Abstract
The purpose of this meta-analysis was to examine the effects of yoga for glycemic control among adults with type 2 diabetes (T2DM). Comprehensive electronic databases searches located 2559 unique studies with relevant key terms. Studies were included if they (1) evaluated a yoga intervention to promote T2DM management, (2) used a comparison group, (3) reported an objective measure of glycemic control at post-intervention, and (4) had follow-up length or post-test of at least 8 weeks from baseline. Independent raters coded participant, design and methodological characteristics and intervention content. Summary effect sizes and 95% confidence intervals (CI) were calculated. Twenty-three studies with 2473 participants (mean age = 53 years; 43% women) met eligibility criteria. Compared with controls, yoga participants were successful in improving their HbA1c (d + = 0.36, 95% CI = 0.16, 0.56; k = 16), FBG (d+ = 0.58, 95% CI = 0.40, 0.76; k = 20), and PPBG (d + = 0.40, 95% CI = 0.23, 0.56; k = 14). Yoga was also associated with significant improvements in lipid profile, blood pressure, body mass index, waist/hip ratio and cortisol levels. Overall, studies satisfied an average of 41% of the methodological quality (MQ) criteria; MQ score was not associated with any outcome (Ps > 0.05). Yoga improved glycemic outcomes and other risk factors for complications in adults with T2DM relative to a control condition. Additional studies with longer follow-ups are needed to determine the long-term efficacy of yoga for adults with T2DM.
Keywords: Meta-analysis, Yoga, Diabetes, Review
1. Introduction
About one out of every eleven adults in the United States currently has diabetes (Center for Disease Control and Prevention, 2016). Type 2 diabetes (T2DM) accounts for 90–95% of all diabetes cases in adults. Diabetes is a major risk factor for heart disease and stroke and is the seventh leading cause of death in the United States (Center for Disease Control and Prevention, 2011). In 2012, the total estimated economic cost of diagnosed diabetes was $245 billion, a 41% increase from 2007 (American Diabetes Association, 2013a).
Controlling blood glucose level is fundamental to the management of T2DM (American Diabetes Association, 2013b). Improved glycemic control is associated with a significant decrease in long-term complications (Skyler et al., 2009; Stettler et al., 2006; UKPDS, 1998). Often pharmacological treatment alone is insufficient to achieve glycemic control; adherence to dietary and physical activity recommendations is advised (Dyson et al., 2011; Knutson et al., 2006; Surwit et al., 2002). However, these lifestyle changes are difficult to achieve and maintain (Kim et al., 2013). One-third of diabetic patients use some type of complementary or alternative medicine (CAM) therapy, and about 3–20% use CAM specifically to treat their diabetes (Bell et al., 2006; Nahin et al., 2012). Yoga, an ancient Indian practice with over 20 million users, is one of the most common CAM therapies used among adults in the United States (Clarke et al., 2015).
Recent studies have demonstrated that yoga improves a variety of symptoms along with physical functioning, depression, neurocognitive functions, and quality of life (D’Silva et al., 2012; Froeliger et al., 2012; Patel et al., 2012; Shapiro et al., 2007). Yoga has received considerable attention in cancer research as an approach for improving quality of life (Levine and Balk, 2012). Studies in cardiac patients have found similar positive effects including reduced blood pressure, cholesterol and body weight (Mamtani and Mamtani, 2005; Okonta, 2012). The benefit of yoga for diabetes management has also been found in recent reviews. Innes and Selfe (2016) showed that yoga may improve glycemic control, lipid levels, and body composition (weight, body mass index) among adults with T2DM. Similarly, Cui et al.’ (2016) meta-analysis reported a pooled weighted mean difference of −23.72 mg/dL (95% CI = −37.78, −9.65) for fasting blood glucose (FBG) and −0.47% (95% CI = −0.87, −0.07) for HbA1c. In another meta-analysis, Kumar et al. (2016) reported beneficial effects of yoga in comparison to standard treatment alone for FBG [Standardized Mean Difference (SMD) −1.40, 95%CI = −1.90, − 0.90] and for HbA1c [SMD −0.64, 95%CI = −0.97, −0.30]. However, this meta-analysis included studies with short follow-up duration (i.e., 40 days). Since HbA1c reflects the average glycemia over the preceding 8–12 weeks (American Diabetes Association, 2016; Nathan et al., 2007), short follow-up duration is insufficient to estimate changes among intervention participants. Furthermore, the authors only examined glycemic parameters. The authors did perform subgroup analysis based on difference in intervention (i.e. breathing practice alone or combination of asanas, breathing and meditation), but no other intervention or sample characteristics were examined as moderators of intervention effect. Finally, a recently published meta-analysis by Vizcaino and Stover (2016) examined lipid profile and blood pressure in addition to the glycemic parameters. The authors found significant decreases in FBG for participants in the yoga condition compared controls (mean difference = −25.72 mg/dL, 95% CI = −40.67, −10.76), but no significant differences for HbA1c and postprandial blood glucose (PPBG). This meta-analysis did not control for the baseline values in their analyses which may have biased the findings.
The purpose of this systematic review and meta-analysis is to examine current evidence on the effect of yoga for diabetes management. Our review updates and extends the scope of the prior meta-analytic reviews in several ways. First, we expand the literature covered and included in this meta-analysis by searching comprehensive list of databases and using an extensive list of search terms. Second, we assess a broad range of outcomes related to glycemic control and other markers of diabetes management including lipid profile, blood pressure, body composition and fasting cortisol. Finally, we examine study (i.e. geographical location, recruitment method), and sample characteristics (e.g. gender) and intervention features (e.g. intervention duration and components) as potential moderators of the intervention effect.
2. Methods
The current systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009). The PRISMA checklist can be found in the Supplemental Materials 1.
2.1. Eligibility criteria
Studies were included if the study (1) examined a yoga intervention to promote T2DM management, (2) used a randomized control trial or quasi-experimental design that included a control or comparison group, (3) included adults ≥18 years of age, (4) reported an objective measure of glycemic control (HbA1c, FBG), (5) provided relevant statistics to calculate between-group effect size, and (6) had follow-up length or post-test of at least 8 weeks from baseline. Studies were excluded if (1) participants did not have T2DM at baseline, (2) participants had type 1 or gestational diabetes, and (3) yoga was not the primary intervention but a part of multimodal intervention (e.g. mindfulness-based stress reduction).
2.2. Information sources and search
Several information sources were used to identify relevant studies: (1) Electronic bibliographic databases (PubMed, PsycInfo, The Cochrane Library, CINAHL, EMBASE, Global Health, Academic Search Premier, PsycARTICLES, Proquest Dissertations and Theses) were searched using a Boolean search strategy: ((yoga) OR (yogasan*) OR (yogi*) OR (yog*) OR (pranayam*) OR (asana*) OR (dhyana) or (vinyasa) or (viniyog*)) NOT ((YOGURT) OR (YOGHURT)) AND ((diabetes) OR (diabetic) OR (NIDDM) OR (“noninsulin-dependent diabetes mellitus”) OR (“noninsulin dependent diabetes mellitus”) OR (“diabetes mellitus”) OR (glycem*) OR (glycaem*) OR (hyperglycem*) OR (hyperglycaem*) OR (glucose*) OR (glycosylated Hb) OR (haemoglobin A) OR (hemoglobin A) OR (“glycated hemoglobin”) OR (HbA1c) OR (A1C) OR (T2DM) OR (“type II diabetes”) OR (“type 2 diabetes”) OR (“diabetes mellitus type 2”) OR (“diabetes mellitus type II”) OR (“type II diabetes mellitus”) OR (“type 2 diabetes mellitus”) OR (T2D) OR (DM2) OR (SUGAR) OR (INSULIN) or (FBS) OR (“fasting blood sugar”) OR (PPBS) OR (“postprandial glucose test”) OR (FPG) OR (“fasting plasma glucose”) OR (PPG) OR (“postprandial blood glucose”) OR (“oral glucose tolerance”) or (metabolic syndrome) OR (“diabetes control”)). The search fields were modified based on the search parameters imposed on each electronic database. No language restrictions were applied. The electronic reference databases were searched in February 2015 and updated in February 2016. We searched the database two months following the end of the calendar year due to the delay in the indexing process of electronic bibliographic databases. This was done to ensure that we retrieved all studies published and/or available through December 31, 2015. (2) Reference lists of manuscripts (including published reviews and included studies) were also reviewed. (3) Finally, we searched the tables of contents of relevant journals (e.g., International Journal of Yoga) for additional studies.
2.3. Study selection
Initial screening involved review of study titles and abstracts for possible inclusion. Full-text manuscripts of potentially relevant records and references from relevant manuscripts were reviewed for final inclusion. When study details or additional information (e.g., results from a pilot study) were published across multiple manuscripts, those manuscripts were linked in the database and represented as a single unit and the manuscript reporting the most complete data was selected as the primary manuscript. Authors were contacted for additional information and/or clarifications when the study details were not reported in full.
2.4. Data collection process and reliability
Two independent coders (HT and RL) extracted study information (e.g., publication year), sample characteristics (e.g., age, gender), design specifics (e.g., recruitment method), intervention procedures (e.g., yoga style, number of classes), and yoga components (e.g., postures, breathing) from each study. The methodological quality of each study was assessed using 15 items (total possible score of 21) adapted from validated measures (Downs and Black, 1998; Jadad et al., 1996; Miller et al., 1995; Miller et al., 2003).
Inter-rater reliability was assessed for all study, sample, design, and intervention characteristics. For categorical variables, raters agreed on 98% of the judgments (mean Cohen’s κ = 0.94; range = 0.55 to 1.00). Reliability for the continuous variables yielded an average intra-class correlation coefficient (ρ) of 0.96 across categories (median = 1.00). Disagreements between the coders were resolved with the help of the third investigator (LAJSS).
2.5. Study outcomes
The primary study outcomes included an objective measure of glycemic control (i.e. HbA1c, FBG, or PPBG). Secondary outcomes included other markers of diabetes management such as lipid profile, systolic and diastolic blood pressure, body composition, and fasting cortisol.
2.6. Summary measures
Effect sizes (d) were calculated as the mean difference between the intervention and the control or comparison group divided by the pretest standard deviation (Cohen, 1998; Morris and DeShon, 2002). Thus, we controlled for baseline when baseline measures were available (Morris and DeShon, 2002). All effect sizes were corrected for sample size bias (Hedges, 1981). Positive effect sizes indicated that participants who received the yoga intervention improved on measures of glycemic control (e.g., HbA1c) as well as lipid profile (e.g., lower total cholesterol), body composition (e.g., lower weight), and blood pressure (e.g., lower diastolic blood pressure) relative to controls. Two independent coders calculated effect sizes for each study, and discrepancies were resolved through discussion.
2.7. Synthesis of results
Weighted mean effect sizes (and the corresponding 95% confidence intervals) were calculated using random-effects procedures (Lipsey and Wilson, 2001). Heterogeneity in effect sizes was assessed by computing Q and the associated degrees of freedom; a significant Q indicates a lack of homogeneity and an inference of heterogeneity. To assess the extent to which outcomes were consistent across studies, the I2 index and its corresponding 95% CIs were calculated (Higgins and Thompson, 2002; Huedo-Medina et al., 2006). The I2 values of 25%, 50%, and 75% are considered to be low, medium, and high heterogeneity (Higgins et al., 2003). Moderator analyses were conducted using a modified weighted regression analysis or the meta-analytic analogue to the ANOVA (following random-effects assumptions) with weights equivalent to the inverse of the variance plus the random variance component for each effect size (Hedges, 1994; Lipsey and Wilson, 2001). All data analyses were conducted with Stata/SE 12.1 (StataCorp, 2013) using published macros (Lipsey and Wilson, 2001; Wilson, 2001).
2.8. Assessment for publication bias
The risk for publication bias was assessed by (a) inspecting funnel plots (Sterne and Egger, 2001), and (b) assessing the degree of asymmetry in the distribution of effect sizes using Begg and Mazumdar’s (1994) and Egger et al.’s (1997) techniques. Trim and fill procedures (Borenstein, 2005; Duval and Tweedie, 2000) were used to estimate and correct for the possibility of missing studies when publication bias was detected using funnel plot asymmetry tests (Begg and Mazumdar, 1994; Egger et al., 1997).
3. Results
Our search strategy revealed 2559 unique records after excluding duplicates. After initial screening of abstracts, 132 manuscripts were selected for full-text review. Of these 132 manuscripts, 98 were excluded because they did not meet inclusion criteria. The final sample included 23 studies. In addition, 11 supplemental manuscripts were retained with intervention details for the 23 included studies (Fig. 1).
Fig. 1.
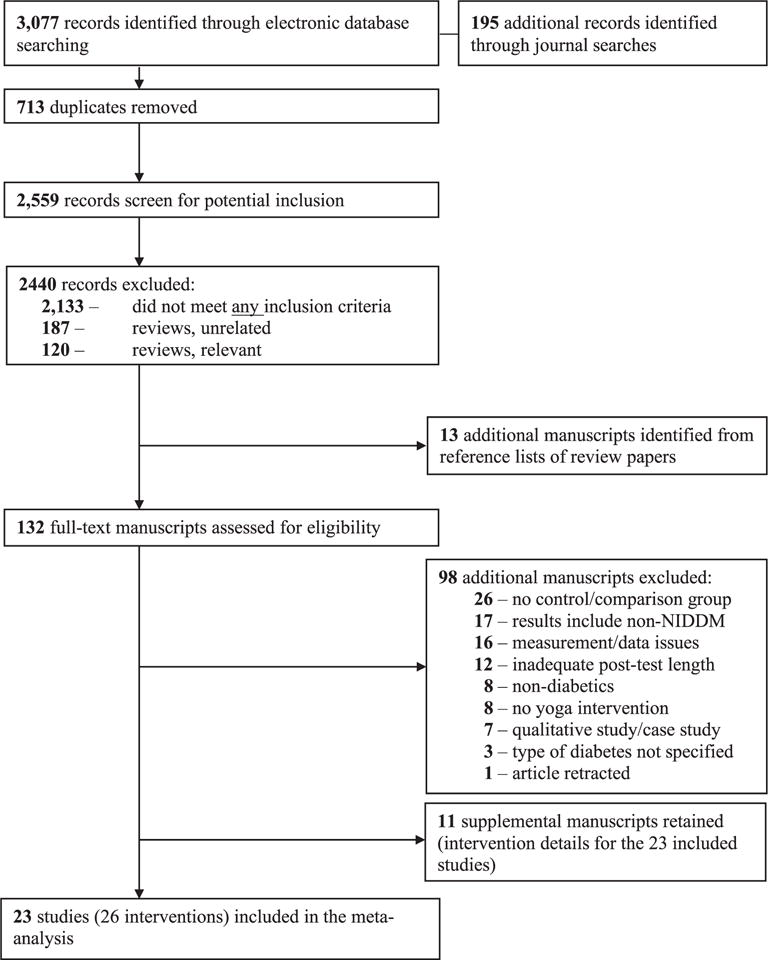
Study retrieval and selection.
3.1. Study and sample characteristics
Details of the 23 studies included in the meta-analysis are provided in Table 1. Studies were published in journals (including one published conference proceeding) from 1992 to 2015 with data collection occurring between 1991 and 2015. The majority of the studies were conducted in India (18), two in London, one in Iran, one in Indonesia, and one in Cuba. The sample of studies included 2473 adults with T2DM who consented to participate (mean age = 53 years; 43% women). Participants were most frequently recruited from clinical settings (e.g., hospital, outpatient clinics; 14/20, 70%); participants were also recruited via the community (e.g., using flyers; 1/20, 5%), yoga centers (2/20, 10%), or from multiple venues (e.g., clinic and community; 3/20, 15%). In most studies (20/23, 87%) participants with medical complications (e.g., coronary artery disease, renal disease) were excluded. Only two studies used HbA1c levels as inclusion criteria: Beena and Sreekumaran (2013) included participants with HbA1c > 8% and Jyotsna et al. (2014) included participants with HbA1c between 6 and 9%. The mean retention rate was 92% (SD = 0.13).
Table 1.
Study, sample, and intervention characteristics of the 23 studies included in the systematic review and meta-analysis.
| Study & Location | Sample | Control | Intervention details
|
Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|
| Weeks | Yoga style (components) | Other components | Total sessions (sessions/week) | Doseb | |||||
| Agrawal et al. (2003); Bikaner, India | N = 200 (77%) 30 F; M age = 52 | TAU | 13 | Not specified – P, B, R, M, sun salutations, health rejuvenation & abdomen exercises | Strict diabetic diet, moderate aerobic exercises & SM & TAU | 78 (5–7) | 4860 | A1c; FBG; C; VLDL; LDL; HDL; trig; SBP; DBP; BMI; W/H ratio | |
| Agte & Tarwadi (2004); Pune, India | N = 87 (75%); 53% F; M age = 55 | TAU | < 1 | SKY yoga – P, B, M | Discussion on stress-free living & nutritional counseling & TAU | 6a (6) | 1440 | A1c; FBG; PPBG; C; HDL; trig | |
| Balaji & Thirumaran (2015); Karikal District, India | N = 30 (100%) | TAU | 10 | SKY yoga – P, B, M, sun salutations | TAU | 60 (6) | 3600 | FBG; C; LDL; HDL; trig | |
| Balaji et al. (2011); Bangalore, Indiad | N = 44 (100%) | TAU | 13 | Not specified – P, B, R, sun salutations | TAU | 91 (7) | 5460 | A1c; FBG; PPBG; C; LDL; HDL; trig; BMI; weight; W/H ratio | |
| Beena & Sreekumaran (2013); Kerala, Indiad | N = 143 (100%); 38% F; M age = 63 | TAU | 13 | Not specified – P, B, R, warm-up exercises | TAU | 78 (6) | 7020 | A1c; FBG; C; LDL; HDL; trig; cortisol | |
| Bindra et al. (2013); Bhopal, India | N = 100 (100%) | TAU | 13 | Not specified – P, B | TAU | NR | NR | A1c; FBG; C; LDL; HDL; trig | |
| Giri et al. (2015); Bali, Indonesia | N = 41 (93%); M age = 45 | RC: Exercise – Walking | 10 | Not specified – P, B, R, M, sun salutations, loosening exercises; chanting | Diabetes & SM education | 25a (2–3) | 1500 | FBG; PPBG; SBP; DBP; BMI; weight | |
|
Gordon et al. (2008b); Havana, Cubac Linked studies: Gordon et al. (2008b); Gordon et al. (2008a); Gordon et al. (2008c) |
N = 327 [218] (71%); 81% F; M age = 64 | TAU | 24 | Hatha yoga – P, B, R, warm-up exercises | Medical & psychological evaluations; instruction on diabetes education, diet, specific treatments & personal care & TAU | 24a (1) | 2880 | A1c; FBG; C; VLDL; LDL; HDL; trig; BMI; cortisol | |
|
Hegde et al. (2011); Mangalore, India Linked studies: Sperandei (2012); Hegde et al. (2012) |
N = 126 (98%); M age = 59 | TAU | 13 | Not specified – P, B, R | TAU | 39 (3) | NR | A1c; FBG; PPBG; SBP; DBP; BMI; W/H ratio; | |
|
Jyotsna et al. (2014); New Delhi, India Linked studies: Jyotsna et al. (2013); Jyotsna et al. (2012) |
N = 120 (100%); M age = 49 | TAU | < 1 | SKY yoga – P, B, R, M, chanting | TAU | 28a (3 In week 1 then 1/week) | 2220 | A1c; FBG; PPBG | |
|
Kumar and Kalidasan (2014a); Tamilnadu, India Linked studies: Kumar and Kalidasan (2014b) |
N= 30 (100%);0% F | TAU | 12 | Not specified – P, R, warm-up exercises | TAU | 72 (6) | 3600 | FBG; PPGB; VLDL; LDL; HDL; SBP; DBP | |
|
Kumar and Kalidasan (2014b); Tamilnadu, India Linked studies: Kumar and Kalidasan (2014a) |
N = 30 (100%); 0% F | TAU | 12 | Not specified – P, B, R, M, prayer, om chanting | TAU | 72 (6) | 4320 | A1c; FBG; PPBG; C; VLDL; LDL; HDL; trig | |
| Kumpatla et al. (2015); Chennai, India | N = 303 (80%); 37% F; M age = 43 | TAU | < 1 | Not specified – P | TAU | 1a (1) | NR | A1c; PPBG; C; VLDL; LDL; HDL; trig; SBP; DBP; BMI; weight | |
| Monro et al. (1992); London, England | N = 21 (100%); 52% F; M age = 55 | TAU | 12 | Not specified – P, B, R | Exercise, diet, relaxation & counseling along with TAU | 60a (5) | 5400 | A1c; FBG | |
| Nagarathna et al. (2012); Bengaluru, India | N = 277 (63%); 31% F; M age = 52 | RC: Exercise, diabetes education, self-monitoring, smoking cessation & SM | 12 | Not specified – P, B, R, M, sun salutations, bandhas & kriyas, loosening exercise | Diabetes education, self-monitoring, smoking cessation, SM, & diet | 87a (5 For 12 weeks then 1/week) | 6840 | A1c; FBG; PPBG; C; VLDL; LDL; HDL; trig | |
| Pardasany et al. (2010); Amritsar, Indiac | N = 45 [30] (100%); 38% F | TAU | 12 | Hatha yoga – P, B | TAU | 36 (3) | NR | A1c; FBG; PPBG; C; LDL | |
| Popli et al. (2014); Delhi, India | N = 130 (100%); 47% F; M age = 43 | TAU | 4 | Not specified – P, B, sun salutations | TAU | 22a (5) | 1299 | A1c; FBG; PPBG | |
|
Rast et al. (2013); Najaf Abad, Iran Linked studies: Rast et al. (2014) |
N = 30 (100%); 100% F | TAU | 8 | Not specified – P, B, R, M | TAU | 24 (3) | 1440 | FBG; C; LDL; HDL; trig | |
|
Shantakumari et al. (2012); Kerala, India Linked studies: Shantakumari et al. (2013) |
N = 100 (100%); 48% F; M age = 45 | TAU | 2 | Not specified – P, B, R, M, sun salutations | TAU | 14a (7) | 840 | FBG; PPBG; C; LDL; HDL; trig; SBP; DBP; BMI; weight; W/H ratio | |
| Sharma et al. (2015); Jaipur, India | N = 80 (100%); M age = 48 | TAU | 12 | Not specified – P, B, R | TAU | 60 (5) | 2150 | A1c; FBG; PPBG | |
| Skoro-Kondza et al. (2009); London, England | N = 59 (100%); 61% F; M age = 60 | Advice & leaflets on healthy lifestyle & wait list | 12 | Not specified – P, B, R | Advice & leaflets on healthy lifestyle | 24a (2) | 2160 | A1c, C, LDL, HDL, trig, BMI, weight, W/H ratio | |
|
Sri et al. (2014); Guntur City, India Linked studies: Sri Santhi et al. (2013) |
N = 60 (100%) | TAU | 26 | Hatha yoga – B | Given Amla everyday plus TAU | 182 (7) | 10,920 | A1c; FBG; PPBG; C; VLDL; LDL; HDL; trig; BMI; weight; W/H ratio | |
| Vaishali et al. (2012); Mangalore, India | N = 60 (95%); 39% F; M age = 65 | EDUC–advice & leaflets on maintaining general healthy lifestyle, regular exercise & conventional hypoglycemic medications | 12 | Not specified – P, B, R | EDUC | 72 (6) | 3780 | A1c; FBG; C; LDL; HDL; trig | |
Notes: N, number of consenting & randomized participants (attrition %); F, proportion female; PT, physical training; P, posture; B, breathing; R, relaxation; M, meditation; A1c, glycated hemoglobin; FBG, fasting blood glucose; PPBG, post prandial blood glucose, C, Total cholesterol; VLDL, very low-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Trig, Triglycerides; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; W/H ratio, waist/hip ratio; OHA, oral hypoglycemic agents; NR, not reported; SKY Yoga, Sudarshan Kriya Yoga; TAU, treatment as usual; SM, stress management; RC, relevant control.
Home practice also advised/encouraged during intervention period.
Estimated number of minutes of intervention excluding measurement.
Non-yoga intervention groups were excluded in two studies: Conventional physical training exercise (Gordon, Morrison, McGrowder, Young, Pena Fraser, et al., 2008) and Tai Chi Chuan (Pardasany et al., 2010).
Two studies assessed their outcomes across multiple subgroups: Balaji et al. (2011) separated intervention participants based on use of (a) oral hypoglycemic agents (OHAs) and insulin and (b) OHAs only; Beena & Sreekumaran (2013) separated intervention participants based on levels of hemoglobin A1c (group 1 = 8.6–9.7%, group 2 = 9.8–10.7%, and group 3 = 10.8–12.7%). Therefore, 26 separate groups (k) were included in the analyses.
3.2. Intervention and control conditions
Details of the intervention and control conditions are provided in Table 1. Few studies (6/23, 26%) identified the style of yoga used in the intervention. Of the six studies reporting the style of yoga intervention, three used hatha yoga (Gordon et al., 2008b; Pardasany et al., 2010; Sri et al., 2014) and three used Sudarshan-kriya yoga (Agte and Tarwadi, 2004; Balaji and Thirumaran, 2015; Jyotsna et al., 2014). The sampled studies reported using several yoga components (not mutually exclusive) including postures (22/23, 96%), breathing exercises (21/23, 91%), relaxation techniques (16/23, 70%), meditation (9/23, 39%), and yogic philosophy (2/23, 9%). The median number of yoga sessions was 50 (range = 1–182); the median duration of each session was 60 min (range = 50–240), with a total dose of 60 h (range = 12–182). The entire yoga intervention was delivered over a median of 12 weeks (ranging from < 1 week – 26 weeks). Home yoga practice was encouraged in 45% of the interventions (10/22).
The control conditions were most often a treatment-as-usual or wait-list control (21/23, 91%). Comparison conditions in the remaining two studies included exercise only (Giri et al., 2015) and exercise plus lifestyle education (i.e., education, stress management, and medication adherence) (Nagarathna et al., 2012). The two exercise comparison conditions were delivered over a median of 11 weeks (range = 10–12) and included a median of 43 sessions (range = 25–60) of 60 min in length with a total median dose of 43 h (range = 25–60).
3.3. Efficacy of the yoga intervention compared to controls
The effect sizes for each outcome were examined for possible outliers prior to analyses using box plots (Emerson and Strenio, 1983). All outlier(s) were removed from the final data analyses for the specific measure indicated (see Table 2 note). The overall weighted mean effect sizes and homogeneity statistics are provided for measures of glycemic control, lipid profile, blood pressure, body composition, and fasting cortisol in Table 2. Forest plots of the overall summary effect sizes for the primary outcomes are found in Figs. 2–4.
Table 2.
The overall weighted mean effect sizes (and homogeneity statistics) of yoga compared to controls.
| Outcome | k | d + random (95% CI) | Q | p | I2 (95% CI) |
|---|---|---|---|---|---|
| Glycemic control | |||||
| Hemoglobin A1ca | 16 | 0.36 (0.16, 0.56) | 48.26 | < 0.001 | 69 (48, 81) |
| Fasting blood glucosea | 20 | 0.58 (0.40, 0.76) | 52.12 | < 0.001 | 64 (41, 77) |
| Post-prandial glucosea | 14 | 0.40 (0.23, 0.56) | 24.70 | 0.025 | 47 (0, 72) |
| Lipid profile | |||||
| Total cholesterola | 18 | 0.51 (0.32, 0.70) | 45.65 | < 0.001 | 63 (38, 78) |
| Very low-density lipoprotein | 7 | 0.49 (0.21, 0.78) | 23.42 | < 0.001 | 74 (45, 88) |
| Low-density lipoprotein | 19 | 0.62 (0.43, 0.81) | 51.22 | < 0.001 | 65 (43, 78) |
| High-density lipoproteina | 17 | 0.49 (0.24, 0.72) | 70.76 | < 0.001 | 77 (64, 86) |
| Triglyceride | 18 | 0.67 (0.40, 0.93) | 92.73 | < 0.001 | 82 (72, 88) |
| Blood pressure | |||||
| Systolic blood pressurea | 4 | 0.22 (0.05, 0.39) | 1.67 | 0.644 | 0 (0, 89) |
| Diastolic blood pressure | 6 | 0.73 (0.25, 1.22) | 41.04 | < 0.001 | 88 (76, 94) |
| Body composition | |||||
| Body mass index | 10 | 0.52 (0.19, 0.85) | 49.78 | < 0.001 | 82 (68, 90) |
| Weight | 7 | 0.28 (−0.07, 0.63) | 18.51 | 0.005 | 68 (28, 85) |
| Waist-to-hip ratioa | 4 | 0.36 (0.17, 0.56) | 0.65 | 0.884 | 0 (0, 70) |
| Fasting cortisol | 4 | 0.64 (0.03, 1.25) | 16.28 | < 0.001 | 82 (52, 93) |
Note. The weighted mean effect sizes (d+) are positive for differences that favor the intervention relative to the comparison group. k, number of interventions; CI, confidence interval; Q, homogeneity statistic; I2, consistency of the effect sizes.
Outliers were detected for hemoglobin A1c (Beena & Sreekumaran, 2013; Popli et al., 2014; Sharma et al., 2015), fasting blood glucose (Balaji & Thirumaran, 2015; Popli et al., 2014; Sharma et al., 2015; Vaishali et al., 2012), and post-prandial glucose (Sharma et al., 2015), total cholesterol (Agte & Tarwadi, 2004), high-density lipoprotein (Balaji & Thirumaran, 2015; Vaishali et al., 2012), systolic blood pressure (Kumar and Kalidasan, 2014a; Shantakumari et al., 2012), and waist-to-hip ratio (Balaji et al., 2011; Sri et al., 2014). The magnitude and direction of the weighted mean ES that included the outlier(s) was generally consistent with the above outcomes (hemoglobin A1c: d+ = 1.06, 95% CI = 0.64, 1.47, k = 21; fasting blood glucose: d+ = 0.90, 95% CI = 0.61, 1.19, k = 24; post-prandial glucose: d+ = 0.65, 95% CI = 0.31, 0.99, k = 15; total cholesterol: d+ = 0.62, 95% CI = 0.38, 0.86, k = 19; high-density lipoprotein: d+ = 0.76, 95% CI = 0.42, 1.10, k = 19; waist-to-hip ratio: d+ = 0.33, 95% CI = 0.15, 0.51, k = 6).
Fig. 2.
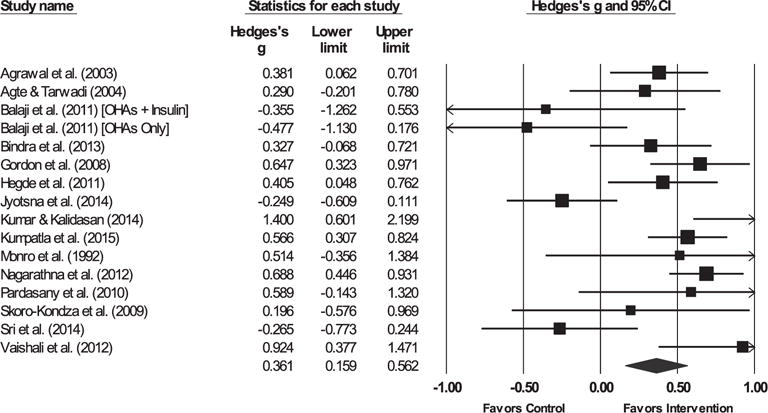
Forest plot for hemoglobin A1c.
Note. The sizes of the boxes are proportional to the weight of each individual study in the analyses.
The summary effect size is represented by a diamond. OHAs, oral hypoglycemic agents.
Fig. 4.
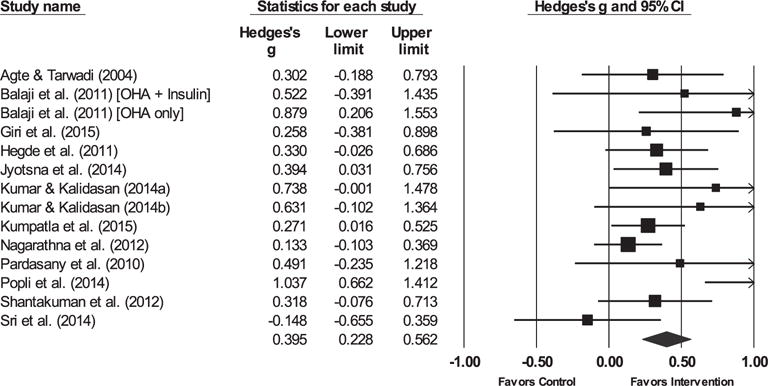
Forest plot for post-prandial glucose.
Note. The sizes of the boxes are proportional to the weight of each individual study in the analyses.
The summary effect size is represented by a diamond. OHAs, oral hypoglycemic agents.
3.3.1. Glycemic control
The yoga intervention was successful in reducing HbA1c, FBG, and post-prandial blood glucose (PPBG) relative to controls. The hypothesis of homogeneity was not supported for HbA1c, FBG, or PPBG (ps < 0.03).
3.3.2. Lipid profile
Compared to controls, participants in the yoga intervention reduced their total cholesterol, very-low density lipoprotein (VLDL), low-density lipoprotein (LDL), and triglyceride levels and increased high-density lipoprotein (HDL) levels. The hypothesis of homogeneity was not supported for any lipid profile measure (ps < 0.001).
3.3.3. Blood pressure
Participants in the yoga interventions decreased both systolic and diastolic blood pressure relative to controls. The hypothesis of homogeneity was not supported for diastolic blood pressure (p < 0.001); however, the hypothesis of homogeneity was supported for systolic blood pressure (Q[3] = 1.67, p = 0.644; I2 = 0) but uncertainty limits were wide (95% CI = 0, 89), exceeding the 50% threshold.
3.3.4. Body composition
Individuals who participated in a yoga intervention had a lower body mass index (BMI) and lower waist-to-hip ratio at post-intervention compared to controls. There was no significant difference in weight between intervention and control participants post-intervention. The hypothesis of homogeneity was not supported for BMI (p < 0.001) or weight (p = 0.005); however, the hypothesis of homogeneity was supported for waist-to-hip ratio (Q[3] = 0.65, p = 0.884; I2 = 0) but uncertainty limits were wide (95% CI = 0, 70) and exceeded the 50% threshold.
3.3.5. Fasting cortisol
Levels of fasting cortisol were lower among intervention vs. control participants at post-intervention. The hypothesis of homogeneity was not supported for fasting cortisol (p < 0.001).
3.4. Moderator analyses
Moderator tests were conducted to determine whether geographical location, recruitment method, sample (proportion women, age), and intervention characteristics (e.g., yoga components, dose) explained the variability in the effect sizes. These tests were conducted only for outcomes with sufficient sample size (k ≥ 5). Therefore, moderator tests were not conducted for the following outcomes: systolic blood pressure, waist-to-hip ratio, and fasting cortisol. Results from the moderator analyses can be found in Supplemental Materials 2.
3.4.1. Glycemic control
Compared to controls, yoga interventions were more successful in lowering FBG in older (vs. younger) patients (B = 0.05, SE = 0.02, p = 0.018; adjusted R2 = 41%), if all or some of the participants were recruited from non-clinic settings (QB[1] = 12.00, p < 0.001; d +clinic = 0.43, 95% CI = 0.23, 0.63; d+non-clinic = 0.96, 95% CI = 0.69, 1.24), and if home practice was not advised or encouraged (QB[1] = 7.15, p = 0.008; d+home practice = 0.31, 95% CI = 0.07, 0.55; d+no home practice = 0.75, 95% CI = 0.54, 0.95). There were no significant moderators of HbA1c and PPBG.
3.4.2. Lipid profile
Yoga interventions (vs. controls) were more successful in reducing total cholesterol when some or all participants were recruited from non-clinic (vs. clinic) settings (QB[1] = 8.00, p = 0.005; d+clinic = 0.48, 95% CI = 0.27, 0.69; d+non-clinic = 1.00, 95% CI = 0.65, 1.36). Compared to controls, interventions were more successful in decreasing LDL when some or all of the participants were recruited from non-clinic (vs. clinic) settings (QB[1] = 5.20, p = 0.023; d+clinic = 0.58, 95% CI = 0.33, 0.83; d+non-clinic = 1.03, 95% CI = 0.67, 1.40) and home practice was not advised or encouraged (QB[1] = 7.77, p = 0.005; d +home practice = 0.31, 95% CI = 0.06, 0.57; d+no home practice = 0.78, 95% CI = 0.57, 0.98). Interventions were less successful in increasing HDL when the intervention advised or encouraged home yoga practice (QB[1] = 8.96, p = 0.003; d+home practice = 0.14, 95% CI = −0.16, 0.43; d+no home practice = 0.73, 95% CI = 0.47, 0.98). Yoga interventions (vs. controls) were more successful in reducing triglycerides levels when yoga intervention components included meditation practices (QB[1] = 8.65, p = 0.003; d+meditation = 1.17, 95% CI = 0.74, 1.60; d +no meditation = 0.35, 95% CI = 0.01, 0.69). There were no significant moderators of VLDL levels.
3.4.3. Body composition
Individuals who participated in a yoga intervention were more likely to have a lower BMI compared to controls when the intervention included relaxation techniques (QB[1] = 5.38, p = 0.020; d +relaxation = 0.70, 95% CI = 0.36, 1.05; d+no relaxation = −0.15, 95% CI = −0.78, 0.48). Compared to controls, the yoga intervention lowered body weight if the intervention content included meditation exercises (QB[1] = 6.36, p = 0.011; d+meditation = 0.76, 95% CI = 0.32, 1.19; d+no meditation = 0.09, 95% CI = −0.20, 0.37). No other significant moderators were found.
3.5. Methodological quality
The studies satisfied an average or 41% (SD = 13%) of the methodological quality (MQ) criteria; total MQ scores ranged from 5 to 13 out of a possible 21 (mean = 8.53, SD = 2.81). Details of the MQ ratings are presented in Table 3. The total MQ score was not associated with any outcome (Ps > 0.05). Furthermore, as shown in Table Supplementary material 3 in the Supplementary Materials, exploratory analyses did not reveal any differences between RCTs or non-RCTs (Ps > 0.14).
Table 3.
Methodological quality items endorsed.
| Item | % |
|---|---|
| Random assignment of participants | 52% |
| Treatment standardized (e.g., manual) | 22% |
| Pretest post-test design | 100% |
| Follow-up rate (k = 17) | |
| ≤70% completed | 6% |
| 70–84% completed | 24% |
| ≥85% completed | 71% |
| Follow-up length | |
| ≤3 months | 87% |
| 3–5 months | 9% |
| ≥6 months | 4% |
| Representative sample | 4% |
| Participant who withdrew after randomization reported (k = 8) | 13% |
| Participants lost to follow-up after treatment reported (k = 9) | 22% |
| Anonymity | 0% |
| Intervention compliance | 35% |
| Treatment fidelity | 0% |
| Collateral verification | 9% |
| Objective measures used | 100% |
| Follow-up assessments blind to group assessment | 17% |
| Analyses controlled for baseline | 22% |
3.6. Risk of publication bias
Tests (graphical and statistical) to assess for the possibility of publication bias were conducted only for the outcomes in which the minimum threshold for the number of studies (i.e., ≥10 studies) was achieved (Lau et al., 2006). Both graphical and statistical tests revealed asymmetries that might be interpreted as small-study effects for FBG, LDL, triglyceride, and BMI. Trim-and-fill procedures were used to estimate an overall effect size (using random-effects procedures) if the “omitted” studies were included. These tests indicated that seven studies assessing FBG, six studies assessing LDL, three studies assessing triglycerides, and four studies assessing BMI might have been omitted. The summary effect size estimates were similar to the original estimates for all outcomes (except for BMI) suggesting that adding the additional studies would not change our overall conclusions (FBG: original d + =0.58, 95% CI = 0.40, 0.76 and estimated d+ =0.38, 95% CI = 0.20, 0.57; LDL: original d+ =0.62, 95% CI = 0.43, 0.81 and estimated d+ = 0.42, 95% CI = 0.22, 0.62; and triglycerides: original d+ = 0.67, 95% CI = 0.40, 0.93 and estimated d+ =0.47, 95% CI = 0.19, 0.75). Trim-and-fill analyses indicated that the estimated summary effect size differed from the original estimate (original: d+ = 0.52, 95% CI = 0.19, 0.85; estimated: d+ =0.15, 95% CI = −0.22, 0.52); therefore, caution should be used when interpreting our findings for BMI. Funnel plots and results of the statistical tests appear in the Supplementary Materials (S4).
4. Discussion
This meta-analysis examined the effects of yoga on glycemic control among adults with T2DM. Twenty-three studies with 2473 adults comparing the yoga intervention to a control or comparison condition were evaluated. Overall, the yoga interventions improved glycemic control (i.e., reduced HbA1c, FBG, PPBG) compared to the control conditions. Participants in the yoga interventions also showed improvements in their lipid profile (e.g., total cholesterol, LDL), blood pressure, BMI, waist/hip ratio, and cortisol levels. The magnitude of the effect sizes ranged from small to large (d+s = 0.22 to 0.73). These findings related to glycemic control are notable given that we restricted our review to studies with post-intervention assessments at least 8 weeks following baseline to allow for sufficient time for the intervention to have an impact on HbA1c. Nonetheless, heterogeneity observed between studies was high, potentially due to methodological differences in the reviewed articles. Our findings are consistent with other meta-analyses assessing the effects of yoga on glycemic control among type 2 diabetics (Cui et al., 2016; Kumar et al., 2016). Consistent to Vizcaino and Stover (2016), we found a positive impact of yoga for lipid profile and blood pressure. We also found significant changes in other markers of diabetes management such as BMI, waist-hip ratio and cortisol.
Our meta-analysis also examined several moderators to explain the variability in the effect sizes. Recruitment setting was significantly associated with glycemic control. Studies that recruited some or all participants primarily from non-clinical setting showed a larger decrease in FBG compared to studies that recruited from clinical settings. Participants recruited from non-clinic setting also had lower total cholesterol and LDL. It is possible that participants recruited from clinics may have had better glycemic control at baseline and therefore had weaker, although still significant, improvements in FBG compared to those recruited from non-clinical setting.
Interventions where yoga practice at home was encouraged were less successful in improving FBG, LDL cholesterol, and HDL cholesterol. However, these findings should be interpreted with caution. Studies with interventions of short duration were more likely to request home practice (r = −0.55, p = 0.02). Furthermore, compliance to yoga practice was reported by only 35% (8/23) studies, where adherence to in-person yoga classes ranged from 50% to 90–95% (Gordon et al., 2008a). Adherence to home practice was reported by only Gordon et al. (2008a) (80–85%). Thus, it is possible that the weaker effects seen among studies promoting home practice were due to poor adherence, short study duration, or both.
When examining the moderator effects of different yoga components, meditation was found to have a significant effect on lowering weight and reducing triglyceride levels; relaxation techniques were associated with reduction in BMI. This finding is consistent with studies conducted in different populations where meditation practice was associated with improvement in diet (Alberts et al., 2012; Daubenmier et al., 2011; Katterman et al., 2014), which could be the reason for improved body composition measures. Similarly, the relaxation techniques used in the yoga practice might help with stress reduction. Prior evidence suggests that stress related activation of the hypothalamic-pituitary-adrenal axis and resulting excess of circulating cortisol leads to abdominal adiposity (Bjorntorp and Rosmond, 2000; Pasquali et al., 2006). Furthermore, numerous studies have shown that stress is associated with increased consumption of energy-dense foods high in sugar and fat, emotional eating and ineffective attempts to control eating (Costarelli and Patsai, 2012; Groesz et al., 2012; Oliver et al., 2000; Tryon et al., 2013). Thus, by reducing stress, yoga practice may positively influence BMI (Block et al., 2009; Smith et al., 2007). Findings from our meta-analysis emphasize the need to include meditation and relaxation components to a yoga practice for diabetics.
It is noteworthy that majority of the studies were conducted in India. Our searches revealed a single study conducted in the United States, but this study used a single-group pretest-posttest design and was therefore not included in the review (Vizcaino, 2013). Geographic location (India vs. other countries) was not found to have effect on any of the examined outcomes in this meta-analysis. But as Cramer et al. (2015) has noted it cannot be denied that familiarity with the yoga tradition could have resulted in favorable outcomes in the studies conducted in India. There is definitely a need to examine the efficacy of yoga as a complementary therapy for diabetes in the context of the American culture and the U.S. healthcare system.
4.1. Limitations
The studies included in this meta-analysis were found to be of low to medium methodological quality. Most of the studies (20/23, 87%) conducted post-test assessments only at the end of the intervention and had no long-term follow-up. There was inadequate reporting of participants that withdrew after randomization or those lost to follow-up. In a large majority of studies, the statistical analysis did not report controlling for baseline values of the outcomes or other characteristics (18/23, 78%). (The effect sizes calculated for the current meta-analysis did account for baseline when baseline values were provided.) The majority of studies reported using a manual with information on yoga components (i.e. postures, breathing, relaxation, etc.) used in the intervention (18/23, 78%). However, none of the studies assessed treatment fidelity. It is noteworthy, though, that the methodological quality score was not associated with any outcome in this meta-analysis.
Insufficient reporting of study details was another major concern. Studies did not provide detailed information on demographics of participants. Only 65% (15/23) reported age and gender, and only three studies reported the length of time since diabetes diagnosis. Particulars about yoga intervention were also not reported. Most of the studies reported on the various yoga components (postures, etc.) included in the trial. However, the style of yoga used was not described. Depending on the style, the intensity of yoga practice may vary; some styles are more vigorous (i.e. Vinyasa, Ashtanga), some are gentle (i.e. Iyengar, Kripalu), some are more focused on spiritual or meditative aspects (i.e. Kundalini). Each style might have different effects on important mediating variables such as aerobic fitness and stress levels, which are in turn associated with diabetic outcomes.
The total duration of intervention was reported in all studies; however, minutes of individual session were not reported (4/23, 17%). Future studies should adhere to standard reporting guidelines such as CONSORT statement (Schulz et al., 2010) for RCTs or TREND statement (Des Jarlais et al., 2004) for nonrandomized trials to avoid incomplete reporting. It is also important that studies record and report any adverse event related to the yoga intervention. In a large-scale survey of yoga class attendees in Japan, 28% reported undesirable symptoms after taking a yoga class; 64% of these adverse events were mild and did not interfere with yoga practice (Matsushita and Oka, 2015). The absence of adverse event reporting precludes a determination of the safety of yoga for patients with chronic conditions such as diabetes.
5. Conclusion
The current meta-analysis revealed that yoga improves glycemic outcomes, lipid profile, blood pressure, and waist/hip ratio in adults with T2DM. However, rigorously designed randomized controlled trials are needed to examine the long-term effects of yoga in this population. There is also a need to study the mechanism by which yoga can affect glycemic control. Future studies should include assessments of behavioral (e.g., diet, physical activity) and psychological (e.g., stress, depression) factors which may act as potential mediators of the effect of yoga for improved clinical outcomes in patients with T2DM.
Supplementary Material
Fig. 3.
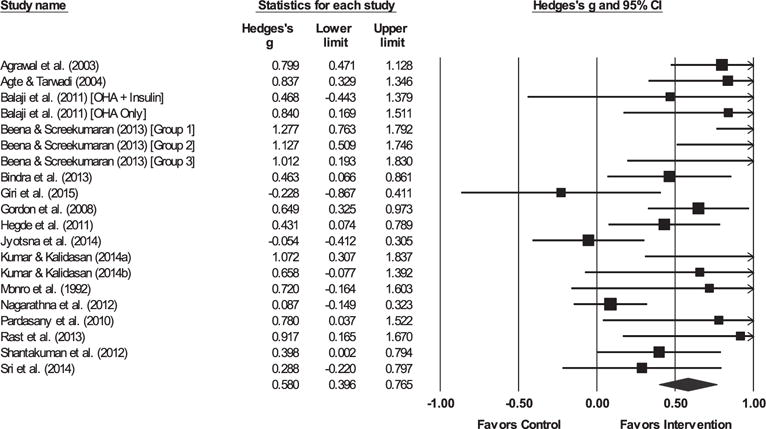
Forest plot for fasting blood glucose.
Note. The sizes of the boxes are proportional to the weight of each individual study in the analyses.
The summary effect size is represented by a diamond. OHAs, oral hypoglycemic agents.
Acknowledgments
The research reported in this paper was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under award number R01-AT008815 to Lori A. J. Scott-Sheldon, PhD (PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ypmed.2017.08.017.
References
- Agrawal RP, Aradhana, Hussain S, Beniwal R, Sabir M, Kochar DK, Kothari RP. Influence of yogic treatment on quality of Life outcomes, glycemic control and risk factors in diabetes mellitus. Int J Diab Dev Countries. 2003;23:130–134. [Google Scholar]
- Agte VV, Tarwadi K. Sudarshan Kriya yoga for treating type 2 diabetes: a preliminary study. Alternative & Complementary Therapies. 2004;10:220–222. [Google Scholar]
- Alberts HJ, Thewissen R, Raes L. Dealing with problematic eating behaviour. The effects of a mindfulness-based intervention on eating behaviour, food cravings, dichotomous thinking and body image concern. Appetite. 2012;58:847–851. doi: 10.1016/j.appet.2012.01.009. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013a;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes–2013. Diabetes Care. 2013b;36(Suppl 1):S11. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Strategies for improving care. Sec. 1. In standards of medical Care in Diabetes - 2016. Diabetes Care. 2016;39(Suppl 1):S6–S12. doi: 10.2337/dc16-S004. [DOI] [PubMed] [Google Scholar]
- Balaji PV, Thirumaran M. Effects of 10 weeks yoga training on blood glucose and lipid profile in type II diabetic patients. Scholars Journal of Applied Medical Sciences. 2015;3:1876–1879. [Google Scholar]
- Balaji PA, Varne SR, Ali SS. Effects of yoga pranayama practices on metabolic parameters and anthropometry in type 2 diabetes. Int Multidiscip Res J. 2011;1:01–04. [Google Scholar]
- Beena RK, Sreekumaran E. Yogic practice and diabetes mellitus in geriatric patients. International journal of yoga. 2013;6:47–54. doi: 10.4103/0973-6131.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- Bell RA, Suerken CK, Grzywacz JG, Lang W, Quandt SA, Arcury TA. Complementary and alternative medicine use among adults with diabetes in the United States. Altern Ther Health Med. 2006;12:16–22. [PubMed] [Google Scholar]
- Bindra M, Nair S, Darotiya S. Influence of pranayamas and yoga-asanas on blood glucose, lipid profile and HbA1c in type 2 diabetes. Int J Pharm Bio Sci. 2013;4:169–172. [Google Scholar]
- Bjorntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S80–5. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among US adults. Am J Epidemiol. 2009;170:181–192. doi: 10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M. Software for publication bias. In: Rothstein H, Sutton AJ, Borenstein M, editors. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Wiley; West Sussex, United Kingdom: 2005. [Google Scholar]
- Center for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- Center for Disease Control and Prevention. Diabetes: Working to Reverse the US Epeidemic: At a Glance 2016. National Center for Chronic Disease Prevention and Health Promotion; 2016. [Google Scholar]
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report. 2015:1–16. [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Erlbaum; New York: 1998. [Google Scholar]
- Costarelli V, Patsai A. Academic examination stress increases disordered eating symptomatology in female university students. Eat Weight Disord. 2012;17:e164–9. doi: 10.1007/BF03325343. [DOI] [PubMed] [Google Scholar]
- Cramer H, Lauche R, Langhorst J, Dobos G. Are Indian yoga trials more likely to be positive than those from other countries? A systematic review of randomized controlled trials. Contemp Clin Trials. 2015;41:269–272. doi: 10.1016/j.cct.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Cui J, Yan JH, Yan LM, Pan L, Le JJ, Guo YZ. Effects of yoga in adults with type 2 diabetes mellitus: a meta-analysis. J Diabetes Investig. 2016 doi: 10.1111/jdi.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Kristeller J, Hecht FM, Maninger N, Kuwata M, Jhaveri K, Lustig RH, Kemeny M, Karan L, et al. Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: an exploratory randomized controlled study. J Obes. 2011;2011:651936. doi: 10.1155/2011/651936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Lyles C, Crepaz N, Group T Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am. J. Public Health. 2004;94:361–366. doi: 10.2105/ajph.94.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Silva S, Poscablo C, Habousha R, Kogan M, Kligler B. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407–423. doi: 10.1016/j.psym.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Dyson PA, Kelly T, Deakin T, Duncan A, Frost G, Harrison Z, Khatri D, Kunka D, McArdle P, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2011;28:1282–1288. doi: 10.1111/j.1464-5491.2011.03371.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson JD, Strenio J. Boxplots and batch comparisons. In: Hoaglin DC, Mosteller F, Tukey JW, editors. Understanding Robust and Exploratory Data Analysis. Wiley; New York, NY: 1983. [Google Scholar]
- Froeliger BE, Garland EL, Modlin LA, McClernon FJ. Neurocognitive correlates of the effects of yoga meditation practice on emotion and cognition: a pilot study. Front Integr Neurosci. 2012;6:48. doi: 10.3389/fnint.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri M, Artanayasa W, Putra A. Effect of yoga on atherosclerosis risk in type 2 diabetes. In: Setemen K, Mahedy K, Sindu G, Suputra P, editors. The 1st International Conference on Innovative Research Across Disciplines. Ukdiksha Press; Kuta, Bali: 2015. pp. 147–152. [Google Scholar]
- Gordon LA, Morrison EY, McGrowder DA, Young R, Garwood D, Zamora EM, Alexander-Lindo RL, Irving RR, Perez Sanz EC. Archives of Medical Science. 2008a. Changes in clinical and metabolic parameters after exercise therapy in patents with type 2 diabetes; pp. 427–437. [Google Scholar]
- Gordon LA, Morrison EY, McGrowder DA, Young R, Pena Fraser YT, Zamora EM, Alexander-Lindo RL, Irving RR. Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. BMC Complementary and Alternative Medicine. 2008b:21. doi: 10.1186/1472-6882-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LA, Morrison EY, McGrowder DA, Penas YF, Zamoraz EM, Garwood D, Alexander-Lindo R, Irving R. Effect of yoga and traditional physical exercise on hormones and percentage insulin binding receptor in patients with type 2 diabetes. Am J Biochem & Biotech. 2008c;4:35–42. [Google Scholar]
- Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, Laraia B, Epel E. What is eating you? Stress and the drive to eat. Appetite. 2012;58:717–721. doi: 10.1016/j.appet.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Behav Stat. 1981;6:107–128. [Google Scholar]
- Hegde SV, Adhikari P, Kotian S, Pinto VJ. Effect of 3-month yoga on oxidative stress in type 2 diabetes with or without complications: a controlled trial. Diabetes Care. 2011;34:2208–2210. doi: 10.2337/dc10-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde SV, Adhikari P, Kotian S, Pinto VJ, D’Souza S, D’Souza V. Response to comment on: Hegde et al. effect of 3-month yoga on oxidative stress in type 2 diabetes with or without complications: a controlled trial. Diabetes Care. 2012;35:e43. doi: 10.2337/dc10-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV. Fixed effects models. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. Russell Sage Foundation; New York: 1994. pp. 285–299. [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- Innes KE, Selfe TK. Yoga for adults with type 2 diabetes: a systematic review of controlled trials. J Diabetes Res. 2016;2016:6979370. doi: 10.1155/2016/6979370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Jyotsna VP, Joshi A, Ambekar S, Kumar N, Dhawan A, Sreenivas V. Comprehensive yogic breathing program improves quality of life in patients with diabetes. Indian J Endocrinol Metab. 2012;16:423–428. doi: 10.4103/2230-8210.95692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyotsna VP, Ambekar S, Singla R, Joshi A, Dhawan A, Kumar N, Deepak KK, Sreenivas V. Cardiac autonomic function in patients with diabetes improves with practice of comprehensive yogic breathing program. Indian J Endocrinol Metab. 2013;17:480–485. doi: 10.4103/2230-8210.111645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyotsna VP, Dhawan A, Sreenivas V, Deepak KK, Singla R. Completion report: effect of comprehensive yogic breathing program on type 2 diabetes: a randomized control trial. Indian journal of endocrinology and metabolism. 2014;18:582–584. doi: 10.4103/2230-8210.137499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katterman SN, Kleinman BM, Hood MM, Nackers LM, Corsica JA. Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: a systematic review. Eat Behav. 2014;15:197–204. doi: 10.1016/j.eatbeh.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Jung TS, Jung JH, Kim SK, Lee SM, Kim KY, Kim DR, Seo YM, Hahm JR. Improvement of glycemic control after re-emphasis of lifestyle modification in type 2 diabetic patients reluctant to additional medication. Yonsei Med J. 2013;54:345–351. doi: 10.3349/ymj.2013.54.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- Kumar U, Kalidasan R. Effect of selected yoga asanas on blood sugar lipid profile and blood pressure parameters among type 2 diabetes mellitus patients. Academic Sports Scholar. 2014a;3 [Google Scholar]
- Kumar U, Kalidasan R. Influences of yogic practices on blood glucose and lipid profile among male type 2 diabetes patients and lipid profile among male type 2 diabetes patients. Int J Multidiscip Educ Res. 2014b;3 [Google Scholar]
- Kumar V, Jagannathan A, Philip M, Thulasi A, Angadi P, Raghuram N. Role of yoga for patients with type II diabetes mellitus: a systematic review and meta-analysis. Complement Ther Med. 2016;25:104–112. doi: 10.1016/j.ctim.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Kumpatla S, Michael C, Viswanathan V. Effect of yogasanas on glyvemic, haemodynamic and lipid profile in newly diagnosed subjects with type 2 diabetes. Int J Diabetes Dev Ctries 2015 [Google Scholar]
- Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Balk JL. Yoga and quality-of-life improvement in patients with breast cancer: a literature review. Int J Yoga Therap. 2012:95–99. [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical Meta-Analysis. Sage; Thousand Oaks, CA: 2001. [Google Scholar]
- Mamtani R, Mamtani R. Ayurveda and yoga in cardiovascular diseases. Cardiol Rev. 2005;13:155–162. [PubMed] [Google Scholar]
- Matsushita T, Oka T. A large-scale survey of adverse events experienced in yoga classes. Biopsychosoc Med. 2015;9:9. doi: 10.1186/s13030-015-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Brown JM, Simpson TL, Handmaker NS, Bien TH, Luckie LF. What works? A methodological analysis of the alcohol treatment outcome literature. In: Hester RK, Miller WR, editors. Handbook of Alcoholism Treatment Approaches: Effective Alternatives. 2nd. Allyn & Bacon; Needham Heights, MA: 1995. pp. 12–44. [Google Scholar]
- Miller WR, Brown JM, Simpson TL, Handmaker NS, Bien TH, Luckie LF. What works? A methodological analysis of the alcohol treatment outcome literature. In: Hester RK, Miller WR, editors. Handbook of Alcohilsm Treatment Approaches: Effective Alternatives. Allyn and Bacon; Boston, MA: 2003. pp. 12–44. [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Monro R, Power J, Coumar A, Nagarathna R, Dandona P. Yoga therapy for NIDDM: a controlled trial. Complement Med Res. 1992;6:66–68. [Google Scholar]
- Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- Nagarathna R, Usharani M, Rao A, Chaku R, Kulkarni R, Nagendra H. Efficacy of yoga based life style modification program on medication score and lipid profile in type 2 diabetes-a randomized control study. International Journal of Diabetes in Developing Countries. 2012;32:122–130. [Google Scholar]
- Nahin RL, Byrd-Clark D, Stussman BJ, Kalyanaraman N. Disease severity is associated with the use of complementary medicine to treat or manage type-2 diabetes: data from the 2002 and 2007 National Health Interview Survey. BMC Complement Altern Med. 2012;12:193. doi: 10.1186/1472-6882-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007;50:2239–2244. doi: 10.1007/s00125-007-0803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonta NR. Does yoga therapy reduce blood pressure in patients with hypertension?: an integrative review. Holist Nurs Pract. 2012;26:137–141. doi: 10.1097/HNP.0b013e31824ef647. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosom Med. 2000;62:853–865. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Pardasany A, Shenoy S, Sandhu JS. Comparing the efficacy of tai chi chuan and hatha yoga in type 2 diabetes mellitus patients on parameters of blood glucose control and lipid metabolism. Indian Journal of Physiotherapy and Occupational Therapy. 2010;4:11–16. [Google Scholar]
- Pasquali R, Vicennati V, Cacciari M, Pagotto U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann N Y Acad Sci. 2006;1083:111–128. doi: 10.1196/annals.1367.009. [DOI] [PubMed] [Google Scholar]
- Patel NK, Newstead AH, Ferrer RL. The effects of yoga on physical functioning and health related quality of life in older adults: a systematic review and meta-analysis. J Altern Complement Med. 2012;18:902–917. doi: 10.1089/acm.2011.0473. [DOI] [PubMed] [Google Scholar]
- Popli U, Subbe CP, Sunil K. Research letter – the role of yoga as a lifestyle medication in treatment of diabetes mellitus: results of a pilot study. Altern Ther. 2014;20:24–26. [PubMed] [Google Scholar]
- Rast S, Hojjati Z, Shabani R. The effect of yoga training on lipid profile and blood glucose in type II diabetic females. Ann Biol Res. 2013;4:128–133. [Google Scholar]
- Rast S, Hojjati Z, Shabani R. The effect of yoga training on blood glucose, insulin and resting heart rate in type II diabetic females. Res J Sport Sci. 2014;2:15–21. [Google Scholar]
- Schulz KF, Altman DG, Moher D, Group C CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- Shantakumari N, Sequeira S, El deeb R. Effect of a yoga intervention on hypertensive diabetic patients. J Adv Intern Med. 2012;1:60–63. [Google Scholar]
- Shantakumari N, Sequeira S, El deeb R. Effects of a yoga intervention on lipid profiles of diabetes patients with dyslipidemia. Indian Heart J. 2013;65:127–131. doi: 10.1016/j.ihj.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D, Cook IA, Davydov DM, Ottaviani C, Leuchter AF, Abrams M. Yoga as a complementary treatment of depression: effects of traits and moods on treatment outcome. Evidence-based complementary and alternative medicine: eCAM. 2007;4:493–502. doi: 10.1093/ecam/nel114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Sharma M, Sharma R, Meena PD, Gupta M, Meena M. Effect of yoga on blood glucose and glycosylated haemoglobin level in diabetes mellitus type-2 patients. Int J Med Sci Educ. 2015;2 [Google Scholar]
- Skoro-Kondza L, Tai SS, Gadelrab R, Drincevic D, Greenhalgh T. Community based yoga classes for type 2 diabetes: an exploratory randomized controlled trial. BMC Health Serv Res. 2009;9 doi: 10.1186/1472-6963-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187–192. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Hancock H, Blake-Mortimer J, Eckert K. A randomised comparative trial of yoga and relaxation to reduce stress and anxiety. Complement Ther Med. 2007;15:77–83. doi: 10.1016/j.ctim.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Sperandei S. Comment on Hegde et al. effect of 3-month yoga on oxidative stress in type 2 diabetes with or without complications: a controlled trial. Diabetes Care. 2012;35:e42. doi: 10.2337/dc11-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sri KVS, Kasturi K, Sivannarayana G. Impact of pranayama and Amla, an approach towards the control of diabetes mellitus. International Journal of PharmTech Research. 2014;6:1157–1161. [Google Scholar]
- Sri Santhi KV, Jalaja Kumari D, Sivannarayana G. Effect of amla, an approach towards the control of diabetes mellitus. Int J Curr Microbiol App Sci. 2013;2:103–108. [Google Scholar]
- StataCorp. Stata/SE, 12.1 for Windows ed. StataCorp LP; College Station, TX: 2013. [Google Scholar]
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- Stettler C, Allemann S, Juni P, Cull CA, Holman RR, Egger M, Krahenbuhl S, Diem P. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006;152:27–38. doi: 10.1016/j.ahj.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Surwit RS, van Tilburg MA, Zucker N, McCaskill CC, Parekh P, Feinglos MN, Edwards CL, Williams P, Lane JD. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25:30–34. doi: 10.2337/diacare.25.1.30. [DOI] [PubMed] [Google Scholar]
- Tryon MS, DeCant R, Laugero KD. Having your cake and eating it too: a habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiol Behav. 2013;114–115:32–37. doi: 10.1016/j.physbeh.2013.02.018. [DOI] [PubMed] [Google Scholar]
- UKPDS. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- Vaishali K, Kumar KV, Adhikari P, UnniKrishnan B. Effects of yoga-based program on glycosylated hemoglobin level serum lipid profile in community dwelling elderly subjects with chronic type 2 diabetes mellitus: a randomized controlled trial. Phys Occup Ther Geriatr. 2012;30:22–30. [Google Scholar]
- Vizcaino M. Hatha yoga practice for type 2 diabetes mellitus patients: a pilot study. Int J Yoga Therap. 2013:59–65. [PubMed] [Google Scholar]
- Vizcaino M, Stover E. The effect of yoga practice on glycemic control and other health parameters in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Complement Ther Med. 2016;28:57–66. doi: 10.1016/j.ctim.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Wilson DB. Meta-Analysis Macros for SAS, SPSS, and Stata 2001 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


