Abstract
Alteration of dynamic range of modulation to cognitive difficulty has been proposed as a salient predictor of cognitive aging. Here we examine in 171 adults (aged 20–94 years) the effects of age on dynamic modulation of BOLD activation to difficulty in parametrically increasing working memory load (0-,2-,3-,4-back conditions). First, we examined parametric increases and decreases in activation to increasing WM load (positive modulation effect and negative modulation effect). Second, we examined the effect of age on modulation to difficulty (WM load) to identify regions that differed with age as difficulty increased (age-related positive and negative modulation effects). Weakened modulation to difficulty with age was found in both the positive-modulation (middle frontal, superior/inferior parietal) and negative-modulation effect (deactivated) regions (insula, cingulate, medial superior frontal, fusiform, and parahippocampal gyri, hippocampus, and lateral occipital cortex). Age-related alterations to positive modulation emerged later in the lifespan than negative modulation. Further, these effects were significantly coupled in that greater up-modulation was associated with lesser down-modulation. Importantly, greater frontal-parietal up-modulation to difficulty and greater down-modulation of deactivated regions was associated with better task accuracy and up-modulation with better working memory span measured outside the scanner. These findings suggest that greater dynamic range of modulation of activation to cognitive challenge is in service of current task performance, as well as generalizing to cognitive ability beyond the scanner task, lending support to its utility as a marker of successful cognitive aging.
Keywords: aging, fMRI, difficulty modulation, coupling, n-back, working memory
1. Introduction
As we age, most fluid cognitive functions decline, including one of the most fundamental cognitive skills, working memory (WM) (Babcock and Salthouse, 1990; Park et al., 2002; Salthouse, 1994; Van der Linden et al., 1994). Both manipulation of items in working memory (Dobbs and Rule, 1989) and the updating of this information (Artuso et al., 2016; Clarys et al., 2009; van der Linden et al., 1994; Hartman et al., 2001) decline with increasing age. The n-back paradigm has been widely utilized in behavioral and functional magnetic resonance (fMRI) studies of WM (Owen et al., 2005; Rottschy et al., 2011), as it requires participants to monitor and flexibly update items kept in WM, and because WM load can be parametrically increased to examine change in BOLD response to increasing cognitive demand. WM robustly engages regions of bilateral dorsolateral prefrontal cortex (DLPFC), posterior parietal cortex (PPC), cingulate gyrus, and lateral cerebellar cortex (Cabeza and Nyberg, 2000). The n-back task consistently activates regions of the cognitive control network: premotor, middle frontal gyrus, anterior cingulate gyrus, and posterior parietal cortex (Owen et al., 2005). In younger adults, increasing WM load is associated with increasing modulation of activation in these fronto-parietal regions (Manoach et al., 1997).
Parametric n-back tasks have been utilized across various populations and contexts (Braver et al., 1997; Callicott et al., 1999; Choo et al., 2005; Cohen, 1997; Druzgal and D’Esposito, 2001; Jansma et al., 2000; Jonides et al., 1997) including the study of normal aging (Cappell et al., 2010; Heinzel et al., 2014, 2016; Mattay et al., 2006; Nagel et al., 2009; Nyberg et al., 2009; Rypma and D’Esposito, 2000; Sala-Llonch et al., 2012; Schulze et al., 2011). In general (with the exception of Kaup et al., 2014 and Wishart et al., 2006), aging studies rely on comparison of extreme age groups (i.e. young vs. old) to examine age differences in modulation to increasing WM load, rather than examining the entire adult lifespan. These comparisons find unilateral prefrontal cortex response in younger adults, but bilateral frontal activation in older adults, with some studies finding greater activation in old compared to younger adults, at lower (e.g., 1-back and 2-back) WM loads (Mattay et al., 2006; Prakash et al., 2012; Reuter-Lorenz et al., 2000; Schneider-Garces et al., 2010). These extreme age-group comparisons, however, omit two important portions of the lifespan, namely middle-age and very old adulthood. In a growing number of recent studies (Ankudowich et al., 2016; Chan et al., 2014; Grady et al., 2006; Kennedy et al., 2015; Kwon et al., 2016; Park et al., 2013; Rieck et al., 2017), middle-age has been illustrated to be an essential piece of information in determining when functional brain changes occur in adulthood. Structurally, the fronto-parietal regions included in the canonical WM network are known to degrade with age around this time in both gray and white matter (Kennedy and Raz, 2009; Raz et al., 2005), and these declines are related to poorer cognition (Kennedy and Raz, 2009; Raz and Rodrigue, 2006).
Recent research has pin-pointed middle-age as a time when important functional changes in modulation to difficulty appear (Kennedy et al., 2015; Rieck et al., 2017). Using a spatial distance judgment paradigm, Rieck et al., 2017 found reduction in dynamic range of BOLD modulation to parametrically increasing difficulty in both fronto-parietal up-modulation (i.e., the ability to increase activation from easier to more difficult conditions) and in down-modulation (i.e., ability to increase deactivation from easier to more difficult conditions) of deactivated regions (such as vmPFC) with age. This reduced dynamic range was evident in early middle-age for down-modulated regions, but not evident until older-age in up-modulated brain regions, suggesting that these modulatory processes follow different aging trajectories. Interestingly, it is increasingly hypothesized that these processes are not independent, but act in synergy. Turner and Spreng (2015) have hypothesized that aging brings about an increased coupling of activation of these “default” (i.e., medial frontal, anterior and posterior cingulate, hippocampal formation) and “executive” (i.e., dorsolateral prefrontal, posterior parietal, cingulate) systems, or DECHA (Default-Executive Coupling Hypothesis of Aging), possibly as an adaptive support for declining fluid abilities with aging. Rieck et al (2017) found significant coupling of up-modulated and down-modulated regions, in that individuals who showed greater modulation in one direction also showed greater modulation in the other direction. Interestingly, greater coupling was associated with higher fluid intelligence. To be able to interpret the nature of alterations in BOLD data, i.e., whether they are beneficial, detrimental, or unrelated to performance, these differences need to be yoked to cognition, ideally to both task performance during scanning and to measures of related cognitive processes assessed outside of the scanner (Grady, 2012).
Here, we aimed to address these issues in the existing literature by examining the full adult lifespan, with substantial sample size, utilizing a richer parametric increase in working memory load than generally used to determine the nature of age-related alterations in dynamic range by yoking BOLD modulation to task performance and out-of-scanner cognition. Thus, the current study sought to characterize age-related differences in modulation to parametrically increasing WM load in a large lifespan sample of healthy adults. Specifically, we aimed to examine when in the lifespan alterations to up-modulation and down-modulation occur, whether and how these shifts in dynamic range are coupled, and whether they are related to task performance and generalize to working memory beyond the scanner environment. To test this, 171 individuals aged 20–94 years old underwent fMRI scanning during a digit n-back paradigm with blocks of incrementally increasing working memory load: 0-, 2-, 3-, 4-back, allowing us to model both age and WM load as continuous variables. We hypothesized that both positive and negative modulation to difficulty would decrease with age, that this modulation would be significantly coupled, and that greater dynamic range of modulation would be associated with better working memory, both during scanning and on a test of WM span.
2. Methods
2.1 Participants
Participants consisted of 171 individuals aged 20–94 (mean age = 53.03 ± 19.13 years; 100 women; 71 men) recruited from the greater Dallas metro area via media advertisements and flyers. All participants received compensation for their time. Prior to enrollment, participants completed a health history screening, telephone, and in-person interviews. All individuals were screened against neurological, psychiatric, metabolic, and cardiovascular disease (except for controlled essential hypertension, n = 35), head trauma with loss of consciousness, diabetes, and cognition-altering medications. To screen for dementia and depression exclusion, participants completed the Mini Mental State Examination (MMSE; Folstein et al., 1975) and the Center for Epidemiological Study Depression Scale (CES-D; Radloff, 1977), with cutoffs < 26 and ≥ 16, respectively (mean MMSE = 29.04 ± 0.86, range 26–30; mean CESD = 4.19 ± 3.79, range 0–16). Participants were fluent English speakers, right-handed, had a minimum of high school education or equivalent (mean education = 15.58 ± 2.49 years), and had normal or corrected-to-normal vision (at least 20/40); when necessary vision was corrected to normal using MRI compatible lenses during the scanning session. See Table 1 for breakdown of sample demographics by age group. Note, however, that age was sampled and analyzed as a continuous variable, unless specified otherwise. All participants provided written informed consent in accord with the University of Texas at Dallas and the University of Texas Southwestern Medical Center institutional review board guidelines.
Table 1.
Participant Demographics by Age Group
| Age | N (M/F) | Edu (SD) | MMSE (SD) | CESD (SD) |
|---|---|---|---|---|
| Younger (20–35) | 42 (18/24) | 15.62 (2.17) | 29.19 (0.94) | 4.48 (3.62) |
| Middle (36–55) | 47 (21/26) | 15.28 (2.52) | 29.26 (0.87) | 4.98 (4.48) |
| Older (56–69) | 38 (16/22) | 15.84 (2.34) | 28.89 (0.76) | 3.40 (2.93) |
| Oldest (70–94) | 44 (16/28) | 15.73 (2.82) | 28.77 (0.83) | 3.77 (3.73) |
|
| ||||
| Total | 171 (71/100) | 15.60 (2.47) | 29.04 (0.87) | 4.19 (3.79) |
Note. Abbreviations: M = male, F = female; Edu = years of education; SD = standard deviation; MMSE = Mini Mental Status Examination; CESD = Center for Epidemiological Study – Depression scale.
2.2 Cognitive Measures
On two separate days, prior to the MRI session, participants underwent extensive cognitive testing spanning a number of cognitive domains (as in Rieck et al., 2017). Because our fMRI task was focused on the domain of working memory, in this study we focused on a similar out-of-scanner cognitive task: the Wechsler Adult Intelligence Scale - Digit Span subtest (WAIS-DS) (Wechsler, 2008). There are three sub-sections of the DS -- Forward, Backward, and Sequencing – that increment in difficulty and thus we use the more taxing span, Sequencing, for analysis in this study to best mirror the difficult nature of the n-back task implemented.
2.3 MRI Protocol
2.3.1. MRI Acquisition
Participants were scanned on a single 3T Philips Achieva scanner equipped with a 32-channel head coil. Blood Oxygenation Level Dependent (BOLD) data were collected using a T2*-weighted echo-planar imaging sequence with 29 interleaved axial slices per volume providing full brain coverage and acquired parallel to the AC-PC line, (64 × 64 × 29 matrix, 3.4 × 3.4 × 5 mm3, FOV = 220 mm2, TE = 30 ms, TR = 1.5 s, flip angle = 60°). High resolution anatomical images were also collected with a T1-weighted MP-RAGE sequence with 160 sagittal slices, 1 × 1 × 1 mm3 voxel size; 256 × 204 × 160 matrix, TR =8.3 ms, TE= 3.8 ms, flip angle = 12°.
2.3.2. fMRI Task Procedure
Using a block design, participants were presented with an n-back task using digits as stimuli. This working memory paradigm required participants to monitor the identity of a series of digits and indicate by button press whether the currently presented stimulus matched the one presented n trials previously. For each trial, participants made a SAME/DIFFERENT response with their index (same) or middle (different) finger to indicate whether the digit was the same or different as n digits ago. Before each block began, a 5 sec cue indicated which type of n-back was about to start: 0-back, 2-back, 3-back, or 4-back, followed by 2 sec of fixation prior to the presentation of digits. For the 0-back trials, participants made a digit identification decision, indicating whether or not the digit on the screen was the one cued at the beginning of the block. Digits (“2–9”) were presented in six pseudo-counterbalanced blocks, for 500 ms with a 2000 ms interstimulus interval using Psychopy v1.77.02 (Peirce 2007; 2009). Each run of data consisted of 8 blocks, including two blocks of each level of difficulty. The blocks were counterbalanced for difficulty within run. Of the 420 trials, 144 were match trials (18 for 0-back and 42 each for 2-,3-,4-back), and 276 were non-match trials (42 0-back and 78 each for 2-,3-,4-back). The 0-back blocks had 10 trials, while the 2-, 3-, and 4-back blocks each had 20 trials. The trials were presented in a pseudo-random order. There were three runs total, yielding a total functional scan time of about 20 minutes.
2.3.4. Task Pre-training Procedure
Participants were trained on the task just prior to entering the scanner to maximize their ability to understand task instructions and to complete the various levels of working memory load. First, trained researchers demonstrated the task, including a real-time on-screen run-through of each level of difficulty, as well as a schematic detailing when participants should respond “same” or “different”. After each demonstration participants completed a brief practice in which they used a duplicate of the scanner button box and were required to perform > 80% accuracy before advancing a level of difficulty. Once the participant completed all levels of difficulty s/he completed a second brief practice to emulate what they would experience in the scanner (cue presentation, timing, etc.). The participants had on average 7.13 (± 2.26; range 5–16) exposures during practice (minimum of 5 because 1-back was also practiced). Participants also completed a post-scan questionnaire providing information about their experience in the experiment.
2.4. fMRI Data Processing
Data preprocessing and statistical analyses were performed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK) along with in-house Matlab R2012b (Mathworks) scripts. Additionally, Art Repair toolbox (Mazaika et al., 2007) was used to identify potential outliers in movement (>2 mm displacement) and intensity shift (>3% deviation from the mean in global intensity spikes) in the EPI images. Runs with >15% of total volumes (~40 volumes) marked as outliers for movement were excluded (excluded n = 3). In order to be included in the analysis, participants were required to have at least two runs (out of three runs total) with quality data. Three additional participants were excluded for the following reasons: poor T1 acquisition (n = 2), no response on > 15% of trials (n = 1). Functional images were adjusted for slice acquisition time and motion correction (using 6 directions of motion-estimates from ArtRepair included as nuisance regressors), and each participant’s T1-weighted anatomical image was used to co-register the functional maps to standardized MNI space. The resulting normalized images were smoothed with an isotropic 8mm FWHM Gaussian kernel.
2.4.1. fMRI Data Analysis
Subject-level and group-level analyses were performed using the general linear model in SPM8. At the individual subject level, contrasts for each level of difficulty were computed (0-, 2-, 3-, 4-back), as well as a linear contrast (using −2.25, −0.25, 0.75, 1.75 weights). For the group-level analyses, age was used as a continuous between-subjects second-level covariate whereas linear modulation was used as a within-subject first-level covariate in a multiple regression model predicting BOLD activity. Two primary contrasts of interest were implemented. First, we examined parametric increases and decreases in activation to increasing WM load (positive modulation effect and negative modulation effect). Second, we examined the effect of age on modulation to difficulty (WM load) to identify regions that differed with age as difficulty increased (age-related positive and negative modulation effects). In addition, we examined the quadratic effect of age on increases and decreases in activation to WM load. To ensure that Type I error rates were correctly controlled at the cluster level (Eklund et al., 2016), cluster corrections were calculated using the Statistical nonParametric Mapping toolbox (SnPM13; http://warwick.ac.uk/snpm) with 5000 permutations to derive the corrected clusterwise threshold. Surface mapping of group effects was conducted using Caret (Van Essen et al., 2001). Marsbar toolbox in SPM8 (Brett et al. 2002), was used when extracting mean parameter estimates from clusters in the positive and negative modulation effects for each participant from their linear contrast of difficulty.
3. Results
3.1. Task Performance
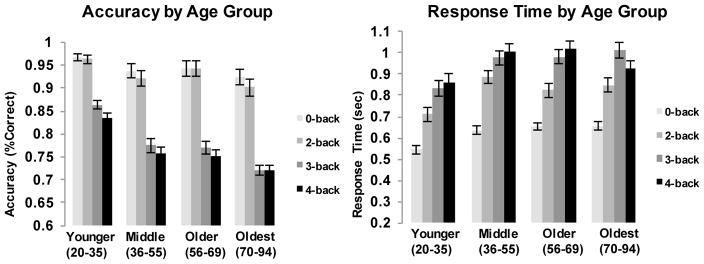
Accuracy and response time (RT) were recorded for all trials. To examine performance differences across WM load levels and potential age effects, two repeated-measures general linear models (GLM) were tested with WM load serving as a 4-level within-subject repeated measure and age (mean centered) as a continuous between-subjects measure predicting either mean accuracy or median RT. For accuracy, we found significant effects of age (F [1,169] = 33.89, p < .001) and WM load (F [3,507] =373.65, p < .001), with accuracy declining as both age and WM load increased. A significant age × WM load interaction (F [3,507] = 12.05, p < .001) indicated that this effect became stronger with increasing age. Response time increased with increasing WM load (F [3,507] = 287.50, p <.001), as well as with age (F [1,169] = 10.60, p =.001), and there was a trend for age x WM load interaction (F [3,507] = 2.61, p =.051). Although we sampled and analyzed age as a continuous variable, for ease of illustration, Figure 1 provides an age group by WM load breakdown for accuracy and RT. Note that all trials were included (i.e., match and non-match).
Figure 1.
fMRI Task Performance by Working Memory Load Level and Age. The left panel illustrates accuracy and the right panel illustrates median response time (in seconds) for each WM load condition broken down by age group (for illustration purposes).
3.2. Neuroimaging Results
3.2.1. Effect of Working Memory Load on BOLD Activation
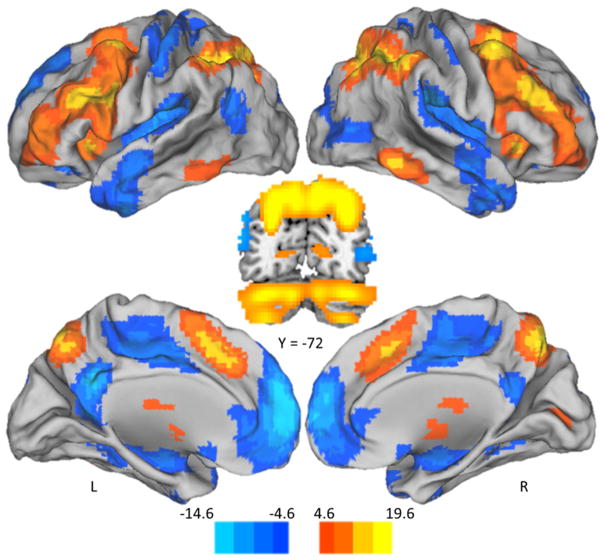
We first sought to establish the brain regions that activate and deactivate in response to parametrically increasing WM load. The positive modulation effect (warm scale in Figure 2; coordinates in Table 2A) represents regions which increased in modulation with increasing WM load, including bilateral anterior cingulate gyrus, dorsolateral prefrontal cortex, middle frontal gyrus, superior precuneus, caudate, cerebellum and posterior parietal cortex, which corresponds well with the canonical cognitive control and working memory networks (Cabeza and Nyberg, 2000). The negative modulation effect (cool scale in Figure 2; coordinates in Table 2B) represents regions which increased in negative modulation (i.e., increased deactivation to difficulty) with increasing WM load and includes posterior cingulate gyrus, medial prefrontal cortex, superior temporal gyrus, and lateral occipital cortex; regions often associated with task-negative and default networks (Grecius et al., 2003).
Figure 2.
Positive Modulation and Negative Modulation Effects of Increasing WM load. Parametric WM load increase from 0- to 4-back was associated with both increased modulation of activation (positive modulation effect in warm color scale), and decreased modulation of activation (negative modulation effect in cool color scale). Positive modulation occurred bilaterally in dorsal cingulate gyrus, dorsolateral prefrontal cortex (middle and inferior frontal gyri) and posterior parietal cortex, superior precuneus, thalamus, midbrain, caudate, and cerebellum. Negative modulation occurred in bilateral posterior cingulate gyrus, bilateral medial prefrontal cortex, bilateral superior temporal gyrus, and bilateral lateral occipital regions. Color-scale indicates t-values. Abbreviations: L – Left, R – Right.
Table 2.
Regions that increased or decreased significantly to increasing working memory load
| A. Positive modulation to difficulty effect | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Label | BA | k | X | Y | Z | t-value | Cluster-Level p fwe |
| L/R posterior parietal/precuneus | 39/40/7 | 4731 | 42 | −42 | 42 | 19.57 | < .001 |
| −39 | −48 | 42 | 18.94 | ||||
| 33 | −66 | 39 | 18.90 | ||||
|
| |||||||
| L/R frontal/subcortical/cerebellum | 6/9 | 16431 | −30 | −63 | −33 | 17.64 | < .001 |
| 30 | −63 | −33 | 16.94 | ||||
| 33 | 12 | 54 | 16.74 | ||||
| B. Negative modulation to difficulty effect | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Label | BA | k | X | Y | Z | t-value | Cluster-Level p fwe |
| L/R medial cortex/temporal | 10/20/47 | 18456 | −3 | 60 | 3 | 14.57 | < .001 |
| −6 | −51 | 27 | 13.09 | ||||
| 45 | −27 | 21 | 11.59 | ||||
Note. The Statistical nonParametric Mapping toolbox v13 (SnPM) was used to calculate cluster thresholds. Abbreviations: BA – Brodmann Area; L – Left; R – Right
3.2.2. Effects of Age on Modulation of Activation to Parametric Increase in Working Memory Load
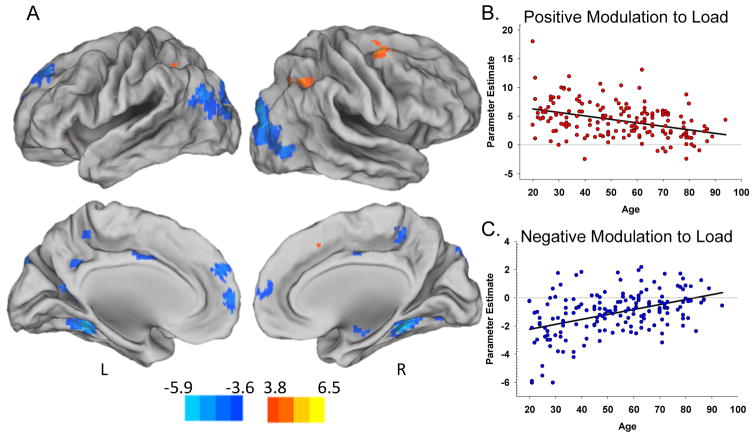
We next sought to examine the effect of age on positive and negative modulation during increasing WM load (i.e., age x WM load interactions). Results indicate a significant weakening of both positive and negative modulation with increasing age. Positive modulation to WM load decreased across the lifespan in right middle frontal gyrus, bilateral superior and inferior parietal lobule, and left supplemental motor area (warm scale in Figure 3A; coordinates in Table 3A). Negative modulation to WM load decreased across the lifespan in left superior frontal gyrus, right insula, bilateral cingulate gyrus, fusiform gyrus, parahippocampal gyrus, and lateral occipital cortex (cool scale Figure 3A; coordinates in Table 3B). Scatterplots in Figure 3 illustrate the decreased dynamic range in both up- (panel B) and down-modulation (panel C) of activation to WM load across the lifespan. In addition to linear age effects, we tested for quadratic age effects during increasing WM load. No significant voxels showed a nonlinear effect. We also included covariates for intracranial volume and sex and the pattern of results was unchanged.
Figure 3.
Effects of aging on positive and negative modulation of activation to difficulty. Increasing age was associated with decreased modulation to difficulty in both regions of positive modulation (warm colors in panel A) and regions of negative modulation (cool colors in panel A). The regions of age-reduced modulation included the rMFG, bilateral superior and inferior parietal, and left supplemental motor area in warm colors, and the superior frontal gyrus, right insula, bilateral cingulate, bilateral fusiform, bilateral parahippocampal gyrus, and lateral occipital regions in cool colors. Panel B scatterplot illustrates the weakened positive modulation effect to difficulty with increasing age, averaged across all warm clusters. Panel C illustrates weakened negative modulation effect (decreased deactivation) with increasing age, averaged across all cool clusters. Color bar indicates t-values. Abbreviations: L – Left; R – Right.
Table 3.
Regions of effect of age on increasing parametric WM Load
| A. Age-Related Decreases in Positive Modulation to WM Load | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Label | BA | k | X | Y | Z | t-value | Cluster-Level p fwe |
| R middle frontal gyrus | 6/8 | 224 | 33 | 3 | 60 | 5.90 | 0.010 |
|
| |||||||
| R angular gyrus | 39 | 319 | 33 | −69 | 51 | 5.07 | 0.006 |
| 36 | −51 | 45 | 4.86 | ||||
|
| |||||||
| L angular gyrus | 39 | 96 | −33 | −54 | 48 | 4.18 | 0.046 |
| −27 | −60 | 39 | 3.96 | ||||
| −30 | −66 | 54 | 3.75 | ||||
|
| |||||||
| L supplementary motor area | 6 | 117 | 0 | 18 | 48 | 4.17 | 0.034 |
| 0 | 6 | 63 | 3.61 | ||||
| B. Age-Related Decreases in Negative Modulation to WM Load | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Label | BA | k | X | Y | Z | t-value | Cluster-Level p fwe |
| R fusiform gyrus | 37 | 281 | 24 | −39 | −12 | 6.54 | 0.010 |
| 24 | −72 | −9 | 4.29 | ||||
|
| |||||||
| L fusiform gyrus | 37 | 203 | −24 | −42 | −12 | 6.43 | 0.015 |
| −39 | −21 | −15 | 3.88 | ||||
|
| |||||||
| L angular gyrus | 39 | 844 | −48 | −75 | 30 | 6.00 | 0.002 |
| −15 | −93 | 36 | 5.51 | ||||
| −30 | −84 | 15 | 4.65 | ||||
|
| |||||||
| R middle occipital gyrus | 19 | 872 | 30 | −90 | 24 | 5.69 | 0.002 |
| 39 | −72 | 6 | 5.67 | ||||
| 21 | −93 | 36 | 5.50 | ||||
|
| |||||||
| L superior frontal gyrus | 9/10 | 636 | −3 | 60 | 6 | 4.95 | 0.002 |
| −15 | 45 | 45 | 4.86 | ||||
| −3 | 54 | 27 | 4.51 | ||||
|
| |||||||
| R hippocampus | 117 | 39 | −6 | −15 | 4.80 | 0.035 | |
| 30 | −9 | −18 | 4.55 | ||||
|
| |||||||
| L precuneus/lingual gyrus | 31 | 127 | −12 | −54 | 12 | 4.79 | 0.030 |
| −9 | −51 | 3 | 3.90 | ||||
| 0 | −51 | 21 | 3.24 | ||||
|
| |||||||
| R insula | 13 | 165 | 42 | −9 | 18 | 4.65 | 0.022 |
| 51 | −3 | 3 | 3.82 | ||||
|
| |||||||
| R/L precuneus | 31 | 127 | 12 | −42 | 54 | 4.26 | 0.030 |
| −12 | −42 | 54 | 3.96 | ||||
|
| |||||||
| L/R middle cingulate gyrus | 24 | 167 | 3 | −6 | 39 | 3.97 | 0.022 |
| −3 | 3 | 36 | 3.91 | ||||
| −9 | −6 | 30 | 3.34 | ||||
|
| |||||||
| R precentral gyrus | 6 | 108 | 45 | −15 | 66 | 3.75 | 0.04 |
| 39 | −6 | −15 | 4.80 | ||||
| 24 | −6 | −15 | 3.92 | ||||
Note. The Statistical nonParametric Mapping toolbox v13 (SnPM) was used to calculate cluster thresholds. Abbreviations: BA – Brodmann Area; L – left; R – Right
3.2.3. Decomposing the Age × WM Load Interactions
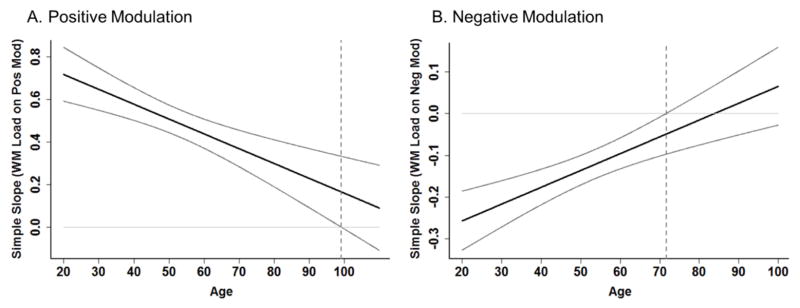
To gauge when in the lifespan failure to modulate begins, we decomposed these significant age x modulation interactions using simple slopes analyses (Preacher, Curran, and Bauer, 2006), with WM load as the focal predictor and age as the moderating variable of positive or negative modulation (see Figure 4). The significant interaction shows that age moderates the effect of WM load on BOLD such that the relationship between WM load and BOLD weakens with increasing age, however, the simple slopes analysis assesses at what specific age this relationship is no longer significant. This is achieved by testing at each point on our continuous age variable whether there is a significant slope between WM load and BOLD, providing bounding areas of significance, or points at which the relationship is or is not significant. Although positive modulation weakens linearly with age, the results of the simple slopes analysis further indicates that up-modulation to WM load remains significant until after age 99 (Figure 4A). In other words, the simple slope (of WM load on positive modulation) decreases across age, but remains significant throughout the age-range sampled in this study (20–94 years) with a predicted age of failure to up-modulate around age 99 (dotted line). The results suggest that fronto-parietal up-modulation to increased WM load occurs successfully throughout the lifespan, but this ability significantly declines with increasing age.
Figure 4.
Positive and Negative Modulation to Difficulty Decrease Differentially across the Lifespan. Simple slopes analysis was used to decompose the significant age x positive modulation and age x negative modulation interactions. A) Aging of positive modulation to difficulty declines linearly with age, and simple slope remains significantly different from zero until around age 99 (dotted line) at which point there would be a failure to modulate (i.e., no difference in BOLD response across difficulty conditions). B) Negative modulation declines with age (decreased deactivation to difficulty) and failure to modulate begins around age 71 (dotted line).
For the age × negative modulation interaction, although modulation weakens linearly across the lifespan, simple slope analysis further indicates the simple slope of WM load on negative modulation becomes non-significant around the age of 71 (dotted line in Figure 4B), indicating that beyond this age, participants on average no longer successfully negatively modulate to increasing WM load (i.e., does not differ from zero). These analyses indicate that down-modulation to WM load fails much earlier in the lifespan than fronto-parietal up-modulation, but that both capacities are retained until at least early old age.
3.3. Relationship between Positively-Modulated and Negatively-Modulated Regions
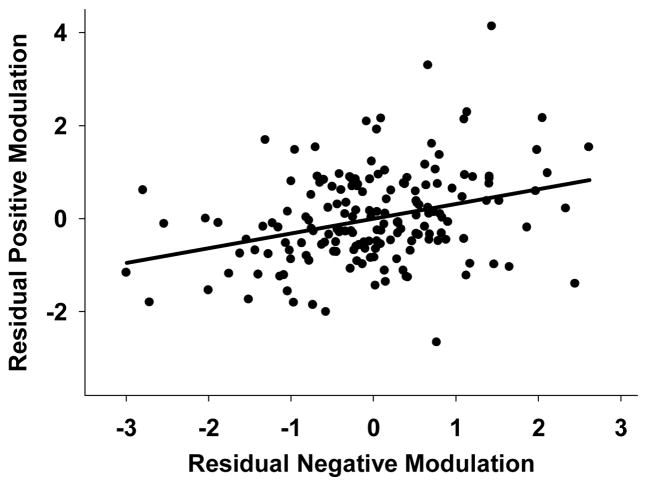
We were interested in determining whether the ability to positively and negatively modulate brain regions was significantly coupled. Partial correlations controlling for the effect of age were calculated between regions exhibiting positive modulation and regions exhibiting negative modulation. Results showed a significant relationship between regions of positive and negative modulation, prage = .32, p < .001, revealing a significant coupling of modulation between these effects beyond the effects of age. The nature of this coupling is illustrated in Figure 5, such that individuals who increase up-modulation in frontal-parietal regions tend to also reduce down-modulation in deactivated regions. This suggests that increased frontal-parietal dynamic range in the service of increasing WM load may come at the expense of decreased dynamic range of deactivated regions.
Figure 5.
Coupling of Positive and Negative Modulation to Difficulty. A significant relationship between BOLD modulation to difficulty was found between regions of positive and negative modulation, beyond the effect of age. Greater positive modulation was associated with decreased negative modulation, suggesting that up-modulating frontal-parietal regions to difficulty comes at the expense of further down-modulating deactivated regions. Modulation values reflect the slope of BOLD activation across the increasing difficulty levels and are residualized for age.
3.4. Effects of Modulation to Difficulty on Cognitive Performance
We next sought to interpret these neuroimaging findings in the context of cognitive performance in order to determine if these age-related alterations to positive and negative modulation are beneficial or detrimental. Parameter estimates were analyzed first by cluster to ensure similar effects across regions of the brain. Because the effects were similar across clusters, we created a mask of all positive effect clusters and all negative effect clusters and extracted parameter estimates for analysis. Following are the results of positive and negative modulation effects on task accuracy during scanning and then results of positive and negative modulation results on a working memory task, given outside the scanner (WAIS-DS).
3.4.1. N-back Task Accuracy
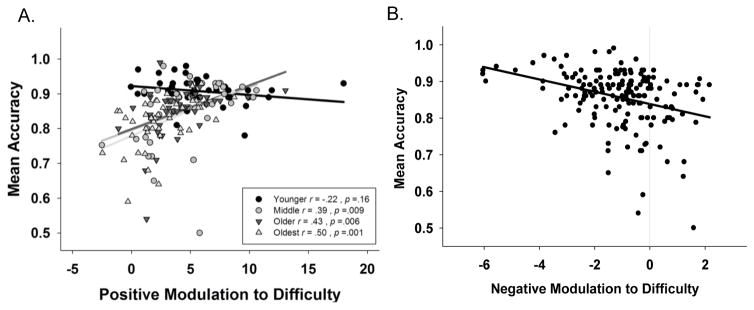
In a GLM, we tested for effects of modulation on mean task accuracy. Age, positive modulation, negative modulation (all continuous, centered variables) and their interactions were entered as predictors of mean task accuracy. We found a significant age × positive modulation interaction (F (1,163) = 9.02, p = 0.003) on accuracy, indicating that the relationship between modulation and accuracy differs by age. Figure 6A illustrates that the interaction is driven by significant association in middle-age and older, but not in younger adults. However, this effect may be due to lesser variance in accuracy in the young and could also be interpreted as the significant main effect of positive modulation (F (1,163) = 28.79, p < 0.001). We also found a significant effect of negative modulation (F (1,163) = 16.53, p < .001), indicating that greater negative modulation (greater deactivation to difficulty) was associated with higher accuracy, regardless of age (Figure 6B). No other interactions were significant including the effect of coupled modulation on accuracy. Notably, this model accounted for 37% of the variance in task accuracy. These findings indicate that both greater up-modulation of frontal-parietal regions to increasing difficulty and increasing modulation in regions of deactivation (greater deactivation) are in the service of more accurate performance, suggesting that greater dynamic range in general is associated with better performance.
Figure 6.
Greater dynamic range of modulation of activation to difficulty predicts higher n-back accuracy. A. Increased frontal-parietal positive modulation to difficulty is associated with increased task accuracy for adults middle-aged and older. B. Greater negative modulation (increase in deactivated regions) to difficulty was also associated with better task accuracy. Modulation values reflect the slope of BOLD activation across the increasing difficulty levels. Accuracy values represent proportion correct across all n-back conditions.
3.4.2. Digit Span Sequencing Performance
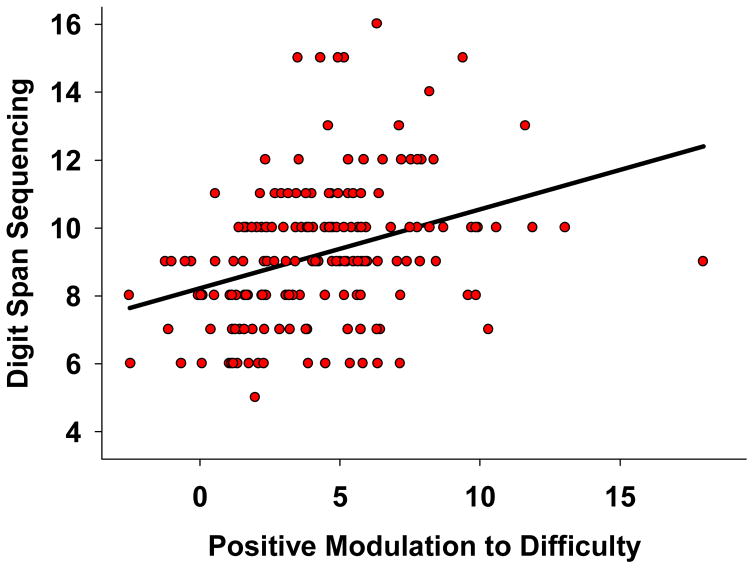
We were next interested in examining whether modulation was predictive of cognitive performance on a WM task beyond the scanner environment. A GLM was specified identically to the previous model, but with DS sequencing score as the dependent variable. We found a significant effect of positive modulation on sequencing (F (1,163) = 10.63, p = .001) with greater positive modulation associated with higher sequencing span, see Figure 7. There was also a marginally significant trend for the age x negative modulation interaction (F (1,163) = 3.86, p = .051 on sequencing span. No other interactions neared significance. This model accounted for 21% of the variance in DS sequencing. These results indicate that greater up-modulation to difficulty in fronto-parietal regions is associated with better WM measured outside of the scanner environment.
Figure 7.
Greater positive modulation to increased working memory load in frontal-parietal regions is associated with better working memory performance beyond the scanner task. Modulation values reflect the slope of BOLD activation across the increasing difficulty levels and the y-axis is number correct.
4. Discussion
The present study examined how the aging brain responds to incrementally increasing WM load during a parametric n-back task in a large, lifespan sample of adults. Across our sample, engagement in a difficult working memory task elicited activation in bilateral fronto-parietal regions, dorsal cingulate gyrus, precuneus, thalamus, midbrain, caudate and cerebellum (positive modulation effect), as well as deactivation (negative modulation effect) in regions such as bilateral posterior cingulate gyrus, medial prefrontal cortex, middle temporal gyrus, and occipital cortex. Increasing age was associated with reduced dynamic range in modulation to increased WM load in right MFG, bilateral superior and inferior parietal lobules, and left supplemental motor area (age-related positive modulation effect), as well as superior frontal gyrus, right insula, bilateral cingulate gyrus, fusiform gyri, parahippocampal gyri, and lateral occipital cortex (age-related negative modulation effect). Negative modulation was altered earlier in the lifespan than positive modulation. Interestingly, we found significant coupling of the up-modulated and down-modulated regions, where up-modulation increases were associated with decreased ability to down-modulate deactivated regions. We also showed that greater dynamic range in modulation (both up-modulation and down-modulation) was associated with better performance during scanning, and up-modulation with better working memory span on the WAIS DS sequencing task, adding support to the literature that increased dynamic range is associated with better cognitive prowess (Rieck et al, 2017).
4.1 How does the brain respond to incrementally increasing WM load?
Across the whole sample, increasing working memory load evoked regions known to be a part of the cognitive control network (bilateral fronto-parietal regions, anterior cingulate gyri, and caudate; Cabeza and Nyberg, 2000; Owen et al., 2005), as well as those known to be part of the default mode or task negative network (bilateral medial prefrontal cortex, posterior cingulate gyrus, middle temporal gyrus, and lateral occipital cortex; Greicius et al., 2003), and these increased parametrically with increasing load in accord with previous studies (Braver et al., 1997; Huang et al., 2016; Jansma et al., 2000; Jonides et al., 1997; Kaup et al., 2014; Manoach et al., 1997; Mattay et al., 2006; McKiernan et al., 2003; Nyberg et al., 2009; Rieck et al., 2017).
4.2 How does modulation to difficulty differ across the lifespan?
Both positive and negative modulation to difficulty decreased with age, such that older individuals were both up-modulating and down-modulating less than their younger counterparts. Decreases in both positive and negative modulation with age are consistent with previous work in our laboratory (Rieck et al., 2017). A number of other researchers have found reliable decreases in negative modulation with age (Park et al., 2010; Persson et al., 2007; Prakash et al., 2012 Turner and Spreng, 2015), and decreased DMN connectivity with age, which may result in decreased negative modulation (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008). Reliable positive modulation decreases with age have also been reported (Kaup et al., 2014; Mattay et al., 2006; Nyberg et al., 2009; Prakash et al., 2012; Rieck et al., 2017).
Both negative and positive modulation to difficulty depended upon age, but evidenced different age-trajectories. Negative modulation to difficulty was altered earlier in the lifespan than was positive modulation. Modulation in the deactivated regions did not begin to fail (i.e., did not differ from zero) until around 70 years of age, although there was a steady decrease in negative modulation across the lifespan. For positive modulation, in our age span, modulation never hit a failing point (differed from zero) and is not estimated to do so until age 99, although it did decline steadily across the lifespan. These findings provide a unique perspective on healthy aging and the ability of older adults to flexibly mobilize brain resources to meet increasing task demands. Most previous reports have not utilized a continuous lifespan age sample that included middle-aged and very old adults, and therefore were not able to explore this relationship. In comparison to two lifespan studies that examined the relationship of differential aging of positive and negative modulation, the current findings are in accord with Rieck et al., (2017), however the reverse was found in a study of subsequent memory effects (Park et al., 2013), where negative subsequent memory effects were seen later in the lifespan than positive subsequent memory effects.
4.4 Coupling of Negative and Positive Modulation
Positively modulated and negatively modulated regions showed a significant coupling. These findings are in accord with the idea that these two networks (portions of the cognitive control network and portions of the “default mode” network) do not function independently of each other, but rather the ability to modulate BOLD activity in one network is related to modulation in the other. The DECHA hypothesis (Turner & Spreng, 2015) further posits that this coupling of activation is altered in older adults, and perhaps as compensation for a declining fluid ability capacity with age. The current results partially support this hypothesis. The nature of the coupling and its association with task performance or cognitive ability is yet to be resolved as only a handful of studies have directly tested these issues to date (e.g., Ng et al., 2016; Rieck et al., 2017; Sambataro et al., 2010; Spreng et al., 2010; Spreng et al., 2014; Spreng et al., 2017). In the current study the coupled modulation was such that as modulation to difficulty in the positive modulation regions increased, there was lesser down-modulation of these deactivated regions. It seems that during the n-back task, up-modulating fronto-parietal regions to increasing WM load comes at a cost of further down-modulating deactivated “default-like” regions. This is in contrast to a recent finding in our lab (in the same participants) where we found that the significant coupling between up- and down-modulated regions stemmed from greater up-modulation associated with greater down-modulation during difficult distance judgments (Rieck et al., 2017). Notably, although both statistically significant relationships, the current n-back coupling association was stronger than coupling of modulation on the distance judgment task. However, coupled modulation significantly predicted performance in the distance judgment study, but in this n-back study it did not. It could be that coupling in the direction we find currently is related to the more difficult nature of the n-back task. In fact, Turner and Spreng (2015) also reported significant positive coupling of functional connectivity between a prefrontal seed and default network regions only on the more difficult levels of a planning task in their older adult group. Interestingly, in tasks that rely more heavily on internal mentation, such as evaluating famous faces or autobiographical memory, this association seems to be stronger (e.g., Spreng et al., 2014; Spreng et al., 2017). It may be the case that true WM tasks, compared to a distance judgment task, may rely more heavily on the cognitive control networks, requiring greater modulation of these regions, and this association may fluctuate over time to meet current needs (Dixon et al., 2017). A further difference between the two tasks is that, while both “default-like”, the regions of deactivation differ. Specifically, we here show greater hippocampal deactivation than we did in the Rieck et al., 2017 distance judgment task. This could stem from the strong working memory nature of this task compared to the distance judgment task. It could also reflect the differential connectivity of this region with other functional subsystems in the default mode network (Buckner et al., 2008), specifically the hippocampal formation connects to the dorsomedial and to the ventromedial PFC, regions we also robustly find deactivate in the Rieck et al., 2017 task.
4.5 What are the cognitive consequences of reduced modulation to difficulty?
A second goal of this study was to determine the nature of decreased dynamic range to task difficulty, i.e., whether it was detrimental, beneficial, or irrelevant to performance. Increased up-modulation of fronto-parietal regions was associated with more accurate performance on the n-back task, as was greater down-modulation of deactivated regions. These findings lend compelling support to the idea that maintaining greater dynamic range (in either direction) of BOLD activation is in the service of better task performance and this ability may be a salient marker of healthy cognitive aging. Indeed, individuals who showed greater positive modulation performed better on the WAIS digit span sequencing task, an increasingly difficult working memory span task. With some possible caveats, these findings appeared to be true for everyone, suggesting that these are important individual difference factors, rather than aging effects per se. However, longitudinal follow-up is required to fully test this notion. Given our wide age range sample, though this provides a unique perspective (cross-sectionally) on the relationship between age, BOLD modulation, and cognitive performance. This is strengthened by the fact that not only does modulation relate to in-the-moment cognitive performance, it also generalizes to the same cognitive domain measured outside the scanning environment, adding to the robustness of this association.
Previous research examining the relationship between modulation (positive and/or negative) and task performance evidenced mixed results and are difficult to interpret given the comparisons made between extreme age groups. Studies found a facilitative relationship between positive modulation and performance for younger adults (Jansma et al., 2000; Leung et al., 2007); only for older adults (Sala-Llonch et al., 2012; Saliasi et al., 2014); for both younger and older adults (Kaup et al., 2014; Nagel et al., 2011); and a differential relationship such that positive modulation predicted accuracy for younger adults, but negative modulation was predictive for older adults (Prakash et al., 2012); or was not associated at all (Cappell et al., 2010). Similarly, mixed associations exist for negative modulation: predictive of behavior in young (Huang et al., 2016); both younger and older adults (Sambataro et al., 2010); related to performance strongly only in older adults (Rieck et al., 2017). Kaup and colleagues (2014), also utilized a continuous age range, and found that both positive and negative modulation was predictive of task performance and this relationship was not age-dependent. The combination of several differences in study design and sample selection may explain the discrepancies across studies. For example, our sample is the largest (N =171) of the published n-back studies (mean N = 35.18) and might have afforded the power to detect these BOLD-performance associations; our sample was screened to be very healthy, and thus, eliminating noise introduced by comorbid aging conditions may have also allowed us to detect these effects. Further, a wide age-range lifespan sample (20–94) that does not omit the middle-age and very old adults likely allowed us more specificity in our models and allowed for use of age slopes (from multiple regression) rather than somewhat arbitrary extreme age cutoff group differences (from group contrasts). Indeed, our age range was the widest of the studies reviewed. We also included a wider range of WM load conditions, increasing to 4-back, which is different from most published studies (most range from 0- to 3-back). Lastly, our pretraining procedure likely helped to stabilize performance and perhaps BOLD response during scanning, and this is not a procedure that all published studies have implemented.
Further, we found no previous reports of greater positive modulation to difficulty during a WM task being associated with better cognitive performance outside of the scanner. In children, greater negative modulation to difficulty during a spatial working memory task was significantly associated with better task performance, while positive modulation to difficulty was more weakly associated (Huang et al., 2016) and in the youngest children, negative modulation to difficulty was predictive of executive function measured outside of the scanner. Rieck and colleagues (2017) found that coupling of positive and negative modulation predicted better fluid reasoning ability outside of the scanner, such that individuals, regardless of age, who could better modulate these two systems, performed better on the task. Extending the association of greater up-modulation to cognitive performance beyond the scanner environment lends strong evidence to the idea that greater dynamic range of modulatory capacity is a salient predictor of cognitive health.
4.6 Conclusions
In sum, by utilizing the entire parametric range of conditions in an n-back design and by sampling a wide age-range of individuals in a large sample, we were able to demonstrate reliable associations among aging, BOLD modulation to difficulty, and the relationships with not only task accuracy, but also out-of-scanner cognition. These findings, taken together suggest that although both positive and negative modulation decrease across the adult lifespan, maintenance of this increased dynamic range is beneficial to both task performance and working memory beyond the scanner environment. Therefore, this study lends further support to growing evidence that greater dynamic range in BOLD modulation is a proxy for the ability to flexibly employ neural resources in response to cognitive demand and may be a salient marker of cognitive ability more broadly (Cappell et al., 2010; Grady et al., 2006; Kaup et al., 2014; Kennedy et al., 2015; Mattay et al., 2006; Nagel et al, 2009; 2011; Park et al., 2010; Persson et al., 2007; Sambataro et al., 2010; Schnieder-Garces et al., 2010; Rieck et al., 2017; Turner and Spreng, 2015).
Highlights.
Dynamic range of modulation of activation to cognitive difficulty declines with age
Frontal-parietal up-modulation declines later than down-modulation of deactivation
Modulation is coupled: as up-modulation increases, down-modulation decreases
Greater up- and down-modulation associated with better cognitive performance
Dynamic range of BOLD modulation has utility as a salient marker of cognitive aging
Acknowledgments
This study was supported in part by grants from the National Institutes of Health R00 AG-03618 and R00 AG-03648. We would like to thank Andy Hebrank for assistance with fMRI task programming, Asha Unni for behavioral task piloting and fMRI data collection, and Marci Horn for cognitive data collection.
Footnotes
Disclosures
No authors have actual or potential conflicts of interest pertaining to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankudowich E, Pasvanis S, Rajah MN. Changes in the modulation of brain activity during context encoding vs. context retrieval across the adult lifespan. Neuroimage. 2016;139:103–113. doi: 10.1016/j.neuroimage.2016.06.022. [DOI] [PubMed] [Google Scholar]
- Artuso C, Cavallini E, Bottiroli S, Palladino P. Updating working memory: memory load matters with aging. Aging Clin Exp Res. 2016 doi: 10.1007/s40520-016-0581-y. [DOI] [PubMed] [Google Scholar]
- Babcock RL, Salthouse TA. Effects of increased processing demands on age differences in working memory. Psychol Aging. 1990;5:421–428. doi: 10.1037//0882-7974.5.3.421. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working Memory. Psychology of learning and motivation. 1974;8:47–89. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. PNAS. 2014;111:E4997–E5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo WC, Lee WW, Venkatraman V, Sheu FS, Chee MWL. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage. 2005;25:579–587. doi: 10.1016/j.neuroimage.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Clarys D, Bugaiska A, Tapia G, Baudouin A. Ageing, remembering, and executive function. Memory. 2009;17:158–168. doi: 10.1080/09658210802188301. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SaRB. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Dixon ML, Andrews-Hanna JR, Spreng RN, Irving ZC, Mills C, Girn M, Christoff K. Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage. 2017;147:632–649. doi: 10.1016/j.neuroimage.2016.12.073. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychol Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. PNAS. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzgal TJ, D’Esposito M. Activity in fusiform face area modulated as a function of working memory load. Brain Res Cogn Brain Res. 2001;10:355–364. doi: 10.1016/s0926-6410(00)00056-2. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman M, Dumas J, Nielsen C. Age Differences in Updating Working Memory: Evidence from the Delayed-Matching-To-Sample Test. Aging, Neuropsychology, and Cognition. 2001;8:14–35. doi: 10.1076/anec.8.1.14.847. [DOI] [Google Scholar]
- Heinzel S, Lorenz RC, Pelz P, Heinz A, Walter H, Kathmann N, Rapp MA, Stelzel C. Neural correlates of training and transfer effects in working memory in older adults. Neuroimage. 2016;134:236–249. doi: 10.1016/j.neuroimage.2016.03.068. [DOI] [PubMed] [Google Scholar]
- Heinzel S, Schulte S, Onken J, Duong QL, Riemer TG, Heinz A, Kathmann N, Rapp MA. Working memory training improvements and gains in non-trained cognitive tasks in young and older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2014;21:146–173. doi: 10.1080/13825585.2013.790338. [DOI] [PubMed] [Google Scholar]
- Huang AS, Klein DN, Leung HC. Load-related brain activation predicts spatial working memory performance in youth aged 9–12 and is associated with executive function at earlier ages. Dev Cogn Neurosci. 2016a;17:1–9. doi: 10.1016/j.dcn.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, van der Wee NJA, Kahn RS. Working memory capacity in schizophrenia: a parametric fMRI study. Schizophrenia Research. 2004;68:159–171. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal Working Memory Load Affects Regional Brain Activation as Measured by PET. J Cogn Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Kaup AR, Drummond SPA, Eyler LT. Brain functional correlates of working memory: reduced load-modulated activation and deactivation in aging without hyperactivation or functional reorganization. J Int Neuropsychol Soc. 2014;20:945–950. doi: 10.1017/S1355617714000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Bischof GN, Hebrank AC, Reuter-Lorenz PA, Park DC. Age trajectories of functional activation under conditions of low and high processing demands: an adult lifespan fMRI study of the aging brain. Neuroimage. 2015;104:21–34. doi: 10.1016/j.neuroimage.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D, Maillet D, Pasvanis S, Ankudowich E, Grady CL, Rajah MN. Context Memory Decline in Middle Aged Adults is Related to Changes in Prefrontal Cortex Function. Cereb Cortex. 2016;26:2440–2460. doi: 10.1093/cercor/bhv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Oh H, Ferri J, Yi Y. Load response functions in the human spatial working memory circuit during location memory updating. NeuroImage. 2007;35:368–377. doi: 10.1016/j.neuroimage.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Schlaug G, Siewert B, Darby DG, Bly BM, Benfield A, Edelman RR, Warach S. Prefrontal cortex fMRI signal changes are correlated with working memory load. Neuroreport. 1997;8:545–549. doi: 10.1097/00001756-199701200-00033. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Mazaika P, Whitfield-Gabrieli S, Reiss A. Artifact repair for fMRI data from high motion clinical subjects. Poster Session Presented at Human Brain Mapping; Chicago, IL. 2007. [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li SC, Nyberg L, Bäckman L, Lindenberger U, Heekeren HR. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J Cogn Neurosci. 2011;23:2030–2045. doi: 10.1162/jocn.2010.21560. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li S-C, Nyberg L, Bäckman L, Lindenberger U, Heekeren HR. Performance level modulates adult age differences in brain activation during spatial working memory. Proc Natl Acad Sci USA. 2009;106:22552–22557. doi: 10.1073/pnas.0908238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KK, Lo JC, Lim JKW, Chee MWL, Zhou J. Reduced functional segregation between the default mode network and the executive control network in healthy older adults: A longitudinal study. Neuroimage. 2016;133:321–330. doi: 10.1016/j.neuroimage.2016.03.029. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Dahlin E, Stigsdotter Neely A, Bäckman L. Neural correlates of variable working memory load across adult age and skill: dissociative patterns within the fronto- parietal network. Scand J Psychol. 2009;50:41–46. doi: 10.1111/j.1467-9450.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Front Hum Neurosci. 2010;3:75. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kennedy KM, Rodrigue KM, Hebrank A, Park DC. An fMRI study of episodic encoding across the lifespan: changes in subsequent memory effects are evident by middle-age. Neuropsychologia. 2013;51:448–456. doi: 10.1016/j.neuropsychologia.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW. Generating Stimuli for Neuroscience Using PsychoPy. Front Neuroinform. 2008;2:10. doi: 10.3389/neuro.11.010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW. PsychoPy--Psychophysics software in Python. J Neurosci Methods. 2007;162:8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Heo S, Voss MW, Patterson B, Kramer AF. Age-related differences in cortical recruitment and suppression: implications for cognitive performance. Behav Brain Res. 2012;230:192–200. doi: 10.1016/j.bbr.2012.01.058. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. doi: 10.3102/10769986031004437. [DOI] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. 1977 doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rieck JR, Rodrigue KM, Boylan MA, Kennedy KM. Age-related reduction of BOLD modulation to cognitive difficulty predicts poorer task accuracy and poorer fluid reasoning ability. NeuroImage. 2017;147:262–271. doi: 10.1016/j.neuroimage.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta- analysis. Neuroimage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Peña-Gómez C, Arenaza-Urquijo EM, Vidal-Piñeiro D, Bargalló N, Junqué C, Bartrés-Faz D. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex. 2012;48:1187–1196. doi: 10.1016/j.cortex.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Saliasi E, Geerligs L, Lorist MM, Maurits NM. Neural correlates associated with successful working memory performance in older adults as revealed by spatial ICA. PLoS ONE. 2014;9:e99250. doi: 10.1371/journal.pone.0099250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. The aging of working memory. Neuropsychology. 1994;8:535–543. doi: 10.1037/0894-4105.8.4.535. [DOI] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. 2010;31:839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Maclin EL, Gratton G, Fabiani M. Span, CRUNCH, and beyond: working memory capacity and the aging brain. J Cogn Neurosci. 2010;22:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze ET, Geary EK, Susmaras TM, Paliga JT, Maki PM, Little DM. Anatomical correlates of age-related working memory declines. J Aging Res. 2011;2011:606871. doi: 10.4061/2011/606871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, DuPre E, Selarka D, Garcia J, Gojkovic S, Mildner J, Luh W-M, Turner GR. Goal-congruent default network activity facilitates cognitive control. J Neurosci. 2014;34:14108–14114. doi: 10.1523/JNEUROSCI.2815-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Lockrow AW, DuPre E, Setton R, Spreng KAP, Turner GR. Semanticized autobiographical memory and the default – executive coupling hypothesis of aging. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, Spreng RN. Prefrontal Engagement and Reduced Default Network Suppression Co-occur and Are Dynamically Coupled in Older Adults: The Default–Executive Coupling Hypothesis of Aging. Journal of Cognitive Neuroscience. 2015;27:2462–2476. doi: 10.1162/jocn_a_00869. [DOI] [PubMed] [Google Scholar]
- Van der Linden M, Brédart S, Beerten A. Age-related differences in updating working memory. Br J Psychol. 1994;85(Pt 1):145–152. doi: 10.1111/j.2044-8295.1994.tb02514.x. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An Integrated Software System for Surface-based Analyses of Cerebral Cortex. Journal of American Medical Informatics Association. 2001;8(5):443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition. San Antonio, TX: Pearson; 2008. [Google Scholar]
- Veltman DJ, Rombouts SARB, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage. 2003;18:247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, Mamourian AC, Belloni DR, Rhodes CH, McAllister TW. Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. Am J Psychiatry. 2006;163:1603–1610. doi: 10.1176/ajp.2006.163.9.1603. [DOI] [PubMed] [Google Scholar]