Abstract
Severity of multiple organ failure is significantly impacted by age and gender in patients with hemorrhagic shock. However, the molecular mechanisms underlying the enhanced organ injury are not fully understood. AMP-activated protein kinase (AMPK) is a pivotal orchestrator of metabolic responses during stress. We investigated whether hemorrhage-induced myocardial injury is age and gender dependent and whether treatment with metformin, an AMPK activator, affords cardioprotective effects. C57/BL6 young (3–5 months) and mature (9–12 months) male and female mice were subjected to hemorrhagic shock by blood withdrawing followed by resuscitation with blood and Lactated Ringer’s solution. Vehicle-treated young and mature mice of both genders had a similar elevation of plasma inflammatory cytokines at 3 hours after resuscitation. However, vehicle-treated male mature mice experienced hemodynamic instability and higher myocardial damage than young male mice, as evaluated by echocardiography, histology and cardiovascular injury biomarkers. There was also a gender-dependent difference in cardiovascular injury in the mature group as vehicle-treated male mice exhibited more severe organ injury than female mice. At molecular analysis, vehicle-treated mature mice of both genders exhibited a marked downregulation of AMPKα activation and nuclear translocation of peroxisome proliferator-activated receptor γ co-activator α when compared with young mice. Treatment with metformin improved cardiovascular function and survival in mature animals of both genders. However, specific cardioprotective effects of metformin were gender-dependent. Metformin did not affect hemodynamic or inflammatory responses in young animals. Thus, our data suggest that targeting metabolic recovery with metformin may be a potential treatment approach in severe hemorrhage in adult population.
Keywords: age, AMP-activated protein kinase (AMPK), hemorrhagic shock, gender, metformin, peroxisome proliferator-activated receptor-γ co-activator α (PGC-1α)
1. Introduction
Clinical studies have reported that adult trauma victims, including patients with hemorrhagic shock, have considerably higher mortality rate than pediatric population [1–3]. Aging is, in fact, a risk factor for development of multiple organ failure and correlates with changes in myocardial function and biochemistry thus affecting the hemodynamic compensation to severe hemorrhage [4–7]. Experimental and clinical studies also suggest that gender-dimorphic immunomodulatory and inflammatory responses may influence the outcome of trauma adult victims [8–12]. For example, women exhibited distinct inflammatory and cardiovascular responses and withstood trauma-related complications of sepsis or circulatory shock with better survival rates than men [11,12]. However, the molecular mechanisms that link age or gender to trauma-induced cardiovascular dysfunction are not fully understood.
Being a mitochondria-rich organ, myocardium is highly dependent on ATP synthesis. Loss of metabolic capacity after mitochondrial damage is a major pathophysiological mechanism of myocardial dysfunction and cardiac failure [13]. At molecular level, AMP-activated protein kinase (AMPK) is a crucial regulator of energy homeostasis which is activated in conditions of energy depletion when there is an intracellular imbalance of high AMP and low ATP levels [14]. AMPK promotes metabolic recovery through activation of peroxisome proliferator-activated receptor γ co-activator α (PGC-1α), a nuclear transcriptional co-activator responsible for mitochondrial biogenesis [14–16]. Several experimental studies have demonstrated that aging-associated loss in AMPK activity may contribute to mitochondrial dysfunction in skeletal muscles and myocardium [17,18]. Among pharmacological activators of this energy pathway, metformin, a widely used anti-hyperglycemic drug, has been shown to indirectly activate AMPK and to maintain metabolic homeostasis [19].
In previous experimental studies, we have demonstrated that AMPK-dependent metabolic mechanisms become dysfunctional with aging and correlate with myocardial and lung injury in mature adult male mice when compared with young animals after hemorrhagic shock [20,21]. To further understand the underlying molecular mechanisms of cardiovascular function during hemorrhagic shock, the purpose of our study was to investigate both age- and gender-dependent mechanisms of the hemodynamic compromise and systemic inflammatory response in a clinically relevant model of hemorrhagic shock in young (3–5 months old) and middle-aged mature (9–12 months old) mice. Also, purpose of our study was to investigate whether pharmacological treatment with metformin would affect AMPK-dependent metabolic pathways and, consequently, ameliorate outcomes of hemorrhagic shock.
2. Methods
2.1. Murine model of hemorrhagic shock
The experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (8th edition, 2011) and had the approval of the Institutional Animal Care and Use Committee. Sixty C57/BL6 male and female young (3–5 months) and mature mice (9–12 months) were obtained from Charles River Laboratories (Wilmington, MA) and were acclimatized for at least 48 h. All mice were allowed free access to water and a maintenance diet in a 12-hour light/dark cycle, with room temperature at 21±2 °C.
Before procedure, each animal was placed on a circulating warming water blanket to maintain body temperature at physiological conditions throughout experiment. Mice were anesthetized with pentobarbital (80 mg/kg) intraperitoneally. Either the left or right femoral artery was cannulated (PE-10 tube) and connected to a blood pressure transducer (PowerLab, ADInstruments, Colorado Springs, CO) for measurement of mean arterial blood pressure (MABP) and heart rate (HR). Hemorrhagic shock was induced by blood removal until mean arterial blood pressure (MABP) reached 30±5 mmHg [20–22]. The mice were kept in this MABP range for 90 minutes by additional blood removal or small volume transfusion. At the end of the shock period, young and mature mice of both genders were randomly assigned to two groups: a vehicle-treated group received vehicle (Normal Saline solution) intra-arterially (IA); a metformin-treated group received metformin (100 mg/kg, IA). Drug treatment was given as single bolus right before resuscitation. Dose of metformin was chosen according to reported pharmacokinetics and pharmacodynamics of the drug in the mice [23] and pilots studies on hemodynamics in our laboratory. Mice were then resuscitated by infusing their shed blood and twice that amount in Lactated Ringer’s solution over a 10-minute period. Mice were further monitored for 3 hours for MABP and HR values. At 3 hours of resuscitation mice were sacrificed and blood, lung, liver and heart were obtained for biochemical assays. In separate groups of mice survival studies were conducted. In these mice, the femoral artery was occluded and the skin incision was closed in layers at 3 hour after resuscitation. Mice were allowed to recover from anesthesia and survival rate was monitored for 7 days. Sham mice were anesthetized and underwent cannulation, but were not hemorrhaged.
2.2. Blood glucose and lactate levels
Arterial whole blood (100 μl) was collected and analyzed by using iStat System analyzer (Abbott Laboratories, Princeton, NJ) for lactate and glucose levels before surgery (baseline), at the end of hemorrhagic hypovolemic phase, and at 3 hours after resuscitation (i.e., at the end of the experiment).
2.3. Echocardiographic assessment
Echocardiographic measurements were obtained before and 3 hours after resuscitation using a VisualSonics 2100 system equipped with a 30 MHz transducer [24]. Ejection fraction (EF), fractional shortening (FS), left ventricle (LV) internal dimensions, including end-diastolic and end-systolic dimensions (LVIDd and LVIDs, respectively), interventricular septal thickness in diastole and systole (IVSd and IVSs, respectively) and LV posterior wall thickness in diastole and systole (LVPWd and LVPWs, respectively) were measured directly.
2.4. Histopathologic analysis
Heart tissue samples were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained with hematoxylin and eosin and evaluated by light microscopy by an independent pathologist. Myocardial injury was determined by the presence of perivascular, endocardial and pericardial edema, neutrophil infiltration and myofibril derangement.
2.5. Plasma cytokines and biomarkers of cardiovascular disease
Plasma levels of interleukin (IL) 1β, IL-10, IL-6, keratinocyte chemoattractant chemokine (KC), tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) and plasma levels of biomarkers of cardiovascular injury, including follistatin, endocan-1 and CXCL16, were evaluated by a multiplex array system according to the manufacture’s protocol (Milliplex, Millipore Corporation, Billerica, MA).
2.6. Myeloperoxidase assay
Myeloperoxidase (MPO) activity was determined as an index of neutrophil accumulation in lung and liver [25]. Tissues were homogenized in a solution containing 0.5% hexa-decyl-trimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged for 30 minutes at 4,000 g at 4°C. An aliquot of the supernatant was allowed to react with a solution of tetra-methyl-benzidine (1.6 mM) and 0.1 mM H2O2. The rate of change in absorbance was measured by spectrophotometry at 650 nm. Myeloperoxidase activity was defined as the quantity of enzyme degrading 1 μmol of hydrogen peroxide/minute at 37°C and expressed in units per 100 mg tissue.
2.7. Cytosol and nuclear extracts
Cardiac tissues were homogenized using a Polytron homogenizer (Brinkman Instruments, West Orange, NY) in a buffer containing 0.32 M sucrose, 10 mM Tris-HCl (pH 7.4), 1 mM EGTA, 2 mM EDTA, 5 mM NaN3, 10 mM b-mercaptoethanol, 20 μM leupeptin, 0.15 μM pepstatin A, 0.2 mM phenylmethanesulfonyl fluoride, 50 mM NaF, 1 mM sodium orthovanadate, and 0.4 nM microcystin. Samples were centrifuged at 1,000 g for 10 minutes at 4°C and the supernatants collected as cytosol extracts. The pellets were then solubilized in Triton buffer (1% Triton X-100, 250 mM NaCl, 50 mM Tris HCl at pH 7.5, 3 mM EGTA, 3 mM EDTA, 0.1 mM phenylmethanesulfonyl fluoride, 0.1 mM sodium orthovanadate, 10% glycerol, 2 mM p-nitrophenyl phosphate, 0.5% NP-40, and 46 μM aprotinin). The lysates were centrifuged at 15,000 g for 30 minutes at 4°C and the supernatant collected as nuclear extracts.
2.8. Western blot analysis
Cytosol and nuclear content of AMPKα1/α2 and its phosphorylated active form pAMPK α1/α2, PGC1-α and sirtuin-1 (SIRT1) were determined by immunoblot analyses. Proteins were loaded on a 10% Bis-Tris gel and separated electrophoretically and transferred to nitrocellulose membranes. For immunoblotting, membranes were blocked with Odyssey blocking buffer and incubated with specific primary antibodies; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was concomitantly probed as loading control. Membranes were washed and incubated with LI-COR secondary antibodies. The immunoreaction was visualized by fluorescence with an Odyssey LI-COR scanner (LI-COR Biotechnology, Lincoln, NE).
2.9. Measurement of nuclear factor-κB (NF-κB) and peroxisome proliferator-activated receptor γ (PPARγ) activity
NF-κB and PPARγ activity was analyzed in cardiac nuclear extracts by TransAM Transcription Factor assay kits specific for the activated form of p65 using the NF-κB consensus site (5′-GGGACTTTCC-3′) or for PPARγ using the PPAR consensus site (5′-AACTAGGTCAAAGGTCA-3′)according to the manufacturer’s protocol (Active Motif, Carlsbad CA).
2.10. Measurement of flavin adenine dinucleotide (FAD)
Cardiac levels of the redox co-factor FAD were determined in homogenized tissues using a Flavin Adenine Dinucleotide (FAD) Assay Kit (Abcam Cambridge, MA). The rate of change in absorbance of an OxiRed probe was measured by spectrophotometry at 570 nm. FAD levels were expressed in pmol/mg tissue.
2.11. Materials
Metformin was obtained from Enzo Life Sciences (Farmingdale, NY) Primary antibodies for AMPKα1/α2 and pAMPKα1/α2 were obtained from Cell Signaling Technology (Danvers, MA). Primary antibodies for SIRT1, GAPDH and PGC1-α were obtained from Abcam (Cambridge, MA). The Odyssey blocking buffer, LI-COR goat anti-rabbit IR-800 and goat anti-mouse IR-680 antibodies, and the 4X Protein Sample Loading Buffer were obtained from LI-COR Biotechnology (Lincoln, NE). The NuPAGE® LDS Sample Buffer (4X), and Western blot gels were purchased from Life Technologies (Grand Island, NY). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
2.12. Statistical analysis
Statistical analysis was performed using SigmaPlot for Windows Version 12.5 (Systat Software, San Jose CA). Data are represented as means ± SD or SEM of n = 3–12 animals for each age and gender group. For multiple group analysis at a single time point, one-way analysis of variance (ANOVA) with Student-Newman-Keuls correction was used. For multiple group analysis at different time points, a two-way ANOVA with Student-Newman-Keuls correction was performed. If data failed to follow a normal distribution, a Mann-Whitney Rank Sum test or an ANOVA on ranks test was performed. Survival rate was analyzed using Kaplan-Meier or Fischer’s exact test. P values less than 0.05 were considered significant.
3. Results
3.1. Age-dependent hypotension after hemorrhagic shock
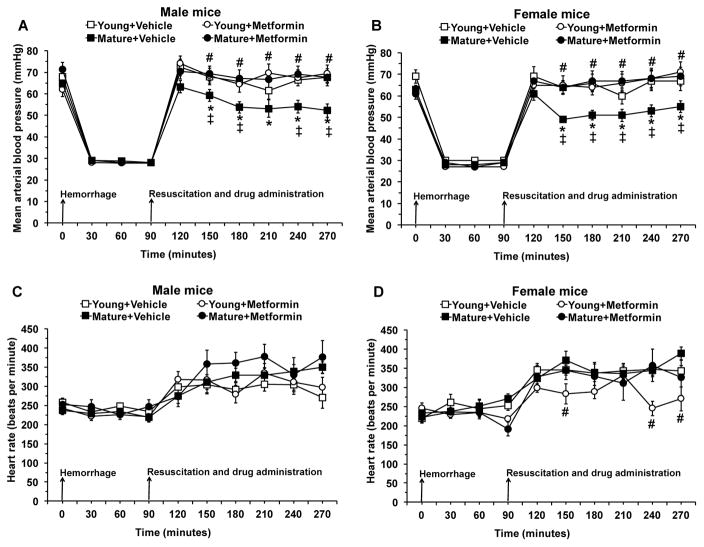
Since the degree of hypoperfusion influences the severity of the inflammatory response, we chose an experimental protocol of pressure-controlled hemorrhage to a target MABP of 30 mmHg in both genders and age groups [22]. After resuscitation with blood and Lactated Ringer’s solution, MABP fully recovered in young vehicle-treated mice of both genders. However, MABP partially recovered and did not reach baseline levels in mature groups of both genders. Treatment with metformin at the time of resuscitation ameliorated MABP in mature mice of both genders when compared with vehicle treatment. Metformin treatment did not affect MABP in young mice. Heart rate was significantly higher after resuscitation when compared to baseline rate at time 0 in all groups of animals (P<0.05). Metformin treatment reduced heart rate in young female mice only (Fig. 1).
Fig. 1.
Effect of in vivo treatment with metformin on mean arterial blood pressure (A and B) and heart rate (C and D) in young and mature mice of both genders subjected to hemorrhage and resuscitation. Data represents the mean ± SD of 10 mice in each group. Vehicle (normal saline) or metformin (100 mg/kg) was administered intra-arterially at the time of resuscitation. Arrows indicate time of induction of hemorrhage and initiation of resuscitation and metformin or vehicle administration. *P<0.05 vs baseline values at time 0 of the same group; ‡P<0.05 vs young group of the same gender; #P<0.05 vs vehicle-treated group of the same age.
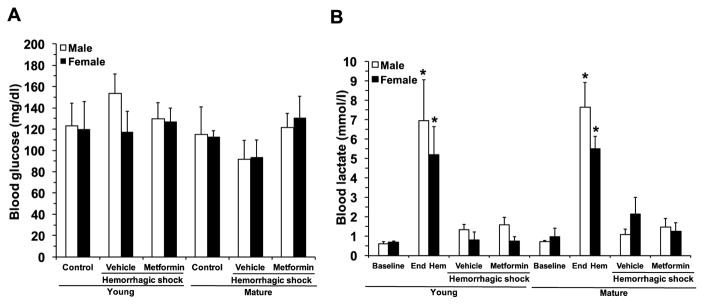
3.2. Effect of metformin treatment on blood levels of lactate and glucose
There were no changes in glucose levels after hemorrhagic shock in vehicle-treated male and female mice of either age groups when compared to baseline control levels. Blood lactate levels were similarly elevated at the end of the hypovolemic phase when compared with baseline values in male and female mice in both age groups, thus confirming a significant hypoperfusion injury in all animals. Blood lactate levels similarly declined and returned to baseline levels at 3 hours after resuscitation in vehicle-treated mice of both genders and ages. Treatment with metformin did not modify glucose or lactate levels in any experimental groups, thus proving that the drug does not impose any risk of hypoglycemia or metabolic acidosis (Fig. 2).
Fig. 2.
Effect of in vivo treatment with metformin on blood levels of glucose (A) and lactate (B) before hemorrhage (control baseline), at the end of the hemorrhagic hypovolemic period (End Hem), and at 3 hours after reperfusion (hemorrhagic shock). Data represents the mean ± SEM of 7 mice for each group. *P<0.05 vs baseline values of age- and gender-matched control mice.
3.3. Age-dependent changes of echocardiography values
To better characterize the age-dependent hemodynamic instability after hemorrhagic shock we also performed echocardiographic measurements (Table 1). Consistent with the age-dependent hypotension, we observed significant echocardiography changes in mature animals. While there were no changes in EF and FS, there was a significant decrease in left ventricle internal dimensions and volumes and a significant increase of the thickness of interventricular septum during systole and diastole in mature mice of both gender animals. Young animals in the female group experienced only a significant decrease in left ventricular internal dimension during diastole. Treatment with metformin restored systolic and diastolic parameters in mature mice of both genders to values like baseline functions. Treatment with metformin did not modify any echocardiographic parameters in young mice.
Table 1.
Echocardiographic parameters in mice before hemorrhage (basal) and at three after resuscitation (HS, hemorrhagic shock).
| Echocardiographic parameters | Young | Mature | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | Metformin | Vehicle | Metformin | ||||||
| Basal | HS | Basal | HS | Basal | HS | Basal | HS | ||
| EF, % | Male | 53.63±2.62 | 48.57±3.15 | 55.50±2.68 | 56.00±4.42 | 50.82±3.07 | 54.00±2.52 | 52.29±1.08 | 55.25±4.59 |
| Female | 42.14±2.70 | 49.60±8.53 | 46.14±2.38 | 52.33±7.67 | 49.00±1.89 | 51.33±2.53 | 54.00±1.26 | 51.33±3.41 | |
| FS, % | Male | 27.50±1.65 | 24.14±1.48 | 28.67±2.12 | 29.29±2.97 | 24.00±1.58 | 27.50±1.66 | 27.50±1.66 | 28.75±2.01 |
| Female | 20.29±1.51 | 25.20±5.08 | 22.57±1.38 | 25.20±4.48 | 25.00±1.21 | 25.50±1.31 | 27.57±0.74 | 26.00±2.07 | |
| LVs, μL | Male | 30.13±2.16 | 31.10±5.91 | 27.37±1.84 | 28.29±5.42 | 42.53±5.13 | 26.25±0.69* | 40.89±2.73 | 32.40±5.74 |
| Female | 35.13±2.35 | 28.06±9.71 | 38.43±5.60 | 24.68±6.42 | 47.54±4.84 | 23.67±5.30* | 38.70±4.44 | 32.37±4.72 | |
| LVd, μL | Male | 64.54±2.44 | 59.04±8.70 | 61.80±2.11 | 60.91±7.08 | 80.28±11.09 | 57.68±2.75* | 85.70±5.60 | 70.15±10.31 |
| Female | 60.71±3.04 | 50.04±9.44 | 63.07±4.59 | 48.45±5.57* | 92.21±6.27 | 46.53±11.10* | 82.94±7.90 | 69.44±5.19 | |
| LV mass, mg | Male | 101.69±2.09 | 96.74±5.61 | 90.92±5.98 | 98.53±5.03 | 119.58±7.88 | 111.63±5.33 | 128.56±5.90 | 127.93±12.69 |
| Female | 79.26±2.59 | 74.06±2.41 | 81.26±2.86 | 73.57±3.10 | 111.84±7.50 | 95.32±8.04 | 112.80±8.00 | 101.43±6.01 | |
| LVPWs, mm | Male | 1.22±0.04 | 1.09±0.04 | 1.13±0.06 | 1.18±0.11 | 1.21±0.11 | 1.47±0.06* | 1.27±0.04 | 1.29±0.06# |
| Female | 0.99±0.05 | 1.07±0.08 | 0.99±0.07 | 1.08±0.08 | 1.07±0.03 | 1.30±0.11* | 1.09±0.02 | 1.11±0.05 | |
| LVPWd, mm | Male | 0.89±0.04 | 0.90±0.04 | 0.78±0.03 | 0.81±0.08 | 0.88±0.04 | 1.01±0.05* | 0.85±0.02 | 0.92±0.01* |
| Female | 0.74±0.04 | 0.77±0.04 | 0.73±0.04 | 0.75±0.03 | 0.80±0.02 | 1.00±0.09* | 0.82±0.02 | 0.84±0.02 | |
| LVIDs, mm | Male | 2.87±0.11 | 2.79±0.20 | 2.71±0.08 | 2.67±0.23 | 3.21±0.26 | 2.67±0.04* | 3.19±0.09 | 2.85±0.27 |
| Female | 2.99±0.09 | 2.60±0.28 | 2.96±0.14 | 2.51±0.24 | 3.38±0.13 | 2.45±0.23* | 3.09±0.15 | 2.86±0.18 | |
| LVIDd, mm | Male | 3.88±0.07 | 3.66±0.22 | 3.79±0.05 | 3.74±0.19 | 4.21±0.26 | 3.68±0.08* | 4.34±0.13 | 3.97±0.19 |
| Female | 3.76±0.08 | 3.42±0.21* | 3.81±0.13 | 3.40±0.16* | 4.48±0.12 | 3.27±0.27* | 4.26±0.18 | 3.84±0.16#* | |
| IVSs, mm | Male | 1.22±0.03 | 1.21±0.05 | 1.25±0.07 | 1.41±0.07 | 1.23±0.02 | 1.39±0.04* | 1.37±0.04 | 1.46±0.05* |
| Female | 1.02±0.03 | 1.14±0.08 | 1.13±0.05 | 1.22±0.08 | 1.24±0.02 | 1.36±0.05 | 1.31±0.07 | 1.34±0.06 | |
| IVSd, mm | Male | 0.88±0.02 | 0.92±0.05 | 0.88±0.04 | 1.00±0.06 | 0.93±0.03 | 1.00±0.03 | 0.97±0.03 | 1.09±0.06 |
| Female | 0.78±0.02 | 0.88±0.07 | 0.80±0.04 | 0.88±0.03 | 0.79±0.01 | 1.04±0.04* | 0.86±0.04 | 0.93±0.03# | |
EF, Ejection fraction; FS, fractional shortening; LV, left ventricle; LVs, LV volume at the end of systole; LVd, LV volume at the end of diastole; LVPWs, LV posterior wall thickness at the end of systole; LVPWd, LV posterior wall thickness at the end of diastole; LVIDs, LV internal diameter at the end of systole; LVIDd, LV internal diameter at the end of diastole; IVSs, Interventricular septum at the end of systole; IVSd, Interventricular septum at the end of diastole. Data represents the mean ± SEM of 5 control mice and 8 vehicle- or metformin-treated mice for each gender and age group.
P < 0.05 vs baseline echocardiographic values for age and gender related animal;
P<0.05 vs vehicle-treated group of the same age and gender.
3.4. Age-dependent myocardial pathological changes
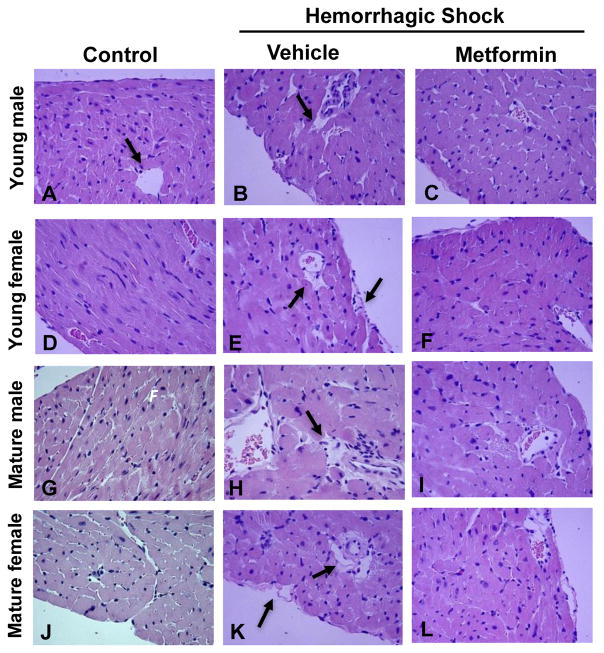
At 3 hours after resuscitation, histological analysis revealed that vehicle-treated mature mice of both genders had more severe myocardial injury, which was characterized by a remarkable perivascular, endocardial and pericardial edema, when compared with young animals. Treatment with metformin ameliorated myocardial damage in both mature and young mice (Fig. 3).
Fig. 3.
Representative histology photomicrographs of heart sections. Normal myocardium architecture in control young male (A) and female (D) and mature male (G) and female mice (J). Myocardial damage in vehicle-treated young male (B) and female (E) and mature male (H) and females (K) after hemorrhagic shock presented with perivascular and endocardial edema (arrows). Significant amelioration of heart architecture in metformin-treated young (C and F) and mature mice (I and L). Magnification x400.
3.5. Age-dependent and gender-dependent changes of cardiovascular disease biomarkers
To evaluate the hemorrhage-induced cardiovascular inflammatory response, a panel of cardiovascular injury markers was measured. At 3 hours after resuscitation, plasma levels of follistatin were significantly increased in all vehicle-treated groups when compared to age- and gender-matched controls; however, the magnitude of elevation was higher in vehicle-treated mature male mice when compared to vehicle-treated young mice and mature females (Fig. 4A). Metformin treatment significantly decreased levels of follistatin in mature groups and left it unchanged in young animals of both genders. Plasma levels of endocan-1 were significantly increased in vehicle-treated mature, but not in young, male mice when compared to age-matched controls. There was no elevation of endocan-1 after hemorrhagic shock in any vehicle-treated female groups. Treatment with metformin did not reduce endocan-1 levels (Fig. 4B). Plasma levels of the chemokine CXCL16 was significantly increased after hemorrhagic shock in all four vehicle-treated groups compared to age-matched controls; however, levels of CXCL16 were lower in vehicle-treated mature female mice when compared to the age-matched male mice. Treatment with metformin significantly reduced the chemokine levels in male mature animals only (Fig. 4C).
Fig. 4.
Effect of in vivo treatment with metformin on plasma levels of follistatin (A), endocan-1 (B) and CXCL16 (C). Data represents the mean ± SEM of 5 control mice, 10 vehicle- or metformin-treated mice for each gender and age group. *P<0.05 vs baseline values of age- and gender-matched control mice; ‡P<0.05 vs young group of the same gender; #P<0.05 vs vehicle-treated group of the same age; ΩP<0.05 vs age-matched male mice.
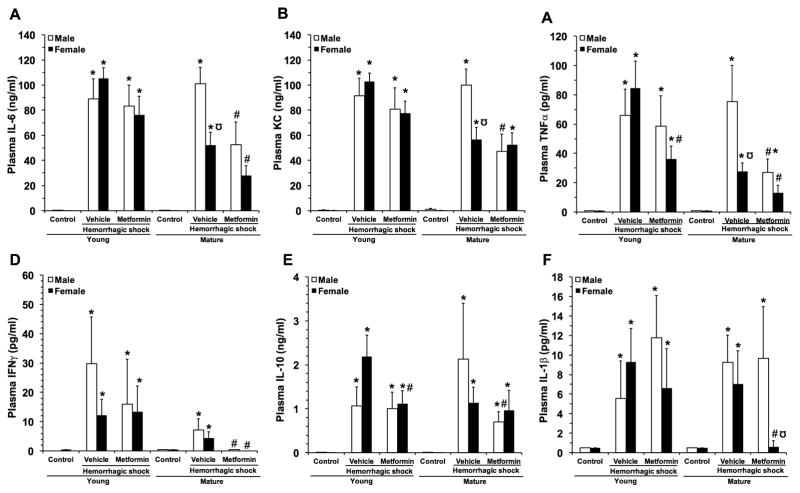
3.6. Age- and gender-dependent effect of metformin on plasma cytokines
To assess extent of the systemic inflammatory response induced by hemorrhagic shock, a panel of plasma cytokines was measured (Fig. 5). At 3 hours after resuscitation, plasma levels of IL-6, KC, TNF-α, IFN-γ, IL-10, IL-1 were significantly increased in all four vehicle-treated groups of animals. Interestingly, IL-6, KC and TNF-α levels were higher in mature male group in comparison with mature female animals. Metformin treatment significantly decreased levels of IL-6, TNF-α, IFN-γ, in both genders of mature mice, KC in mature males and IL-10 in mature females only. In young female animals, metformin had only effect on decreasing levels of KC, IL-10 and TNF-α without affecting young males.
Fig. 5.
Effect of in vivo treatment with metformin on plasma levels of IL-6 (A), KC (B), TNFα (C), IFNγ (D), IL-10 (E) and IL-1β (F). Data represents the mean ± SEM of 5 control mice and 10 vehicle- or metformin-treated mice for each gender and age group. *P<0.05 vs baseline values of age- and gender-matched control mice; ‡P<0.05 vs young group of the same gender; #P<0.05 vs vehicle-treated group of the same age; ΩP<0.05 vs age-matched male mice.
3.7. Age-dependent neutrophil infiltration in liver and lung
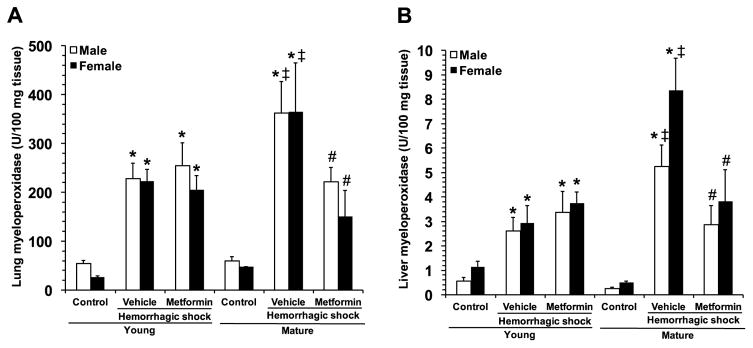
To evaluate injury in other major organs, we measured MPO activity, as an index of neutrophil infiltration. MPO activity increased in lung and liver in all vehicle-treated groups after hemorrhagic shock when compared to gender- and age-matched controls (Fig. 6). However, vehicle-treated mature male and female animals had higher lung neutrophil infiltration after hemorrhagic shock when compared with vehicle-treated young mice. Metformin treatment significantly decreased MPO activity compared to vehicle treatment in mature mice of both genders but not in young mice.
Fig. 6.
Myeloperoxidase activity in lungs and liver. Data represents the mean ± SEM of 5 control mice and 10 vehicle- or metformin-treated mice for each gender and age group. *P<0.05 vs baseline values of age- and gender-matched control mice; ‡P<0.05 vs young group of the same gender; #P<0.05 vs vehicle-treated group of the same age.
3.8. Age- and gender-dependent activation of AMPKα
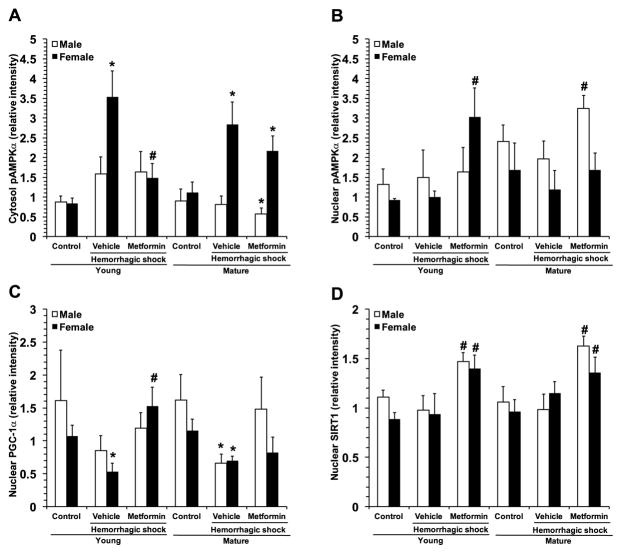
Because of the central role of AMPK in metabolic regulation and its potential impact on inflammatory pathways, we evaluated the phosphorylation of the catalytic α-subunit in the heart and the nuclear translocation of its substrate, the PGC-1α (Fig. 7). After hemorrhagic shock, pAMPKα tended to increase in both cytosol and nuclear compartments in vehicle-treated young male, but not in mature adult mice, where instead a slight decline of the active protein was observed when compared to basal levels of age-matched control mice (Fig. 7A and B). This event correlated with a significant nuclear downregulation of PGC-1α in vehicle-treated mature mice (Fig. 7C). Interestingly, in the female group, pAMPKα was significantly elevated in the cytosol, but not the nuclear compartment, whereas nuclear PGC-1α was significantly downregulated in both vehicle-treated young and mature mice (Fig. 7). Treatment with metformin did not affect cytosol or nuclear activation of AMPK in the young male mice; however, it favored translocation of pAMPKα from the cytosol to nucleus in male mature mice. In the young female group, treatment with metformin significantly reduced pAMPKα in the cytosol, while increasing levels in the nucleus. In the female mature group, treatment with metformin slightly but not significantly reduced pAMPKα in the cytosol, but restored levels in the nucleus, which were maintained similarly to basal levels of age-matched controls (Fig. 7A and B). Treatment with metformin restored PGC-1α nuclear translocation to basal levels in young and mature adult mice of both genders (Fig. 7C).
Fig. 7.
Image analyses of expression of cytosolic pAMPKα (A), nuclear pAMPKα (B), nuclear PGC-1α (C) and nuclear SIRT1 (D). Data represents the mean ± SEM of 4 control mice and 6 vehicle- or metformin-treated mice for each gender and age group. *P<0.05 vs baseline values of age- and gender-matched control mice; #P<0.05 vs vehicle-treated group of the same age.
3.9. Age- and gender-dependent nuclear translocation of SIRT1
Since sirtuin 1 (SIRT1) may participate in activating PGC-1α (26), we evaluated the nuclear translocation of this deacetylase (Fig. 7D). There were no changes in nuclear SIRT1 expression after hemorrhagic shock in vehicle-treated mice of either age or gender when compared to baseline control levels. Treatment with metformin significantly increased SIRT1 nuclear translocation in male and female mice of both age groups when compared with vehicle treatment (Fig. 7D).
3.10. Effect of metformin treatment on NF-κB activation and PPARγ activation in the heart
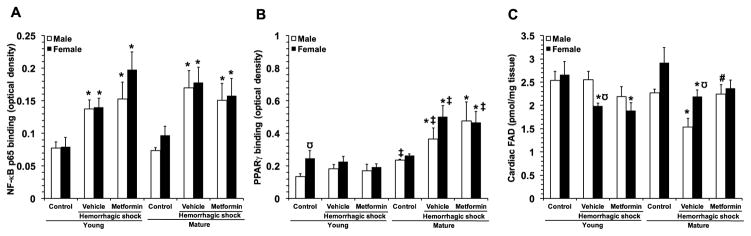
Since synthesis of pro-inflammatory cytokines is regulated by NF-κB transcription [27] we determined the effect of metformin on the activity of this pro-inflammatory nuclear transcription factor. At 3 hours after resuscitation NF-κB was significantly increased in both genders and age groups when compared to matched controls. Treatment with metformin did not affect NF-κB activation in any groups of animals (Fig. 8A). Since the nuclear receptor PPARγ has been shown to be a key transcriptional factor in regulating oxidative metabolism as well as inflammation [28], we also determined the effect of metformin on the activity of this nuclear receptor. In the young groups, there were no changes in PPARγ DNA binding activity after hemorrhagic shock in vehicle-treated mice of either gender when compared to baseline control levels; however, young control female mice demonstrated higher basal levels than age-matched male control mice. Treatment with metformin did not affect PPARγ activation in the young groups. Interestingly, in the mature groups, PPARγ activity significantly increased in vehicle-treated mice of both genders, when compared to basal levels of age-matched control mice. Treatment with metformin did not affect activation of PPARγ activation in mature mice of either gender (Fig. 8B).
Fig 8.
Activity of the p65 subunit of NF-κB (A) and PPARγ (B) in cardiac nuclear extracts at 3 hours after resuscitation. Data represents the mean ± SEM of 3 control mice and 6 vehicle- or metformin-treated mice for each gender and age group. (C) Cardiac content of FAD. Data represents the mean ± SEM of 4 control mice and 7 vehicle- or metformin-treated mice for each gender and age group. *P<0.05 vs baseline values of age- and gender-matched control mice; ‡P<0.05 vs young group of the same gender; #P<0.05 vs vehicle-treated group of the same age; Ω P<0.05 vs age-matched male mice.
3.9. Effect of metformin on cardiac levels of FAD
Since metformin has been shown to interfere directly with the mitochondrial electron transport chain [29–30], we determined the effect of metformin on the cardiac levels of the complex II redox co-factor FAD. In the young groups, there was a significant reduction in FAD levels after hemorrhagic shock in vehicle-treated female, but not male mice when compared to age-matched control mice. Treatment with metformin did not affect FAD content in the young groups. Interestingly, in the mature groups, FAD content significantly decreased in vehicle-treated mice of both genders, when compared to basal levels of age-matched control mice; however, vehicle-treated female mice demonstrated higher FAD content than mature male mice at 3 hours after resuscitation. Treatment with metformin restored FAD content at baseline values
3.11. Effect of metformin on survival
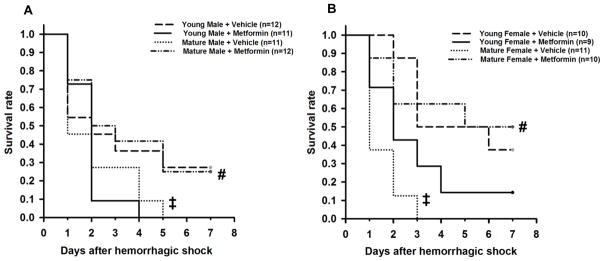
To evaluate the therapeutic potential of metformin, we tested the effect of the drug on survival up to 7 days after hemorrhagic shock (Fig. 9). Vehicle-treated mature animals of both genders had higher mortality rate by 7 days when compared with the gender-matched young groups. Treatment with metformin significantly ameliorated survival only in mature groups of both genders. Interestingly, in the young male, but not female mice, treatment with metformin significantly reduced survival rate when compared with vehicle treatment (P=0.037 at χ2 test).
Fig. 9.
Effect of metformin on survival rates of young and mature male and female mice subjected to hemorrhagic shock. Vehicle or metformin (100 mg/kg intra-arterially) was administered as single bolus at the time of resuscitation. ‡P<0.05 vs young group of the same gender; #P<0.05 vs vehicle-treated group of the same age.
4. Discussion
Although not described in pediatric population, gender dimorphism in multiple organ failure incidence and related death has been observed after trauma in adult populations [8–12,31]. Among the adult population, aging also strongly correlates with high morbidity and mortality and appears to be a risk factor for poor outcomes independently of injury severity after severe trauma with hemorrhagic shock [2–5,32].
We have previously reported that in murine models of hemorrhagic shock the degree of severity and pathophysiologic characteristics of myocardial and lung injury are age-dependent [20,21]. In this study, we have extended our investigation to gender-related responses and we have demonstrated that male gender is also a variable for heightened cardiovascular inflammation and injury. Interestingly, this gender dimorphism was only evident in middle-aged mature adult animals. We also demonstrated that treatment with the AMPK activator metformin provided hemodynamic stability, a significant improvement of myocardial damage, reduction of circulating biomarkers of cardiovascular injury as well as amelioration of survival. However, metformin was ineffective in improving survival in young mice. Surprisingly, it appeared that metformin could potentially be detrimental in young male mice.
Maladaptive decompensatory hypotension after severe hemorrhage is a deleterious event that leads to insufficient oxygen delivery to organs contributing to multiple organ failure and cardiovascular shock [33]. In our study, in order to avoid differences in hypoperfusion we adopted a model of pressure-controlled hemorrhage, which is associated with severe inflammation and organ injury in rodents [22]. We observed that all experimental groups experienced a similar degree of hypoperfusion, as confirmed by similar changes in blood levels of lactate, a reliable biomarker of tissue hypoxia and anaerobic metabolism [34]. Furthermore, all experimental groups experienced a similar systemic inflammation, as confirmed by similar changes in plasma levels of cytokines. Despite this, we observed that early hemodynamic compensatory responses after blood and fluid resuscitation were efficient in the young animals only and were not influenced by the gender. On the contrary, only vehicle-treated mature animals of both genders experienced severe hypotension and alterations of echocardiography parameters, thus suggesting an age-related but gender-independent decline of the cardiac reserve. The age-dependent cardiac dysfunction was characterized by specific decrease in LV volumes and dimensions, as well as increase of thickness of the LVPW. This latter change well paralleled with the age-dependent severe perivascular edema in the myocardium at histological analysis. Edema of the myocardium extending to the endocardium is an early event of ischemia and reperfusion injury in humans [35]. These pathophysiological changes are well consistent with the occurrence of vasoplegia, third spacing and capillary leak [36]. Of clinical importance, metformin provided significant improvement of these hemodynamic parameters in mature animals of both genders, thus suggesting the re-establishment of preload and effective circulatory volume and the decrease of myocardial swelling. Surprisingly, we observed distinct gender-related differences in cardiovascular injury biomarkers and pro-inflammatory cytokines between vehicle-treated mature male and female mice after hemorrhagic shock. For example, IL-6, KC and TNFα, but not IL-10, IL-1β and IFNγ, were significantly higher in the mature male mice than the mature female animals. Similarly, follistatin, endocan-1 and CXCL16 were significantly higher in the mature male mice than female mice. Follistatin is a cardiokine induced in response to ischemic insults and has been shown to promote myocardial hypertrophy and heart failure [37]. Endocan-1 is a proteoglycan secreted by the vascular endothelium in response to inflammatory cytokines and well reflects endothelial dysfunction [38]. CXCL16 is a chemokine secreted by immune-competent cells and involved in neutrophil recruitment to injured vascular wall [39]. The young group of both genders also experienced some cardiac injury; however, at lower degree than the mature mice, as demonstrated by the lower elevation of follistatin and endocan-1. Thus altogether, our results provide further evidence that age is a risk factor of the hemodynamic instability in hemorrhagic shock, further supporting the clinical findings observed in humans older than 55 years old [2–4,32]. Our data also suggest that female gender in mature age may be a protective factor against hemorrhage-induced cardiovascular inflammation and remodeling and may involve diverse immune responses Interestingly, treatment with metformin attenuated this inflammatory response, but the anti-inflammatory effects were more prominent in the female mature mice than the male group. Furthermore, these gender-dependent changes appeared specific for the cardiovascular system since lung and liver injury, as assessed by neutrophil infiltration, appeared affected by age only.
In evaluating the molecular mechanisms of metformin, we investigated the contribution of AMPK signaling pathway. AMPK is a serine/threonine protein kinase, which serves as a cellular energy sensor, regulating ATP levels through activation of catabolic and inhibition of anabolic processes, including mitochondrial biogenesis. It consists of three subunits: one catalytic α-subunit, which is activated by phosphorylation, and two regulatory β- andγ subunits [14,16,40]. Consistent with our previous findings [20], we observed that there was an age-dependent dysregulation of AMPK activation in the cytosol and nuclear compartments in hearts of male mice after hemorrhagic shock. On the contrary, in female mice of both ages pAMPKα was significantly elevated in the cytosol, but not the nuclear compartment. In both genders, however, there was a reduction of PGC-1α nuclear translocation. Treatment with metformin did not cause any further increase of pAMPK, but induced only a nucleo-cytoplasmatic redistribution and promoted nuclear translocation of PGC-1α, thus also suggesting maintenance of mitochondrial quality control.
An intriguing finding of our study was that metformin did not afford any beneficial effects in the young groups of animals subjected to hemorrhagic shock. These data are in apparent discrepancy with our previous data demonstrating that another AMPK activator, AICAR, was able to provide beneficial effects in both young and adult male mice [20,21]. It must be noted, however, that the molecular mechanisms of metformin may extend beyond the activation of the AMPK metabolic pathway. For example, there is extensive evidence that SIRT1 shares several targets with AMPK and may increase the nuclear PGC-1α activity by deacetylation [16,26]. In our study, metformin treatment increased SIRT1 expression in all experimental groups, thus ruling out any age or gender specific effect of metformin on SIRT1 nuclear translocation.
One mechanism by which PGC1α modulates mitochondrial function is through interaction with transcriptional factors, including PPARγ, that directly controls downstream target genes [28,41]. We have previously demonstrated that PPARγ plays an important protective role in hemorrhagic shock, since PPARγ ligands are able to reduce organ injury and improve cardiovascular function in male rodents [42–44]. In the present study, we found that PPARγ activity was upregulated in an age-dependent, but not gender-dependent manner after hemorrhagic shock. However, treatment with metformin had no effect on PPARγ activation.
Several studies have also demonstrated that metformin can inhibit the inflammatory response by inhibition of NF-κB [45,46], which is a key regulator of the inflammatory response [27]. In our study, we observed that NF-κB activity similarly increased in the heart of all vehicle-treated groups after hemorrhagic shock in a gender and age-independent manner. Interestingly, treatment with metformin did not affect NF-κB activation in the heart of any groups. Although we could not find any effect of metformin on NF-κB or PPARγ activation, we cannot rule out other potential anti-inflammatory effects of the drug. For example, it has been recently reported that metformin may directly bind the pro-inflammatory alarmin high mobility group box 1, thus neutralizing the deleterious effects of this alarmin [47]. Therefore, further investigation is warranted to understand the distinct age-dependent and gender-dependent anti-inflammatory mechanisms of the drug.
Several studies have reported that metformin selectively blocks the reverse electron flow through the electron respiratory chain Complex I, without affecting the downstream oxidative phosphorylation machinery [29,30]. This inhibitor effect on complex I has been associated with potential redirection of substrate flux feeding into complex II and increased mitochondrial function in conditions of stress [48]. In our study, we observed gender differences in complex II oxidative capacity only in the young groups, since female mice exhibited lower cardiac levels of the complex II co-factor FAD than young males after hemorrhagic shock. Surprisingly with aging, male mice demonstrated a greater sensitivity to hemorrhage-induced mitochondrial dysfunction compared to females. Treatment with metformin restored cardiac levels of FAD in both male and female mature mice, thus supporting the hypothesis that metformin can shift oxidative metabolism in the electron respiratory chain for energy homeostasis. Furthermore, inhibition of complex I by metformin has also been associated with reduced production of mitochondrial reactive oxygen species, which are potent mediators of cell injury [29], especially in conditions of aging [49]. Therefore, this mechanism could also explain the beneficial effects of metformin in mature, but not young mice, in our model. Taken together, our data further support that maintenance of AMPK activation and mitochondrial function during stress well associates with hemodynamic stability and anti-inflammatory effects in aging animals in shock.
Another interesting finding of our study is that gender-related differences were found in 9–12 months old mice only. At this age, both male and female mice are considered retired breeders, which have passed their peak reproductive age [50]. Therefore, their comparison with a younger group of mice (3–5 months old) may be more relevant to gender differences found in epidemiological studies with patient populations >50 years old. Several clinical and experimental studies support the hypothesis that estrogen may play a protective role in trauma and hemorrhagic shock and may explain the gender differences observed in patients and animal models [8,9,12,33,51]. In our study, the presence of gender-difference in a mature middle-age group of mice most probably with declining levels of estrogens, and, on the contrary, the absence of gender-difference in a young group of mice at full peak of sexual maturation would argue against the involvement of sex hormones. The possible role of estrogens and other sex hormones in the cardiovascular protective mechanisms of metformin certainly needs further investigation.
In conclusion, our data suggest that during hemorrhagic shock cardiovascular injury manifests with age- and gender-dependent characteristics and severity. Despite these age- and gender-dependent differences, metformin may represent a therapeutic strategy in an aging population. Whether the cardiovascular protective effects of metformin are secondary to activation of restorative metabolic pathways or interference with sex hormones needs to be further investigated.
HIGHLIGHTS.
Severity of multiple organ failure and cardiovascular injury during hemorrhagic shock is age- and gender-dependent.
Metformin, an activator of AMP-activated kinase, improves hemodynamic recovery in aging male and female mice after hemorrhage.
Specific cardioprotective and anti-inflammatory effects of metformin are gender-dependent.
Acknowledgments
Funding Support: This work was supported by grants from the National Institutes of Health (NIH R01 GM-067202 and R01 GM-115973 to B.Z.).
This work was supported by grants from the National Institutes of Health (NIH) (R01 GM-067202 and R01 GM-115973) to Basilia Zingarelli.
ABBREVIATIONS
- AMPK
AMP-activated protein kinase
- ANOVA
one-way analysis of variance
- EF
ejection fraction
- FAD
flavin adenine dinucleotide
- FAD
flavin adenine dinucleotide dehydrogenase
- FS
fractional shortening
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HR
heart rate
- IA
intra-arterially
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-10
interleukin-10
- IVSd
interventricular septal thickness in diastole
- IVSs
interventricular septal thickness in systole
- KC
keratinocyte chemoattractant chemokine
- LVd
end-diastolic left ventricle volume
- LVs
end-systolic left ventricle volume
- LVIDd
end- diastolic left ventricle internal dimension
- LVIDs
end-systolic left ventricle internal dimension
- LVPWd
left ventricle posterior wall thickness in diastole
- LVPWs
left ventricle posterior wall thickness in systole
- MPO
myeloperoxidase
- MABP
Mean arterial blood pressure
- NF-κB
nuclear factor-κB
- PGC-1α
peroxisome proliferator-activated receptor-γ co-activator α
- PPARγ
peroxisome proliferator-activated receptor-γ
- SIRT1
sirtuin-1
- TNF-α
tumor necrosis factor-α
Footnotes
Conflict of interest: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mitchell RJ, Curtis K, Chong S, Holland AJ, Soundappan SV, Wilson KL, Cass DT. Comparative analysis of trends in paediatric trauma outcomes in New South Wales, Australia. Injury. 2013;44(1):97–103. doi: 10.1016/j.injury.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Curtis KA, Mitchell RJ, Chong SS, Balogh ZJ, Reed DJ, Clark PT, D’Amours S, Black DA, Langcake ME, Taylor CB, McDougall P, Cameron PA. Injury trends and mortality in adult patients with major trauma in New South Wales. Med J Aust. 2012;197(4):233–237. doi: 10.5694/mja11.11351. [DOI] [PubMed] [Google Scholar]
- 3.Sammy I, Lecky F, Sutton A, Leaviss J, O’Cathain A. Factors affecting mortality in older trauma patients-A systematic review and meta-analysis. Injury. 2016;47(6):1170–1183. doi: 10.1016/j.injury.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129(1):39–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 5.Vanzant EL, Hilton RE, Lopez CM, Zhang J, Ungaro RF, Gentile LF, Szpila BE, Maier RV, Cuschieri J, Bihorac A, Leeuwenburgh C, Moore FA, Baker HV, Moldawer LL, Brakenridge SC, Efron PA Inflammation and Host Response to Injury Investigators. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care. 2015;19(1):77. doi: 10.1186/s13054-015-0788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folkow B, Svanborg A. Physiology of cardiovascular aging. Physiol Rev. 1993;73(4):725–764. doi: 10.1152/physrev.1993.73.4.725. [DOI] [PubMed] [Google Scholar]
- 7.Böning A, Rohrbach S, Kohlhepp L, Heep M, Hagmüller S, Niemann B, Mühlfeld C. Differences in ischemic damage between young and old hearts--Effects of blood cardioplegia. Exp Gerontol. 2015;67:3–8. doi: 10.1016/j.exger.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Choudhry MA, Bland KI, Chaudry IH. Gender and susceptibility to sepsis following trauma. Endocr Metab Immune Disord Drug Targets. 2006;6(2):127–135. doi: 10.1016/j.injury.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Childs EW, Tharakan B, Hunter FA, Smythe WR. 17beta-estradiol mediated protection against vascular leak after hemorrhagic shock: role of estrogen receptors and apoptotic signaling. Shock. 2010;34(3):229–235. doi: 10.1097/SHK.0b013e3181d75b50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majetschak M, Christensen B, Obertacke U, Waydhas C, Schindler AE, Nast-Kolb D, Schade FU. Sex differences in posttraumatic cytokine release of endotoxin-stimulated whole blood: relationship to the development of severe sepsis. J Trauma. 2000;48(5):832–840. doi: 10.1097/00005373-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Kamran M, Kamal E, Momen M. Association of gender with outcomes in critically ill patients. Crit Care. 2012;16(3):R92. doi: 10.1186/CC11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48(5):932–937. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115(3):547–555. doi: 10.1172/JCI24405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carling D, Viollet B. Beyond energy homeostasis: the expanding role of AMP-activated protein kinase in regulating metabolism. Cell Metab. 2015;21(6):799–804. doi: 10.1016/j.cmet.2015.05.005. doi.org/10.1016/j.cmet.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci USA. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantó C, Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5(2):151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J. AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell. 2010;9(4):592–606. doi: 10.1111/j.1474-9726.2010.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benoit V, Bruno G, Nieves S, Jocelyne L, Marc F, Fabrizio A. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122(6):253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsiukevich D, Piraino G, Klingbeil LR, Hake PW, Wolfe V, O’Connor M, Zingarelli B. The AMPK activator Aicar ameliorates age-dependent myocardial injury in murine hemorrhagic shock. Shock. 2017;47(1):70–78. doi: 10.1097/SHK.0000000000000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klingbeil LR, Kim P, Piraino G, O’Connor M, Hake PW, Wolfe V, Zingarelli B. Age-dependent changes of AMPK metabolic pathways in the lung in a mouse model of hemorrhagic shock. Am J Respir Cell Mol Biol. 2017 Jan 13; doi: 10.1165/rcmb.2016-0118OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majde JA. Animal models for hemorrhage and resuscitation research. J Trauma. 2003;54(5 Suppl):S100–S105. doi: 10.1097/01.TA.0000064503.24416.F4. [DOI] [PubMed] [Google Scholar]
- 23.Wilcock C, Bailey CJ. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica. 1994;24(1):49–57. doi: 10.3109/00498259409043220. [DOI] [PubMed] [Google Scholar]
- 24.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, Khuchua Z. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem. 2011;286(2):899–908. doi: 10.1074/jbc.M110.171439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14(3):157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 26.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 27.Zingarelli B, Sheehan M, Wong HR. Nuclear factor-κB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31(1 Suppl):S105–S111. doi: 10.1097/01.CCM.0000042465.21096.B5. [DOI] [PubMed] [Google Scholar]
- 28.Zingarelli B, Cook JA. Peroxisome proliferator-activated-receptor-γ is a new therapeutic target in sepsis and inflammation. Shock. 2005;23(5):393–399. doi: 10.1097/01.shk.0000160521.91363.88. [DOI] [PubMed] [Google Scholar]
- 29.Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38(1):33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- 30.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez MC, Efron PA, Ozrazgat-Baslanti T, Zhang J, Cuschieri J, Maier RV, Minei JP, Baker HV, Moore FA, Moldawer LL, Brakenridge SC. Sex-based differences in the genomic response, innate immunity, organ dysfunction, and clinical outcomes after severe blunt traumatic injury and hemorrhagic shock. J Trauma Acute Care Surg. 2016;81(3):478–485. doi: 10.1097/TA.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauaia A, Moore EE, Johnson JL, Chin TL, Banerjee A, Sperry JL, Maier RV, Burlew CC. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. J Trauma Acute Care Surg. 2014;76(3):582–592. doi: 10.1097/TA.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: latest results in hemorrhagic shock. Crit Care. 2008;12(4):218. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caputo ND, Kanter M, Fraser R, Simon R. Comparing biomarkers of traumatic shock: the utility of anion gap, base excess, and serum lacate in the ED. Am J Emerg Med. 2015;33(9):1134–1139. doi: 10.1016/j.ajem.2015.04.085. [DOI] [PubMed] [Google Scholar]
- 35.Ota S, Tanimoto T, Hirata K, Orii M, Shiono Y, Shimamura K, Ishibashi K, Yamano T, Ino Y, Kitabata H, Yamaguchi T, Kubo T, Imanishi T, Akasaka T. Assessment of circumferential endocardial extent of myocardial edema and infarction in patients with reperfused acute myocardial infarction: a cardiovascular magnetic resonance study. Int Heart J. 2014;55(3):234–238. doi: 10.1536/ihj.13-297. doi.org/10.1536/ihj.13-297. [DOI] [PubMed] [Google Scholar]
- 36.Herget-Rosenthal S, Saner F, Chawla LS. Approach to hemodynamic shock and vasopressors. Clin J Am Soc Nephrol. 2008;3(2):546–553. doi: 10.2215/CJN.01820407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masayuki S, Noriyuki O. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Cell Biology. 2011;108(43):899–906. doi: 10.1073/pnas.1108559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox LA, van Eijk LT, Ramakers BP, Dorresteijn MJ, Gerretsen J, Kox M, Picckers P. Inflammation-induced increases in plasma endocan levels are associated with endothelial dysfunction in humans in vivo. Shock. 2015;43(4):322–326. doi: 10.1097/SHK.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 39.Ueland T, Gullestad L, Nymo SH, Yndestad A, Aukrust P, Askevold ET. Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta. 2015;443:71–77. doi: 10.1016/j.cca.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Arad M, Seidman CE. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100(4):474–488. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 41.Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res. 2010;107(7):825–38. doi: 10.1161/CIRCRESAHA.110.223818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zingarelli B, Hake PW, O’Connor M, Burroughs TJ, Wong HR, Solomkin JS, Lentsch AB. Lung injury after hemorrhage is age dependent: role of peroxisome proliferator-activated receptor gamma. Crit Care Med. 2009;37(6):1978–1987. doi: 10.1097/CCM.0b013e31819feb4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chima RS, Hake PW, Piraino G, Mangeshkar P, Denenberg A, Zingarelli B. Ciglitazone ameliorates lung inflammation by modulating the inhibitor kappaB protein kinase/nuclear factor-kB pathway after hemorrhagic shock. Crit Care Med. 2008;36(10):2849–2857. doi: 10.1097/ccm.0b013e318187810e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zingarelli B, Chima RS, O’Connor M, Piraino G, Denenberg A, Hake PW. Liver apoptosis is age dependent and is reduced by activation of peroxisome proliferator-activated receptor-γ in hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2010;298(1):G133–G141. doi: 10.1152/ajpgi.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schönbeck U, Libby P. Metformin inhibits proinflammatory responses and nuclear factor-κB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26(3):611–617. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- 46.Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med. 2011;89(7):667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horiuchi T, Sakata N, Narumi Y, Kimura T, Hayashi T, Nagano K, Liu K, Nishibori M, Tsukita S, Yamada T, Katagiri H, Shirakawa R, Horiuchi H. Metformin directly binds the alarmin HMGB1 and inhibits its proinflammatory activity. J Biol Chem. 2017 doi: 10.1074/jbc.M116.769380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kristensen JM, Larsen S, Helge JW, Dela F, Wojtaszewski JF. Two weeks of metformin treatment enhances mitochondrial respiration in skeletal muscle of AMPK kinase dead but not wild type mice. PLOS One. 2013;8(1):e53533. doi: 10.1371/journal.pone.0053533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 2005;19(3):419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- 50.Königsberg M, López-Diazguerrero NE, Rivera-Martinez LP, González-Puertos VY, González-Vieira R, Gutiérrez-Ruiz MC, Zentella A. Physiological deterioration associated with breeding in female mice: a model for the study of senescence and aging. Comp Biochem Physiol A Mol Integr Physiol. 2007;146(4):695–701. doi: 10.1016/j.cbpa.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Yu HP, Chaudry IH. The role of estrogen and receptor agonists in maintaining organ function after trauma-hemorrhage. Shock. 2009;31(3):227–237. doi: 10.1097/SHK.0b013e31818347e7. [DOI] [PMC free article] [PubMed] [Google Scholar]