Abstract
Background
The hepatitis C virus (HCV) is a major cause of global morbidity and mortality, with conflicting evidence regarding a possible association with psoriasis.
Objective
To determine the prevalence of HCV in psoriasis patients, compared to controls, and determine the incidence of hepatic decompensation in HCV+ psoriasis patients compared to HCV+ controls.
Methods
Cross-sectional and cohort studies were conducted in The Health Improvement Network (THIN).
Results
In fully adjusted models, a statistically significant increase in prevalence was seen in the adults with psoriasis (OR: 1.24, 95% CI 1.10 – 1.40). A “dose-response” of HCV prevalence with increasing psoriasis severity was not observed. HCV+ patients with psoriasis had a non-statistically significant increased incidence of hepatic decompensation compared to HCV+ individuals without psoriasis (aHR: 1.58, 95% CI: 0.90– 2.77). The risk was highest, and statistically significant, in those with moderate to severe psoriasis (aHR: 21.51, 95% CI: 7.58–61.03).
Conclusions
These results demonstrate a higher prevalence of HCV in adults with psoriasis and a higher rate of hepatic decompensation in HCV+ individuals with moderate-severe psoriasis.
Introduction
The hepatitis C virus (HCV) causes a blood-borne infection of the liver that represents a major cause of global morbidity and mortality. Approximately 170 million people are chronically infected with HCV worldwide, with 3 to 4 million new cases developing every year1. Chronic hepatitis develops in 70–80% of people with acute HCV infection, and, can lead to hepatic decompensation, including development of hepatocellular carcinoma and liver failure. In addition to effects on hepatocytes, the virus localizes in skin and lymphoid tissue, leading to systemic immune dysregulation in a subset of patients2. Furthermore, recent research confirms up-regulation of inflammatory cytokines in the skin of HCV+ patients with psoriasis3.
This evidence suggests a plausible mechanism for an increased prevalence of psoriasis in adults with HCV (Fig.1); however, the precise relationship is incompletely understood. Some cross-sectional studies have shown higher rates of HCV infection in adults with psoriasis as compared to the general population; however, others have not demonstrated a statistically significant association4–10. Moreover, results of existing studies are difficult to interpret because of design limitations including small sample sizes and inability to evaluate psoriasis severity.
Figure 1.
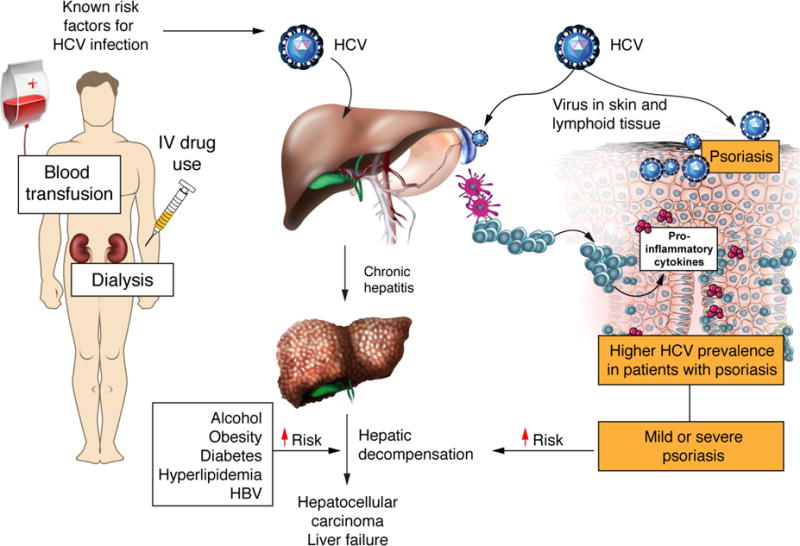
Conceptual Model for the Increase Prevalence of Psoriasis in Adults with HCV Infection
Understanding the epidemiology of HCV infection in patients with psoriasis has significant clinical implications. First, it is important to identify high risk patients so appropriate screening can be implemented to reduce transmission as well as HCV-associated morbidity and mortality which can now be prevented with curative anti-viral pharmacotherapy11. Second, many systemic psoriasis treatments are either immunosuppressive or hepatotoxic; therefore, it is particularly important to determine the epidemiology of HCV in patients with more severe psoriasis who are likely to require systemic therapy.
The objective of this study was to determine the prevalence of HCV in patients with psoriasis of varying severity and to evaluate HCV-related liver outcomes among HCV+ psoriasis patients compared to HCV+ patients without psoriasis. We hypothesize individuals with psoriasis will have higher rates of HCV compared to those without psoriasis and that HCV+ psoriasis individuals will have a higher incidence of hepatic decompensation.
Methods
Study Design
We conducted a population-based, cross-sectional study to investigate the lifetime prevalence of HCV in psoriasis patients compared to controls. Data was collected between January 1, 1994 and June 30, 2012. We also conducted a cohort study to examine the incidence of hepatic decompensation in HCV+ psoriasis patients compared to HCV+ patients without psoriasis. Start date was the latest of the following: registration with a clinical practice plus 180 days, date at which the clinical practice implemented Vision software, date of psoriasis diagnosis or start of therapy for psoriasis patients with moderate-to-severe psoriasis (or corresponding visit date for the control patients), and diagnosis of HCV. Censoring occurred when patients developed a diagnosis of a hepatic decompensation event, died, transferred out of the practice, or reached the end of the data collection period.
Data Source
Data was from The Health Improvement Network (THIN), an electronic medical records database in the United Kingdom (UK) which has been used extensively for epidemiological work and previously validated for the study of psoriasis12 and HCV13. THIN contains information for over eleven million individuals in the UK and is broadly representative of the population14, 15. A subset of psoriasis patients in THIN also belongs to a prospective cohort called the Incident Heath Outcomes and Psoriasis Events (iHOPE) cohort. Patients in iHOPE have objective measures of psoriasis severity (i.e., percentage of body surface area affected) recorded by their GP, as previously described16.
Study Population
The study population in THIN included patients ages 18–89. Patients with a history of HIV or organ transplant were excluded. Up to 5 patients without psoriasis (e.g., unexposed controls) were randomly selected for each patient with psoriasis, matched on GP practice and start date (+/− 180 days), to ensure that patients were observed by similar providers during similar time periods.
The study population in iHOPE was patients with psoriasis ages 25–64, randomly sampled from THIN. Questionnaires were sent to their GP to ascertain the severity of psoriasis by body surface area (BSA) which was categorized as mild (<3% BSA), moderate (3–10% BSA), or severe (>10% BSA). Each iHOPE patient was matched with up to ten randomly chosen controls without psoriasis, as available, and matched on practice and age category.
Exposure Definitions
Diagnoses are recorded in THIN using a READ diagnostic code scheme17. Patients were identified as having psoriasis if they had at least one READ code for psoriasis (PPV 90%12). Psoriasis severity was based on treatment. Psoriasis patients who had a code for phototherapy (ultraviolet A, ultraviolet B, and/or psoralen and ultraviolet A), methotrexate, cyclosporine, oral retinoids (acitretin or etretinate), etanercept, infliximab, adalimumab, or ustekinumab were identified as having moderate-severe psoriasis. In iHOPE, psoriasis severity was determined by survey of the patients’ GP regarding the body surface area affected.
Outcomes
Patients were classified as having HCV if they received a READ diagnostic code for HCV (PPV 86%13), at any point during the study period. Hepatic decompensation was defined as one READ code for psoriasis AND at least one READ code for HCV, prior to a READ code for hepatic decompensation: ascites, variceal hemorrhage, spontaneous bacterial peritonitis, hepatic encephalopathy, or hepatocellular carcinoma13.
Statistical Analyses
Statistical analysis was performed in STATA 14.0 (StatCorp, College Station, TX). Statistical significance was determined by two-sided p values at a significance level < 0.05. Descriptive statistics were used to summarize baseline characteristics. Chi-squared tests were used for categorical variables and one-way analysis of variance (ANOVA) for continuous variables, based on psoriasis severity groups. We descriptively reported incidence rates for hepatic decompensation. Logistic regression was used to compare the prevalence of HCV in the psoriasis groups (overall, mild, and severe as defined by treatment in THIN; mild, moderate, and severe as defined by % of BSA involved in iHOPE) versus patients without psoriasis. Cox proportional hazards regression was used to compare the incidence of hepatic decompensation in the psoriasis groups (overall, mild, and moderate-to-severe) versus controls in THIN. Initial multivariable models included age, gender, BMI, observation time (for the cross-sectional study only), smoking history, alcohol use, Townsend score, and known risk factors for the outcomes. Known risk factors for HCV infection include IV drug use, history of dialysis and history of a blood transfusion. Risk factors for hepatic decompensation include obesity, alcohol use, diabetes, hyperlipidemia and concomitant hepatitis B infection. Each covariate was sequentially removed individually, beginning with the highest p-value. If the point estimate of the psoriasis variable was not altered by a minimum of 10%, the covariate was removed in order to create the most parsimonious model. Sensitivity analyses assessed the robustness of the results.
Results
We identified 201,358 patients with psoriasis and 961,888 matched controls in THIN. Among psoriasis patients, 188,664 had mild psoriasis and 12,694 had moderate-severe psoriasis. Baseline characteristics of this study population are summarized in Table 1. Among patients with moderate-severe psoriasis, the most commonly prescribed therapies were methotrexate (69.3%), phototherapy (26.2%), cyclosporine (10.4%), oral retinoids (6.1%) and biologics (0.95%) (Table 2).
Table 1.
Baseline Demographics in THIN1
| Controls | Mild Psoriasis | Moderate-severe Psoriasis | |
|---|---|---|---|
|
| |||
| N | 961,888 | 188,664 | 12,694 |
|
| |||
| Age, mean in years (SD) | 48.39 (17.15) | 45.64 (17.16) | 46.20 (15.10) |
|
| |||
| Female Sex, N (%) | 540,005 (56.14) | 97,769 (51.82) | 6,153 (48.47) |
|
| |||
| Cohort time, years (SD) | 10.10 (4.82) | 8.33 (5.16) | 9.35 (5.02) |
|
| |||
| Smoking History, N (%) | |||
| Never | 443,229 (46.08) | 69,791 (36.99) | 4,621 (36.40) |
| Ever | 496,096 (51.58) | 114,444 (60.66) | 7,949 (62.62) |
| Missing | 22,563 (2.35) | 4,429 (2.35) | 124 (0.98) |
|
| |||
| Drinking History, N (%) | |||
| Never | 94,976 (9.87) | 16,985 (9.00) | 1,210 (9.53) |
| Ever | 762,890 (79.31) | 150,053 (79.53) | 10,390 (81.85) |
| Missing | 104,022 (10.81) | 21,626 (11.46) | 1,094 (8.62) |
|
| |||
| Body Mass Index, Mean (SD) | 27.00 (5.72) | 27.26 (5.90) | 28.55 (6.44) |
|
| |||
| Body Mass Index, N (%) | |||
| Underweight/Normal | 345,580 (35.93) | 64,804 (34.35) | 3,951 (28.29) |
| Overweight | 300,335 (31.22) | 57,815 (30.64) | 4,010 (31.59) |
| Obese | 212,891 (22.13) | 44,292 (23.48) | 4,080 (32.14) |
| Missing | 103,082 (10.72) | 21,751 (11.53) | 1,013 (7.98) |
|
| |||
| Townsend Score, N (%) | |||
| 1st Quintile | 241,061 (25.06) | 43,677 (23.15) | 3,049 (24.02) |
| 2nd Quintile | 203,781 (21.19) | 38,297 (20.30) | 2,659 (20.95) |
| 3rd Quintile | 192,114 (19.97) | 38,274 (20.29)34,331 | 2,575 (20.29) |
| 4th Quintile | 168,741 (17.54) | (18.20)24,721 | 2,235 (17.61) |
| 5th Quintile | 115,859 (12.04) | (13.10)9,364 (4.96) | 1,613 (12.71) |
| Missing | 40,332 (4.19) | 563 (4.44) | |
|
| |||
| History of IVDU2, N (%) | 133 (0.01) | 45 (0.02) | 4 (0.03) |
|
| |||
| History of dialysis3, N (%) | 946 (0.10) | 151 (0.08) | 23 (0.18) |
|
| |||
| History of blood transfusion, N (%) | 7,887 (0.82) | 1,397 (0.74) | 138 (1.09) |
All p values are <0.001 unless otherwise reported.
p = 0.002
p = 0.001
Table 2.
Treatment Characteristics of Patients with Moderate-Severe Psoriasis
| Treatment | N1 (%) |
|---|---|
| Any | 12,694 |
| Phototherapy | |
| Any | 3,329 (26.22) |
| UVA/UVB | 2,395 (18.87) |
| PUVA | 934 (7.36) |
| Oral systemic | |
| Any | 9,984 (78.65) |
| Methotrexate | 8,796 (69.29) |
| Cyclosporine | 1,315 (10.36) |
| Oral Retinoids | 778 (6.13) |
| Biologic | |
| Any | 121 (0.95) |
| Etanercept | 47 (0.37) |
| Adalimumab | 60 (0.47) |
| Infliximab | 14 (0.11) |
| Ustekinumab | 2 (0.02) |
All treatments reported for any patient are included
Patients with psoriasis had a higher lifetime prevalence of HCV as compared to control patients: 0.17% (95% CI: 0.16 – 0.19) vs 0.12% (95% CI: 0.11 – 0.13) (p< 0.001). In fully adjusted models, controlling for age, sex, and observation time, psoriasis overall (aOR: 1.24, 95% CI: 1.10 – 1.40) and mild psoriasis (aOR: 1.25, 95% CI: 1.11 – 1.42) were associated with an increased prevalence of HCV. The prevalence of HCV in the moderate-severe group was similar to the control group (aOR: 1.01, 95% CI: 0.63 – 1.63) (Fig. 2). Sensitivity analyses excluding patients with a history of any systemic therapy (methotrexate, cyclosporine, oral retinoids, and biologics) increased the point-estimate of the odds ratio in the moderate-severe psoriasis group (OR: 1.93, 95% CI: 0.96 – 3.88) (Fig. 2).
Figure 2.
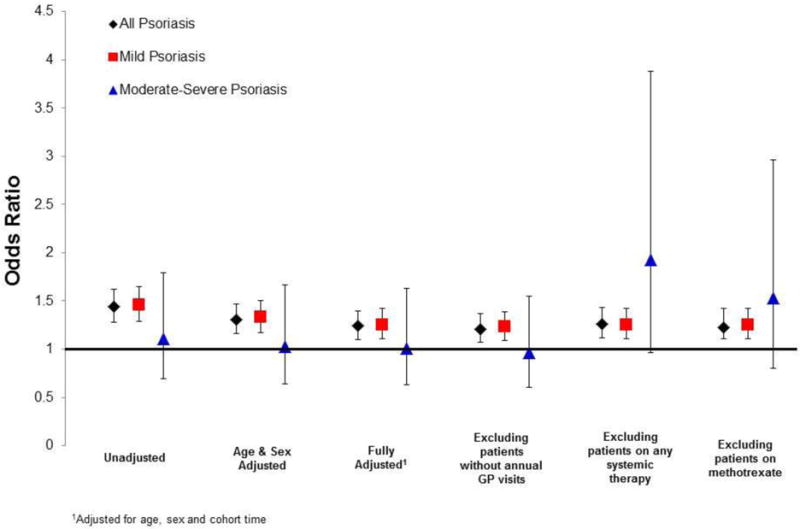
Prevalence of HCV infection in psoriasis patients compared to matched controls in THIN
In the iHOPE cohort, we identified 8,756 patients with psoriasis and 87,495 controls. Patient demographics were similar to those in THIN (Table 3). Patients with psoriasis had a higher lifetime prevalence of HCV as compared to control patients: 0.21% (95% CI: 0.11 – 0.30%) vs 0.11% (95% CI: 0.09 – 0.14%, p = 0.02). Based on disease severity, patients with mild psoriasis (BSA <3%) had a prevalence of 0.24%, (95% CI: 0.21 – 0.43) and those with moderate psoriasis (BSA 3–10%) had a prevalence of 0.22%, (95% CI: 0.09 – 0.46%). There were no cases of HCV in the severe psoriasis (BSA >10%) group (95% CI: 0 – 0.34%). In fully adjusted models, controlling for age, sex, smoking status and observation time, only mild psoriasis (aOR: 1.88, 95% CI: 1.01 – 3.51%) was associated with an increased prevalence of HCV. The prevalence did not reach statistical significance in the overall psoriasis group (aOR: 1.52, 95% CI: 0.92 – 2.51%) or the moderate group (aOR: 1.57, 95% CI: 0.73 – 3.39%) however point estimates of association were consistent with an increased prevalence of HCV as well. These results remained robust to sensitivity analyses.
Table 3.
Baseline Demographics in iHOPE1
| Controls | Mild Psoriasis | Moderate Psoriasis | Severe Psoriasis | |
|---|---|---|---|---|
|
| ||||
| N | 87,495 | 4,538 | 3,132 | 1,086 |
|
| ||||
| Age, mean in yrs (SD)2 | 45.31 (11.08) | 45.67 (11.22) | 45.26 (11.02) | 44.50 (10.86) |
|
| ||||
| Female Sex, N (%) | 46,304 (52.92) | 2,369 (52.20) | 1,481 (47.29) | 478 (44.01) |
|
| ||||
| Cohort time, years (SD)3 | 13.53 (6.75) | 13.42 (6.77) | 13.79 (6.61) | 13.75 (6.70) |
|
| ||||
| Smoking History, N (%) | ||||
| None | 42,548 (48.63) | 1,751 (38.59) | 1,072 (34.23) | 384 (35.36) |
| Current | 21,295 (24.34) | 1,344 (29.62) | 1,040 (33.21) | 364 (33.52) |
| Former | 22,289 (25.47) | 1,404 (30.94) | 992 (31.67) | 331 (30.48) |
| Missing | 1,363 (1.56) | 39 (0.86) | 28 (0.89) | 7 (0.64) |
|
| ||||
| Drinking History, N (%) | ||||
| None | 8,640 (9.87) | 386 (8.51) | 255 (8.14) | 92 (8.47) |
| Current | 63,475 (72.55) | 3,393 (74.77) | 2,301 (73.47) | 786 (72.38) |
| Former | 5,191 (5.93) | 297 (6.54) | 214 (6.83) | 81 (7.46) |
| Missing | 10,189 (11.65) | 462 (10.18) | 362 (11.56) | 127 (11.69) |
|
| ||||
| Body Mass Index, Mean (SD) | 27.06 (5.72) | 27.61 (6.00) | 28.02 (6.13) | 29.06 (6.68) |
|
| ||||
| Body Mass Index, N (%) | ||||
| Underweight/Normal | 31,569 (36.08) | 1,529 (33.69) | 956 (30.52) | 270 (24.86) |
| Overweight | 27,289 (31.19) | 1.430 (31.51) | 980 (31.29) | 317 (29.19) |
| Obese | 19,450 (22.23) | 1,170 (25.78) | 874 (27.91) | 365 (33.61) |
| Missing | 9,187 (10.50) | 409 (9.01) | 322 (10.28) | 134 (12.34) |
|
| ||||
| Townsend Score, N (%) | ||||
| 1st Quintile | 21,260 (24.30) | 1,014 (22.34) | 714 (22.80) | 224 (20.63) |
| 2nd Quintile | 17,786 (20.33) | 934 (20.58) | 585 (18.68) | 199 (18.32) |
| 3rd Quintile | 17,399 (19.89) | 923 (20.34) | 652 (20.82) | 211 (19.43) |
| 4th Quintile | 15,130 (17.29) | 787 (17.34) | 572 (18.26) | 221 (20.35) |
| 5th Quintile | 11,052 (12.63) | 604 (13.31) | 449 (14.34) | 176 (16.21) |
| Missing | 4,868 (5.56) | 276 (6.08) | 160 (5.11) | 55 (5.06) |
|
| ||||
| History of IVDU, N (%)3 | 26 (0.03) | 2 (0.04) | 1 (0.03) | 0 (0.00) |
|
| ||||
| History of dialysis, N (%)3 | 25 (0.03) | 1 (0.02) | 0 (0.00) | 1 (0.09) |
|
| ||||
| History of blood transfusion, N (%)3 | 343 (0.39) | 17 (0.37) | 8 (0.26) | 4 (0.37) |
All p values are <0.001 unless otherwise reported.
p = 0.012
p > 0.05
In THIN, among HCV+ individuals, 338 psoriasis patients and 1,193 controls developed hepatic decompensation (sTable 1). The incidence of hepatic decompensation was 6.19 events per 1000 person-years in controls and 9.33 events per 1000 person-years in patients with psoriasis. After adjusting for age and sex, the hazard ratios for hepatic decompensation were 1.58 (95% CI: 0.90 – 2.77) in all patients with psoriasis, 1.23 (95% CI: 0.66 – 2.28) in mild psoriasis and 21.51 (95% CI: 7.58 – 61.03) in moderate-severe psoriasis (Table 4).
Table 4.
Incidence of Hepatic Decompensation in THIN
| Controls | All Psoriasis | Mild Psoriasis | Moderate – Severe Psoriasis | |
|---|---|---|---|---|
| Mean follow-up time, yrs (SD) | 5.89 (4.64) | 5.26 (4.52) | 5.34 (4.60) | 3.64 (2.15) |
| Median (IQR) | 4.67 (1.91,9.17) | 3.74 (1.57,7.61) | 3.74 (1.56,7.74) | 4.05 (1.98, 4.72) |
| Total person-years | 7091 | 1822 | 1764 | 59 |
| New events, N (%) | 49 (4.02) | 17 (4.83) | 13 (3.88) | 4 (23.53) |
| Incidence per 1000 person yrs (95% CI) | 6.91 (5.22–9.14) | 9.33 (5.80–15.01) | 7.37 (4.28–12.69) | 68.30 (25.63–181.98) |
| Hazard Ratio1 (95% CI) | Reference | 1.58 (0.90–2.77) | 1.23 (0.66 – 2.28) | 21.51 (7.58–61.03) |
Adjusted for age and sex
Discussion
Results from our large, population-based study in the U.K. demonstrate a higher prevalence of HCV in patients with psoriasis compared with those without psoriasis (OR: 1.24, 95% CI: 1.10 – 1.40). A statistically significant relationship between HCV and psoriasis severity was not observed. Importantly, the background prevalence of HCV infection in our study (0.12 – 0.17%) is consistent with what has been previously reported for the general population in THIN, providing an external measure of validity13. Our study is, to our knowledge, the largest population-based analysis of HCV prevalence in adults with psoriasis and the first to evaluate the impact of psoriasis severity on HCV prevalence and HCV outcomes such as hepatic decomposition. Cohen et al previously reported a significant association between HCV and psoriasis using a population-based sample of 12,502 patients with psoriasis and 24,287 controls from Israel7. This risk differed by smoking status: OR = 1.93 (95% CI: 1.30–2.67) in smokers and OR = 2.22 (95% CI: 1.63–3.04) in non-smokers. Kanada et al examined 6,532 adults, including 162 with psoriasis, from the National Health and Nutrition Examination Survey in the United States and did not find a significant association between HCV and psoriasis (OR = 0.24, 95% CI: 0.03 – 2.01)9. Other studies assessing this relationship were single-institutional case series, limiting generalizability of results4–6, 8, 10.
We also found a statistically significant, increased rate of hepatic decompensation in HCV+ individuals with moderate-severe psoriasis (HR: 21.51, 95% CI: 7.58 – 61.03), a finding that has not previously been reported. The overall rate of hepatic decompensation in our study was 6.19 events per 1000 person-years in controls and 9.33 events per 1000 person-years in patients with psoriasis, which is consistent with previous reports of approximately 3 – 53 per 1000 person-years18–20. This increased risk of hepatic decompensation in patients with moderate-severe psoriasis suggests recognition and treatment of HCV in this population is important to reduce risk of subsequent liver failure.
Our study has several strengths compared to previous research. Our analysis of more than 200,000 psoriasis patients and almost 1,000,000 controls is orders of magnitude greater in size than prior studies. This large sample with practice-matched, date-matched controls and extensive series of sensitivity analyses minimizes observation bias that may be more apparent in other observational studies. Using THIN, we were also able to examine known risk factors for HCV infection (IV drug use, dialysis, blood transfusion) and hepatic decompensation (obesity, alcohol use, diabetes, hyperlipidemia and concomitant hepatitis B infection). Moreover, psoriasis and HCV diagnoses have been previously validated in THIN, minimizing misclassification12, 13.
There are also some limitations. Given the cross-sectional design of the HCV prevalence study, we are unable to determine the directionality of the relationship between HCV infection and psoriasis; however, the exact timing of HCV infection is often difficult to determine, because many people are diagnosed years after being infected. In general, the pathophysiology of concordant HCV infection and psoriasis is not well understood, but is has been previously proposed HCV infection may lead to the development of psoriasis in genetically susceptible individuals3. Furthermore, blocking tumor necrosis factor, a well-established treatment mechanism for psoriasis, has also been shown to be an effective adjuvant treatment for HCV infection21, further suggesting a shared inflammatory pathway. Also, it is difficult to know if the lack of relationship between HCV infection and severity of psoriasis is real or due to the low prevalence of HCV in the UK, resulting in limited statistical power to detect a relationship. One previous study of 90 adults with untreated psoriasis did show an association between Psoriasis Area Severity Index (PASI) and quantity of HCV-mRNA22, but additional and larger studies are needed to better understand the association between psoriasis severity and HCV infection.
In summary, HCV is a viral infection of the liver that leads to significant morbidity, including liver failure and hepatocellular carcinoma. In this study, HCV was more prevalent among psoriasis patients. Our study also suggests that more severe psoriasis is associated with an increased risk of hepatic decompensation among HCV+ individuals. Further research is necessary to better understand the pathophysiology and shared immunology between HCV and psoriasis. Dermatologists should be aware of this increased prevalence of HCV to screen all psoriasis patients with HCV risk factors prior to initiation of hepatotoxic or immunosuppressive medications. Earlier identification of HCV+ individuals will give more patients access to curative therapy, reducing the complications of chronic infection and decreasing transmission.
Supplementary Material
Acknowledgments
We are indebted to Vincent Lo Re, MD MSCE, Assistant Professor of Medicine and Senior Scholar in the Center for Clinical Epidemiology at the University of Pennsylvania for his assistance in conceptualizing this study design and expertise in hepatitis.
Funding/Support: This work was supported by an unrestricted grant from Pfizer to the Trustees of the University of Pennsylvania (JMG), a medical dermatology fellowship from the National Psoriasis Foundation (MHN), NIH Training Grants T32-GM075766 (MHN) and T32-AR007465-32 (SKG), and K24-AR064310 36 (JMG), K23-AR063764 (AO), and K23-AR068433 (JT), from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Financial Disclosures: In the previous 12 months, Dr. Gelfand served as a consultant for Abbvie, Astrazeneca, Celgene Corp, Coherus, Eli Lilly, Janssen Biologics (formerly Centocor), Sanofi, Merck, Novartis Corp, Valeant, and Pfizer Inc., receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Amgen, Eli Lilly, Janssen, Novartis Corp, Regeneron, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis. Dr. Gelfand is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma. Dr. Ogdie has served as consultant for Novartis and Pfizer and has received payment for continuing medical education work related to psoriatic arthritis. Dr. Takeshita received a research grant from Pfizer, to the trustees of the University of Pennsylvania, for work that is unrelated to what is presented in this manuscript and has received payment for continuing medical education work related to psoriasis.
Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- BSA
body surface area
- GP
general practioner
- HCV
hepatitis C virus
- iHOPE
Incident Health Outcomes and Psoriasis Events
- THIN
The Health Improvement Network
- UK
United Kingdom
Footnotes
Conflict of Interest Dr. Noe, Dr. Shin, and Ms. Grewal have no financial interests to disclose.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Antonelli A, Ferrari SM, Ruffilli I, Fallahi P. Cytokines and HCV-related autoimmune disorders. Immunol Res. 2014;60(2–3):311–9. doi: 10.1007/s12026-014-8569-1. [DOI] [PubMed] [Google Scholar]
- 3.Chun K, Afshar M, Audish D, Kabigting F, Paik A, Gallo R, et al. Hepatitis C may enhance key amplifiers of psoriasis. J Eur Acad Dermatol Venereol. 2016 doi: 10.1111/jdv.13578. [DOI] [PubMed] [Google Scholar]
- 4.Chouela E, Abeldano A, Panetta J, Ducard M, Neglia V, Sookoian S, et al. Hepatitis C virus antibody (anti-HCV): prevalence in psoriasis. Int J Dermatol. 1996;35(11):797–9. doi: 10.1111/j.1365-4362.1996.tb02977.x. [DOI] [PubMed] [Google Scholar]
- 5.Taglione E, Vatteroni ML, Martini P, Galluzzo E, Lombardini F, Delle Sedie A, et al. Hepatitis C virus infection: prevalence in psoriasis and psoriatic arthritis. J Rheumatol. 1999;26(2):370–2. [PubMed] [Google Scholar]
- 6.Andrade DL, de Oliveira Mde F, de Souza TF, Lima RA, Bomfim EA, Rego VR, et al. A study about hepatitis C virus infection in patients with psoriasis in a Brazilian reference center. Acta Gastroenterol Latinoam. 2012;42(4):285–90. [PubMed] [Google Scholar]
- 7.Cohen AD, Weitzman D, Birkenfeld S, Dreiher J. Psoriasis associated with hepatitis C but not with hepatitis B. Dermatology. 2010;220(3):218–22. doi: 10.1159/000286131. [DOI] [PubMed] [Google Scholar]
- 8.Imafuku S, Nakayama J. Profile of patients with psoriasis associated with hepatitis C virus infection. J Dermatol. 2013;40(6):428–33. doi: 10.1111/1346-8138.12112. [DOI] [PubMed] [Google Scholar]
- 9.Kanada KN, Schupp CW, Armstrong AW. Association between psoriasis and viral infections in the United States: focusing on hepatitis B, hepatitis C and human immunodeficiency virus. J Eur Acad Dermatol Venereol. 2013;27(10):1312–6. doi: 10.1111/j.1468-3083.2012.04563.x. [DOI] [PubMed] [Google Scholar]
- 10.Kanazawa K, Aikawa T, Tsuda F, Okamoto H. Hepatitis C virus infection in patients with psoriasis. Arch Dermatol. 1996;132(11):1391–2. doi: 10.1001/archderm.132.11.1391. [DOI] [PubMed] [Google Scholar]
- 11.Bourliere M, Bronowicki JP, de Ledinghen V, Hezode C, Zoulim F, Mathurin P, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS) Lancet Infect Dis. 2015;15(4):397–404. doi: 10.1016/S1473-3099(15)70050-2. [DOI] [PubMed] [Google Scholar]
- 12.Seminara NM, Abuabara K, Shin DB, Langan SM, Kimmel SE, Margolis D, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol. 2011;164(3):602–9. doi: 10.1111/j.1365-2133.2010.10134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo Re V, 3rd, Haynes K, Forde KA, Localio AR, Schinnar R, Lewis JD. Validity of The Health Improvement Network (THIN) for epidemiologic studies of hepatitis C virus infection. Pharmacoepidemiol Drug Saf. 2009;18(9):807–14. doi: 10.1002/pds.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.THIN Database. [Available from: https://www.ucl.ac.uk/pcph/research-groups-themes/thin-pub/database.
- 15.Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–5. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 16.Yeung H, Takeshita J, Mehta NN, Kimmel SE, Ogdie A, Margolis DJ, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–9. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chisholm J. The Read clinical classification. BMJ. 1990;300(6732):1092. doi: 10.1136/bmj.300.6732.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. Jama. 2000;284(4):450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 19.Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002;97(11):2886–95. doi: 10.1111/j.1572-0241.2002.07057.x. [DOI] [PubMed] [Google Scholar]
- 20.Bruno S, Zuin M, Crosignani A, Rossi S, Zadra F, Roffi L, et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol. 2009;104(5):1147–58. doi: 10.1038/ajg.2009.31. [DOI] [PubMed] [Google Scholar]
- 21.Zein NN. Etanercept as an adjuvant to interferon and ribavirin in treatment-naive patients with chronic hepatitis C virus infection: a phase 2 randomized, double-blind, placebo-controlled study. J Hepatol. 2005;42(3):315–22. doi: 10.1016/j.jhep.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Gabr SA, Berika MY, Alghadir AH. Apoptosis and clinical severity in patients with psoriasis and HCV infection. Indian J Dermatol. 2014;59(3):230–6. doi: 10.4103/0019-5154.131377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


