Abstract
The domestic chicken, Gallus gallus, serves as an excellent model for the study of a wide range of ocular diseases and conditions. The purpose of this manuscript is to outline some anatomic, physiologic, and genetic features of this organism as a robust animal model for vision research, particularly for modeling human retinal disease. Advantages include a sequenced genome, a large eye, relative ease of handling and maintenance, and ready availability. Relevant similarities and differences to humans are highlighted for ocular structures as well as for general physiologic processes. Current research applications for various ocular diseases and conditions, including ocular imaging with spectral domain optical coherence tomography, are discussed. Several genetic and non-genetic ocular disease models are outlined, including for pathologic myopia, keratoconus, glaucoma, retinal detachment, retinal degeneration, ocular albinism, and ocular tumors. Finally, the use of stem cell technology to study the repair of damaged tissues in the chick eye is discussed. Overall, the chick model provides opportunities for high-throughput translational studies to more effectively prevent or treat blinding ocular diseases.
Keywords: Chick, Eye, Ocular disease, Animal model, Stem cell, Optical coherence tomography, Mutant
1. Introduction
Animal models are critical to vision research for numerous ocular diseases and conditions, including retinal detachment, retinal degeneration, glaucoma, and corneal injuries. Currently, multiple species including non-human primates, rodents, felines, and certain avian species are being used for ocular research purposes; all have advantages and disadvantages. However, the chick is perhaps an underutilized model animal with many advantages in cost, size, and ease of handling compared to other models. This review will focus on our experience with modeling human retinal disease using the chick model system and discuss its advantages and disadvantages for vision research.
2. Basic Anatomy and Physiology
2.1 Overview
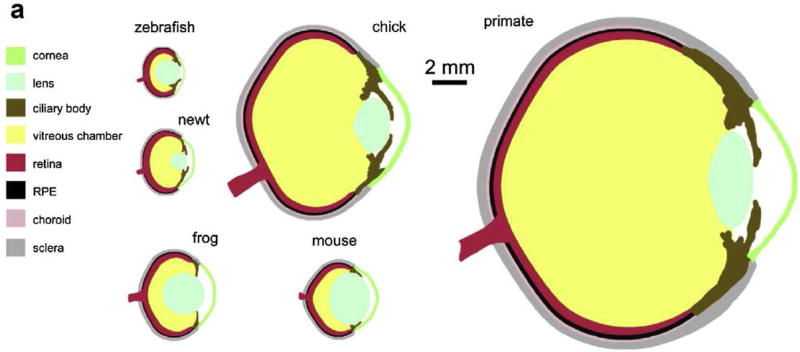
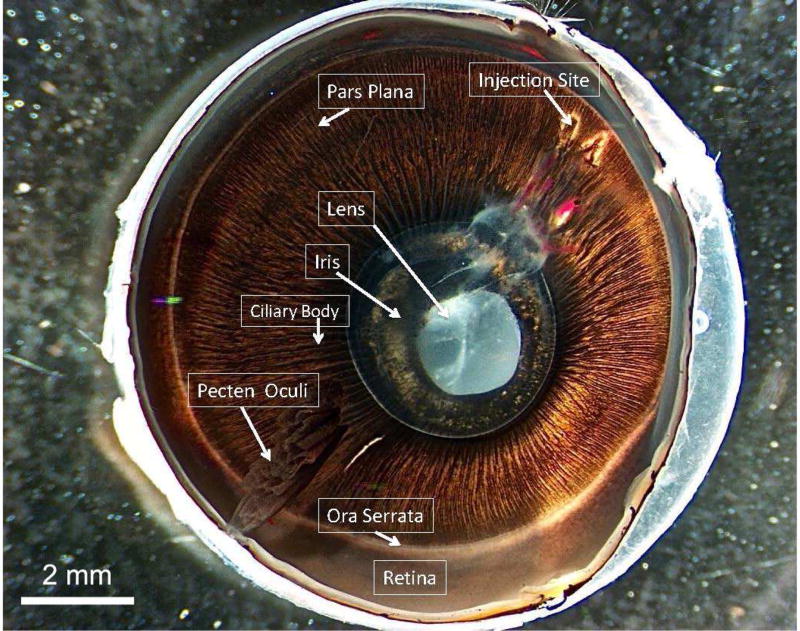
The chick is a diurnal bird originating from jungle fowl (Eriksson et al., 2008). Like most avian species, the chick relies heavily on vision for everything from predator evasion to food acquisition. With this highly developed visual system, the chick eye is relatively large compared to its overall size. The mean axial globe length of the chick eye, 12–13 mm, (Montiani-Ferreira et al., 2003; Troilo et al., 1995) is approximately half that of the human eye. Avian species exhibit this relatively large eye size by dedicating up to 50% of the cranial volume to the eye, compared to approximately 5% in humans (Waldvogel, 1990). Table 1 provides anatomical comparisons between chick and human eyes, and Figure 1 compares the chick to several other species used in vision research. Figures 2 and 3 are images of hemisected chick eyes, also allowing for an overview of anatomical structures. Specific categories of chick ocular anatomic and physiologic details are presented in sections 2.2 through 2.9.
Table 1.
Comparative Anatomy
| Gallus gallus Chickens | Humans | References | |
|---|---|---|---|
|
| |||
| Relative Size |
|
|
(Waldvogel, 1990) |
|
| |||
| Cornea |
|
|
Chicken: (Fowler et al., 2004; Glasser et al., 1994; Jones et al., 2007; Kanski, 1994; Richey et al., 2011) |
| Human: (Grewal et al., 2010; Rüfer et al., 2005) | |||
|
| |||
| Sclera |
|
|
(Coulombre and Coulombre, 1973; Koch, 1973; Rada et al., 1991) |
|
| |||
| Choroid |
|
|
Chicken: (Jones et al., 2007; Koch, 1973; Nava et al., 2016; Wood, 1917) |
| Human: (Ho et al., 2011) | |||
|
| |||
| Iris |
|
|
(Koch, 1973) |
|
| |||
| Lens |
|
|
Chicken: (Iribarren et al., 2014; Samuelson, 1991) |
| Human: (Schachar, 2004) | |||
|
| |||
| Photoreceptors |
|
|
Chicken: (Bowmaker, 2008; Bueno et al., 2011; Dartnall et al., 1983; Hart, 2001; Headington et al., 2011; Kram et al., 2010; Meyer and May, 1973; Nava et al., 2016; Okano et al., 1992) |
| Human: (Bowmaker, 2008; Curcio et al., 1990; Jonas et al., 1992; Kashani et al., 2010; Okano et al., 1992; Wells-Gray et al., 2016) | |||
|
| |||
| Optic Nerve and Inner Retina |
|
|
Chicken: (Fischer et al., 2005, 2010a, 2010b; Morcos and Chan-Ling, 2000; Rager and Rager, 1978) |
| Human: (Curcio and Allen, 1990). | |||
|
| |||
| Vasculature |
|
|
(Jones et al., 2007; Waldvogel, 1990) |
Wavelength values from Okano et al. (1992)
Wavelength values from Dartnall et al. (1983)
Non-astrocytic Inner Retinal Glia-like
Figure 1.
Comparative ocular anatomy of chick and different vertebrate classes (Reprinted from Experimental Eye Research, 123, Fischer, A.J., Bosse, J.L., El-Hodiri, H.M., Reprint of: The ciliary marginal zone (CMZ) in development and regeneration of the vertebrate eye, 115–120., Copyright (2014), with permission from Elsevier. (Fischer et al., 2014a)). Scale bar 2 mm.
Figure 2.
Photograph of hemisected chick eye showing the anterior cap as viewed from below the lens. Scale bar 2 mm.
Figure 3.
Photograph of posterior portion of hemisected chick eye showing pecten oculi with surrounding retina, choroid, and sclera. Scale bar 2 mm.
2.2 Cornea
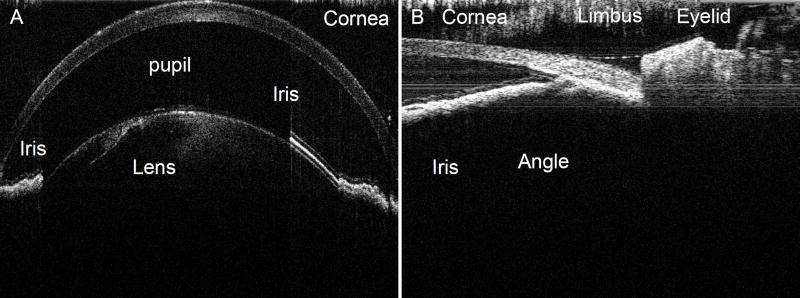
The chick cornea is a transparent structure composed of five distinct layers: epithelium (outermost), Bowman’s layer, stroma, Descemet's membrane, and endothelium (innermost) (Jones et al., 2007; Kanski, 1994; Ritchey et al., 2011). The normal, average respective adult corneal diameter and thickness measurements are 9.1mm and 405µm (by histology and optical coherence tomography (OCT)) in chick (Fowler et al., 2004) compared with 11.5–12.5mm (Rüfer et al., 2005) and 518–558µm (as measured via ultrasound, Scheimpflug imaging, and OCT by Grewal et al. (2010)) in humans. Figure 4 shows a representative anterior segment spectral domain OCT image of the chick. Bowman’s layer has been shown to play a key role in corneal wound healing (Fowler et al., 2004), but rabbit and rodent models used in corneal wound healing studies have severely underdeveloped Bowman’s layers (Hayashi et al., 2002). In contrast, the chick has a true Bowman’s layer, which is an advantage for trauma or corneal refractive studies, particularly studies of epithelial debridement in which epithelial cell re-growth is evaluated (Fowler et al., 2004; Ritchey et al., 2011). Chick corneas are thinner than those of other animals, but the relative thickness ratios of chick corneal layers are very similar to those observed in human corneas (Fowler et al., 2004; Ritchey et al., 2011). Furthermore, the chick eye, like the human, has a more stable blood-aqueous barrier than some species, such as the rabbit, which produces prominent fibrin material with minimal tissue manipulation (Fowler et al., 2004).
Figure 4.
SD-OCT imaging of chick anterior segment of the eye. A) Anterior segment scan showing cross-sectional image centered on the dilated pupil (Scale bar 500 µm). There is asymmetric dilation, greater on the left side of the image. B) Open irido-corneal angle in un-dilated chick. Images were obtained with the Envisu SD-OCT system using an anterior imaging lens (Scale bar 100 µm).
2.3 Sclera
Extending laterally from the limbus, the avian sclera is in many respects analogous to the primate sclera. Distinguishing features include the presence of both fibrous and cartilaginous scleral layers and the presence of scleral ossicles (Coulombre and Coulombre, 1973; Koch, 1973; Rada et al., 1991). A layer of hyaline cartilage is located internally, with a thin fibrous layer of type I collagen and proteoglycans situated externally. The hypocellular fibrous layer is similar in composition to the primate sclera (Rada et al., 1991). The two layers respond differently during ocular growth, with the cartilaginous layer incorporating additional glycosaminoglycans (GAGs) and expanding, while the fibrous layer became thinner (Gottlieb et al., 1990; Marzani and Wallman, 1997). Of note, the fibrous sclera of mammals also becomes thinner under growth-inducing conditions (Funata and Tokoro, 1990) and decreases GAG incorporation in the extracellular matrix (Norton and Rada, 1995). In both chicks and primates, thinning of the fibrous sclera is more pronounced in the posterior sclera (Gottlieb et al., 1990; Norton and Rada, 1995). Interestingly, Marzani and Wallman (1997) demonstrated that fibrous sclera exposed to ocular growth-inducing stimuli was able to induce increased GAG uptake and expansion of control cartilaginous sclera when the two scleral tissue types were cultured together, while cartilaginous sclera exposed to the same growth stimuli could not produce changes in control fibrous sclera. Thus, the fibrous layer appears to play an important role in driving scleral physiology and ocular growth. The anterior aspect of the sclerotic coat is composed of the ossicles (Koch, 1973). The bony ossicles are developed from intramembranous ossification and occupy a region from the corneal limbus to the equator of the eye. Numbering 13–14 in chickens, they form an overlapping ring around the eye (Coulombre and Coulombre, 1973; Koch, 1973). Ossicles provide support and protection to the eye, serve as an attachment site for parts of the ciliary muscle, and may provide mechanical support for the mechanism of corneal accommodation (Glasser et al., 1994).
2.4 Iris
Posterior to the cornea sits the pigmented iris that regulates the amount of light entering the eye. The mature iris is composed primarily of striated muscle but surprisingly contains both muscarinic and nicotinic acetylcholine receptors (Jones et al., 2007; Koch, 1973; Pilar et al., 1987), while adrenergic receptors are found on the dilator muscle (Hoffman et al., 1980). In humans, the dilator muscle has alpha1 adrenergic receptors, while the sphincter muscle has muscarinic, but no nicotinic, receptors (Gupta et al., 2014; Reibaldi et al., 1984). These differences require a different pharmacologic strategy to dilate the chick pupil. In humans, sympathomimetics (e.g., phenylephrine 2.5%) are frequently combined with muscarinic antagonists (tropicamide or atropine) to dilate the pupil. In chicks, nicotinic receptor antagonists are critical. For example, Pilar et al. (1987) demonstrated in ex vivo studies that the nicotinic antagonist alpha bungarotoxin reduced pupil contraction force more than atropine 0.5% in postnatal birds and that the combination of drugs effectively blocked electrically-induced pupil constriction, more than nicotinic antagonist alone. Moderate dilation for ophthalmic procedures requiring bright illumination has been achieved with the nicotinic antagonist turbocurarine in 0.1% benzalkonium-containing solution (Cebulla et al., 2012). However, a triple combination of this agent with a muscarinic antagonist and adrenergic agonist might improve dilation responses.
For some time it was thought that avian species lack a true consensual pupillary reflex, and that instead the consensual response may occur as light reaches the contralateral retina through transillumination (Jones et al., 2007; Levine, 1955; Schaeffel et al., 1986). Interestingly, in some avian species, transillumination can be distinctly noted on exam. A detailed anatomical study in pigeons by Chard and Gundlach (1937) demonstrated only 1 mm of bone separated the two orbits, providing further credence to the transillumination theory. Schaeffel et al. (1986) demonstrated that longer wavelengths of light passed through the lamina interorbitalis in chicks, while shorter wavelengths did not. Additionally, they observed that shorter wavelengths, <577 nm, did not cause any consensual pupillary response. More recent work has demonstrated the presence of a pupillary reflex in the eyes with a transected optic nerve after exposing the contralateral eye to a light stimulus, definitively proving the presence of a true consensual reflex (Li and Howland, 1999).
2.5 Ciliary Body
The ciliary body, located posterior to the iris, is made up of two striated muscles: the anterior and posterior sclerocorneal muscles (Jones et al., 2007; Murphy et al., 1995). While in humans the ciliary body is responsible for accommodation, its exact role in avian lenticular accommodation is still debated (See section 2.7). As in humans, the ciliary body of avian species also plays a role in aqueous humor production and outflow in addition to holding the lens in place via radial zonular fiber attachments (Jones et al., 2007; Koch, 1973; Lauber, 1987; Murphy et al., 1995).
In the avian eye, aqueous humor is produced by the ciliary epithelium and flows into the anterior chamber via the pupillary opening. Like in humans, the aqueous humor is drained primarily by the trabecular meshwork. The aqueous has multiple functions including supplying nutrients and oxygen to avascular structures in the anterior chamber, namely the lens and cornea, and providing pressure support to help shape the cornea (Jones et al., 2007; Kinnear et al., 1974; Koch, 1973).
2.6 Lens
The adult chick lens measures approximately 3.5 mm in thickness and 5.9 mm in anterior radius of curvature (Iribarren et al., 2014), compared with human lens dimensions of approximately 3.9 mm in thickness and 10.5 mm in anterior radius of curvature (Schachar, 2004). The lenses of avian species exhibit several differences from those of primates. For one, an annular pad surrounds the lens cortex and serves as an attachment point for ciliary muscles (Koch, 1973; Murphy et al., 1995). This allows direct force to be applied to the lens. This force may in part be responsible for accommodation in birds. The avian lens exhibits more flexibility than its human equivalent (Samuelson, 1991), and, when coupled with the fast action of the striated ciliary muscle (Briggs 1991), allows for more rapid accommodation in birds (Jones et al., 2007).
2.7 Accommodation
In humans, accommodation occurs through lenticular changes as a result of ciliary muscle contraction. Ciliary muscle contraction leads to laxity of the zonular fibers allowing for “rounding up” of the lenticular apparatus. This mechanism can produce, on average, up to 16 diopters (D) of accommodation, allowing for a focal distance of 6.25 cm. By comparison, stimulation of the Edinger-Westphal nucleus in chickens may generate between 19 D (Troilo and Wallman 1987) and 25 D (Glasser et al., 1995) of accommodation. Total natural accommodation in chickens is 15–17 D, as measured by Schaeffel and Howland (1987) using infrared photoretinoscopy and photokeratometry. Accommodation in chicks is estimated to occur at a rate of 80 D/s (Schaeffel and Feldkaemper, 2015), much faster than corresponding human estimates of 10 D/s (Schaeffel et al., 1993). The mechanism of accommodation in the chick has been debated. It is thought that accommodation is mediated through both lenticular and corneal changes, contributing 60% and 40%, respectively (Glasser et al., 1994; Troilo and Wallman, 1987).
Several theories exist regarding the exact mechanism of avian lenticular accommodation. One early theory maintained that contraction of the ciliary body against the lens equator causes axial thickening of the lens (Meyer, 1977; Pumphery, 1961; Walls, 1963). Glasser et al. (1995) proposed that peripheral circular and oblique muscle fibers of the iris contract, forcing the ciliary body to push against the annular pad at equatorial plane of the lens, altering the lens shape. Ostrin et al. (2011) demonstrated that, following iridectomy in chicks, nicotinic receptor mediated accommodation was not significantly different between iridectomized and control chicks, suggesting the iris does not drive accommodation. Given the diversity of avian species, it is likely that different mechanisms of lenticular accommodation exist in birds. At this point, however, the mechanism of lenticular accommodation in chickens remains unclear.
Corneal changes account for up to 40% of accommodation in the chicken eye. Glasser et al. (1994) showed that corneal accommodation occurs through ciliary muscle contraction, which produces a force on the inner lamella of the cornea, causing peripheral flattening and increased central curvature. A subsequent study by (Murphy et al., 1995) demonstrated that the ciliary muscle anatomy in chickens is consistent with the Glasser et al. (1994) mechanism. In summary, accommodation in the chick is thought to be dependent on lenticular changes as well as by changes in corneal curvature induced by the ciliary muscle.
2.8 Retina and Pecten Oculi
The chick retina has a similar organization to humans and other mammals, but with some major differences. While primates, certain avian species, and some lizards and fishes have foveal contours, the chick retina lacks a fovea (Schwab, 2012; Slijkerman et al., 2015), but instead exhibits an afoveate area centralis situated 2 mm from the dorsal edge of the optic disc (Morris, 1982). The chick retina has similar layer organization to the mammalian retina, with 3 nuclear layers: the ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL).
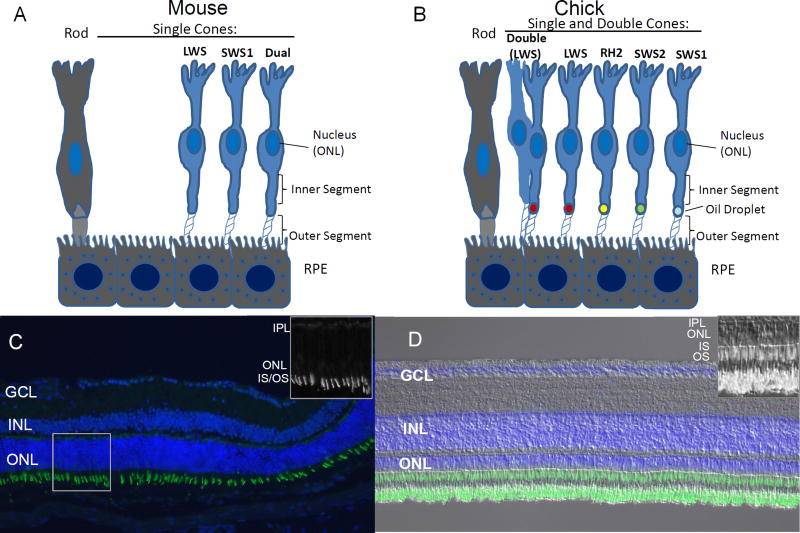
Spectral domain OCT imaging has demonstrated that the relative thickness of chick INL to ONL is greater than in mammals (Moayed et al., 2011). Figure 5 demonstrates the comparative differences in INL and ONL thickness in chick and mouse and types of photoreceptors. To gain more insight into these differences, we analyzed DAPI or Draq5 stained sections from central regions of chick and mouse retinas (n=6 for each species), finding INL/ONL ratios of 4.41 ± 0.62 in the chick and 0.76 ± 0.09 in the mouse (unpublished observation). This finding demonstrates that many more interneurons exist in the INL of the chick retina relative to photoreceptors in the ONL. There are more photoreceptors in the mouse retina relative to the chick and relative to interneurons in the INL. The greater number of interneurons per photoreceptor in the chick suggests that there is more image processing or change detection occurring within the retina before ganglion cells send signals to the brain.
Figure 5.
Comparative retinal anatomy of mouse vs. chick. A) Schematic of mouse dichromatic retinal photoreceptors. Single cone photoreceptors have either long (LWS) or very short (SWS1) wavelength sensitive opsin. Dual photoreceptors express both pigments. B) Schematic of chick tetrachromatic retinal photoreceptors. Double cone photoreceptors function in the perception of motion. Single cone photoreceptors have either long (LWS), medium (RH2), short (SWS2), or very short (SWS1) wavelength sensitive opsin. Oil droplets with 4 different transmission wavelengths are located at the junction of the photoreceptor inner and outer segment. C) Photomicrograph of healthy C57bl/6 mouse and (D) chick retina show the relative differences in nuclear layer thickness between the two species, with a thicker INL to ONL ratio in chick. Both photomicrographs are shown at 200× magnification and stained with DAPI (blue nuclei) and (LWS/RH2) opsin (green, C and D) and Nomarski (D). Inset (C) and (D) detail of the LWS/RH2 opsin channel in box area. IS = inner segment OS = outer segment, ONL = outer nuclear layer, INL = inner nuclear layer, IPL= inner plexiform layer, GCL = ganglion cell layer, RPE = retinal pigment epithelium. Scale bar 50 µm.
Differences in the retinal photoreceptors and the presence of accompanying colored oil droplets also set the chicken eye apart from its human counterpart. To date, six types of photoreceptors have been characterized in the chicken retina on the basis of gene expression and oil droplet absorption (Bowmaker, 2008; Bowmaker et al., 1997; Hart, 2001; Kram et al., 2010; Yokoyama, 2000). Four are composed of single cones giving rise to tetrachromic vision while a fifth photoreceptor type composed of a “double-cone” is responsible for achromic perception of movement (Hart 2001; Jones et al., 2007; Kram et al., 2010). Bowmaker (2008) uses current opsin family nomenclature for photoreceptor pigments, grouping them into five evolutionarily distinct opsin gene families: LWS, RH2, SWS2, SWS1, and RH1. Associated wavelengths of maximal absorption (αmax) listed were determined by Okano et al. (1992). These chick cone photopigments are composed of long (LWS (571 nm) chick red), medium (RH2 (508 nm) chick green), short (SWS2 (455 nm) chick blue), and extreme short (SWS1 (415 nm) chick violet) wavelength sensitive opsins (Bowmaker, 2008; Hart, 2001; Okano et al., 1992). The LWS opsin is also found in double cone photoreceptors, which are the most abundant subtype in the chicken retina, comprising almost 41% of the cone photoreceptors (Kram et al., 2010). By comparison, humans have only trichromatic vision with three cone photopigments (two LWS subtypes, L (558 nm) red, and M (531 nm) green, and an SWS1 (419 nm) blue-sensitive) (Bowmaker, 2008; Dartnall et al., 1983). Additionally, a rod photoreceptor is responsible for night vision. As in humans, chicken rod photoreceptors express rhodopsin-1 (RH1) with αmax around 500 nm. (Bowmaker, 2008; Bruhn and Cepko, 1996; Okano et al., 1992). Kram et al. (2010) demonstrated that individual photoreceptor subtypes are not randomly arranged; instead, they display a striking degree of regularity in their spatial organization. The exact mechanism by which the patterning takes place is unknown, but it is theorized that lateral inhibition and photoreceptor specific signaling inhibit the development of the same receptor subtypes in a nearby area (Kram et al., 2010).
Photoreceptor oil droplets are found in most, if not all, avian species, some lizards, anurans, and some fishes (Bowmaker, 2008), but are not present in placental mammals. In chickens, the oil droplets are colored (red, orange-yellow, yellow-green, by light microscopy (Bowmaker and Knowles, 1977)), due to the presence of carotenoid pigments in all but the SWS2 cone type, which contains clear oil droplets (Bowmaker, 2008; Goldsmith et al., 1984; Hart, 2001; Kram et al., 2010; Niessner et al., 2011). Oil droplets are found at the distal end of the inner segment of cone photoreceptors (Fig. 5), but are lacking in rods (Bowmaker et al., 1997). Light passes through the oil droplets before reaching the cone opsins. Many functions of these droplets have been reviewed by Hart (2001), including protection from ultraviolet radiation, increased visual acuity, and detection of magnetic fields. Importantly, oil droplets function as long-pass cut-off filters, allowing only certain wavelengths of light to pass through, thus altering the spectral sensitivity of photoreceptors, decreasing chromatic aberration, and enhancing spectral discrimination (Beason and Loew, 2008; Bowmaker, 1997; Hart, 2001; Hart, 2006; Porter et al., 2014; Stavenga and Wilts, 2014; Walls, 1963). Yellow filters are particularly important in filtering scattered short-wavelength light that creates glare, creating sharper images (Walls, 1963). The presence of several different filter colors also presents an advantage in discrimination of various hues, e.g. yellow filters improve discrimination in shades of green by filtering blue light (Hart, 2001; Walls, 1963). Thus, the spatial arrangement of particular oil droplet and cone types in certain regions of the retina allow avian species to have specialized, region-specific hue discrimination (Hart, 2001; Walls, 1963). For chick oil droplets, the 50% transmission wavelengths are 575, 520, 497, and 454 nm, blocking light below these wavelengths (Bowmaker and Knowles 1977). Of course, filtering light decreases the range of wavelengths over which an individual photoreceptor pigment can detect the light. Thus, oil droplets allow for better chromic discrimination at the trade-off of decreased sensitivity (Collin et al., 2009; Govardovskii, 1983; Hart, 2001; Vorobyev, 2003). The light lost by filtering may be offset by the lens-like function of the droplets that alter the refractive index of longer light wavelengths and focus light to the outer segment (Stavenga and Wilts, 2014).
It is thought that a common ancestor to birds and mammals had developed an advanced visual system containing oil droplets, but that these oil droplets (and double cones) were lost during some part of mammalian evolution, possibly during a period when mammals were primarily nocturnal (Collin, 2010; Collin et al., 2009; Walls, 1963). Evidence for this is found in fossil records from the Mesozoic Era, showing mammals that were primarily small insectivores (Heesy and Hall, 2010; Luo, 2007). Nocturnal behavior was thought to remove the evolutionary need for better color perception and acuity, eventually resulting in the loss of cone types. Moreover, as most, but not all, oil droplets absorb light, they were thought to hinder night vision, further supporting their loss in early nocturnal mammals. In fact, many mammals today have only dichromic vision (Two LWS subtypes, L and M cone photopigment types), with humans having re-evolved trichromic vision (L, M, and SWS1 cone photopigment types) in response to pressure to develop better vision in a diurnal cycle (Jacobs, 1996).
Both chicken and human retinas are cone-rich, highlighting the importance of color vision in both species. In chickens this is manifested in a 3:2 cone to rod ratio overall (Meyer and May, 1973), while the human retina exhibits a 1:20 ratio of cones to rods (Curcio et al., 1990). Cone density varies within both chicken and human retinas. In both species, areas corresponding to central vision contain a high density of cones that decreases gradually in areas of increasing eccentricity (Bueno et al., 2011; Chui et al., 2008; Kram et al., 2010). In the chick, the highest density of cones occurs in the afoveate area centralis (a roughly circular rod-free region approximately 3 mm in diameter (as calculated from the figures in Weller et al. (2009)), which provides for high acuity color vision (Bueno et al., 2011; Morris, 1982). Adaptive optics studies (Bueno et al., 2011; Headington et al., 2011) and histological investigation (Kram et al., 2010) demonstrate estimates for chick cone density varying from 15,000 to 36,000 cones/mm2 in the central retina. In comparison, despite being more rod-dominated overall, adaptive optics (Wells-Gray et al., 2016) and histological (Jonas et al., 1992) studies estimate human cone density to be between 140,000 and 188,000 cones/mm2 at the fovea. In peripheral retinal locations, chick cone densities are approximately 8,000 cones/mm2 (Bueno et al., 2011), while corresponding human estimates range from 6,000 to 7,000 cones /mm2 (Chui et al., 2008; Jonas et al., 1992)
As mentioned, novel adaptive optics imaging techniques have been used to characterize the cellular properties of the chick retina in vivo. It is worth noting that, in addition to the aforementioned studies focused on measuring cone densities, adaptive optics has also been successfully used to measure chick photoreceptor pointing properties, describing the alignment of their longitudinal axes, in vivo (Walker et al., 2015). This application may be useful in future studies of chick eye development.
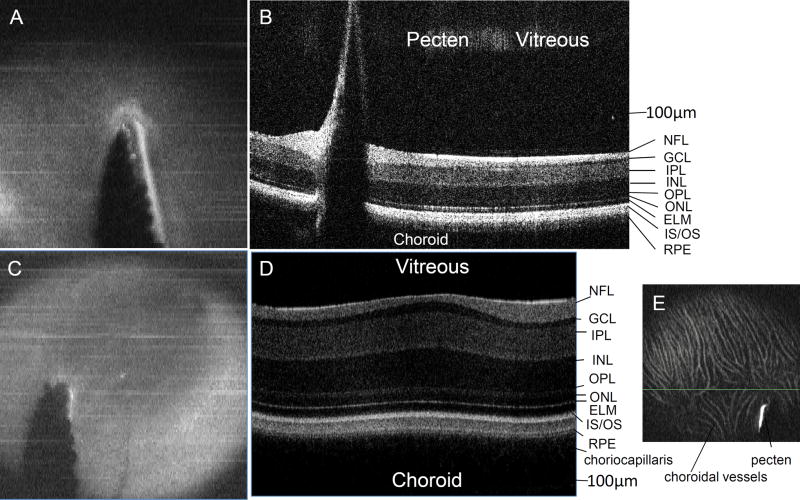
Avian species lack retinal vasculature, and instead have a pecten oculi. The pecten (Fig. 6), unique to avian species, begins from the optic nerve head and extends out into the vitreous. It is a highly pleated and vascular structure, but its exact function is still debated (Jones et al., 2007; Mann, 1924).
Figure 6.
SD-OCT imaging of chick posterior ocular segment. A) SD-OCT En face view centered on pecten oculi. B) Corresponding SD-OCT B-scan showing cross-sectional view of pecten and retinal layers. C) SD-OCT En face view of pecten oculi and area centralis. D) Corresponding cross-sectional SD-OCT area centralis image. E) SD-OCT En face view centered anterior to the pecten oculi at the level of the choroidal vasculature. NFL (Nerve Fiber layer), GCL (Ganglion cell layer), IPL (Inner plexiform layer), INL (Inner nuclear layer), OPL (Outer plexiform layer), ONL (Outer nuclear layer), ELM (External Limiting Membrane), IS/OS (Inner segment/ outer segment junction), RPE (Retinal pigment epithelium complex). Images were obtained with the Envisu system (840nm) with the General Retina bore. Scale bars 100 µm.
In chickens, the pecten is directly connected to the inner nerve layer (Fig. 6) (Seaman and Storm, 1963). The lack of vessels overlying the retina decreases the amount of light scattered before reaching the photoreceptors, improving visual acuity. As the chicken retina lacks direct vascularization, the inner retina is classically thought to receive nutrients, at least in part, from the pecten via diffusion through the vitreous (Jones et al., 2007; Waldvogel, 1990). While the pecten is located >1 cm from certain retinal neurons, casting doubt on the ability of nutrients to effectively diffuse across such a distance, Pettigrew et al. (1990) demonstrated that the saccadic oscillations observed in birds may aid in propelling nutrients to more distant regions. The underlying choriocapillaris supplies oxygenation and nutrients to the outer retina (Evans and Martin, 1993).
Initial experiments with surgical ablation of the pecten in pigeons led to degeneration of the inner retinal layers (Wingstrand and Munk, 1965). However, the optic nerve head derives some of its vascular supply from arterioles at the base of the pecten; therefore, the retinal atrophy may have been caused by disruption of these vessels rather than destruction of the pecten (Brach, 1975). When using electrocautery to ablate the pecten, Brach (1975) found no retinal degradation and preservation of vision. These findings suggest the pecten may have functions apart from nutritional support of the retina.
The pecten oculi of many avian species contain a high density of melanin. Unlike many other pigments which have a much more limited range of absorption, melanin can absorb near infrared, visible, and ultraviolet light (Goodman and Bercovich, 2008; Hu et al., 2008). The fact that high levels of the enzymes catalase, carbonic anhydrase, and alkaline phosphatase are found within the pecten suggest metabolic activity, leading some to suggest that the melanin is involved in creating metabolic energy from absorbed light (Brach, 1977; Goodman and Bercovich, 2008). Specific hypotheses for this process include increased recycling of metabolic intermediates coupled to the energy absorbing function of melanin and the use of melanin and catalase to produce oxygen in the retina from toxic intermediates in response to light-induced NADPH stimulation (Goodman and Bercovich, 2008). Though these actions would be a small addition to the total metabolism, they may confer a competitive advantage, especially to avian species in environments with scarce resources or those undergoing long migratory flights with little rest.
2.9 Choroid
The avian choroid is a multi-layered, pigmented, and well vascularized tissue (Jones et al., 2007; Koch, 1973; Wood, 1917). Anteriorly the choroid becomes continuous with the iris. The proximal layers of the choroid contain the choriocapillaris (Fig. 6) and are responsible for supplying the outer retina with nutrients and oxygen (Evans and Martin, 1993; Jones et al., 2007; Koch 1973). The lamina basalis, the innermost layer, separates the retinal pigment epithelium from the underlying choriocapillaris (De Stefano and Mugnaini, 1997; Evans and Martin, 1993; Jones et al., 2007). Interestingly, the choroid may also serve as an ocular cooling system, dissipating heat from light and metabolic processes in the eye (Auker et al., 1982; De Stefano and Mugnaini, 1997; Jones et al., 2007; Parver et al., 1980).
2.10 Optic Nerve and Inner Retina
The chick optic nerve head (ONH) has a relatively linear shape with the pecten inserted basally (Brach, 1977; Seaman and Storm, 1963). Thus, the pecten limits ONH visualization with non-invasive imaging techniques. High-quality histological images of the optic nerve head of the embryonic chick have been published by Halfter et al., (2001). Relatively little work has been done on the chick ONH. May (2008) provides comparisons of what is known about the optic nerves and inner retinas of various species used in glaucoma research. As in humans, the chick optic nerve does have a lamina cribrosa, though this structure is described by Morcos and Chan-Ling (2000) as poorly developed without much specialization of connective tissue. The chick also lacks retinal vessels at the optic disc. The chick retina contains approximately 2.4 million ganglion cells (Rager and Rager, 1978), as compared to an estimated 700,000 – 1.5 million in human retinas (Curcio and Allen, 1990). According to Ehrlich and Morgan (1980) in the chick, 30–35% of ganglion cell layer neurons in both central and peripheral locations appear to be amacrine cells, while humans demonstrate a higher concentration of amacrine cells in the periphery 80%, with only 3% at the fovea (Curcio and Allen, 1990). The chick retina does not appear to have astrocytes in the inner retina (Schuck et al., 2000) as seen in humans.
Studies to explore neuroglial and gliovascular biological mechanisms have characterized the special glia of the chick optic nerve head (see Fischer et al., 2010a; Schuck et al., 2000; Stanke et al., 2010). The chick has a population of peripapillary glia which are hypothesized to play a role in limiting the development of capillaries into the avascular retina (Schuck et al., 2000). Stanke et al. (2010) identified Pax2 expression in the peripapillary glia as well as in the glia of chick optic nerve. The chick optic nerve head macroglia include a novel glial cell type termed “NIRG” (non-astrocytic inner retinal glia-like) which are also present in the retina. Although the morphology of NIRG cells in the inner plexiform layer is typical of astrocytes (Rompani and Cepko, 2010), they are positive for Nkx2.2, Olig2, and nestin and negative for Pax2, S100-beta, and GFAP unlike astrocytes (Fischer et al., 2010a; Stanke et al., 2010). NIRG cells are present in chick ONH, and may be present in the ONH of canines and non-human primates, but not mice and guinea pigs (Fischer et al., 2010a).
2.11 Visual Acuity
Visual acuity in animal models is often described using techniques to measure visual spatial resolution. Johnson (1914) initially measured visual acuity in an adult chicken by observing its choices between two food boxes after providing a signal light. This study estimated the chicken’s visual acuity to be approximately 25% that of the human. Studies in young chicks (Over and Moore, 1981; Schmid and Wildsoet 1998) suggest that chicks demonstrate useful visual acuity within the first few days post-hatch. Over and Moore (1981) estimated the visual acuity of the chick to be approximately 1.5 cycles per degree (c/d) by post-hatch day 5 using a protocol that required chicks to jump to a grating. This study found that most of the improvement in spatial acuity occurred between post-hatch days 1 (1.17 c/d) and day 2 (1.39 c/d) and that beyond this there was minimal improvement from day 2 to day 25. DeMello et al. (1991) used a different method where adult chickens were trained to peck at targets when they became illuminated. The highest visual acuity recorded among their 6 subjects was 6.2 c/d. More recently, Schmid and Wildsoet (1998) studied the spatial acuity and contrast sensitivity in chicks using an optokinetic nystagmus (OKN) drum, with following of the drum by head turn indicating a positive visual response. Using this technique, spatial acuity was estimated to improve from 6.0–7.7 c/d at post-hatch days 2 or 4 to 7.7–8.6 c/d by day 8. For comparison, measurements of spatial acuity in adult humans with normal Snellen visual acuity were concentrated between 16 and 29 c/d in a recent study by Dakin and Turnbull (2016).
Temporal visual acuity, another important component of visual perception, is a measure of how quickly an organism can process visual stimuli. Lisney et al. (2011) used a behavioral technique wherein birds were trained to peck when provided with light stimuli to measure the flicker fusion responses of adult chickens. This study determined the critical flicker fusion frequency (CFF), the frequency at which a flickering light is perceived to be continuous, to be an average of 87 Hz in the chicken. Lisney et al. (2012) then used electroretinography (ERG) techniques to repeat measurements of CFF in chickens, finding that the ERG responses were in phase with the stimulus up to 105 Hz, although it did not seem chicks could consciously perceive stimuli at this rate. Similar findings have been noted in humans, with CFF between 53 and 87 Hz as measured using ERG (Bowles and Kraft, 2012). Recent work by Davis et al. (2015) also indicates that humans have the ability to perceive flicker artifacts at rates well above the reported CFF, perhaps up to rates of 500 Hz. Davis et al. (2015) suggest that saccadic eye movements are responsible for this ability to detect high frequency flicker.
Chicks also have the ability to perceive a wide spectrum of colors using the cone photopigment types described in Section 2.8. Viets et al. (2016) reviews the photoreceptor patterns present in the retinas of various species, including the chick, and describes the resultant color perception abilities of each species, noting that the chick has excellent color vision.
2.12 Electrophysiology
The electroretinogram (ERG) is a measure of light-evoked electrophysiologic responses used in functional evaluation of the retina. Subsequent sections of this review describe the use of ERG to characterize induced disease models (Section 3.3) and hereditary mutant models, particularly those with retinal degeneration (Sections 4.3, 4.5, 4.6, and 4.8). Both pattern and full field ERGs have identifiable wave forms that can be measured in the chick. Ostrin et al. (2016) studied pattern ERGs (PERGs) in normal and experimental chicks after optic nerve transection. Interestingly, chicks with transected optic nerves still demonstrate meaningful PERGs, indicating it is unlikely that retinal ganglion cells play a role in the chick PERG. Importantly, the PERG may also have some utility in estimating the visual acuity in the chicken. Using PERG, Ostrin et al. (2016) estimated chick visual acuity to be approximately 2.2 cycles/degree, representing notably less than described by OKN testing methods (See Section 2.11). Ostrin et al. (2016) speculate that that inherent differences in the visual pathways activated by PERG and OKN testing may be responsible for the difference estimation of visual acuity. Schmid et al. (2013) used multifocal ERG (mfERG) to study retinas of normal chicks and those with form-deprivation myopia. In normal chicks there was minimal variation of the mfERG across the central retina, suggesting relatively uniform cone density. In chicks undergoing form-deprivation myopia, significant alterations were noted in mfERG waveforms compared to controls. Perhaps most interestingly, the effects of form-deprivation treatment on the mfERG were notable as early as 2 hours after treatment initiation.
ERG has also been used for specific investigation of chick rod function. Scaheffel et al. (1991) used ERG to demonstrate that rod function in chicks is under diurnal control, with function endogenously turned off during the day. Similar results have also been noted in the quail (Manglapus et al., 1998). Dark adaptation prior to ERG in chicks did not result in activation of rod function during normal daytime hours, but rod activity was noted during nighttime hours (Schaeffel et al., 1991).
3. Current Research Applications: Chick Models for Human Diseases and Conditions
Chick models have been used for the study of several major ocular diseases and conditions, as detailed below in sections 3.1–3.5. Table 2 provides an overview of the induced disease models described herein.
Table 2.
Induced Ocular Models
| Process/Disease/Condition (section) |
Method | References |
|---|---|---|
| Corneal wound healing, refractive surgery, transplantation (3.1) |
|
|
| Open angle glaucoma (decreased aqueous outflow, elevated IOP) (3.2) | Exposure to continuous light post hatch | (Frankelson et al., 1969; Jensen and Matson, 1957; Kinnear et al., 1974; Lauber, 1987; Lauber et al., 1961, 1965, 1985; Li et al., 1995; Smith et al., 1969; Wahl et al., 2016) |
| Glaucoma (retinal ganglion cell death) (3.2) | Intravitreal injection colchicine | (Fischer et al., 1999a; Fischer and Reh, 2002) |
| Rhegmatogenous retinal detachment (3.3) | Sub-retinal injections of hyaluronic acid or saline | (Cebulla et al., 2012) |
| Retinal excitotoxic damage (many retinal disorders) (3.3) | Intravitreal injection NMDA | (Fischer et al., 1998; Fischer and Reh, 2002) |
| Chemical retinal detachment/folds (3.3) | Intravitreal injection NMDA plus IGF-1 | (Fischer et al., 2010b) |
| Gestational diabetes (3.3) | Inject embryo with glucose | (Zhang et al., 2016) |
| Diabetic retinopathy (type 1) (3.3) | Inject embryo with streptozotocin | (Shi et al., 2014; Yoshiyama et al., 2005) |
| Ocular Tumors (Uveal Melanoma, Retinoblastoma) (3.4) |
|
|
| Myopia (3.5) |
|
(Cohen et al., 2011; Hodos and Kuenzel, 1984; Schaeffel et al., 1988; Troilo et al., 1987; Wallman et al., 1978; Westbrook et al., 1995; Wildsoet, 2003; Wildsoet and Schmid 2000) |
| Hyperopia (3.5) |
|
(Cohen et al., 2011; Li et al., 1995; Schaeffel et al., 1988; Wahl et al., 2015; Wildsoet, 2003; Wildsoet and Schmid, 2000) |
3.1 Corneal Disease
This chick cornea is well-suited for study of corneal wound healing, opacification, and refractive surgery, and transplantation, particularly given the anatomical similarities with humans. The chick Bowman's layer, as in humans, is composed primarily of type V collagen intermixed with smaller amounts of type I collagen (Fowler et al., 2004; Gordon et al., 1994). Linsenmayer et al. (1998) provides a detailed review of chick corneal development and the collagen molecules involved. In the chick and the human, Bowman's layer plays a key role in corneal epithelial healing and adhesion. Fowler et al. (2004) used the chick to compare post-mechanical debridement re-epithelialization rates in corneas with preserved or laser-disrupted Bowman’s layer and observed significantly faster re-epithelialization with Bowman's layer preserved. Moreover, after performing photorefractive keratectomy (PRK) on chick corneas, this study demonstrated histologically that irregularities between the stroma and Bowman’s membrane correlated with the development of corneal haze, a major postoperative concern for laser refractive surgeries. In chick models, corneal haze has been shown to be proportional to the density of stromal cells, the bulk of which stain positive for the smooth muscle actin marker found in myofibroblasts (Fowler et al., 2004; Martínez-García et al., 2006). This is consistent with work in post-LASIK human corneas by Dawson et al. (2005) that showed corneal haze in the flap margin with heavy stromal cellularity, most notably composed of myofibroblasts.
Ritchey et al. (2011) studied wound healing following incision of chicken corneas and demonstrated epithelial and stromal cell changes resulting in cellular migration to the site of injury, re-innervation of the wound site by the ophthalmic division of the trigeminal nerve, and migration of CD45-expressing monocytes to the wound site. Martínez-García et al. (2006) performed PRK in hens, producing similar changes including proliferation of epithelial cells at the limbus and initial apoptosis of some stromal cells followed by stromal proliferation 24 hours after PRK. The presence of macrophages was also seen at 12 hours post-op. These changes are similar to those found in rabbit models of corneal injury (Wilson et al., 2001). The chicken cornea has also been developed as a model for corneal haze, and can be used for future study on the pathophysiology and potential treatment of the condition (Gómez et al., 2001; Merayo-Lloves et al., 2001). In sum, the similarities in anatomy and mechanics of healing make the chicken an ideal model to study corneal injuries and healing following mechanical and laser procedures, such as refractive surgery (Fowler et al., 2004; Martínez-García et al., 2006; Ritchey et al., 2011).
The corneal endothelial layer is responsible for maintaining corneal deturgescence and plays a role in a variety of human diseases, most notably Fuch’s endothelial dystrophy. Importantly, damage and loss of endothelial cells from multiple pathologies often renders donor corneas non-optimal as transplants. Given the current demand for corneal transplantation, the possibility of using cultured endothelial cells to rebuild denuded corneas is being explored. Insler and Lopez (1991) transplanted human corneas, which had undergone endothelial cell denudement and replacement with culture-grown human fetal endothelial cells, into non-human primate subjects. They found that over 65% of corneas deturgesed and remained clear over 12 months. However, limited availability of human endothelial cells and inherent difficulties in using primate subjects make this a less than ideal research model. Hence, the development of a chicken model is underway. Mangioris et al. (2011) demonstrated that corneal endothelial cells can be harvested and cultured from chicken embryos. They were able to transplant these endothelial cells into host corneas at the same developmental stage, though few endothelial cells successfully incorporated into the host corneas. Those that did incorporate showed good adhesion to Descemet’s membrane. As the techniques for transferring cultured cells improve, the chicken cornea may serve as an important model for corneal transplant using cultured endothelial cells.
3.2 Glaucoma
A chicken model for induced open-angle glaucoma, known as the light-induced glaucoma (LIG) or continuous light (CL) chick, (reviewed by (Bouhenni et al., 2012)) has been present for some time (Jensen and Matson, 1957; Lauber et al., 1961). Exposure to continuous light after hatching has been shown to produce decreased aqueous humor outflow and a shallow anterior chamber, leading to increased intraocular pressure (IOP) (Smith et al., 1969). Kinnear et al. (1974) found that IOP was initially low in these chicks and later became elevated around 16 to 20 weeks post hatch. Despite the fact that these chicks have increased axial length compared to controls, they exhibit significant hyperopia due to decreased corneal curvature (Li et al., 1995). Eventually, retinal detachment or other pathology occurs leading to complete blindness (Lauber, 1987). A recent experiment by Wahl et al. (2016) provided further description of the IOP in chicks exposed to continuous light (CL). The study demonstrated that CL chicks did not have diurnal fluctuation in IOP present in control chicks. Like Kinnear et al. (1974), they found that CL chicks initially had lower IOP than control chicks at early time points (post-hatch day 4 and week 4).
The iridiocorneal angle appears narrowed in the LIG model; however, both daily miotics, including isoflurophate, demecarium bromide, and ecothiophate iodide, (Lauber et al., 1965), which open up the trabecular meshwork, and iridectomy (Frankelson et al., 1969) failed to produce significant reductions in IOP. This indicates that acute angle pathology does not contribute to increased IOP in this model (Lauber, 1987). Importantly, the LIG model may have a role in translational glaucoma research. The effect of multiple IOP-lowering drugs which are effective in human glaucoma, including acetazolamide (Lauber et al., 1965), timolol, and pilocarpine (Lauber et al., 1985), similarly decrease IOP in the LIG model. Thus, the LIG model may specifically be useful to evaluate glaucoma drug candidates for future translation to the clinic. Furthermore, given the large eye size, stable blood-aqueous barrier, ease of handling, and low cost, LIG chicks might also serve as a reasonable model for glaucoma surgical procedure/device studies.
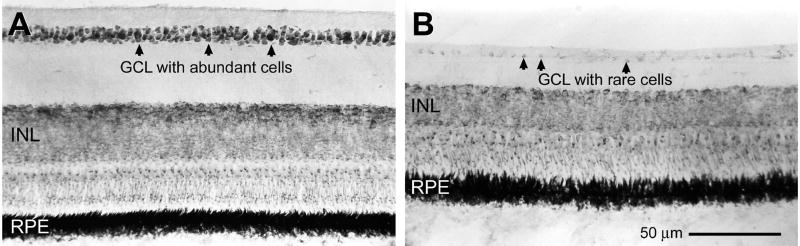
In addition to outflow models of glaucoma, a colchicine model (Fig. 7) has been established which selectively depletes retinal ganglion cells (Fischer et al., 1999a; Fischer and Reh, 2002). This model may be useful for studying mechanisms of RGC loss and neuroprotection.
Figure 7.
A) Photomicrograph of healthy chicken retina stained with toludine blue at 200× magnification. B) Photomicrograph of chicken retina at 200× magnification on day 14 after treatment with colchicine illustrating loss of ganglion cell nuclei. Scale bar 50 µm.
3.3 Retinal Disease
The chick retina’s cone predominance, large eye size, and similar cellular responses to retinal damage as occur in the human make it an advantageous research model. Several models to study retinal damage have been created. Intravitreal injection of the N-methyl-D-aspartate (NMDA) toxin causes excitotoxic damage in the retina, with neuronal apoptosis concentrated in the inner retina (Fischer et al., 1998; Fischer and Reh, 2002). Several human diseases have a significant component of excitotoxic damage with elevated glutamate, including retinal ischemia, vascular occlusion, diabetic retinopathy, and glaucoma (reviewed in (Ishikawa, 2013; Osborne et al., 2004); see (Romano et al., 1998) for review of excitotoxic damage in the chick retina). Thus, the NMDA model is relevant for studying these mechanisms of retinal disease. In addition, combinations of intravitreal NMDA and IGF-1 (Fischer et al., 2010b) or NMDA and IL-6 (Fischer et al., 2015) produce chemical injury with resulting retinal detachment (RD)/retinal folds (Fig. 8).
Figure 8.
Chemically-induced retinal folds. A) Photomicrograph at 200× magnification of healthy chicken retina and (B) NMDA6 treated retina stained with toludine blue. C) NMDA6 treated eye, En-face and (D, E) cross-sectional SD-OCT images of retinal folds. The B-scans (D, E) correspond to the green line and below in (C). Images were obtained with the Envisu system with the General Retina bore. Scale bar 50 µm for (A), (B), and (C). Scale bar 100 µm for (D) and (E).
A model for rhegmatogenous RD has also been by established by Cebulla et al. (2012) using subretinal injections of hyaluronic acid to detach the retina. Despite some anatomic differences in the retina compared with humans and other mammals, the biologic responses in chick RD were similar (Cebulla et al., 2012; Fisher et al., 2005; Lewis et al., 2002; Sethi et al., 2005). Retinal responses to RD included Müller glia proliferation, migration, and increased expression of the intermediate filaments, photoreceptor apoptosis and opsin mistrafficking, outer segment degeneration, loss of outer nuclear layer thickness in detached areas, blunting of the retinal pigment epithelium (RPE), and macrophage accumulation in areas of damage were observed (Cebulla et al., 2012). Additionally, the time-course of these changes, particularly with the preservation of the ONL until seven to 14 days post-detachment, fits well with what is known about human retinal detachments (Davidorf et al., 1975; Diederen et al., 2007; Hassan et al., 2002; Ross, 2002; Ross and Kozy, 1998; Salicone et al., 2006), in contrast to some experimental RD models like rabbits which have more rapid and severe photoreceptor degeneration (Fisher et al., 2005; Lewis et al., 2002). In addition, the cone responses in RD could be evaluated in chicks, including observing a relative loss of SWS1-opsin in RD areas with heavy degeneration compared with levels of LWS/RH2 opsin or calbindin (Cebulla et al., 2012).
Other models for RD present some inherent challenges when compared with the chick model. For example, ground squirrel RD models, commonly used for cone research, do not show the same robust response of Müller glia seen in human and chick RD (Linberg et al., 2002). Moreover, chicks may have advantages over other large mammals, particularly in ease of handling and low cost.
Diabetes is another major disease that needs animal modeling for study. A model has been developed in embryonic chicks for the study of the macular thinning seen in offspring of mothers with gestational diabetes (Tariq et al., 2010; Zhang et al., 2016). Zhang et al. (2016) developed this model by injecting glucose into chick embryos and have subsequently demonstrated specific changes in expression of signaling molecules in the retina, including Pax6, related to these morphological changes.
Streptozotocin, a chemical with selective toxicity for pancreatic beta cells, is commonly used to create diabetic animal models. In contrast to the rat, adult chicks have been reported to be resistant to streptozotocin-induction of diabetes (Simon and Dubois, 1980; Stellenwerf and Hazelwood, 1979). However, chick embryos appear amenable to streptozotocin diabetes induction (Shi et al., 2014; Yoshiyama et al., 2005). Yoshiyama et al. (2005) injected streptozotocin into the albumen of fertilized eggs day 14 of incubation and showed decreased insulin and elevated glucose levels at day 17. Shi et al. (2014) then demonstrated that injection of streptozotocin in the amniotic layer at embryonic day 12 produced typical ocular sequelae of diabetes, including cataract, retinal thinning, ERG abnormalities, and insulin-related signaling changes that were similar to those reported in the streptozotocin rat model. Direct effects of the drug rather than an indirect effect on the insulin-producing cells have not been excluded in this model.
Finally, as described in the subsequent section on the chicken ocular mutants, chicken models are used for the study of inherited retinal disease (IRD). The utility of animal models for IRD research is largely dependent on the similarity of a species’ genome to the human genome. Slijkerman et al. (2015) assessed the utility of the chicken model by comparing amino acid sequences of human proteins known to be associated with IRD to those in their avian counterparts. They found 65% average sequence identity, less similar than all of the mammals studied, which notably included mice (79%) and macaques (92%), but more similar than the zebrafish (54%). This suggests that the chicken model may not be useful in studying certain forms of IRD. On the other hand, the ability to reproduce large numbers of chicks more quickly than their mammalian counterparts provides key advantages in studying IRD.
3.4 Ocular Cancer
Embryonic chicks have also been used in the study of ocular cancers. Kalirai et al. (2015) reviewed the chick embryo’s specific applications in the study of uveal melanoma. They describe the ability to incorporate human uveal melanoma xenografts into embryonic chicks prior to their immune system maturation, allowing for the study of metastasis in vivo. One method for introduction of the uveal melanoma xenografts involves injecting the cells into the chorioallantoic membrane (CAM) of the chick embryo. This CAM method (reviewed by (Deryugina and Quigley, 2008)), first conducted with uveal melanoma cells by Luyten et al. (1993), is advantageous because of the relative ease of access to the CAM, translating to a high embryo survival rate. The CAM’s vascularity also allows for study of the dissemination of tumor cells to various tissues (Kalirai et al., 2015). Bérubé et al. (2005) used this CAM model to study matrix metalloproteinase-2 (MMP-2) activity in several uveal melanoma cell lines and determined that MMP-2 activity was increased in the vicinity of uveal melanoma tissue. Kalirai et al. (2015) also describe their own experiments with injecting uveal melanoma cells directly into the embryonic chick vascular system with subsequent incorporation of tumor cells into ocular and liver tissues. This approach may be useful in future studies of the metastatic behavior of uveal melanoma. Additionally, Busch et al. (2008) have transplanted murine melanoma cells directly into the optic cups of embryonic chicks and observed their malignant growth potential in the developing eye, including invasion of periocular tissue and the lens. Further, they were able to determine that invasive behavior of the melanomas was dependent on bone morphogenic protein-2 (BMP-2) expression and that invasive properties were eliminated in the presence of noggin, a BMP antagonist and morphogenetic signaling factor which has dorsalizing effects that inhibit mesoderm formation, including bone.
Retinoblastoma has also been studied using CAM assays in chick embryos. Busch et al. (2015) used this technique to investigate the metastatic behaviors of several different retinoblastoma cell lines and found that some lines were able to disseminate throughout the embryo, and all lines were able to extravasate from the blood vessels. Embryonic chicks have also been used in gene expression studies of the Disabled-1 (Dab1) signaling pathway believed to be implicated in the development of retinoblastomas (Katyal et al., 2011; Katyal and Godbout, 2004).
Beyond their utility in ocular oncology, CAM models also have broad applicability for studying general biological processes. They are cost-effective and offer potential for high-throughput analyses for novel therapeutics for cancer, angiogenesis and wound healing. See Ribatti (2016) for a review.
3.5 Myopia and Hyperopia
Humans are born with hyperopia, which resolves naturally in most cases as the eye grows after birth and undergoes emmetropization. Refer to Iribarren (2015) for a comprehensive review of refractive development in humans. Refractive errors are common in humans and deserve study. Significant hyperopia and myopia both impact quality of vision and can increase the risk of amblyopia. Of particular concern is the growth in the frequency and severity of myopia in recent years, with as many as 80–90% of school age children in some Asian countries suffering from some degree of myopia (Morgan et al., 2012). Even in western countries the estimated prevalence is 20–25% of the population (Lawrence and Azar, 2002; Sperduto et al., 1983) with some studies indicating prevalence as high as 41% in young adults (Jones and Luensmann, 2012; Vitale et al., 2008). More importantly, high myopia, greater than 6 D, can predispose to severe vision loss, retinal detachment, degeneration of the sclera and retinal pigment epithelium, as well as neovascularization and other abnormalities (Jones and Luensmann, 2012; Morgan et al., 2012). For more detailed review of pathologic myopia, refer to Ohno-Matsui et al. (2016). The increased prevalence and potential for vision-threatening changes highlight the need to better study and understand the basis of myopia.
The chick has provided important insight into the process of emmetropization and development of refractive errors. It is well-known that vision deprivation can alter eye growth and emmetropization. The chick model has perhaps been the most widely studied model because of the ease in handling, relatively large eyes, and relatively rapid rate of ocular growth during adolescent development. Most chicks are born with some degree of refractive error and hyperopia. As the chick grows, increases in the axial length and other anatomic changes lead to emmetropization (Wallman et al., 1981). These changes are very similar to the changes the human eye undergoes in the first two years of life. Interestingly, it appears that normal retinal function plays a large role in emmetropization (Troilo et al., 1987; Westbrook et al., 1995). Deprivation of central vision in chicks can lead to increased axial length and a high degree of myopia, known as form-deprivation myopia (Hodos and Kuenzel, 1984; Wallman et al., 1978). Figure 9 demonstrates a form-deprivation myopia model. Schaeffer and Feldkaemper (2015) reviewed the use of the chick as a model for myopia, and offered comparisons to other animal models. Hyperopia and myopia can also be induced by altering the refractive power of the eye with plus and minus lenses respectively (Schaeffel et al., 1988). Induced refractive changes like formdeprivation myopia remain naturally reversible during the chick’s emmetropization period. Wildsoet and Schmid (2000) found that myopia quickly resolved in young chicks when form deprivation stimulus was removed, but it remained present in formdeprivation chicks whose myopia was subsequently treated with corrective lenses, supporting a vision-driven emmetropization process. Wildsoet (2003) also demonstrated that chicks with induced hyperopic defocus were able to undergo similar active emmetropization to resolve refractive errors after removal of the defocusing stimulus. However, some degree of emmetropization can take place in chicks even when the optic nerve has been transected, indicating local retinal control of emmetropization (Troilo, 1990; Wildsoet and Schmid, 2000; Wildsoet 2003). Wildsoet and Schmid (2000) further elaborated that, while form-deprivation myopia did resolve after removal of defocusing stimulus in chicks with transected optic nerves, these chicks experienced more variability in their refractive error changes, implying that vision is needed for the most accurate emmetropization. Wildsoet (2003) demonstrated that chicks with induced hyperopia were also able to undergo emmetropization after optic nerve transection.
Figure 9.
Model for form-deprivation myopia. A) Control chick eyes. B) Chick eyes treated with injection of IL-6 followed by goggles obscuring central vision for 7 days showing resulting increase in globe size in treated eyes. Scale bar 5 mm.
Hyperopia can also be induced by exposing chicks to continuous light (CL) after hatching (Li et al., 1995; Wahl et al., 2015). Exposure of chicks to increased light intensity with normal, 12-hour light-dark cycles also produces hyperopia in growing chicks (Cohen et al., 2011), while exposure to decreased light intensity on normal cycles produces myopia. These phenomena are explained by the role of the pineal gland and melatonin secretion in emmetropization (Li and Howland 2003). The chick pineal gland appears to be a directly light-sensitive organ, as Li and Howland (2003) demonstrated that placing a hood over the heads of chicks whose eyes were exposed to CL protected those chicks from developing hyperopia. This was hypothesized to be due to maintenance of normal melatonin secretion rhythms in hooded chicks. Additionally, Wahl et al. (2011) demonstrated that giving daily melatonin eye drops to CL chicks was protective against development of hyperopia. Additionally, they found that administering a melatonin hormone blocker, luzidone, caused normal day-night cycle chicks to develop hyperopia.
In chickens and other models including primates, different areas of the visual field respond independently to a defocusing stimulus. Studies using a contact lens with a clouded hemi-field showed differential growth of the sclera in the area with clouding (Troilo et al., 1987; Wallman et al., 1987), even following optic nerve transection (Troilo, 1990). Even when central vision is corrected by refractive lenses, the periphery may remain relatively hyperopic. It is thought that this remaining peripheral stimulus for growth may contribute to increasing eye size and central myopia (Schippert and Schaeffel, 2006). A recent study by Tepelus et al. (2012) tested this hypothesis in chickens using radial refractive lenses, which had more positive power in the periphery than centrally. At least for one type of radial refractive lens, findings indicated inhibition of both central and peripheral eye growth. The use of these chick models for refractive error is facilitated by a recent Iribarren et al. (2014) study, which established a modified version of Bennett’s equation for calculation of lens power. The introduction of a standardized method for this calculation allows widely available biometric techniques to be employed in chick experiments.
While the specifics of the environmental and genetic interplay that cause myopia in humans is still unknown, significant research has been done on the molecular signaling responsible for vision-guided eye growth (Also discussed in section 5.1). Some of the endogenous signaling molecules (reviewed by Wallman and Winawer (2004)) that have been determined to be involved in chick vision-guided eye growth and myopia development include dopamine (Lauber, 1991; Stone et al., 1989), gamma-aminobutyric acid (GABA) (Chebib et al., 2009; Christian et al., 2013; Stone et al., 2003), insulin-like growth factor-1 (IGF1) and fibroblast growth factor-2 (FGF2) (Ritchey et al., 2012b), N-methyl-D-aspartic acid (NMDA) (Fischer et al., 1998; Fischer et al., 1997), retinoids (Fischer et al., 1999b), and glucagon (Feldkaemper and Schaeffel, 2002; Fischer et al., 1999c; Fischer, 2005; Fischer et al., 2008), which may be one of the most critical signaling molecules (Fischer, 2011). Colchicine has also been determined to affect eye growth and development of myopia (Fischer et al., 1999b). Colchicine’s effect is likely mediated by its ability to destroy bullwhip neurons in the retina, which are thought to mediate ocular growth through glucagon release (Fischer et al., 2008). It should be noted that neurons that produce glucagon or related neuropeptides have not been detected in the retinas of primates (Fischer, unpublished observations). Murine retinas also lack glucagon signaling, although other similar neuropeptides have been detected (Mathis and Schaeffel, 2007). Thus, the mechanisms involving the growth-slowing effects of glucagon in the chick are likely mediated by a different mechanism for detecting defocus than in primates. It is also notable that the chick choroid’s ability to rapidly gain thickness in response to imposed myopic defocus (Wallman et al., 1995; Wildsoet and Wallman, 1995) has not been observed in primate models. This choroidal response is notable as the choroid is situated next to the RPE, which Rymer and Wildsoet (2005) suggest plays a critical role in signaling and regulation of eye growth. Nevertheless, evidence suggests that similar principles regulate retina/vision-guided ocular growth and emmetropization in warm-blooded vertebrates, but the players within the retina are different (Schaeffel and Feldkaemper, 2015).
Further use of chick models in the study of myopia has identified candidate genes that undergo differential expression during eye growth. Ashby and Feldkaemper (2010) used microarray technology with real-time RT-PCR confirmation to investigate gene expression changes specific to the amacrine cell layers of chicks subjected to myopia and hyperopia-inducing lenses. Differential gene expression after plus vs. minus lens treatment identified candidates for future study.
4. Chicken Hereditary Ocular Conditions
The chicken genome, 1.23 × 109 base pairs (bp), is approximately 40% of the size of the 3.1 × 109 bp human genome. A total of 16362 coding genes has been characterized. A list of hereditary conditions reported in chickens is available at the On-line Mendelian Inheritance in Animals (OMIA) website, http://omia.angis.org.au/home/. These chickens could be useful in the study of the biology and genetics of various ocular diseases, as well as for gene editing studies. As of November 2016, a total of 212 hereditary traits have been reported in OMIA. Out of these, 13 have significant ocular phenotypes as summarized in Table 3. The literature about these mutants has not been standardized, and we use the OMIA nomenclature to standardize names used herein. It should be noted that in chickens, contrary to humans, the males are the homogametic sex (ZZ) while females are the heterogametic sex (ZW). Thus, females are affected by sex-linked genes rather than males. Many of these hereditary conditions have been reviewed by Hocking and Guggenheim (2014). In sections 4.1 to 4.12 below, we describe 13 ocular mutant models and examples of the research that has been performed using them.
Table 3.
Chicken Hereditary Ocular Conditions
| Name (symbol; OMIA #; Section #) |
Gene | Disease/Condition Model |
Inheritance | References |
|---|---|---|---|---|
| Blindness Enlarged Globe (beg; 001367-9031; 4.9) | Unknown | Retinal Degeneration, Pathologic Myopia, Iris Neovascularization | Autosomal Recessive | (Boote et al., 2009; Morgan et al., 2013) |
| Chick albino (ca; 000369-9031; 4.1) | Tyrosinase (TYR) | Ocular Albinism | Autosomal Recessive | (Jeffery and Williams, 1994; Rymer et al., 2007) |
| Coloboma (co; 000219-9031; 4.4) | Unknown | Coloboma | Z-linked | (Abbott et al., 1970; Robb et al., 2013) |
| Delayed Amelanosis, Smyth Line (DAM; 000034-9031; 4.3) | Unknown | Vitiligo, Vogt-Koyanagi-Harada Disease, Sympathetic Ophthalmia, Chediak-Higashi syndrome | Multifactorial | (Boyle et al., 1987; Fite et al., 1982–1983, 1983, 1986; Fulton et al., 1983; Jang et al., 2014; Lahiri and Bailey, 1993; Shi and Erf, 2012; Shi et al., 2012; Smyth et al., 1981; Smyth and McNeil, 1999; Wick et al., 2006) |
| Microphthalmia-4 (mi-4; 000650-9031; 4.11) | Unknown | Microphthalmia | Unknown | (Somes, 1992) |
| Pink-eye (pk; 001700-9031; 4.2) | Unknown | Possibly Ocular Albinism | Autosomal Recessive | (Brumbaugh et al., 1973; Brumbaugh and Lee, 1975; Warren, 1940) |
| Pop-eye (pop; 001253-9031; 4.7) | Unknown | Keratoconus | Z-linked | (Bitgood and Whitley, 1986) |
| Sleepy Eye (se; 000921-9031; 4.12) | Unknown | None | Autosomal Recessive | (Somes, 1968; Carefoot, 1993) |
| Retinal Degeneration (GUCY1*; 001245-9031; 4.5) | Retinal Guanylate cyclase-1 (GC1) | Leber’s Congenital Amaurosis Type 1 (LCA1) | Autosomal Recessive | (Baehr and Frederick, 2009; Chung and Traboulsi, 2009; Semple-Rowland and Cheng, 1999; Semple-Rowland et al., 1998, 2001; Ulshafer and Allen, 1985; Ulshafer et al., 1984; Verrier et al., 2011; Williams et al., 2006) |
| Retinal Dysplasia and Degeneration (rdd; 001366-9031; 4.6) | Multiple PDZ domain protein (MPDZ) | Retinitis Pigmentosa, LCA | Z-linked | (Ali et al., 2011; Beattie et al., 2012; Burt et al., 2003; Finnegan et al., 2010; McKibbin et al., 2014; Randall et al., 1983) |
| Retinopathy, Globe Enlarged (rge; 001368-9031; 4.8) | Guanine nucleotide binding protein 3 (GNB3) | Pathologic Myopia (early), Retinal Degeneration (late), ON bipolar cell dysfunction | Autosomal Recessive | (Boote et al., 2009; Curtis et al., 1988; Inglehearn et al., 2003; Montiani-Ferreira et al., 2003, 2005; Tummala et al., 2006; Ritchey et al., 2010, 2012a) |
| Retinal degeneration, Smoky Joe (SJ; 001869-9031; 4.10) | Unknown | Cataracts, Buphthalmos, Iridodonesis, Phthisis Bulbi, Retinal Degeneration | Autosomal Recessive | (Baxter et al., 2014; Salter et al., 1997; Tran et al., 2013) |
| Visual Impairment (GSN/1; 001352-9031; 4.12) | Unknown | None | Unknown | Shibuya et al. (2002) |
4.1 Albino Chick (ca)
Rymer et al. (2007) showed albino chicken mutants shared some of the same ocular features found in human albinos including red eyes (due to lack pigment and showing of the underlying choroidal vessels), decreased visual acuity, and increased refractive abnormalities. It is important to note that this mutant strain lacked the nystagmus that characterizes albinism in humans. Additionally, this strain was previously demonstrated to lack the decreased cell density of the neural retina observed in albino mammals (Jeffery and Williams, 1994). Despite this, ca chicks share many of the other ocular abnormalities as albino humans including photophobia, decreased visual acuity, and refractive errors. Thus, this strain serves as a good model for the study of ocular pathology resulting from albinism.
4.2 Pink Eye (pk)
First described by Warren (1940), this autosomal recessive mutation causes decreased melanin deposition in ocular tissues as well as feathers. Further studies in the pk mutant (Brumbaugh et al., 1973; Brumbaugh and Lee, 1975) demonstrated a structural defect in the premelanosomes, rendering them unable to effectively bind melanin intermediates. Although the pk mutant may be useful in the study of ocular albinism, this model has not been studied further in recent decades.
4.3 Delayed Amelanosis (DAM)
The delayed amelanosis (DAM) chicken, also known as the Smyth Line (SL) chicken, was first described by Smyth et al. (1981) as a disease model for vitiligo. This strain exhibits marked loss of melanin in its feathers and develops blindness within two weeks of the appearance of amelanotic feathers. Histopathologic analysis initially demonstrates the destruction of choroidal melanocytes and inflammatory cell infiltration in the choroid (Fite et al., 1982–1983). As vision deteriorates, choroidal involvement is followed by depigmentation of retinal pigment epithelium (RPE), loss of apical processes in the RPE, and subsequent photoreceptor cell death (Fite et al., 1982–1983, 1983; Smyth et al., 1981). Evidence suggests that impaired phagocytosis in the RPE may be responsible for the retinal degeneration that occurs in the DAM strain (Lahiri and Bailey, 1993). Degenerative changes of the retina begin at the base of the pecten oculi and progress to involve the central retina, occasionally sparing some areas. Fulton et al. (1983) used ERG to describe variability in the visual sensitivity of DAM mutants that correlated directly with the degree of histopathological disease involvement. This mutant strain has been suggested as a model for further study of several inherited diseases involving ocular pigmentation, including vitiligo, Vogt-Koyanagi-Harada disease, Chediak-Higashi syndrome, and sympathetic ophthalmia (Fite et al., 1982–1983).
Successful treatment of Smyth Line vitiligo has been demonstrated with administration of cyclosporine (Fite et al., 1986) and corticosterone (Boyle et al., 1987), producing decreased cutaneous and ocular manifestations of autoimmune amelanosis. As reviewed by Wick et al. (2006), DAM chicks have been used extensively as a model for the study the genetic basis and pathophysiology of vitiligo. DAM chicks are a convenient model due to their early disease expression at approximately six to ten weeks post-hatch (Smyth and McNeil, 1999). Recent studies of DAM chicks have led to more detailed characterization of the autoimmune, cytokine-mediated disease activity (Shi and Erf, 2012) and have narrowed in on various possible genetic markers of the disease (Jang et al., 2014) as well as potential changes in gene regulation correlated with disease activity (Shi et al., 2012).
4.4 Coloboma (co)
First described by Abbott et al. (1970), the coloboma (co) chick demonstrates a wide variety of malformations inherited through sex-linked genes. Chicks hatch with ocular coloboma as well as skeletal malformations, commonly including shortened limbs. The clinical characteristics of these chicks are similar to those seen in human coloboma cases (Hocking and Guggenheim, 2014). Recent progress has been made toward identification of the responsible mutation, which is lethal for female embryos (Robb et al., 2013). Three candidate genes have been identified as targets for further investigation.
4.5 Retinal Degeneration (GUCY1*)
Another mutant strain being studied is the retinal degeneration or GUCY1* chick by OMIA nomenclature, formerly known as the rc or rd chick (Semple-Rowland and Cheng, 1999). Other studies (Semple-Rowland et al., 2001; Williams et al., 2006; Verrier et al., 2011) have referred to their strain as GUCY1*B. This mutant demonstrates loss of retinal guanylate cyclase-1 (RetGC1) activity and serves as a model for Leber’s Congenital Amaurosis Type 1 (LCA1), which is caused by mutation in the human orthologue GUCY2D (Chung and Traboulsi, 2009; Perrault et al., 1996). Typically inherited as an autosomal recessive condition, LCA1 (reviewed by (Boye, 2016; den Hollander et al., 2008)) is characterized by loss of visual function in infancy, nystagmus, and apparent roving eye movements. In the corresponding chick model with mutation in GUCY1*, affected chicks are blind at hatch and ERG measurements are diminished or even non-recordable upon hatching (Ulshafer et al., 1984). However, histological studies have shown no significant pathology until 7–10 days post-hatch (Baehr and Frederick, 2009; Semple-Rowland et al., 1998; Ulshafer and Allen, 1985). Like LCA1 in humans, the GUCY1* chick exhibits autosomal recessive inheritance with homozygous mutations resulting in loss of function of RetGC1 in rods and cones, resulting in significantly lower levels of cyclic guanosine monophosphate, a signal transduction mediator in photoreceptors (Baehr and Frederick, 2009; Semple-Rowland et al., 1998). Initial pathology is confined to photoreceptors with eventual loss of the photoreceptor layer by six to eight months post-hatch (Semple-Rowland et al., 1998; Ulshafer and Allen, 1985).
This model has already been used to study gene therapy. Williams et al. (2006) introduced a functional guanylate cyclase gene into GUCY1*B mutants using a lentivirus vector (Also described in section 5.2). Six of the seven subjects studied showed improvements in ERG responses and vision, based on optokinetic responses and vision-guided behavior. Verrier et al. (2011) also demonstrated restoration of vision in GUCY1*B chicks treated with bicistronic lentivral transgenes for functional guanylate cyclase, although visual improvements were transient and incomplete.
4.6 Retinal Dysplasia and Degeneration (rdd/MPZD)
Still another mutant strain serves as a model for retinitis pigmentosa. The phenotypic features of rdd chicks were described by Randall et al. (1983) as progressive degeneration of the retina with a diminished number of photoreceptors. Burt et al., (2003) determined that the z-linked mutation rdd leads to progressive vision loss from hatch with blindness resulting around 15 weeks. A significant reduction in photoreceptor numbers is noted in mutant chicks by embryonic day 18; by three weeks post-hatch, ERG recordings are flat (Burt et al., 2003). Spectral domain OCT demonstrates progressive thinning of the retina and the choroid of rdd mutants when compared to wild type chicks (McKibbin et al., 2014). Progressive loss of lamination of the outer retina also occurs. Histopathological findings include pigment changes and atrophy of the pecten oculi (Burt et al., 2003).
Recently, (Ali et al., 2011) identified the defective MPDZ gene located on chicken chromosome Z implicated in the rdd chicken model in which a C→T substitution resulted in a premature stop codon. In wild-type birds the full-length MPDZ protein is found in the retinal outer limiting membrane, while the full-length MPDZ protein was absent in the retinas of rdd chickens. This localization to the outer limiting membrane is important for two reasons. First, the outer layers of the retina, are the earliest sites of observed pathology in rdd chicks (Finnegan et al., 2010). Second, CRB1, a protein known to interact with MPDZ, is also located there. Mutations in CRB1 are known to cause retinitis pigmentosa (den Hollander et al., 1999) and LCA (Cremers et al., 2002) in humans. Evaluation of human retinal degeneration patients only revealed heterozygous changes in MPDZ, although deletions of exons, introns, or promoters could not be ruled out; MPDZ variants acting as modifiers could also not be excluded (Ali et al., 2011).
In addition to identifying a null mutation present in the rdd chicks, further progress is also being made at the level of protein expression. Finnegan et al. (2010) utilized gel electrophoresis, gel imaging, and mass spectrometry to profile protein expression in wild-type and rdd mutant chickens. The group was able to identify seven proteins whose expressions were significantly different between the wild-type and rdd chicken retinas. The most notable variation was that of the protein Secernin 1. Interestingly, the total expression of Secernin 1 was not different between wild-type and rdd chickens. However, resolution of the protein along an acid-base axis revealed the differential expression of two isoforms of Secernin 1, possibly the result of post-translational modifications including phosphorylation. Although the exact role of Secernin 1 and the other differentially expressed proteins is not known, the use of proteomics is a promising tool for further elucidation of the molecular mechanisms underlying retinal degeneration. Lam et al. (2006) have established a proteome database specific to the chick retina to facilitate further work in this field. Further characterization of the biomolecular differences between rdd and wild-type chicks has been investigated by Beattie et al. (2012) using Raman microscopy techniques.
4.7 Pop-eye (pop)
Bitgood and Whitley (1986) described a sex-linked mutant strain causing keratoconus. The keratoconus is not immediately detectable post-hatch, but is discernible after a few weeks of growth. This Pop-eye mutant, as it was eventually called, is associated with an isolated increase in anterior chamber depth. The authors suggested that the Pop-eye strain may serve as a good model for studying ocular refractive errors as well as potential treatment modalities for keratoconus; however, our literature search did not reveal further ocular studies using the Pop-eye mutant.
4.8 Retinopathy, Globe Enlarged (rge)
The retinopathy, globe enlarged (rge) mutant strain was first described in a commercial line of chickens found in the United Kingdom (Curtis et al., 1988; Inglehearn et al., 2003; Montiani-Ferreira et al., 2005). At birth, rge chicks have poor visual acuity but normal ocular dimensions (Montiani-Ferreira et al., 2003). ERG abnormalities are noted within 12 hours of hatching. As they age, they experience increased ocular growth, leading to increased axial and radial dimensions. Chicks go blind around 30 days post-hatch although gross abnormalities of the retina are not seen early in the disease process. Histopathological analysis showed significant thinning of the retina compared to controls by 14 days post-hatch, with gradual thinning continuing throughout the disease process. Around post-hatch day 90, gross retinal degeneration is seen (Montiani-Ferreira et al., 2005). Montiani-Ferreira et al. (2003) suggest photoreceptor dysfunction, rather than photoreceptor loss, is the primary driver of vision loss in rge chickens.
In addition to progressive retinal degeneration and dysfunction described above, the rge chick eye is also characterized by progressive globe enlargement, likely secondary to the underlying retinal dysfunction (Montiani-Ferreira et al., 2003). This enlargement is related to flattening and thickening of the cornea (Boote et al., 2009), similar to corneal growth changes seen in chicks undergoing constant light exposure (Wahl et al., 2009). Both radial globe diameter and corneal radius of curvature were significantly increased in rge chicks as early as one month post-hatch (Montiani-Ferreira et al., 2003). To further characterize these changes, Boote et al. (2009) examined the ultrastructure of rge corneas using transmission electron microscopy and X-ray scattering techniques. They found that the organization of collagen fibrils in the central area of the corneas displayed an orthogonal pattern in both rge mutants and controls. However, significant differences were seen in the peripheral cornea. In the normal chick cornea, collagen fibers take on an annular, circumferential pattern in the periphery. This pattern was preserved in one month post-hatch rge mutants but by three months post-hatch significant disruptions could be seen. Instead of an annular pattern, the rge chicks instead displayed corneal fibrils in superior-inferior and medial-lateral orthogonal planes, similar to those of the central cornea. These corneal changes observed in rge mutants also makes them an interesting model for further study on the impact of ultrastructure on corneal shape, in addition to the above applications to retinal disease.
Tummala et al. (2006) demonstrated that a deletion of a single amino acid (D153del) in guanine nucleotide-binding protein beta-3 (GNB3), results in the rge phenotype. The gene, GNB3, is expressed in ON-type bipolar cells and both rod and cone photoreceptors (Dhingra et al., 2012). The wild type products aid in the opening of cyclic guanosine monophosphate-gated channels (Ritchey et al., 2012a). Ritchey et al. (2010) demonstrated complete absence of the GNB3 protein in rge chicks. Globe enlargement in the rge strain allows for study of ocular growth, which is important for understanding emmetropization and certain refractive errors. In fact, Ritchey et al. (2012a) used rge mutants to show that emmetropization and increased ocular growth due to form-deprivation and hyperopic defocus using lenses does not require high acuity vision or GNB3 mediated signaling. At the same time, growth responses to myopic defocus appeared to be dependent on visual acuity and/or GNB3 mediated signaling.
Human retinal abnormalities have also been linked to GNB3 mutation. Recently, Vincent et al. (2016) used genetic sequencing to identify four patients with newly-described GNB3 mutations. Three of the four individuals presented with symptoms consistent with Congenital Stationary Night Blindness (CSNB). Three were family members, including one homozygous (c.1017G>A [p.Trp339*]/[p.Trp339*]) family member and two compound heterozygous siblings (c.1017G>A [p.Trp339*]/c.170_172delAGA [p.Lysdel57]). The fourth individual demonstrated a sporadic homozygous mutation (c.200C>T [p.Ser67Phe]). One of the siblings mentioned was asymptomatic, and the other three individuals had experienced night-blindness beginning in childhood. None demonstrated nystagmus, and all had best-corrected visual acuity ≥20/30, along with normal retinal thickness via OCT and normal funduscopic exams. All four individuals demonstrated rod ON bipolar cell dysfunction on ERG. Arno et al. (2016) identified another specific mutation (c.124C>T [p.Arg42Ter]) in the GNB3 gene linked to inherited retinopathy. This patient presented as a child with nystagmus, a normal funduscopic exam, mild OCT abnormalities in the macula, and ERG abnormalities. While further data is needed for comparison to the phenotypic findings in the rge chick, these findings underscore the utility of the rge chick in studying inherited retinal disease in humans.
4.9 Blindness Enlarged Globe (beg)
Similar to the rge mutant, the blindness enlarged globe (beg) mutant is characterized by a developmental retinal dystrophy followed by subsequent globe enlargement; however, one key difference is that beg mutants are blind at hatch (Morgan et al., 2013). Retinal changes in beg mutants include the development of intracellular spaces, loss of photoreceptors, and clumping of the retinal pigment epithelium. Morgan et al. (2013) examined the corneal structure of beg mutants in analogous fashion to the Boote et al. (2009) investigation of rge mutant corneas (described in section 4.8). They found no significant difference between control and beg corneas at one month post-hatch, despite the fact that these birds are blind at hatch. This was different from rge mutants who do show significant ultrastructural changes at one month post-hatch even though they are just beginning to lose their vision at that time (Boote et al., 2009). In terms of the actual collagen fibril orientation, Morgan et al. (2013) observed similar findings to the Boote et al. (2009) rge study: beg mutants had orthogonal patterns in the central cornea similar to controls; however, the annular pattern present in the peripheral cornea of controls was disrupted in beg mutants. This disarray of the peripheral cornea was progressive out to nine months of age, the latest time point studied.
4.10 Smoky Joe (SJ)
The Smoky Joe strain, also exhibiting an autosomal recessive inheritance pattern (Hocking and Guggenheim, 2014), remains largely uncharacterized. This strain was first described by Salter et al. (1997). Interestingly, not all homozygous SJ chicks display the same phenotype. Moreover, the exact genetic mechanism causing the SJ phenotypes has not yet been elucidated. While some subjects retain vision at hatch, by eight weeks all subjects are blind (Tran et al., 2013). Furthermore, these chicks are plagued with a wide range of ocular pathology including buphthalmos, cataracts, iridodonesis, and phthisis bulbi with resultant retinal degeneration (Salter et al., 1997). Additionally, although both eyes are always affected in some way, the changes are not necessarily congruent between the two eyes.
A natural dichotomy exists between SJ mutants: those that are blind at hatch and those that are not. Tran et al. (2013) attempted to characterize the differences in embryonic retinal development between these two groups. They found that the total number of retinal cells was reduced in birds who were blind at hatch. A delay in the formation of the three nuclear layers (inner nuclear layer, outer nuclear layer, and ganglion cell layer) was also noted in birds blind at hatch. In terms of specific cell types, Tran et al. (2013) found that amacrine cells and rod photoreceptor cells were reduced both in terms of total number and in proportion to other cell types in blind hatchlings. This observation implicates these two cell types as possible sites of early pathologic change; however, one important limitation was noted by the authors. They pointed out that only the number of cells, and not functionality, was investigated.
The SJ strain was used recently to examine the role of light exposure in the sexual maturation of the chick (Baxter et al., 2014). The researchers used blind SJ chicks to model how chicks would mature in the absence of light detection in the retina, and they determined that the chicks’ ability to detect light did not affect their sexual maturation. They did, however, determine that the type of light exposure significantly influenced maturation.
4.11 Microphthalmia-4 (mi-4)
As described by Somes (1992), the mi-4 mutant chick demonstrates severe reduction in eye size (2–30% of normal size) in 80% of affected chicks. It manifests bilaterally in 2/3 of affected chicks, and demonstrates no atypical effects on comb or neck feathers. These mutant chicks typically die in embryo or in the first week of life. The associated mutation is believed to be transmitted by autosomal recessive inheritance with near 100% penetrance in females and near zero in males. To our knowledge, this mutant has not appeared again in the literature since its discovery.
4.12 Other Hereditary Ocular Conditions
Two other unique ocular mutants, the Sleepy Eye (se) and Visual Impairment (GSN/1) chicks are identified in the OMIA database. The human disease correlates for these conditions are less clear than for previously described chick lines. The se chick was first identified by Somes (1968). These chicks hatched with their lower eyelids partially closed, and this defect persisted throughout their lives. Carefoot (1993) investigated the mutation present in se chicks and described its location on chromosome 1. The GSN/1 strain was first identified by Shibuya et al. (2002). These chicks demonstrated decreased visual acuity without obvious pathology on funduscopic exam. Pathologic exam demonstrated a reduction in the number of retinal ganglion cells and a reduction in the nerve fibers present in the optic tectum. To our knowledge, the se and GSN/1 chicks have not been used for further studies.
5. Gene Therapy and Retinal Regeneration
5.1 Stem cells
Advancements in stem cell technology may hold the potential to cure previously untreatable conditions. Endogenous stem cells exist in the circumferential marginal zone (CMZ) of undamaged chicken retinas (Fischer and Reh, 2000). These cells may, in part, be responsible for increases in retinal size during ocular growth and emmetropization (Discussed in section 3.5). Indeed, the proliferation of neural stem cells found at the far peripheral edge of the retina increases with increased rates of ocular growth and with introduction of growth factors including epidermal growth factor, insulin-like growth factor, and insulin (Fischer and Reh, 2000). Fischer et al. (2005) also demonstrated that growth appears to be modulated by large glucagon containing neurons, named bullwhip cells. Increased glucagon levels lead to progenitor cell suppression, while antagonists have the opposite effect. Fischer et al. (2005) theorized that the vision-induced cues for retinal growth may be communicated to the stem cells via glucagon signaling from the bullwhip neurons. Notably, NMDA-induced cytotoxicity did not increase rates of growth, possibly indicating that this pool of stem cells does not take part in retinal repair (Fischer and Reh, 2000).
Fischer (2011) also focused on using chick retinas to demonstrate the regenerative potential of the retina. In damaged chick retinas, Müller glia, a cell type derived from neuroepithelial stem cells and distinct from the CMZ progenitor cells present in fish, amphibians, and birds, undergo de-differentiation to acquire progenitor phenotype, expressing Pax6, Chx10 (Fischer and Reh, 2001), Six3 (Fischer, 2005), Notch1 (Ghai et al., 2010; Hayes et al., 2007), and other factors found in retinal progenitor cells including Klf4 (Todd and Fischer, 2015) and asc11a (Fischer and Reh, 2001). Interestingly, these de-differentiated cell types are not found in the intact retina, possibly indicating that damage may induce signaling that causes Müller glia to revert (Fischer, 2011). Alternatively, given the finding that Müller glia-derived progenitor cells will go through multiple rounds of mitosis ex vivo compared to a single round of division in vivo (Fischer and Reh, 2001), Fischer (2011) posits that the intact retina suppresses Müller progenitor cells. Although all Müller glia-derived progenitor cells have the ability to generate new neurons, only a small fraction of them do. The mechanism of this inhibition remains unknown. In past studies, similar Müller glia-derived progenitor cells have been found in fish models (Bernardos et al., 2007; Fausett and Goldman, 2006). Unlike the chicken and mammalian models, in fish these cells give rise to a significant number of new neuronal cells (Montgomery et al., 2010).
Significant progress has been made in identifying the cell signaling pathways that orchestrate the transition of Müller glia into Müller glia-derived progenitor cells (MGPCs). The zebrafish retina has an exceptional regenerative capacity that is regulated by a complex network of signaling pathways which includes Notch, Wnt, MAPK, Jak/Stat, PI3k/AKT, and TGFβ (Conner et al., 2014; Lenkowski et al., 2013; Nelson et al., 2012; Ramachandran et al., 2011; Wan et al., 2014; Zhao et al., 2014). In contrast, the chick retina has little or no capacity for functional regeneration, yet large numbers of proliferating MGPCs can be stimulated to form after damage or FGF2-stimulation (Fischer et al., 2014b; Fischer and Reh, 2001). Similar to the zebrafish, a network of cell-signaling pathways that influence MGPC-formation is being uncovered in the chick model system. The network of pathways that control the formation of MGPCs is known to include Notch-, Wnt-, MAPK-, Jak/Stat-, mTor-, Hedgehog-, and glucocorticoid-signaling (Fischer et al., 2009a, 2009b, Gallina et al., 2015, 2014; Ghai et al., 2010; Hayes et al., 2007; Todd et al., 2016; Todd and Fischer, 2015; Zelinka et al., 2016). In the mammalian retina, Müller glia retain a severely limited capacity to become MGPCs and this process requires damage followed by growth factor stimulation, or transgenic overexpression of the proneural transcription factor ascl1 (Karl et al., 2008; Ooto et al., 2004; Ueki et al., 2015). Similar to the fish and avian retina, MAPK and Wnt have been implicated in driving MGPC formation in the rodent retina (reviewed by (Hamon et al., 2016)).
Stem cells may not always be able to directly replace dead or damaged retinal cells, but they may provide an environment that prolongs the life-spans and improves functionality of diseased cells. Stanke and Fischer (2010) showed embryonic cells from chicks 7, 10, and 11 days after conception had the ability to prolong retinal ganglion cell life in retinas treated with colchicine, a drug known to damage and induce apoptosis in retinal ganglion cells (Fig. 7). The cellular factors responsible for the differential survival could perhaps be developed therapeutically for retinal disease.
In addition, significant investigation has been conducted in the chick to determine the role of the RPE in retinal regeneration. Wang et al. (2010) and Belecky-Adams et al. (2008) review the RPE’s ability to differentiate into retinal neurons in the embryonic chick and suggest that RPE reprogramming may offer an approach for regenerating damaged retinal cells. Interestingly, one of the studies reviewed therein (Liang et al., 2006) describes the ability of in vitro cultured RPE cells to differentiate into retinal neurons after transplantation into embryonic chicks. Of note, the regenerative potential of the RPE is not unique to the chick and has been demonstrated to a limited extent in mammals. Zhao et al. (1995) demonstrated that the early embryonic rodent RPE could transdifferentiate into neural retina. Sakami et al. (2008) demonstrated that activin, a signaling molecule from the TGFβ family, inhibits the ability of the RPE cells to transdifferentiate after early embryonic stages in rodents and subsequently found that activin receptor blockade was able to delay this inhibition. Other species like the newt appear to have even more robust regenerative potential, as RPE cells from adult newts have been demonstrated to transform into retinal neurons and ganglion cells (reviewed by (Grigoryan and Markitantova, 2016)).
5.2 Gene Manipulation
One key challenge in chick research has been the induction of transgenic chicks, which has proven more difficult than transducing rodents. However, the field of gene manipulation has expanded into chick models since the completion of sequencing of the chicken genome (Hillier et al., 2004). New methods have resulted in improvements for inducing gain-of-function and loss-of-function mutations, quantifying changes in gene expression, and manipulating embryonic stem cells (reviewed by (Stern, 2005; Streit et al., 2013)). Given the well-documented similarities between chick and human embryonic development, ease of access to chick embryos, large size of developing chick embryos, and economic efficiency of chick research, the chick model is ideal for expanded gene manipulation applications. Several different techniques for gene manipulation have already been used to successfully target the retina in embryonic chick models (Summarized in Table 4). Several of these techniques have been reviewed by Doran et al. (2016), Hocking and Guggenheim (2014), and Vergara and Canto-Soler (2012). This section focuses primarily on gene manipulation techniques applied specifically to the chick retina.
Table 4.
Published Chick Retina Gene Manipulation Techniques
| Technique | Goal | Chick mutant model (Gene) |
Method | References |
|---|---|---|---|---|
| Lentiviral Vector | ||||
| pTYF-EF1a-GC1-IRES-eGFP | Insert functional gene | GUCY1* (GC1 guanylate cyclase-1) | Inject ED 2† | (Williams et al., 2006) |
Bicistronic vector constructs:
|
Insert functional gene, co-express two proteins | GUCY1*(G C1 guanylate cyclase-1) | Inject ED 2 | (Verrier et al., 2011) |
| Retroviral RCAS†† | ||||
DNA plasmids:
|
Insert functional gene | N/A | Inject specific pathogen free eggs ED3 | (Haynes et al., 2007; Jason R Spence et al., 2007; Jason R. Spence et al., 2007; Vergara and Canto-Soler, 2012) |
RNAi:
|
Knock-down gene expression | N/A | Inject specific pathogen free eggs ED6–7 | (Harpavat and Cepko, 2006) |
| Electroporation | ||||
| Morpholinos | Knock-down gene expression | N/A | Electroporate HH††† 10–12, 9–12, 11 | (Betancur et al., 2010; Canto-Soler and Adler, 2006; Mende et al., 2008) |
| DNA plasmids | Insert functional gene | N/A | Electroporate ED5 | (Vergara et al., 2013) |
| RNAi | Knock-down gene expression | N/A | Electroporate ED5 | (Vergara et al., 2013) |
| EGFP†††† | Labeling | N/A | Electroporate HH 14–16 | (Lance-Jones et al., 2012) |
| Sonoporation | ||||
| DNA plasmid (pCAX-eGFP) | Insert functional gene in retinal neurons | N/A | Sonoporate ED7 Retinal cultures: 20kHz (0.5–2.5W) | (Fischer et al., 2006) |
ED= embryonic day
RCAS=Replication-Competent Avian sarcoma-leukosis virus (ASLV) long terminal repeat (LTR) with a Splice acceptor vector
HH= Hamburger Hamilton Stage
EGFP=Enhanced Green Fluorescent Protein
Retroviral vectors were introduced to chick eye research more than two decades ago, with the use replication-incompetent viruses as lineage tracers to study developing extrinsic eye muscles in chicks (Wahl et al., 1994). Retroviral vectors were also among the first methods used for gene transduction in embryonic chick retinas. As mentioned in section 4.5, Williams et al. (2006) used a lentiviral vector in rd (GUCY1*B) mutant chick embryos to transfer a gene coding for retinal guanylate cyclase (RetGC1) to the embryos, resulting in, functional, ERG, and histopathological improvements in the chicks’ retinal disease post-hatch. However, they found their intervention was able to slow but not prevent retinal degeneration, as treated chicks still progressed to blindness. In this experiment, it seemed that gene transduction was not consistent enough across the retina to achieve a lasting result. Verrier et al. (2011) also used lentiviral systems to deliver modified transgenes to rd (GUCY1*B) chick embryos, attempting to improve on the gene transduction levels from the Williams et al. (2006) work. The experiments of Verrier et al. (2011) resulted in similar, temporary restoration of vision in the chicks and little improvement in gene transduction levels. Semple-Rowland and Berry (2014) describe further experience with lentiviral vectors in the developing chick retina, identifying several bicistronic genes they have transduced effectively and their downstream actions. In addition, Semple-Rowland and Berry, (2014) provides detailed description of their methods for preparation and delivery of vectors as well as hatching of these chicks.
Replication-Competent Avian sarcoma-leukosis virus (ASLV) long terminal repeat (LTR) with a Splice acceptor vector (RCAS) retroviral systems have also proven to be useful vectors for the study of embryonic chick eye development, as reviewed by Vergara and Canto-Soler (2012). While RCAS has been primarily used to deliver transgenes for gain-of-function studies, an RCAS gene knock-down technique using RNA interference (RNAi) has also been utilized in the chick retina by Harpavat and Cepko (2006).
Electroporation has been a critical gene manipulation tool in studies of the developing chick eye. Electroporation has been used to facilitate morpholino incorporation in several gene knock-down studies (Betancur et al., 2010; Canto-Soler and Adler, 2006; Mende et al., 2008). Electroporation has also been used in chick embryos to facilitate enhanced green fluorescent protein (EGFP) labeling for study of the developing ocular adnexa, specifically the abducens and oculomotor nerves (Lance-Jones et al., 2012). Vergara et al. (2013) successfully used electroporation for genetic manipulation of ex vivo cultured retinal cells. This particular electroporation method was used for both gain-of-function experiments using plasmid transfection and loss-of-function experiments using RNAi molecule transfection. While reviewed thoroughly by Vergara and Canto-Soler (2012), it should be noted that development of effective retinal cell culture techniques, described initially in (Adler et al., 1982; Hyndman and Adler, 1982), provide the foundation required for use of these advanced gene manipulation techniques. Although the embryonic retina is receptive to electroporation-mediated gene transfer, the sclera of post-hatch chicks imposes significant electrical resistance and prevents electroporation of the retina in vivo (Fischer, unpublished observations).
Ultrasound-mediated transfer of genetic material, or sonoporation, has also been used successfully in chick embryos. Fischer et al. (2006) used sonoporation to transfect chick retinal neurons with plasmid DNA and compared this method with adenoviral vector transfection. Sonoporation was a more effective means of transfection and proved to be relatively simple and inexpensive.
A new selective gene editing technology ready for use in retinal gene manipulation studies in the chick is the CRISPR-Cas9 system. This tool allows for more specific genome editing than other options. A recent study by Ranganathan et al. (2014) demonstrated modifications to the CRISPR-Cas9 system that allow for an expanded number of target gene sites in chicks. As a proof of concept, they demonstrated their system could target the MERTK gene, but they did not describe experiments that actively manipulated the chick genome. This MERTK target has significance for future use of the CRISPR-Cas9 system in retina research, as mutations in this gene have been previously linked to development of retinal dystrophy in rats (D’Cruz et al., 2000). More recently, Oishi et al. (2016) further applied the CRISPR-Cas9 system, using chick primordial germ cells, to create the first germline chimeric roosters with mutagenized ovalbumin or ovomucoid egg white genes. Chimeric chicks were then bred to obtain heterozygous and homozygous mutant animals. The authors speculate that modification of their CRISPR-Cas9 methods will allow for broad applications in genomic manipulation, including knock-in mutation induction, large genomic deletions, and modification of transcription, which would present obvious opportunities for inherited retinal disease research and other lines of investigation.
While this array of tools applied to the chick eye research represents a significant repertoire, other gene manipulation tools successful in chick research not specific to the eye could be adapted to eye research. These methods include the use of adeno-associated viral vectors to transfer genetic material to embryonic chicks (Matsui et al., 2012), in vitro culture and modification of primordial germ cells that can be introduced to chick embryos to produce transgenic chickens (Macdonald et al., 2010; Tyack et al., 2013; van de Lavoir et al., 2006), transposons capable of creating transgenic chickens (Macdonald et al., 2012; Park and Han, 2012), homologous recombination techniques to produce gene knockouts (Schusser et al., 2013), and transcription activator-like effector nucleases (TALENs) to knockout targeted genes (Park et al., 2014).
6. Comparisons of the Chick and Mouse Ocular Research Models
The chick and the mouse are both commonly used animal models for ocular research. While a complete description of the mouse as a model for ocular research is beyond the scope of this review, Table 5 offers relative, practical comparisons between the two animal models, describing their utility in ocular research. These comparisons are derived from the research experience of the authors of this review. Many of the key advantages of the chick mentioned in Table 5, including its relative ease of ocular manipulation, relative ease of drug delivery to the anterior and posterior chambers, and the larger amount of available tissue, are direct results of the larger eye size in the chick. Its low cost and easy handling are advantages for potential high-throughput studies. Key advantages in mouse models relate primarily to the relative ease of genetic manipulation in the mouse and the wide variety of available mutants, including inducible Cre-lox systems, for modeling human disease.
Table 5.
Relative Comparisons of Chick and Mouse Ocular Research Models
| Feature | Chick* | Mouse* |
|---|---|---|
| Cost | + | ++ |
| Ease of Handling | ++ | ++ |
| Ease of Ocular Manipulation | +++ | + |
| Ease of Genetic Manipulation | + | +++ |
| Availability of Advanced Imaging Techniques | +++ | +++ |
| Ease of Drug Delivery to Anterior and Posterior Chambers | +++ | + |
| Amount of Available Tissue | +++ | + |
| Similarity of Visual Acuity to Humans | +++ | + |
| Availability of Mutant Models for Human Disease | + | +++ |
| Accessibility of Antibodies and Reagents | ++ | +++ |
| Other Notable Differences |
|
|
+ = Low, ++ = Medium, +++ = High; This scale is based on unpublished observations from the authors’ experience.
Chorioallantoic Membrane
While a thorough description of chick models for human ocular diseases and conditions has been provided herein, detailed reviews of mouse models of human disease exist already in the literature. Some of these reviews include Graw (1999) and Marchitti et al. (2008) for mutants used in studying cataract and lens development, Graw (2003) for congenital ocular defects, Kao (2006) and Marchitti et al. (2008) for corneal disease, Lindsey and Weinreb (2005) for induced mutant glaucoma models, Pang et al. (2015) for viral vector-induced glaucoma models, Chakraborty and Pardue (2015) for mutant models used to study refractive development, Baehr and Frederick (2009) for naturally occurring mutant models of outer retinal disease, Dalke and Graw (2005) for natural and induced mutant models for congenital retinal disease, Nair and Vemuganti (2015) for retinoblastoma mutant models, and Yang et al. (2008) for uveal melanoma xenograft models. A detailed database of murine mutants and genetic diseases and conditions reported in mice can be found at the Jackson Laboratory Mouse Genome Informatics (MGI) website, http://www.informatics.jax.org/. Further information regarding murine models for human disease can be found via the Online Mendelian Inheritance in Man (OMIM) website, https://www.omim.org/.
Another notable advantage of the chick model (Table 5) is that the chick is much more visual than rodents which are nocturnal in nature (Schmid and Wildsoet, 1998). For example, the daytime vision of chicks is far superior to rodents and therefore is more directly relevant to human in the study of vision. Although both chick and mouse lack an anatomic fovea, the presence of an analogous structure (area centralis in the chick) to the human fovea is also advantageous. The higher central density of cones in chick compared with rodents may have advantages in better modeling human macular disease. Finally, the chick chorioallantoic membrane (CAM) model (See Section 3.4 for detail on the CAM model) provides another advantageous system for studying ocular cancer therapeutics and metastatic mechanisms without the immunosuppression, cost, and time required for murine xenograft models.
7. Conclusions and Future Directions
The chick has been utilized in vision research for over a century. Recent work has broadly expanded the potential utility of chick as a model system for ocular, particularly retinal, diseases and conditions. Despite being a non-mammalian animal model, chick retinal damage responses have surprising similarity to those in humans, especially when compared with other commonly-used mammalian models. Given the similarity of retinal damage responses to humans, low cost, ease of handling, capability for retinal imaging, ready availability, sequenced genome and ability for genetic manipulation, we expect the popularity of the chick model to expand to support high-throughput, translational studies in the future. In particular, chick models may be used for trials of ocular pharmaceuticals including new chemotherapeutic, anti-fibrotic, anti-angiogenic and IOP-lowering agents, models for developing new surgical techniques, further studies of stem cell properties, and novel models for human ocular diseases and conditions.
Highlights.
The chick is a robust model for ocular disease research.
Model advantages include low cost and ease of handling.
The chick has a cone-rich retina with similar cellular damage responses to humans.
The chick has potential for ocular genetics research and manipulation.
The chick has potential for use in translational ophthalmic research.
Acknowledgments
Funding: This work was supported by the National Institutes of Health X; the X; the X, and the X. This paper does not necessarily reflect the views of the X.
The authors would like to thank Bioptigen (acquired by Leica) for use of the Envisu SD-OCT system to obtain high-resolution images of the chick eye. The authors had full access to all of the cited materials and take responsibility for the integrity of the interpretations of these sources. The authors prepared the figures and wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no relevant financial disclosures.
References
- Abbott UK, Craig RM, Bennett EB. Sex-linked coloboma in the chicken. J. Hered. 1970;61:95–105. doi: 10.1093/oxfordjournals.jhered.a108059. [DOI] [PubMed] [Google Scholar]
- Adler R, Magistretti PJ, Hyndman AG, Shoemaker WJ. Purification and cytochemical identification of neuronal and non-neuronal cells in chick embryo retina cultures. Dev. Neurosci. 1982;5:27–39. doi: 10.1159/000112659. [DOI] [PubMed] [Google Scholar]
- Ali M, Hocking PM, McKibbin M, Finnegan S, Shires M, Poulter JA, Prescott K, Booth A, Raashid Y, Jafri H, Ruddle JB, Mackey DA, Jacobson SG, Toomes C, Lester DH, Burt DW, Curry WJ, Inglehearn CF. Mpdz null allele in an avian model of retinal degeneration and mutations in human leber congenital amaurosis and retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2011;52:7432–7440. doi: 10.1167/iovs.11-7872. [DOI] [PubMed] [Google Scholar]
- Arno G, Holder GE, Chakarova C, Kohl S, Pontikos N, Fiorentino A, Plagnol V, Cheetham ME, Hardcastle AJ, Webster AR, Michaelides M UK Inherited Retinal Disease Consortium. Recessive Retinopathy Consequent on Mutant G-Protein β Subunit 3 (GNB3) JAMA Ophthalmol. 2016;3:25–28. doi: 10.1001/jamaophthalmol.2016.1543. [DOI] [PubMed] [Google Scholar]
- Ashby RS, Feldkaemper MP. Gene expression within the amacrine cell layer of chicks after myopic and hyperopic defocus. Investig. Ophthalmol. Vis. Sci. 2010;51:3726–3735. doi: 10.1167/iovs.09-4615. [DOI] [PubMed] [Google Scholar]
- Auker CR, Parver LM, Doyle T, Carpenter DO. Choroidal blood flow. I. Ocular tissue temperature as a measure of flow. Arch. Ophthalmol. 1982;100:1323–1326. doi: 10.1001/archopht.1982.01030040301020. [DOI] [PubMed] [Google Scholar]
- Baehr W, Frederick JM. Naturally occurring animal models with outer retina phenotypes. Vision Res. 2009;49:2636–2652. doi: 10.1016/j.visres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M, Joseph N, Osborne VR, Bédécarrats GY. Red light is necessary to activate the reproductive axis in chickens independently of the retina of the eye. Poult. Sci. 2014;93:1289–1297. doi: 10.3382/ps.2013-03799. [DOI] [PubMed] [Google Scholar]
- Beason RC, Loew ER. Visual pigment and oil droplet characteristics of the bobolink (dolichonyx oryzivorous) a new world migratory bird. Vision Res. 2008;48:1–8. doi: 10.1016/j.visres.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Beattie JR, Finnegan S, Hamilton RW, Ali M, Inglehearn CF, Stitt AW, McGarvey JJ, Hocking PM, Curry WJ. Profiling retinal biochemistry in the MPDZ mutant retinal dysplasia and degeneration chick: a model of human RP and LCA. Invest. Ophthalmol. Vis. Sci. 2012;53:413–420. doi: 10.1167/iovs.11-8591. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Haynes T, Wilson JM, Del Rio-Tsonis K. The chick as a model for retina development and regeneration. In: Tsonis PA, editor. Animal models in eye research. first. Academic Press; London: 2008. pp. 102–119. [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bérubé M, Deschambeault A, Boucher M, Germain L, Petitclerc E, Guérin SL. MMP-2 expression in uveal melanoma: differential activation status dictated by the cellular environment. Mol. Vis. 2005;11:1101–1111. [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, Sauka-Spengler T. Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3570–3575. doi: 10.1073/pnas.0906596107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitgood JJ, Whitley RD. Pop-eye: an inherited Z-linked keratoglobus in the chicken. J. Hered. 1986;77:123–125. doi: 10.1093/oxfordjournals.jhered.a110183. [DOI] [PubMed] [Google Scholar]
- Boote C, Hayes S, Young RD, Kamma-Lorger CS, Hocking PM, Elsheikh A, Inglehearn CF, Ali M, Meek KM. Ultrastructural changes in the retinopathy, globe enlarged (rge) chick cornea. J. Struct. Biol. 2009;166:195–204. doi: 10.1016/j.jsb.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhenni RA, Dunmire J, Sewell A, Edward DP. Animal models of glaucoma. J. Biomed. Biotechnol. 2012:1–11. doi: 10.1155/2012/692609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles KE, Kraft TW. ERG critical flicker fusion frequency assessment in humans. In: LaVail MM, Ash JD, Anderson RE, Hollyfield JG, Grimm C, editors. Retinal degenerative diseases. Springer; New York: 2012. pp. 503–509. [Google Scholar]
- Bowmaker JK. Evolution of vertebrate visual pigments. Vision Res. 2008;48:2022–2041. doi: 10.1016/j.visres.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Bowmaker JK, Heath LA, Wilkie SE, Hunt DM. Visual pigment and oil droplets from six classes of photoreceptor in the retinas of birds. Vision Res. 1997;37:2183–2194. doi: 10.1016/s0042-6989(97)00026-6. [DOI] [PubMed] [Google Scholar]
- Bowmaker JK, Knowles A. The visual pigments and oil droplets of the chicken retina. Vision Res. 1977;17:755–764. doi: 10.1016/0042-6989(77)90117-1. [DOI] [PubMed] [Google Scholar]
- Boye SE. A Mini-review: Animal Models of GUCY2D Leber Congenital Amaurosis (LCA1) Adv. Exp. Med. Biol. 2016;854:253–258. doi: 10.1007/978-3-319-17121-0_34. [DOI] [PubMed] [Google Scholar]
- Boyle ML, Pardue SL, Smyth JR. Effect of corticosterone on the incidence of amelanosis in Smyth delayed amelanotic line chickens. Poult. Sci. 1987;66:363–367. doi: 10.3382/ps.0660363. [DOI] [PubMed] [Google Scholar]
- Brach V. The functional significance of the avian pecten: a review. Condor. 1977;79:321–327. [Google Scholar]
- Brach V. The effect of intraocular ablation of the pecten oculi of the chicken. Invest. Ophthalmol. 1975;14:166–168. [PubMed] [Google Scholar]
- Briggs FN. Muscle, physiology and biochemistry. In: Dulbecco R, editor. Encyclopedia of human biology. Vol. 5. Academic Press; New York: 1991. pp. 229–242. [Google Scholar]
- Bruhn SL, Cepko CL. Development of the pattern of photoreceptors in the chick retina. J. Neurosci. 1996;16:1430–1439. doi: 10.1523/JNEUROSCI.16-04-01430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh JA, Lee KW. The gene action and function of two dopa oxidase positive melanocyte mutants of the fowl. Genetics. 1975;81:333–347. doi: 10.1093/genetics/81.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh J, Bowers R, Lee K. Histochemical evidence that peroxidase does not affect melanin formation in feather melanocytes. Yale J. Biol. Med. 1973;46:523–534. [PMC free article] [PubMed] [Google Scholar]
- Bueno JM, Giakoumaki A, Gualda EJ, Schaeffel F, Artal P. Analysis of the chicken retina with an adaptive optics multiphoton microscope. Biomed. Opt. Express. 2011;2:1637–1648. doi: 10.1364/BOE.2.001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt DW, Morrice DR, Lester DH, Robertson GW, Mohamed MD, Simmons I, Downey LM, Thaung C, Bridges LR, Paton IR, Gentle M, Smith J, Hocking PM, Inglehearn CF. Analysis of the rdd locus in chicken: a model for human retinitis pigmentosa. Mol. Vis. 2003;9:164–170. [PubMed] [Google Scholar]
- Busch C, Drews U, Eisele SR, Garbe C, Oppitz M. Noggin blocks invasive growth of murine B16-F1 melanoma cells in the optic cup of the chick embryo. Int. J. Cancer. 2008;122:526–533. doi: 10.1002/ijc.23139. [DOI] [PubMed] [Google Scholar]
- Busch M, Philippeit C, Weise A, Dünker N. Re-characterization of established human retinoblastoma cell lines. Histochem. Cell Biol. 2015;143:325–338. doi: 10.1007/s00418-014-1285-z. [DOI] [PubMed] [Google Scholar]
- Canto-Soler MV, Adler R. Optic cup and lens development requires Pax6 expression in the early optic vesicle during a narrow time window. Dev. Biol. 2006;294:119–132. doi: 10.1016/j.ydbio.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Carefoot WC. Further studies of linkage and mappings of the loci of genes in group 3 on chromosome 1 of the domestic fowl. Br. Poult. Sci. 1993;34:205–209. doi: 10.1080/00071669308417576. [DOI] [PubMed] [Google Scholar]
- Cebulla CM, Zelinka CP, Scott MA, Lubow M, Bingham A, Rasiah S, Mahmoud AM, Fischer AJ. A Chick Model of Retinal Detachment: Cone Rich and Novel. PLoS One. 2012;7:1–12. doi: 10.1371/journal.pone.0044257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Pardue MT. Molecular and biochemical aspects of the retina on refraction. In: Hejtmancik JF, Nickerson JM, editors. Progress in molecular biology and translational science: Molecular biology of eye disease. Vol. 134. 2015. pp. 249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard RD, Gundlach RH. The structure of the eye in the homing pigeon. J. Comp. Psychol. 1937;25:249–272. [Google Scholar]
- Chebib M, Hinton T, Schmid KL, Brinkworth D, Qian H, Matos S, Kim HL, Abdel-Halim H, Kumar RJ, Johnston GA, Hanrahan JR. Novel, potent, and selective GABAC antagonists inhibit myopia development and facilitate learning and memory. J. Pharmacol. Exp. Ther. 2009;328:448–457. doi: 10.1124/jpet.108.146464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian PG, Harkin DG, Schmid KL. GABAergic Agents Modify the Response of Chick Scleral Fibroblasts to Myopic and Hyperopic Eye Cup Tissues. Curr. Eye Res. 2013;39:172–187. doi: 10.3109/02713683.2013.834941. [DOI] [PubMed] [Google Scholar]
- Chui TYP, Song H, Burns SA. Individual variations in human cone photoreceptor packing density: variations with refractive error. Invest. Ophthalmol. Vis. Sci. 2008;49:4679–4687. doi: 10.1167/iovs.08-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DC, Traboulsi EI. Leber congenital amaurosis: Clinical correlations with genotypes, gene therapy trials update, and future directions. J. AAPOS. 2009;13:587–592. doi: 10.1016/j.jaapos.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Belkin M, Yehezkel O, Solomon AS, Polat U. Dependency between light intensity and refractive development under light-dark cycles. Exp. Eye Res. 2011;92:40–46. doi: 10.1016/j.exer.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Collin SP. Evolution and ecology of retinal photoreception in early vertebrates. Brain. Behav. Evol. 2010;75:174–185. doi: 10.1159/000314904. [DOI] [PubMed] [Google Scholar]
- Collin SP, Davies WL, Hart NS, Hunt DM. The evolution of early vertebrate photoreceptors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2925–2940. doi: 10.1098/rstb.2009.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner C, Ackerman KM, Lahne M, Hobgood JS, Hyde DR. Repressing Notch Signaling and Expressing TNF Are Sufficient to Mimic Retinal Regeneration by Inducing Muller Glial Proliferation to Generate Committed Progenitor Cells. J. Neurosci. 2014;34:14403–14419. doi: 10.1523/JNEUROSCI.0498-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombre AJ, Coulombre JL. The skeleton of the eye. II. Overlap of the scleral ossicles of the domestic fowl. Dev. Biol. 1973;33:257–267. doi: 10.1016/0012-1606(73)90136-x. [DOI] [PubMed] [Google Scholar]
- Cremers FPM, van den Hurk JA, den Hollander AI. Molecular genetics of Leber congenital amaurosis. Hum. Mol. Genet. 2002;11:1169–1176. doi: 10.1093/hmg/11.10.1169. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Allen KA. Topography of ganglion cells in human retina. J. Comp. Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human Photoreceptor Topography. J. Comp. Neurol. 1990;523:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Curtis R, Baker JR, Curtis PE, Johnston A. An inherited retinopathy in commercial breeding chickens. Avian Pathol. 1988;17:87–99. doi: 10.1080/03079458808436430. [DOI] [PubMed] [Google Scholar]
- D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Dakin SC, Turnbull PRK. Similar contrast sensitivity functions measured using psychophysics and optokinetic nystagmus. Sci. Rep. 2016;6:1–14. doi: 10.1038/srep34514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalke C, Graw J. Mouse mutants as models for congenital retinal disorders. Exp. Eye Res. 2005;81:503–512. doi: 10.1016/j.exer.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dartnall HJ, Bowmaker JK, Mollon JD. Human visual pigments: microspectrophotometric results from the eyes of seven persons. Proc. R. Soc. Lond. B Biol. Sci. 1983;220:115–130. doi: 10.1098/rspb.1983.0091. [DOI] [PubMed] [Google Scholar]
- Davidorf FH, Havener WH, Lang JR. Macular vision following retinal detachment surgery. Ophthalmic Surg. 1975;6:74–81. [PubMed] [Google Scholar]
- Davis J, Hsieh Y-H, Lee H-C. Humans perceive flicker artifacts at 500 Hz. Sci. Rep. 2015;5:1–4. doi: 10.1038/srep07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DG, Kramer TR, Grossniklaus HE, Waring GO, Edelhauser HF. Histologic, ultrastructural, and immunofluorescent evaluation of human laser-assisted in situ keratomileusis corneal wounds. Arch. Ophthalmol. 2005;123:741–756. doi: 10.1001/archopht.123.6.741. [DOI] [PubMed] [Google Scholar]
- DeMello LR, Foster TM, Temple W. Discriminative performance of the domestic hen in a visual acuity task. J. Exp. Anal. Behav. 1992;58:147–157. doi: 10.1901/jeab.1992.58-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano ME, Mugnaini E. Fine structure of the choroidal coat of the avian eye. Vascularization, supporting tissue and innervation. Anat. Embryol. (Berl) 1997;195:393–418. doi: 10.1007/s004290050060. [DOI] [PubMed] [Google Scholar]
- Den Hollander AI, Roepman R, Koenekoop RK, Cremers FPM. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Den Hollander AI, ten Brink JB, de Kok YJ, van Soest S, van den Born LI, van Driel MA, van de Pol DJ, Payne AM, Bhattacharya SS, Kellner U, Hoyng CB, Westerveld A, Brunner HG, Bleeker-Wagemakers EM, Deutman AF, Heckenlively JR, Cremers FP, Bergen AA. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12) Nat. Genet. 1999;23:217–221. doi: 10.1038/13848. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem. Cell Biol. 2008;130:1119–1130. doi: 10.1007/s00418-008-0536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Ramakrishnan H, Neinstein A, Fina ME, Xu Y, Li J, Chung DC, Lyubarsky A, Vardi N. Gβ3 Is Required for Normal Light ON Responses and Synaptic Maintenance. J. Neurosci. 2012;32:11343–11355. doi: 10.1523/JNEUROSCI.1436-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederen RMH, La Heij EC, Kessels AGH, Goezinne F, Liem ATA, Hendrikse F. Scleral Buckling Surgery after Macula-Off Retinal Detachment. Worse Visual Outcome after More than 6 Days. Ophthalmology. 2007;114:705–709. doi: 10.1016/j.ophtha.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Doran TJ, Cooper CA, Jenkins KA, Tizard MLV. Advances in genetic engineering of the avian genome: “Realising the promise.”. Transgenic Res. 2016;25:1–13. doi: 10.1007/s11248-016-9926-8. [DOI] [PubMed] [Google Scholar]
- Ehrlich D, Morgan IG. Kainic acid destroys displaced amacrine cells in post-hatch chicken retina. Neurosci. Lett. 1980;17:43–48. doi: 10.1016/0304-3940(80)90059-2. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Larson G, Gunnarsson U, Bed’hom B, Tixier-Boichard M, Stromstedt L, Wright D, Jungerius A, Vereijken A, Randi E, Jensen P, Andersson L. Identification of the Yellow skin gene reveals a hybrid origin of the domestic chicken. PloS Genet. 2008;4 doi: 10.1371/journal.pgen.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HE, Martin GR. Organa sensuum [Organa Sensoria] In: Baumel JJ, King AS, Breazile JE, editors. Handbook of Avian Anatomy. Nuttall Ornithological Club; Cambridge: 1993. pp. 585–611. [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J. Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis. Neurosci. 2002;19:755–766. doi: 10.1017/s0952523802196064. [DOI] [PubMed] [Google Scholar]
- Finnegan S, Robson J, Hocking PM, Ali M, Inglehearn CF, Stitt A, Curry WJ. Proteomic profiling of the retinal dysplasia and degeneration chick retina. Mol. Vis. 2010;16:7–17. [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ. Müller glia, vision-guided ocular growth, retinal stem cells, and a little serendipity: The cogan lecture. Investig. Ophthalmol. Vis. Sci. 2011;52:7705–7710. doi: 10.1167/iovs.11-8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ. Neural regeneration in the chick retina. Prog. Retin. Eye Res. 2005;24:161–182. doi: 10.1016/j.preteyeres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Bosse JL, El-Hodiri HM. Reprint of: The ciliary marginal zone (CMZ) in development and regeneration of the vertebrate eye. Exp. Eye Res. 2014a;123:115–120. doi: 10.1016/j.exer.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, McGuire JJ, Schaeffel F, Stell WK. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat. Neurosci. 1999c;2:706–712. doi: 10.1038/11167. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Morgan IG, Stell WK. Colchicine causes excessive ocular growth and myopia in chicks. Vision Res. 1999a;39:685–697. doi: 10.1016/s0042-6989(98)00178-3. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Omar G, Walton NA, Verrill TA, Unson CG. Glucagon-Expressing Neurons within the Retina Regulate the Proliferation of Neural Progenitors in the Circumferential Marginal Zone of the Avian Eye. J. Neurosci. 2005;25:10157–10166. doi: 10.1523/JNEUROSCI.3247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Pickett Seltner RL, Poon J, Stell WK. Immunocytochemical characterization of quisqualic acid- and N-methyl-D- aspartate-induced excitotoxicity in the retina of chicks. J. Comp. Neurol. 1998;393:1–15. [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci. 2001;4:247–52. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina. Dev. Biol. 2002;251:367–379. doi: 10.1006/dbio.2002.0813. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev. Biol. 2000;220:197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Ritchey ER, Scott MA, Wynne A. Bullwhip neurons in the retina regulate the size and shape of the eye. Dev. Biol. 2008;317:196–212. doi: 10.1016/j.ydbio.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Ritchey ER, Sherwood P. Mitogen-activated protein kinase-signaling regulates the ability of Müller glia to proliferate and protect retinal neurons against excitotoxicity. Glia. 2009a;57:1538–1552. doi: 10.1002/glia.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Tuten W. Mitogen-activated protein kinase-signaling stimulates Müller glia to proliferate in acutely damaged chicken retina. Glia. 2009b;57:166–181. doi: 10.1002/glia.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Zelinka C, Sherwood P. A novel type of glial cell in the retina is stimulated by insulin-like growth factor 1 and may exacerbate damage to neurons and Müller glia. Glia. 2010b;58:633–649. doi: 10.1002/glia.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Seltner RL, Stell WK. Opiate and N-methyl-D-aspartate receptors in form-deprivation myopia. Vis. Neurosci. 1998;15:1089–1096. doi: 10.1017/s0952523898156080. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Seltner RL, Stell WK. N-methyl-D-aspartate-induced excitotoxicity causes myopia in hatched chicks. Can. J. Ophthalmol. 1997;32:373–377. [PubMed] [Google Scholar]
- Fischer AJ, Stanke JJ, Omar G, Askwith CC, Burry RW. Ultrasound-mediated gene transfer into neuronal cells. J. Biotechnol. 2006;122:393–411. doi: 10.1016/j.jbiotec.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Wallman J, Mertz JR, Stell WK. Localization of retinoid binding proteins, retinoid receptors, and retinaldehyde dehydrogenase in the chick eye. J. Neurocytol. 1999b;28:597–609. doi: 10.1023/a:1007071406746. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Gallina D, Scott MA, Todd L. Reactive microglia and macrophage facilitate the formation of Müller glia-derived retinal progenitors. Glia. 2014b;62:1608–1628. doi: 10.1002/glia.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Milani-Nejad N. Reactive retinal microglia, neuronal survival, and the formation of retinal folds and detachments. Glia. 2015;63:313–327. doi: 10.1002/glia.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Scott MA. Heterogeneity of glia in the retina and optic nerve of birds and mammals. Plos One. 2010a;5:1–15. doi: 10.1371/journal.pone.0010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SK, Lewis GP, Linberg KA, Verardo MR. Cellular remodeling in mammalian retina: Results from studies of experimental retinal detachment. Prog. Retin. Eye Res. 2005;24:395–431. doi: 10.1016/j.preteyeres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Fite KV, Montgomery N, Whitney T, Boissy R, Smyth JR. Inherited retinal degeneration and ocular amelanosis in the domestic chicken (Gallus domesticus) Curr. Eye Res. 1982–1983;2:109–115. doi: 10.3109/02713688208997684. [DOI] [PubMed] [Google Scholar]
- Fite KV, Pardue S, Bengston L, Hayden D, Smyth JR. Effects of cyclosporine in spontaneous, posterior uveitis. Curr. Eye Res. 1986;5:787–796. doi: 10.3109/02713688609000020. [DOI] [PubMed] [Google Scholar]
- Fite KV, Whitney T, Montgomery N, Smyth JR. Behavioral and central visual correlates of inherited retinal degeneration in the domestic chicken (Gallus domesticus) Exp. Neurol. 1983;79:729–745. doi: 10.1016/0014-4886(83)90037-7. [DOI] [PubMed] [Google Scholar]
- Fowler WC, Chang DH, Roberts BC, Zarovnaya EL, Proia AD. A new paradigm for corneal wound healing research: the white leghorn chicken (Gallus gallus domesticus) Curr. Eye Res. 2004;28:241–250. doi: 10.1076/ceyr.28.4.241.27837. [DOI] [PubMed] [Google Scholar]
- Frankelson EN, Lauber JK, Boyd TA. The role of angle closure in light-induced avian glaucoma. Can. J. Ophthalmol. 1969;4:59–63. [PubMed] [Google Scholar]
- Funata M, Tokoro T. Scleral change in experimentally myopic monkeys. Graefes Arch. Clin. Exp. Ophthalmol. 1990;228:174–179. doi: 10.1007/BF00935729. [DOI] [PubMed] [Google Scholar]
- Fulton AB, Fite KV, Bengston L. Retinal degeneration in the delayed amelanotic (DAM) chicken: an electroretinographic study. Curr. Eye Res. 1983;2:757–763. doi: 10.3109/02713688209020008. [DOI] [PubMed] [Google Scholar]
- Gallina D, Zelinka C, Fischer AJ. Glucocorticoid receptors in the retina, Muller glia and the formation of Muller glia-derived progenitors. Development. 2014;141:3340–3351. doi: 10.1242/dev.109835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Zelinka CP, Cebulla CM, Fischer AJ. Activation of glucocorticoid receptors in Müller glia is protective to retinal neurons and suppresses microglial reactivity. Exp. Neurol. 2015;273:114–125. doi: 10.1016/j.expneurol.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Zelinka C, Fischer AJ. Notch Signaling Influences Neuroprotective and Proliferative Properties of Mature Muller Glia. J. Neurosci. 2010;30:3101–3112. doi: 10.1523/JNEUROSCI.4919-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser A, Murphy CJ, Troilo D, Howland HC. The mechanism of lenticular accommodation in chicks. Vision Res. 1995;35:1525–1540. doi: 10.1016/0042-6989(94)00211-4. [DOI] [PubMed] [Google Scholar]
- Glasser A, Troilo D, Howland HC. The mechanism of corneal accommodation in chicks. Vision Res. 1994;34:1549–1566. doi: 10.1016/0042-6989(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Goldsmith TH, Collins JS, Licht S. The cone oil droplets of avian retinas. Vision Res. 1984;24:1661–1671. doi: 10.1016/0042-6989(84)90324-9. [DOI] [PubMed] [Google Scholar]
- Gómez S, Herreras JM, Merayo J, García M, Argüeso P, Cuevas J. Effect of hyaluronic acid on corneal haze in a photorefractive keratectomy experimental model. J. Refract. Surg. 2001;17:549–554. doi: 10.3928/1081-597X-20010901-08. [DOI] [PubMed] [Google Scholar]
- Goodman G, Bercovich D. Melanin directly converts light for vertebrate metabolic use: Heuristic thoughts on birds, Icarus and dark human skin. Med. Hypotheses. 2008;71:190–202. doi: 10.1016/j.mehy.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Foley JW, Birk DE, Fitch JM, Linsenmayer TF. Type V collagen and Bowman’s membrane. Quantitation of mRNA in corneal epithelium and stroma. J. Biol. Chem. 1994;269:24959–24966. [PubMed] [Google Scholar]
- Gottlieb MD, Joshi HB, Nickla DL. Scleral changes in chicks with form-deprivation myopia. Curr. Eye Res. 1990;9:1157–1165. doi: 10.3109/02713689009003472. [DOI] [PubMed] [Google Scholar]
- Govardovskiĭ VI. On the role of oil drops in colour vision. Vision Res. 1983;23:1739–1740. doi: 10.1016/0042-6989(83)90192-x. [DOI] [PubMed] [Google Scholar]
- Graw J. Cataract mutations and lens development. Prog. Retin. Eye Res. 1999;18:235–267. doi: 10.1016/s1350-9462(98)00018-4. [DOI] [PubMed] [Google Scholar]
- Graw J. The genetic and molecular basis of congenital eye defects. Nat. Rev. Genet. 2003;4:876–888. doi: 10.1038/nrg1202. [DOI] [PubMed] [Google Scholar]
- Grewal DS, Brar GS, Grewal SPS. Assessment of central corneal thickness in normal, keratoconus, and post-laser in situ keratomileusis eyes using Scheimpflug imaging, spectral domain optical coherence tomography, and ultrasound pachymetry. J. Cataract Refract. Surg. 2010;36:954–964. doi: 10.1016/j.jcrs.2009.12.033. [DOI] [PubMed] [Google Scholar]
- Grigoryan EN, Markitantova YV. Cellular and molecular preconditions for retinal pigment epithelium (RPE) natural reprogramming during retinal regeneration in urodela. Biomedicines. 2016;4:1–18. doi: 10.3390/biomedicines4040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Agarwal R, Srivastava S. Textbook on Clinical Ocular Pharmacology & Therapeutics. JP Medical Ltd.; 2014. Chapter 6: Mydriatics and Cycloplegics; p. 354. [Google Scholar]
- Halfter W, Dong S, Balasubramani M, Bier ME. Temporary disruption of the retinal basal lamina and its effect on retinal histogenesis. Dev. Biol. 2001;238:79–96. doi: 10.1006/dbio.2001.0396. [DOI] [PubMed] [Google Scholar]
- Hamon A, Roger JE, Yang X-J, Perron M. Müller glial cell-dependent regeneration of the neural retina: An overview across vertebrate model systems. Dev. Dyn. 2016;245:727–738. doi: 10.1002/dvdy.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpavat S, Cepko CL. RCAS-RNAi: a loss-of-function method for the developing chick retina. BMC Dev. Biol. 2006;6:1–7. doi: 10.1186/1471-213X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart NS. The visual ecology of avian photoreceptors. Prog. Retin. Eye Res. 2001;20:675–703. doi: 10.1016/s1350-9462(01)00009-x. [DOI] [PubMed] [Google Scholar]
- Hart NS, Lisney TJ, Collin SP. Cone photoreceptor oil droplet pigmentation is affected by ambient light intensity. J. Exp. Biol. 2006;209:4776–4787. doi: 10.1242/jeb.02568. [DOI] [PubMed] [Google Scholar]
- Hassan TS, Sarrafizadeh R, Ruby AJ, Garretson BR, Kuczynski B, Williams GA. The effect of duration of macular detachment on results after the scleral buckle repair of primary, macula-off retinal detachments. Ophthalmology. 2002;109:146–152. doi: 10.1016/s0161-6420(01)00886-7. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Osawa T, Tohyama K. Comparative observations on corneas, with special reference to Bowman’s layer and Descemet’s membrane in mammals and amphibians. J. Morphol. 2002;254:247–258. doi: 10.1002/jmor.10030. [DOI] [PubMed] [Google Scholar]
- Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Dev. Biol. 2007;312:300–311. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes T, Gutierrez C, Aycinena J-C, Tsonis PA, Del Rio-Tsonis K. BMP signaling mediates stem/progenitor cell-induced retina regeneration. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20380–20385. doi: 10.1073/pnas.0707202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headington K, Choi SS, Nickla D, Doble N. Single Cell Imaging of the Chick Retina with Adaptive Optics. Curr. Eye Res. 2011;36:947–957. doi: 10.3109/02713683.2011.587934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesy CP, Hall MI. The nocturnal bottleneck and the evolution of mammalian vision. Brain. Behav. Evol. 2010;75:195–203. doi: 10.1159/000314278. [DOI] [PubMed] [Google Scholar]
- Hillier LW, Miller W, Birney E, Warren W, Hardison RC, Ponting CP, Bork P, Burt DW, Groenen MAM, Delany ME. Sequencing and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- Ho JH, Branchini L, Regatiari C, Krishnan C, Fujimoto JG, Duker JS. Anlaysis of normal peripaillary choroidal thickness via spectral domain optical coherence tomography. Ophthalmology. 2011;118:2001–2007. doi: 10.1016/j.ophtha.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking PM, Guggenheim JA. The chick as an animal model of eye disease. Drug Discov. Today Dis. Model. 2014;10:e225–e230. [Google Scholar]
- Hodos W, Kuenzel WJ. Retinal-image degradation produces ocular enlargement in chicks. Investig. Ophthalmol. Vis. Sci. 1984;25:652–659. [PubMed] [Google Scholar]
- Hoffman DW, Marchi M, Giacobini E. Norepinephrine uptake in aging adrenergic nerve terminals. Neurobiol. Aging. 1980;1:65–68. doi: 10.1016/0197-4580(80)90026-3. [DOI] [PubMed] [Google Scholar]
- Hu DN, Simon JD, Sarna T. Role of ocular melanin in ophthalmic physiology and pathology. Photochem. Photobiol. 2008;84:639–644. doi: 10.1111/j.1751-1097.2008.00316.x. [DOI] [PubMed] [Google Scholar]
- Hyndman AG, Adler R. Neural retina development in vitro. Effects of tissue extracts on cell survival and neuritic development in purified neuronal cultures. Dev. Neurosci. 1982;5:40–53. doi: 10.1159/000112660. [DOI] [PubMed] [Google Scholar]
- Inglehearn CF, Morrice DR, Lester DH, Robertson GW, Mohamed MD, Simmons I, Downey LM, Thaung C, Bridges LR, Paton IR, Smith J, Petersen-Jones S, Hocking PM, Burt DW. Genetic, ophthalmic, morphometric and histopathological analysis of the Retinopathy Globe Enlarged (rge) chicken. Mol. Vis. 2003;9:295–300. [PubMed] [Google Scholar]
- Insler MS, Lopez JG. Heterologous transplantation versus enhancement of human corneal endothelium. Cornea. 1991;10:136–48. doi: 10.1097/00003226-199103000-00009. [DOI] [PubMed] [Google Scholar]
- Iribarren R. Crystalline lens and refractive development. Prog. Retin. Eye Res. 2015;47:86–106. doi: 10.1016/j.preteyeres.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Iribarren R, Rozema JJ, Schaeffel F, Morgan IG. Calculation of crystalline lens power in chickens with a customized version of Bennett’s equation. Vision Res. 2014;96:33–38. doi: 10.1016/j.visres.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Ishikawa M. Abnormalities in glutamate metabolism and excitotoxicity in the retinal diseases. Scientifica (Cairo) 2013;2013:1–13. doi: 10.1155/2013/528940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH. Primate photopigments and primate color vision. Proc. Natl. Acad. Sci. U.S.A. 1996;93:577–581. doi: 10.1073/pnas.93.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H-M, Erf GF, Rowland KC, Kong B-W. Genome resequencing and bioinformatic analysis of SNP containing candidate genes in the autoimmune vitiligo Smyth line chicken model. BMC Genomics. 2014;15:1–21. doi: 10.1186/1471-2164-15-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery G, Williams A. Is abnormal retinal development in albinism only a mammalian problem? Normality of a hypopigmented avian retina. Exp. Brain Res. 1994;100:47–57. doi: 10.1007/BF00227278. [DOI] [PubMed] [Google Scholar]
- Jensen LS, Matson WE. Enlargement of avian eye by subjecting chicks to continuous incandescent illumination. Science. 1957;125:741. doi: 10.1126/science.125.3251.741. [DOI] [PubMed] [Google Scholar]
- Johnson HM. Visual pattern discrimination in the vertebrates – II: comparative visual acuity in the dog, the monkey and the chick. J. Anim. Behav. 1914;4:340–361. [Google Scholar]
- Jonas JB, Schneider U, Naumann GOH. Count and density of human retinal photoreceptors. Graefe’s Arch. Clin. Exp. Ophthalmol. 1992;230:505–510. doi: 10.1007/BF00181769. [DOI] [PubMed] [Google Scholar]
- Jones D, Luensmann D. The prevalence and impact of high myopia. Eye Contact Lens. 2012;38:188–196. doi: 10.1097/ICL.0b013e31824ccbc3. [DOI] [PubMed] [Google Scholar]
- Jones MP, Pierce KE, Ward D. Avian Vision: A Review of Form and Function with Special Consideration to Birds of Prey. J. Exot. Pet Med. 2007;16:69–87. [Google Scholar]
- Kalirai H, Shahidipour H, Coupland SE, Luyten G. Use of the Chick Embryo Model in Uveal Melanoma. Ocul. Oncol. Pathol. 2015;1:133–140. doi: 10.1159/000370151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanski JJ. Clinical ophthalmology: a systematic approach. 3. Butterworth-Heinemann; 1994. [Google Scholar]
- Kao WW-Y. Ocular surface tissue morphogenesis in normal and disease states revealed by genetically modified mice. Cornea. 2006;25:S7–S19. doi: 10.1097/01.ico.0000247207.55520.a4. [DOI] [PubMed] [Google Scholar]
- Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc. Natl. Acad. Sci. U.S.A. 2008;105:19508–19513. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani AH, Zimmer-Galler IE, Shah SH, Dustin L, Do DV, Eliott D, Haller JA, Nguyen QD. Retinal thickness analysis by race, gender, and age using stratus OCT. Am. J. Ophthalmol. 2010;149:496–502. doi: 10.1016/j.ajo.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katyal S, Glubrecht DD, Li L, Gao Z, Godbout R. Disabled-1 alternative splicing in human fetal retina and neural tumors. PloS One. 2011;6 doi: 10.1371/journal.pone.0028579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katyal S, Godbout R. Alternative splicing modulates Disabled-1 (Dab1) function in the developing chick retina. EMBO J. 2004;23:1878–1888. doi: 10.1038/sj.emboj.7600185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear A, Lauber JK, Boyd TA. Genesis of light-induced avian glaucoma. Invest. Ophthalmol. 1974;13:872–875. [PubMed] [Google Scholar]
- Koch T. Anatomy of the chicken and domestic birds. 1. Iowa State University Press; Ames, Iowa: 1973. [Google Scholar]
- Kram YA, Mantey S, Corbo JC. Avian cone photoreceptors tile the retina as five independent, self-organizing mosaics. PloS One. 2010;5:1–26. doi: 10.1371/journal.pone.0008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri D, Bailey CF. A Comparison of Phagocytosis by the Retinal Pigment Epithelium in Normal and Delayed Amelanotic Chickens. Exp. Eye Res. 1993;56:625–634. doi: 10.1006/exer.1993.1080. [DOI] [PubMed] [Google Scholar]
- Lam TC, Li KK, Lo SCL, Guggenheim JA, To CH. A chick retinal proteome database and differential retinal protein expressions during early ocular development. J. Proteome Res. 2006;5:771–784. doi: 10.1021/pr050280n. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C, Shah V, Noden DM, Sours E. Intrinsic properties guide proximal abducens and oculomotor nerve outgrowth in avian embryos. Dev. Neurobiol. 2012;72:167–185. doi: 10.1002/dneu.20948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber JK. Review: avian models for experimental myopia. J. Ocul. Pharmacol. 1991;7:259–276. [PubMed] [Google Scholar]
- Lauber JK. Light-induced avian glaucoma as an animal model for human primary glaucoma. J. Ocul. Pharmacol. 1987;3:77–100. doi: 10.1089/jop.1987.3.77. [DOI] [PubMed] [Google Scholar]
- Lauber JK, McGinnis J, Boyd J. Influence of miotics, Diamox and vision occluders on light-induced buphthalmos in domestic fowl. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1965;120:572–575. doi: 10.3181/00379727-120-30593. [DOI] [PubMed] [Google Scholar]
- Lauber JK, McLaughlin MA, Chiou GC. Timolol and pilocarpine are hypotensive in light-induced avian glaucoma. Can. J. Ophthalmol. 1985;20:147–152. [PubMed] [Google Scholar]
- Lauber JK, Shutze JV, McGinnis J. Effects of exposure to continuous light on the eye of the growing chick. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1961;106:871–872. doi: 10.3181/00379727-106-26505. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Azar DT. Myopia and models and mechanisms of refractive error control. 2002;15:127–133. doi: 10.1016/s0896-1549(01)00002-5. [DOI] [PubMed] [Google Scholar]
- Lenkowski JR, Qin Z, Sifuentes CJ, Thummel R, Soto CM, Moens CB, Raymond PA. Retinal regeneration in adult zebrafish requires regulation of TGFβ signaling. Glia. 2013;61:1687–1697. doi: 10.1002/glia.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. Consensual pupillary response in birds. Science. 1955;122:690. doi: 10.1126/science.122.3172.690. [DOI] [PubMed] [Google Scholar]
- Lewis GP, Charteris DG, Sethi CS, Fisher SK. Animal models of retinal detachment and reattachment: identifying cellular events that may affect visual recovery. Eye (Lond) 2002;16:375–387. doi: 10.1038/sj.eye.6700202. [DOI] [PubMed] [Google Scholar]
- Li T, Howland HC. A true neuronal consensual pupillary reflex in chicks. Vision Res. 1999;39:897–900. doi: 10.1016/s0042-6989(98)00197-7. [DOI] [PubMed] [Google Scholar]
- Li T, Howland HC. The effects of constant and diurnal illumination of the pineal gland and the eyes on ocular growth in chicks. Invest. Ophthalmol. Vis. Sci. 2003;44:3692–3697. doi: 10.1167/iovs.02-0990. [DOI] [PubMed] [Google Scholar]
- Li T, Troilo D, Glasser A, Howland HC. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Res. 1995;35:1203–1209. doi: 10.1016/0042-6989(94)00231-a. [DOI] [PubMed] [Google Scholar]
- Liang L, Yan R-T, Ma W, Zhang H, Wang S-Z. Exploring RPE as a source of photorecptors: differentiation and integration of transdifferentiating cells grafted into embryonic chick eyes. Invest. Ophthalmol. Vis. Sci. 2006;47:5066–5074. doi: 10.1167/iovs.06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linberg KA, Sakai T, Lewis GP, Fisher SK. Experimental retinal detachment in the cone-dominant ground squirrel retina: morphology and basic immunocytochemistry. Vis. Neurosci. 2002;19:603–619. doi: 10.1017/s095252380219506x. [DOI] [PubMed] [Google Scholar]
- Lindsey JD, Weinreb RN. Elevated intraocular pressure and transgenic applications in the mouse. J. Glaucoma. 2005;14:318–320. doi: 10.1097/01.ijg.0000169411.09258.f6. [DOI] [PubMed] [Google Scholar]
- Lisney TJ, Ekesten B, Tauson R, Hastad O, Odeen A. Using electroretinograms to assess flicker fusion frequency in domestic hens Gallus gallus domesticus. Vision Res. 2012;62:125–133. doi: 10.1016/j.visres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Lisney TJ, Rubene D, Rozsa J, Lovile H, Hastad O, Odeen A. Behaviorual assessment of flicker fusion frequency in chicken Gallus gallus domesticus. 2011;51:1324–1332. doi: 10.1016/j.visres.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Luo Z-X. Transformation and diversification in early mammal evolution. Nature. 2007;450:1011–1019. doi: 10.1038/nature06277. [DOI] [PubMed] [Google Scholar]
- Luyten GP, Mooy CM, De Jong PT, Hoogeveen AT, Luider TM. A chicken embryo model to study the growth of human uveal melanoma. Biochem. Biophys. Res. Commun. 1993;192:22–29. doi: 10.1006/bbrc.1993.1376. [DOI] [PubMed] [Google Scholar]
- Macdonald J, Glover JD, Taylor L, Sang HM, McGrew MJ. Characterisation and germline transmission of cultured avian primordial germ cells. PloS One. 2010;5:1–10. doi: 10.1371/journal.pone.0015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald J, Taylor L, Sherman A, Kawakami K, Takahashi Y, Sang HM, McGrew MJ. Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E1466–E1472. doi: 10.1073/pnas.1118715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangioris G, Chiodini F, Dosso A. New strategy to study corneal endothelial cell transplantation: the chick cornea model. Cornea. 2011;30:1461–1464. doi: 10.1097/ICO.0b013e31821821fe. [DOI] [PubMed] [Google Scholar]
- Manglapus MK, Uchiyama H, Buelow NF, Barlow RB. Circadian rhythms of rod-cone dominance in the Japanese quail retina. J. Neurosci. 1998;18:4775–4784. doi: 10.1523/JNEUROSCI.18-12-04775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann IC. The function of the pecten. Br. J. Ophthalmol. 1924;8:209–226. doi: 10.1136/bjo.8.5.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García MC, Merayo-Llovés J, Blanco-Mezquita T, Mar-Sardaña S. Wound healing following refractive surgery in hens. Exp. Eye Res. 2006;83:728–735. doi: 10.1016/j.exer.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest. Ophthalmol. Vis. Sci. 1997;38:1726–1739. [PubMed] [Google Scholar]
- Mathis U, Schaeffel F. Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007;245:267–275. doi: 10.1007/s00417-006-0282-x. [DOI] [PubMed] [Google Scholar]
- Matsui R, Tanabe Y, Watanabe D. Avian Adeno-Associated Virus Vector Efficiently Transduces Neurons in the Embryonic and Post-Embryonic Chicken Brain. PloS One. 2012;7:1–13. doi: 10.1371/journal.pone.0048730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CA. Comparative anatomy of the optic nerve head and inner retina in non-primate animal models used for glaucoma research. Open Ophthalmol. J. 2008;2:94–101. doi: 10.2174/1874364100802010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckibbin M, Ali M, Inglehearn C, Shires M, Boyle K, Hocking PM. Spectral domain optical coherence tomography imaging of the posterior segment of the eye in the retinal dysplasia and degeneration chicken, an animal model of inherited retinal degeneration. Vet. Ophthalmol. 2014;17:113–119. doi: 10.1111/vop.12051. [DOI] [PubMed] [Google Scholar]
- Mende M, Christophorou NAD, Streit A. Specific and effective gene knock-down in early chick embryos using morpholinos but not pRFPRNAi vectors. Mech. Dev. 2008;125:947–962. doi: 10.1016/j.mod.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Merayo-Lloves J, Yáñez B, Mayo A, Martín R, Pastor JC. Experimental model of corneal haze in chickens. J. Refract. Surg. 1995;17:696–699. doi: 10.3928/1081-597X-20011101-11. [DOI] [PubMed] [Google Scholar]
- Meyer DB. Handbook on sensory physiology. In: Crescitelli F, editor. The Avian Eye and Its Adaptations. Springer; Berlin: 1977. pp. 549–611. [Google Scholar]
- Meyer DB, May HC. The topographical distribution of rods and cones in the adult chicken retina. Exp. Eye Res. 1973;17:347–355. doi: 10.1016/0014-4835(73)90244-3. [DOI] [PubMed] [Google Scholar]
- Moayed AA, Hariri S, Song ES, Choh V, Bizheva K. In vivo volumetric imaging of chicken retina with ultrahigh-resolution spectral domain optical coherence tomography. Biomed. Opt. Express. 2011;2:1268–1274. doi: 10.1364/BOE.2.001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JE, Parsons MJ, Hyde DR. A novel model of retinal ablation demonstrates that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors. J. Comp. Neurol. 2010;518:800–814. doi: 10.1002/cne.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiani-Ferreira F, Fischer A, Cernuda-Cernuda R, Kiupel M, DeGrip WJ, Sherry D, Cho SS, Shaw GC, Evans MG, Hocking PM, Petersen-Jones SM. Detailed histopathologic characterization of the retinopathy, globe enlarged (rge) chick phenotype. Mol. Vis. 2005;11:11–27. [PubMed] [Google Scholar]
- Montiani-Ferreira F, Li T, Kiupel M, Howland H, Hocking P, Curtis R, Petersen-Jones S. Clinical features of the retinopathy, globe enlarged (rge) chick phenotype. Vision Res. 2003;43:2009–2018. doi: 10.1016/s0042-6989(03)00303-1. [DOI] [PubMed] [Google Scholar]
- Morcos Y, Chan-Ling T. Concentration of astrocytic filaments at the retinal optic nerve junction is coincident with the absence of intra-retinal myelination: comparative and developmental evidence. J. Neurocytol. 2000;29:665–678. doi: 10.1023/a:1010835404754. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Ohno-Matsui K, Saw S-M. Myopia. Lancet. 2012;379:1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- Morgan SR, Dooley EP, Hocking PM, Inglehearn CF, Ali M, Sorensen TLM, Meek KM, Boote C. An X-ray scattering study into the structural basis of corneal refractive function in an avian model. Biophys. J. 2013;104:2586–2594. doi: 10.1016/j.bpj.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris VB. An afoveate area centralis in the chick retina. J. Comp. Neurol. 1982;210:198–203. doi: 10.1002/cne.902100210. [DOI] [PubMed] [Google Scholar]
- Murphy CJ, Glasser A, Howland HC. The anatomy of the ciliary region of the chicken eye. Investig. Ophthalmol. Vis. Sci. 1995;36:889–896. [PubMed] [Google Scholar]
- Nair RM, Vemuganti GK. Transgenic models in retinoblastoma research. Ocu. Oncol. Pathol. 2015;1:207–213. doi: 10.1159/000370157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava DR, Antony B, Zhang L, Abramoff MD, Wildsoet CF. Novel method using 3-dimensional segmentation in spectral domain-optical coherence tomography imaging in the chick reveals defocus-induced regional and time-sensitive asymmetries in the choroidal thickness. Vis. Neurosci. 2016;33:1–10. doi: 10.1017/S0952523816000067. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Gorsuch RA, Bailey TJ, Ackerman KM, Kassen SC, Hyde DR. Stat3 defines three populations of müller glia and is required for initiating maximal müller glia proliferation in the regenerating zebrafish retina. J. Comp. Neurol. 2012;520:4294–4311. doi: 10.1002/cne.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessner C, Denzau S, Gross JC, Peichl L, Bischof HJ, Fleissner G, Wiltschko W, Wiltschko R. Avian Ultraviolet/Violet cones identified as probable magnetoreceptors. PloS One. 2011;6:1–8. doi: 10.1371/journal.pone.0020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog. Retin. Eye. Res. 2016;52:156–187. doi: 10.1016/j.preteyeres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Oishi I, Yoshii K, Miyahara D, Kigami H, Tagami T. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Kojima D, Fukada Y, Shichida Y, Yoshizawa T. Primary structures of chicken cone visual pigments: vertebrate rhodopsins have evolved out of cone visual pigments. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5932–5936. doi: 10.1073/pnas.89.13.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN, Casson RJ, Wood JPM, Chidlow G, Graham M, Melena J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Ostrin LA, Liu Y, Choh V, Wildsoet CF. The role of the iris in chick accommodation. Investig. Ophthalmol. Vis. Sci. 2011;52:4710–4716. doi: 10.1167/iovs.10-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin LA, Choh V, Wildsoet CF. The pattern ERG in chicks – Stimulus dependence and optic nerve section. Vision Res. 2016;128:45–52. doi: 10.1016/j.visres.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Over R, Moore D. Spatial acuity of the chicken. Brain Res. 1981;211:424–426. doi: 10.1016/0006-8993(81)90967-7. [DOI] [PubMed] [Google Scholar]
- Pang I-H, Millar JC, Clark AF. Elevation of intraocular pressure in rodents using viral vectors targeting the trabecular meshwork. Exp. Eye Res. 2015;141:33–41. doi: 10.1016/j.exer.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Han JY. piggyBac transposition into primordial germ cells is an efficient tool for transgenesis in chickens. Proc. Natl. Acad. Sci. U.S.A. 2012;109:9337–9341. doi: 10.1073/pnas.1203823109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Lee HJ, Kim KH, Kim J-S, Han JY. Targeted gene knockout in chickens mediated by TALENs. Proc. Natl. Acad. Sci. U.S.A. 2014;111:1–6. doi: 10.1073/pnas.1410555111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parver LM, Auker C, Carpenter DO. Choroidal blood flow as a heat dissipating mechanism in the macula. Am. J. Ophthalmol. 1980;89:641–646. doi: 10.1016/0002-9394(80)90280-9. [DOI] [PubMed] [Google Scholar]
- Perrault I, Rozet JM, Calvas P, Gerber S, Camuzat A, Dollfus H, Châtelin S, Souied E, Ghazi I, Leowski C, Bonnemaison M, Le Paslier D, Frézal J, Dufier JL, Pittler S, Munnich A, Kaplan J. Retinal-specific guanylate cyclase gene mutations in Leber’s congenital amaurosis. Nat. Genet. 1996;14:461–464. doi: 10.1038/ng1296-461. [DOI] [PubMed] [Google Scholar]
- Pettigrew JD, Wallman J, Wildsoet CF. Saccadic oscillations facilitate ocular perfusion from the avian pecten. Nature. 1990;343:362–363. doi: 10.1038/343362a0. [DOI] [PubMed] [Google Scholar]
- Pilar G, Nunez R, McLennan IS, Meriney SD. Muscarinic and nicotinic synaptic activation of the developing chicken iris. J. Neurosci. 1987;7:3813–3826. doi: 10.1523/JNEUROSCI.07-12-03813.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ML, Kingston ACN, McCready R, Cameron EG, Hofmann CM, Suarez L, Olsen GH, Cronin TW, Robinson PR. Characterization of visual pigments, oil droplets, lens and cornea in the whooping crane grus americana. J. Exp. Biol. 2014;217:3883–3890. doi: 10.1242/jeb.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumphery RJ. Biology and Comparative Physiology of Birds. Academic Press; New York: 1961. Sensory Organs: Vision; pp. 5–68. [Google Scholar]
- Rada JA, Thoft RA, Hassell JR. Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Dev. Biol. 1991;147:303–312. doi: 10.1016/0012-1606(91)90288-e. [DOI] [PubMed] [Google Scholar]
- Rager G, Rager U. Systems-matching by degeneration: A quantitative electron microscopic study of the generation and degeneration of retinal ganglion cells in the chicken. Exp. Brain Res. 1978;33:65–78. doi: 10.1007/BF00238795. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Zhao X-F, Goldman D. Ascl1a/Dkk/ -catenin signaling pathway is necessary and glycogen synthase kinase-3 inhibition is sufficient for zebrafish retina regeneration. Proc. Natl. Acad. Sci. U.S.A. 2011;108:15858–15863. doi: 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CJ, Wilson MA, Pollock BJ, Clayton RM, Ross AS, Bard JBL, McLachlan I. Partial retinal dysplasia and subsequent degeneration in a mutant strain of domestic fowl (rdd) Exp. Eye Res. 1983;37:337–347. doi: 10.1016/0014-4835(83)90171-9. [DOI] [PubMed] [Google Scholar]
- Ranganathan V, Wahlin K, Maruotti J, Zack DJ. Expansion of the CRISPR–Cas9 genome targeting space through the use of H1 promoter-expressed guide RNAs. Nat. Commun. 2014;5:4516. doi: 10.1038/ncomms5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibaldi A, Lograno MD, Avitabile T. Iris receptors of fresh human eyes. Ann. Ophthalmol. 1984;16:746–748. [PubMed] [Google Scholar]
- Ribatti D. The chick chorioallanotic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016;141:70–77. doi: 10.1016/j.mod.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Ritchey ER, Bongini RE, Code KA, Zelinka C, Petersen-Jones S, Fischer AJ. The pattern of expression of guanine nucleotide-binding protein β3 in the retina is conserved across vertebrate species. Neuroscience. 2010;169:1376–1391. doi: 10.1016/j.neuroscience.2010.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey ER, Code K, Zelinka CP, Scott Ma, Fischer AJ. The chicken cornea as a model of wound healing and neuronal reinnervation. Mol. Vis. 2011;17:2440–2454. [PMC free article] [PubMed] [Google Scholar]
- Ritchey ER, Zelinka C, Tang J, Liu J, Code KA, Petersen-Jones S, Fischer AJ. Vision-guided ocular growth in a mutant chicken model with diminished visual acuity. Exp. Eye Res. 2012a;102:59–69. doi: 10.1016/j.exer.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey ER, Zelinka CP, Tang J, Liu J, Fischer AJ. The combination of IGF1 and FGF2 and the induction of excessive ocular growth and extreme myopia. Exp. Eye Res. 2012b;99:1–16. doi: 10.1016/j.exer.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb EA, Antin PB, Delany ME. Defining the Sequence Elements and Candidate Genes for the Coloboma Mutation. PloS One. 2013;8:1–15. [Google Scholar]
- Rompani SB, Cepko CL. A common progenitor for retinal astrocytes and oligodendrocytes. J. Neurosci. 2010;30:4970–4980. doi: 10.1523/JNEUROSCI.3456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross WH. Visual recovery after macula-off retinal detachment. Eye. 2002;16:440–446. doi: 10.1038/sj.eye.6700192. [DOI] [PubMed] [Google Scholar]
- Ross WH, Kozy DW. Visual recovery in macula-off rhegmatogenous retinal detachments. Ophthalmology. 1998;105:2149–2153. doi: 10.1016/S0161-6420(98)91142-3. [DOI] [PubMed] [Google Scholar]
- Rüfer F, Schröder A, Erb C. White-to-white corneal diameter: normal values in healthy humans obtained with the Orbscan II topography system. Cornea. 2005;24:259–261. doi: 10.1097/01.ico.0000148312.01805.53. [DOI] [PubMed] [Google Scholar]
- Rymer J, Choh V, Bharadwaj S, Padmanabhan V, Modilevsky L, Jovanovich E, Yeh B, Zhang Z, Guan H, Payne W, Wildsoet CF. The albino chick as a model for studying ocular developmental anomalies, including refractive errors, associated with albinism. Exp. Eye Res. 2007;85:431–442. doi: 10.1016/j.exer.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymer J, Wildsoet CF. The role of the retinal pigment epithelium in eye growth regulation and myopia: A review. Vis. Neurosci. 2005;22:251–261. doi: 10.1017/S0952523805223015. [DOI] [PubMed] [Google Scholar]
- Sakami S, Etter P, Reh TA. Activin signaling limits the competence for retinal regeneration from the pigmented epithelium. Mech. Dev. 2008;125:106–116. doi: 10.1016/j.mod.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salicone A, Smiddy WE, Venkatraman A, Feuer W. Visual Recovery after Scleral Buckling Procedure for Retinal Detachment. Ophthalmology. 2006;113:1734–1742. doi: 10.1016/j.ophtha.2006.03.064. [DOI] [PubMed] [Google Scholar]
- Salter DW, Payne WS, Ramsey DT, Blair M, Render JA. A new inherited ocular anomaly in pigmented white Leghorn chickens. J. Vet. Diagnostic Investig. 1997;9:407–409. doi: 10.1177/104063879700900411. [DOI] [PubMed] [Google Scholar]
- Samuelson D. Ophthalmic anatomy. In: Gelatt KN, Gilger BC, Kern TJ, editors. Veterinary Ophthalmology. third. Lippincott, Williams & Wilkins; Baltimore, MD: 1991. pp. 31–150. [Google Scholar]
- Schachar RA. Central surface curvatures of postmortem-extracted intact human crystalline lenses: Implications for understanding the mechanism of accommodation. Ophthalmology. 2004;111:1699–1704. doi: 10.1016/j.ophtha.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Feldkaemper M. Animal models in myopia research. Clin. Exp. Optom. 2015;98:507–517. doi: 10.1111/cxo.12312. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Howland H. Corneal accommodation in chick and pigeon. J. Comp. Physiol. A. 1987;160:375–384. doi: 10.1007/BF00613027. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Howland H, Farkas L. Natural accommodation in the growing chicken. Vision Res. 1986;26:1977–1993. doi: 10.1016/0042-6989(86)90123-9. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Rohrer B, Lemmer T, Zrenner E. Diurnal control of rod function in the chicken. Vis. Neurosci. 1991;6:641–653. doi: 10.1017/s0952523800002637. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Wilhelm H, Zrenner E. Inter-individual variability in the dynamics of natural accommodation in humans: relation to age and refractive errors. J. Physiol. 1993;461:301–320. doi: 10.1113/jphysiol.1993.sp019515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippert R, Schaeffel F. Peripheral defocus does not necessarily affect central refractive development. Vision Res. 2006;46:3935–3940. doi: 10.1016/j.visres.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Rayner CL, Brown B. Hemi-field and full-field form-deprivation induce timing changes in multifocal ERG responses in chick. Ophthalmic Physiol. Opt. 2013;33:257–266. doi: 10.1111/opo.12055. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Assessment of visual acuity and contrast sensitivity in the chick using and optokinetic nystagmus paradigm. Vision Res. 1998;38:2629–2634. doi: 10.1016/s0042-6989(97)00446-x. [DOI] [PubMed] [Google Scholar]
- Schuck J, Gerhardt H, Wolburg H. The peripapillary glia of the optic nerve head in the chicken retina. Anat. Rec. 2000;259:263–275. doi: 10.1002/1097-0185(20000701)259:3<263::AID-AR40>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Schusser B, Collarini EJ, Yi H, Mettler S, Fesler J, Pedersen D. Immunoglobulin knockout chickens via efficient homologous recombination in primordial germ cells. Proc. Natl. Acad. Sci. U.S.A. 2013;110:20170–20175. doi: 10.1073/pnas.1317106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab IR. Evolution’s witness: How eyes evolved. Oxford University Press; New York: 2012. p. 147. [Google Scholar]
- Seaman AR, Storm H. A correlated light and electron microscope study on the pecten oculi of the domestic fowl (Gallus domesticus) Exp. Eye Res. 1963;2:163–172. doi: 10.1016/s0014-4835(65)80003-3. [DOI] [PubMed] [Google Scholar]
- Semple-Rowland SL, Berry J. Use of lentiviral vectors to deliver and express bicistronic transgenes in developing chicken embryos. Methods. 2014;66:466–473. doi: 10.1016/j.ymeth.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple-Rowland SL, Cheng KM. Rd and rc chickens carry the same GC1 null allele (GUCY1*) Exp. Eye Res. 1999;69:579–581. doi: 10.1006/exer.1999.0743. [DOI] [PubMed] [Google Scholar]
- Semple-Rowland SL, Lee NR, Van Hooser JP, Palczewski K, Baehr W. A null mutation in the photoreceptor guanylate cyclase gene causes the retinal degeneration chicken phenotype. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1271–1276. doi: 10.1073/pnas.95.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple-Rowland SL, Tepedino M, Coleman JE. Pinopsin mRNA levels are significantly elevated in the pineal glands of chickens carrying a null mutation in guanylate cyclase-1. Brain Res. Mol. Brain Res. 2001;97:51–58. doi: 10.1016/s0169-328x(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Sethi CS, Lewis GP, Fisher SK, Leitner WP, Mann DL, Luthert PJ, Charteris DG. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 2005;46:329–342. doi: 10.1167/iovs.03-0518. [DOI] [PubMed] [Google Scholar]
- Shi F, Erf GF. IFN-γ, IL-21, and IL-10 Co-Expression in Evolving Autoimmune Vitiligo Lesions of Smyth Line Chickens. J. Invest. Dermatol. 2012;132:642–649. doi: 10.1038/jid.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Kong B-W, Song J, Lee J, Dienglewicz RL, Erf GF. Understanding mechanisms of vitiligo development in Smyth line of chickens by transcriptomic microarray analysis of evolving autoimmune lesions. BMC Immunol. 2012;13:1–15. doi: 10.1186/1471-2172-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Ko ML, Huang CC, Park SY, Hong MP, Wu C, Ko GY. Chicken embryos as a potential new model for early onset type I diabetes. J Diabetes Res. 2014;2014:1–10. doi: 10.1155/2014/354094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Yamazaki H, Mizutani M, Nunoya T, Tajima M, Satou T. Hereditary visual impairment in a new mutant strain of chicken, GSN/1. Acta Neuropathol. 2002;103:137–144. doi: 10.1007/s004010100442. [DOI] [PubMed] [Google Scholar]
- Simon J, Dubois MP. Failure of a sublethal streptozotocin dose to induce diabetes in the chicken. Horm. Metab. Res. 1980;12:631–632. doi: 10.1055/s-2007-999217. [DOI] [PubMed] [Google Scholar]
- Slijkerman RWN, Song F, Astuti GDN, Huynen MA, van Wijk E, Stieger K, Collin RWJ. The pros and cons of vertebrate animal models for functional and therapeutic research on inherited retinal dystrophies. Prog. Retin. Eye Res. 2015;48:137–159. doi: 10.1016/j.preteyeres.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Slingsby C, Wistow GJ, Clark AR. Evolution of crystallins for a role in the vertebrate eye lens. Protein Sci. 2013;22:367–380. doi: 10.1002/pro.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, Becker B, Podos S. Light-induced angle-closure glaucoma in the domestic fowl. Invest. Ophthalmol. 1969;8:213–221. [PubMed] [Google Scholar]
- Smyth JR, Boissy RE, Fite KV. The DAM chicken: a model for spontaneous postnatal cutaneous and ocular amelanosis. J. Hered. 1981;72:150–156. [PubMed] [Google Scholar]
- Smyth JRJ, McNeil M. Alopecia areata and universalis in the Smyth chicken model for spontaneous autoimmune vitiligo. J. Investig. Dermatol. Symp. Proc. 1999;4:211–215. doi: 10.1038/sj.jidsp.5640213. [DOI] [PubMed] [Google Scholar]
- Somes RG. Microphthalmia-4: a sex-influenced inherited eye condition of the chicken. J. Hered. 1992;83:152–155. doi: 10.1093/oxfordjournals.jhered.a111178. [DOI] [PubMed] [Google Scholar]
- Somes RG. Sleepy-eye, an eyelid mutant of the fowl. J. Hered. 1968;59:375–378. doi: 10.1093/oxfordjournals.jhered.a107752. [DOI] [PubMed] [Google Scholar]
- Spence JR, Aycinena J-C, Del Rio-Tsonis K. Fibroblast growth factor-hedgehog interdependence during retina regeneration. Dev. Dyn. 2007;236:1161–1174. doi: 10.1002/dvdy.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Madhavan M, Aycinena J-C, Del Rio-Tsonis K. Retina regeneration in the chick embryo is not induced by spontaneous Mitf downregulation but requires FGF/FGFR/MEK/Erk dependent upregulation of Pax6. Mol. Vis. 2007;13:57–65. [PMC free article] [PubMed] [Google Scholar]
- Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch. Ophthalmol. 1983;101:405–407. doi: 10.1001/archopht.1983.01040010405011. [DOI] [PubMed] [Google Scholar]
- Stanke JJ, Fischer AJ. Embryonic retinal cells and support to mature retinal neurons. Investig. Ophthalmol. Vis. Sci. 2010;51:2208–2218. doi: 10.1167/iovs.09-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke J, Moose HE, El-Hodiri HM, Fischer AJ. Comparative study of pax2 expression in glial cells in the retina and optic nerve of birds and mammals. J. Comp. Neurol. 2010;518:1–26. doi: 10.1002/cne.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga DG, Wilts BD. Oil droplets of bird eyes: microlenses acting as spectral filters. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:1–8. doi: 10.1098/rstb.2013.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellenwerf WA, Hazelwood RL. Peripheral utilization of a glucose load after alloxan and streptozotocin in the rat and chicken: a comparison. Gen. Comp. Endocrinol. 1979;39:131–140. doi: 10.1016/0016-6480(79)90218-1. [DOI] [PubMed] [Google Scholar]
- Stern CD. The chick: A great model system becomes even greater. Dev. Cell. 2005;8:9–17. doi: 10.1016/j.devcel.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc. Natl. Acad. Sci. U.S.A. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Liu J, Sugimoto R, Capehart C, Zhu X, Pendrak K. GABA, experimental myopia, and ocular growth in chick. Investig. Ophthalmol. Vis. Sci. 2003;44:3933–3946. doi: 10.1167/iovs.02-0774. [DOI] [PubMed] [Google Scholar]
- Streit A, Tambalo M, Chen J, Grocott T, Anwar M, Sosinsky A, Stern CD. Experimental approaches for gene regulatory network construction: The chick as a model system. Genesis. 2013;51:296–310. doi: 10.1002/dvg.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq YM, Samarawickrama C, Li H, Huynh SC, Burlutsky G, Mitchell P. Retinal thickness in the offspring of diabetic pregnancies. Am. J. Ophthalmol. 2010;150:883–887. doi: 10.1016/j.ajo.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Tepelus TC, Vazquez D, Seidemann A, Uttenweiler D, Schaeffel F. Effects of lenses with different power profiles on eye shape in chickens. Vision Res. 2012;54:12–19. doi: 10.1016/j.visres.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Todd L, Fischer AJ. Hedgehog signaling stimulates the formation of proliferating Müller glia-derived progenitor cells in the chick retina. Development. 2015;142:2610–2622. doi: 10.1242/dev.121616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Squires N, Suarez L, Fischer AJ. Jak/Stat signaling regulates the proliferation and neurogenic potential of Müller glia-derived progenitor cells in the avian retina. Sci. Rep. 2016;6:1–16. doi: 10.1038/srep35703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Bedecarrats GY, Choh V. Inner retinal cell development is impaired in Smoky Joe chickens. Poult. Sci. 2013;92:1322–1330. doi: 10.3382/ps.2012-02848. [DOI] [PubMed] [Google Scholar]
- Troilo D. Experimental studies of emmetropization in the chick. Ciba Found. Symp. 1990;155:89–102. doi: 10.1002/9780470514023.ch6. [DOI] [PubMed] [Google Scholar]
- Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr. Eye Res. 1987;6:993–999. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- Troilo D, Li T, Glasser A, Howland HC. Differences in eye growth and the response to visual deprivation in different strains of chicken. Vision Res. 1995;35:1211–1216. doi: 10.1016/0042-6989(94)00230-j. [DOI] [PubMed] [Google Scholar]
- Troilo D, Wallman J. Changes in corneal curvature during accommodation in chicks. Vision Res. 1987;27:241–247. doi: 10.1016/0042-6989(87)90186-6. [DOI] [PubMed] [Google Scholar]
- Tummala H, Ali M, Getty P, Hocking PM, Burt DW, Inglebearn CF, Lester DH. Mutation in the guanine nucleotide-binding protein β-3 causes retinal degeneration and embryonic mortality in chickens. Investig. Ophthalmol. Vis. Sci. 2006;47:4714–4718. doi: 10.1167/iovs.06-0292. [DOI] [PubMed] [Google Scholar]
- Tyack SG, Jenkins KA, O’Neil TE, Wise TG, Morris KR, Bruce MP, McLeod S, Wade AJ, McKay J, Moore RJ, Schat KA, Lowenthal JW, Doran TJ. A new method for producing transgenic birds via direct in vivo transfection of primordial germ cells. Transgenic Res. 2013;22:1257–1264. doi: 10.1007/s11248-013-9727-2. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Wilken MS, Cox KE, Chipman L, Jorstad N, Sternhagen K, Simic M, Ullom K, Nakafuku M, Reh TA. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proc. Natl. Acad. Sci. U.S.A. 2015;112:13717–13722. doi: 10.1073/pnas.1510595112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulshafer RJ, Allen C, Dawson WW, Wolf ED. Hereditary retinal degeneration in the Rhode Island Red chicken. I. Histology and ERG. Exp. Eye Res. 1984;39:125–35. doi: 10.1016/0014-4835(84)90003-4. [DOI] [PubMed] [Google Scholar]
- Ulshafer RJ, Allen CB. Hereditary retinal degeneration in the Rhode Island Red chicken: ultrastructural analysis. Exp. Eye Res. 1985;40:865–877. doi: 10.1016/0014-4835(85)90131-9. [DOI] [PubMed] [Google Scholar]
- Van de Lavoir M-C, Diamond JH, Leighton PA, Mather-Love C, Heyer BS, Bradshaw R, Kerchner A, Hooi LT, Gessaro TM, Swanberg SE, Delany ME, Etches RJ. Germline transmission of genetically modified primordial germ cells. Nature. 2006;441:766–769. doi: 10.1038/nature04831. [DOI] [PubMed] [Google Scholar]
- Vergara MN, Canto-Soler MV. Rediscovering the chick embryo as a model to study retinal development. Neural Dev. 2012;7:22–40. doi: 10.1186/1749-8104-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara MN, Gutierrez C, O’Brien DR, Canto-Soler MV. Ex vivo electroporation of retinal cells: A novel, high efficiency method for functional studies in primary retinal cultures. Exp. Eye Res. 2013;109:40–50. doi: 10.1016/j.exer.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier JD, Madorsky I, Coggin WE, Geesey M, Hochman M, Walling E, Daroszewski D, Eccles KS, Ludlow R, Semple-Rowland SL. Bicistronic Lentiviruses Containing a Viral 2A Cleavage Sequence Reliably Co-Express Two Proteins and Restore Vision to an Animal Model of LCA1. PloS One. 2011;6:1–12. doi: 10.1371/journal.pone.0020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viets K, Eldred KC, Johnston RJ. Mechanisms of photoreceptor patterning in vertebrates and invertebrates. 2016;32:638–659. doi: 10.1016/j.tig.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, Audo I, Tavares E, Maynes JT, Tumber A, Wright T, Li S, Michiels C, GNB3 Consortium. Condroyer C, MacDonald H, Verdet R, Sahel J-A, Hamel CP, Zeitz C, Heon E. Biallelic mutations in GNB3 cause a unique form of autosomal-recessive congenital stationary night blindness. Am. J. Human Genet. 2016;98:1011–1019. doi: 10.1016/j.ajhg.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale S, Ellwein L, Cotch MF, Ferris FL, Sperduto R. Prevalence of refractive error in the United States, 1999–2004. Arch. Ophthalmol. 2008;126:1111–1119. doi: 10.1001/archopht.126.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyev M. Coloured oil droplets enhance colour discrimination. Proc. R. Soc. London B Biol. Sci. 2003;270:1255–1261. doi: 10.1098/rspb.2003.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl C, Li T, Choden T, Howland H. Morphometrics of corneal growth in chicks raised in constant light. J. Anat. 2009;214:355–361. doi: 10.1111/j.1469-7580.2008.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl B, Li T, Howland H. Plasticity in the growth of the chick eye: Emmetropization achieved by alternate morphologies. Vision Res. 2015;110:15–22. doi: 10.1016/j.visres.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl C, Li T, Howland HC. Intraocular pressure fluctuations of growing chick eyes are suppressed in constant light conditions. Exp. Eye Res. 2016;148:52–54. doi: 10.1016/j.exer.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Wahl C, Li T, Takagi Y, Howland H. The effects of light regimes and hormones on corneal growth in vivo and in organ culture. J. Anat. 2011;219:766–775. doi: 10.1111/j.1469-7580.2011.01429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldvogel JA. The Bird’s Eye View. Am. Sci. 1990;78:342–353. [Google Scholar]
- Walker MK, Blanco L, Kivlin R, Choi SS, Doble N. Measurement of the photoreceptor pointing in the living chick eye. Vision Res. 2015;109:59–67. doi: 10.1016/j.visres.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, Adams JI, Trachtman JN. The eyes of young chickens grow toward emmetropia. Invest. Ophthalmol. Vis. Sci. 1981;20:557–561. [PubMed] [Google Scholar]
- Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–51. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Walls GL. The vertebrate eye and its adaptive radiation. Hafner; New York: 1963. [Google Scholar]
- Wan J, Zhao X-F, Vojtek A, Goldman D. Retinal Injury, Growth Factors, and Cytokines Converge on β-Catenin and pStat3 Signaling to Stimulate Retina Regeneration. Cell Rep. 2014;9:285–297. doi: 10.1016/j.celrep.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-Z, Ma W, Yan RT, Mao W. Generating retinal neurons by reprogramming retinal pigment epithelial cells. Expert Opin. Biol. Ther. 2010;10:1227–1239. doi: 10.1517/14712598.2010.495218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DC. Inheritance of pinkeye in the fowl. J. Hered. 1940;31:291–292. [Google Scholar]
- Weller C, Lindstrom SH, De Grip WJ, Wilson M. The area centralis in the chicken retina contains efferent target amacrine cells. Vis. Neurosci. 2009;N26:249–254. doi: 10.1017/S0952523808080917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells-Gray EM, Choi SS, Bries A, Doble N. Variation in rod and cone density from the fovea to the mid-periphery in healthy human retinas using adaptive optics scanning laser ophthalmoscopy. Eye (Lond) 2016;30:1135–1143. doi: 10.1038/eye.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook AM, Crewther SG, Beresford JA, Allen M, Keller I, Crewther DP. Formoguanamine-induced inhibition of deprivation myopia in chick is accompanied by choroidal thinning while retinal function is retained. Vision Res. 1995;35:2075–2088. doi: 10.1016/0042-6989(94)00282-q. [DOI] [PubMed] [Google Scholar]
- Wick G, Andersson L, Hala K, Gershwin ME, Selmi C, Erf GF, Lamont SJ, Sgonc R. Avian Models with Spontaneous Autoimmune Diseases. Adv. Immunol. 2006;92:71–117. doi: 10.1016/S0065-2776(06)92002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildoset CF. Neural pathways subserving negative lens-induced emmetropization in chicks – Insights from selective lesions of the optic nerve and ciliary nerve. Curr. Eye Res. 2003;27:371–385. doi: 10.1076/ceyr.27.6.371.18188. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF, Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact and sectioned optic nerves. Vision Res. 2000;40:3273–3282. doi: 10.1016/s0042-6989(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- Williams ML, Coleman JE, Haire SE, Aleman TS, Cideciyan AV, Sokal I, Palczewski K, Jacobson SG, Semple-Rowland SL. Lentiviral expression of retinal guanylate cyclase-1 (RetGC1) restores vision in an avian model of childhood blindness. PLoS Med. 2006;3:0904–0917. doi: 10.1371/journal.pmed.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Hong J, Lee J. The Corneal Wound Healing Response. Prog. Retin. Eye Res. 2001;20:625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Wingstrand KG, Munk O. The pecten oculi of the pigeon with particular regard to its function. Det K. Danske Vidensk. Selsk. Biol. Skr. 1965;14:1–64. [Google Scholar]
- Wood CA. The fundus oculi of birds, especially as viewed by the ophthalmoscope: a study in comparative anatomy and physiology. Lakeside Press; Chicago: 1917. [Google Scholar]
- Yang H, Fang G, Huang X, Yu J, Hsieh CL, Grossniklaus HE. In-vivo xenograft murine human uveal melanoma model develops hepatic micrometastases. Melanoma Res. 2008;18:95–103. doi: 10.1097/CMR.0b013e3282f628df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S. Molecular evolution of vertebrate visual pigments. Prog. Retin. Eye Res. 2000;19:385–419. doi: 10.1016/s1350-9462(00)00002-1. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Sugiyama T, Kanke M. Experimental diabetes model in chick embryos treated with streptozotocin. Biol. Pharm. Bull. 2005;28:1986–1988. doi: 10.1248/bpb.28.1986. [DOI] [PubMed] [Google Scholar]
- Zelinka CP, Volkov L, Goodman ZA, Todd L, Palazzo I, Bishop WA, Fischer AJ. mTor signaling is required for the formation of proliferating Müller glia-derived progenitor cells in the chick retina. Development. 2016;143:1859–1873. doi: 10.1242/dev.133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S-J, Li Y-F, Tan R-R, Tsoi B, Huang W-S, Huang Y-H, Tang X-L, Hu D, Yao N, Yang X, Kurihara H, Wang Q, He R-R. A new gestational diabetes mellitus model: hyperglycemia-induced eye malformation via inhibition of Pax6 in the chick embryo. Dis. Model. Mech. 2016;9:177–186. doi: 10.1242/dmm.022012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Thornquist SC, Barnstable CJ. In vitro transdifferentiation of embryonic rat retinal pigment epithelium to neural retina. Brain Res. 1995;677:300–310. doi: 10.1016/0006-8993(95)00163-k. [DOI] [PubMed] [Google Scholar]
- Zhao X-F, Wan J, Powell C, Ramachandran R, Myers MG, Goldman D. Leptin and IL-6 Family Cytokines Synergize to Stimulate Müller Glia Reprogramming and Retina Regeneration. Cell Rep. 2014;9:272–284. doi: 10.1016/j.celrep.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]