in the mouse. Arthritis Rheum. 2002;46:2339–2348. doi: 10.1002/art.10472. [DOI] [PubMed] [Google Scholar]
- Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Khmaladze I, Jia H, Bajtner E, Nandakumar KS, Blom T, Mo JA, Holmdahl R. Pathogenic autoreactive B cells are not negatively selected toward matrix protein collagen II. J Immunol. 2011;187:4451–4458. doi: 10.4049/jimmunol.1101378. [DOI] [PubMed] [Google Scholar]
- Casellas R, Zhang Q, Zheng NY, Mathias MD, Smith K, Wilson PC. Igkappa allelic inclusion is a consequence of receptor editing. J Exp Med. 2007;204:153–160. doi: 10.1084/jem.20061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AG, Mackin KM, Foster MH. Genetic elimination of alpha3(IV) collagen fails to rescue anti-collagen B cells. Immunol Lett. 2011;141:134–139. doi: 10.1016/j.imlet.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart ML, Jankowska-Gan E, Huang G, Roenneburg DA, Keller MR, Torrealba JR, Rhoads A, Kim B, Bobadilla JL, Haynes LD, Wilkes DS, Burlingham WJ, Greenspan DS. Interleukin-17-dependent autoimmunity to collagen type V in atherosclerosis. Circulation Research. 2010;107:1106–1116. doi: 10.1161/CIRCRESAHA.110.221069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Re V, De Vita S, Marzotto A, Rupolo M, Gloghini A, Pivetta B, Gasparotto D, Carbone A, Boiocchi M. Sequence analysis of the immunoglobulin antigen receptor of hepatitis c virus-associated non-hodgkin lymphomas suggests that the malignant cells are derived from the rheumatoid factor-producing cells that occur mainly in type II cryoglobulinemia. Blood. 2000;96:3578–3584. [PubMed] [Google Scholar]
- Forster M, Raposo B, Ekman D, Klaczkowska D, Popovic M, Nandakumar KS, Lindvall T, Hultqvist M, Teneva I, Johannesson M, Ahlqvist E, Holmdahl R. Genetic control of antibody production during collagen-induced arthritis development in heterogeneous stock mice. Arthritis Rheum. 2012;64:3594–3603. doi: 10.1002/art.34658. [DOI] [PubMed] [Google Scholar]
- Foster MH. Basement membranes and autoimmune diseases. Matrix Biol. 2017:57–58. 149–168. doi: 10.1016/j.matbio.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MH, Buckley ES, Chen BJ, Hwang KK, Clark AG. Uncommon structural motifs dominate the antigen binding site in human autoantibodies reactive with basement membrane collagen. Molecular Immunol. 2016;76:123–133. doi: 10.1016/j.molimm.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Sampson J, Li H, Jiang X, Totrov M, Wang XH, Williams C, O’Neal T, Volsky B, Li L, Cardozo T, Nyambi P, Zolla-Pazner S, Kong XP. Human anti-V3 HIV-1 monoclonal antibodies encoded by the VH551/Vl lambda genes define a conserved antigenic structure. PLoS One. 2011;6:e27780. doi: 10.1371/journal.pone.0027780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Holmdahl R, Jansson L, Larsson A, Jonsson R. Arthritis in DBA/1 mice induced with passively transferred type II collagen immune serum. Immunohistopathology and serum levels of anti-type II collagen auto-antibodies. Scand J Immunol. 1990;31:147–157. doi: 10.1111/j.1365-3083.1990.tb02754.x. [DOI] [PubMed] [Google Scholar]
- Kalinina O, Doyle-Cooper CM, Miksanek J, Meng W, Prak EL, Weigert MG. Alternative mechanisms of receptor editing in autoreactive B cells. Proc Natl Acad Sci U S A. 2011;108:7125–7130. doi: 10.1073/pnas.1019389108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A, Schulze DH, Bona C, Kelsoe G. Murine V kappa gene expression does not follow the VH paradigm. J Exp Med. 1989;169:1859–1864. doi: 10.1084/jem.169.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler F, Hug E, Eschbach C, Meixlsperger S, Hobeika E, Kofer J, Wardemann H, Jumaa H. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity. 2008;29:912–921. doi: 10.1016/j.immuni.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Lerner RA, Glassock RJ, Dixon FJ. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967;126:989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Jiang Y, Prak E, Radic M, Weigert M. Editors and editing of anti-DNA receptors. Immunity. 2001;15:947–957. doi: 10.1016/s1074-7613(01)00251-5. [DOI] [PubMed] [Google Scholar]
- Li SW, Prockop DJ, Helminen H, Fassler R, Lapvetelainen T, Kiraly K, Peltarri A, Arokoski J, Lui H, Arita M, et al. Transgenic mice with targeted inactivation of the col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995;9:2821–2830. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- Liang Z, Chen C, Mohan C. Molecular signatures of anti-nuclear antibodies: Contributions of specific light chain residues and a novel New Zealand Black V kappa 1 germline gene. J Immunol. 2003;171:3886–3894. doi: 10.4049/jimmunol.171.7.3886. [DOI] [PubMed] [Google Scholar]
- Lindh I, Snir O, Lonnblom E, Uysal H, Andersson I, Nandakumar KS, Vierboom M, t Hart B, Malmstrom V, Holmdahl R. Type II collagen antibody response is enriched in the synovial fluid of rheumatoid joints and directed to the same major epitopes as in collagen induced arthritis in primates and mice. Arthritis Res & Therapy. 2014;16:R143. doi: 10.1186/ar4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Velez MG, Humann J, Rowland S, Conrad FJ, Halverson R, Torres RM, Pelanda R. Receptor editing can lead to allelic inclusion and development of B cells that retain antibodies reacting with high avidity autoantigens. J Immunol. 2005;175:5067–5076. doi: 10.4049/jimmunol.175.8.5067. [DOI] [PubMed] [Google Scholar]
- McLean GR, Cho CW, Schrader JW. Autoreactivity of primary human immunoglobulins ancestral to hypermutated human antibodies that neutralize HCMV. Molecular Immunol. 2006;43:2012–2022. doi: 10.1016/j.molimm.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Meyers KE, Kinniry PA, Kalluri R, Neilson EG, Madaio MP. Human Goodpasture anti-alpha3(IV)NC1 autoantibodies share structural determinants. Kidney Int. 1998;53:402–407. doi: 10.1046/j.1523-1755.1998.00827.x. [DOI] [PubMed] [Google Scholar]
- Mo JA, Bona CA, Holmdahl R. Variable region gene selection of immunoglobulin G-expressing B cells with specificity for a defined epitope on type II collagen. Eur J Immunol. 1993;23:2503–2510. doi: 10.1002/eji.1830231019. [DOI] [PubMed] [Google Scholar]
- Mo JA, Holmdahl R. The B cell response to autologous type II collagen: Biased V gene repertoire with V gene sharing and epitope shift. J Immunol. 1996;157:2440–2448. [PubMed] [Google Scholar]
- Nandakumar KS, Holmdahl R. Efficient promotion of collagen antibody induced arthritis (CAIA) using four monoclonal antibodies specific for the major epitopes recognized in both collagen induced arthritis and rheumatoid arthritis. J Immunol Methods. 2005;304:126–136. doi: 10.1016/j.jim.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Nandakumar KS, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: Description of the disease and the influence of age, sex, and genes. Am J Pathol. 2003;163:1827–1837. doi: 10.1016/S0002-9440(10)63542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer KO, Leinonen A, Boutaud A, Borza DB, Todd P, Gunwar S, Langeveld JP, Hudson BG. The Goodpasture autoantigen. Mapping the major conformational epitope(s) of alpha3(IV) collagen to residues 17–31 and 127–141 of the NC1 domain. J Biol Chem. 1999;274:11267–11274. doi: 10.1074/jbc.274.16.11267. [DOI] [PubMed] [Google Scholar]
- Phelps RG, Rees AJ. The HLA complex in Goodpasture’s disease: A model for analyzing susceptibility to autoimmunity. Kidney Int. 1999;56:1638–1653. doi: 10.1046/j.1523-1755.1999.00720.x. [DOI] [PubMed] [Google Scholar]
- Raposo B, Dobritzsch D, Ge C, Ekman D, Xu B, Lindh I, Forster M, Uysal H, Nandakumar KS, Schneider G, Holmdahl R. Epitope-specific antibody response is controlled by immunoglobulin V(H) polymorphisms. J Exp Med. 2014;211:405–411. doi: 10.1084/jem.20130968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnelid J, Lysholm J, Engstrom-Laurent A, Klareskog L, Heyman B. Local anti-type II collagen antibody production in rheumatoid arthritis synovial fluid. Evidence for an HLA-DR4-restricted IgG response. Arthritis Rheum. 1994;37:1023–1029. doi: 10.1002/art.1780370707. [DOI] [PubMed] [Google Scholar]
- Sackey FN, Congdon KL, Brady GF, Hopfer H, Zhang Y, Mackin KM, Clark AG, Foster MH. Shared variable domain elements among anti-collagen antibodies reactive with Goodpasture epitopes. In: Vogel FL, Zimmermann LF, editors. Autoimmunity: Role, Regulation and Disorder. Nova Science Publishers, Inc.; Hauppauge, NY: 2008. [Google Scholar]
- Saus J, Wieslander J, Langeveld J, Quinones S, Hudson B. Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem. 1988;263:13374–13380. [PubMed] [Google Scholar]
- Stuart JM, Dixon FJ. Serum transfer of collagen-induced arthritis in mice. J Exp Med. 1983;158:378–392. doi: 10.1084/jem.158.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski A, Klareskog L, Carlsten H, Herberts P, Koopman WJ. Secretion of antibodies to types I and II collagen by synovial tissue cells in patients with rheumatoid arthritis. Arthritis Rheum. 1989;32:1087–1092. doi: 10.1002/anr.1780320906. [DOI] [PubMed] [Google Scholar]
- Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148:2103–2108. [PubMed] [Google Scholar]
- Uysal H, Bockermann R, Nandakumar KS, Sehnert B, Bajtner E, Engstrom A, Serre G, Burkhardt H, Thunnissen MM, Holmdahl R. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J Exp Med. 2009;206:449–462. doi: 10.1084/jem.20081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez MG, Kane M, Liu S, Gauld SB, Cambier JC, Torres RM, Pelanda R. Ig allotypic inclusion does not prevent B cell development or response. J Immunol. 2007;179:1049–1057. doi: 10.4049/jimmunol.179.2.1049. [DOI] [PubMed] [Google Scholar]
- Watson CT, Breden F. The immunoglobulin heavy chain locus: Genetic variation, missing data, and implications for human disease. Genes Immun. 2012;13:363–373. doi: 10.1038/gene.2012.12. [DOI] [PubMed] [Google Scholar]
- Wiehe K, Easterhoff D, Luo K, Nicely NI, Bradley T, Jaeger FH, Dennison SM, Zhang R, Lloyd KE, Stolarchuk C, Parks R, Sutherland LL, Scearce RM, Morris L, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Sinangil F, Phogat S, Michael NL, Kim JH, Kelsoe G, Montefiori DC, Tomaras GD, Bonsignori M, Santra S, Kepler TB, Alam SM, Moody MA, Liao HX, Haynes BF. Antibody light-chain-restricted recognition of the site of immune pressure in the RV144 HIV-1 vaccine trial is phylogenetically conserved. Immunity. 2014;41:909–918. doi: 10.1016/j.immuni.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley DT, Briggaman RA, O’Keefe EJ, Inman AO, Queen LL, Gammon WR. Identification of the skin basement-membrane autoantigen in epidermolysis bullosa acquisita. N Engl J Med. 1984;310:1007–1013. doi: 10.1056/NEJM198404193101602. [DOI] [PubMed] [Google Scholar]
- Worni-Schudel IM, Clark AG, Chien T, Hwang KK, Chen BJ, Foster MH. Recovery of a human natural antibody against the noncollagenous-1 domain of type IV collagen using humanized models. J Trans Med. 2015;13:185–196. doi: 10.1186/s12967-015-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su SC, Hecox DB, Brady GF, Mackin KM, Clark AG, Foster MH. Central tolerance regulates B cells reactive with Goodpasture antigen alpha3(IV)NC1 collagen. J Immunol. 2008;181:6092–6100. doi: 10.4049/jimmunol.181.9.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Stahl SJ, Watts NR, DiMattia MA, Steven AC, Wingfield PT. A cell-penetrating antibody fragment against HIV-1 rev has high antiviral activity: Characterization of the paratope. J Biol Chem. 2014;289:20222–20233. doi: 10.1074/jbc.M114.581090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YR, Takeda S, Rajewsky K. Gene targeting in the Ig kappa locus: Efficient generation of lambda chain-expressing B cells, independent of gene rearrangements in Ig kappa. The EMBO Journal. 1993;12:811–820. doi: 10.1002/j.1460-2075.1993.tb05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


