Abstract
Estrogen is the major mitogenic stimulus of mammary gland development during puberty wherein ER signaling acts to induce abundant PR expression. PR signaling, in contrast, is the primary driver of mammary epithelial cell proliferation in adulthood. The high circulating levels of progesterone during pregnancy signal through PR, inducing expression of the prolactin receptor (PRLR). Cooperation between PR and prolactin (PRL) signaling, via regulation of downstream components in the PRL signaling pathway including JAKs and STATs, facilitates the alveolar morphogenesis observed during pregnancy. Indeed, these pathways are fully integrated via activation of shared signaling pathways (i.e. JAKs, MAPKs) as well as by the convergence of PRs and STATs at target genes relevant to both mammary gland biology and breast cancer progression (i.e. proliferation, stem cell outgrowth, tissue cell type heterogeneity). Thus, rather than a single mediator such as ER, transcription factor cascades (ER>PR>STATs) are responsible for rapid proliferative and developmental programming in the normal mammary gland. It is not surprising that these same mediators typify uncontrolled proliferation in a majority of breast cancers, where ER and PR are most often co-expressed and may cooperate to drive malignant tumor progression. This review will primarily focus on the integration of PR and PRL signaling in breast cancer models and the importance of this cross-talk in cancer progression in the context of mammographic density. Components of these PR/PRL signaling pathways could offer alternative drug targets and logical complements to anti-ER or anti-estrogen-based endocrine therapies.
Keywords: estrogen, progesterone, breast, cancer, prolactin, receptor, kinase
Introduction
Transcription factor cascades orchestrate mammary gland development
The mammary gland is unique among organ systems in that it develops primarily after birth, undergoing extensive postnatal development characterized by massive epithelial cell proliferation that occurs over a relatively short time interval. The ovarian steroid hormones, estrogen and progesterone, and the pituitary peptide hormone, prolactin (PRL), acting through their specific receptors, are critical for this process. Extensive genetic and tissue recombination studies in mouse models have revealed that estrogen receptor alpha (ER) signaling in mammary epithelial cells (MEC) is required for pubertal ductal elongation, while progesterone receptor (PR) actions in MEC are essential for ductal side branching and alveologenesis. During pregnancy, prolactin receptor (PRLR) signaling drives further mammary alveologenesis and is required for the ultimate goal of milk production and secretion. Although these hormone receptor-dependent signaling pathways dominate distinct developmental stages, the mammary gland also exhibits stage-dependent sensitivity to each hormone. Notably, although ER and PR are segregated, that is, expressed in separate normal mammary epithelial cell populations [1, 2], extensive paracrine crosstalk occurs between ER and PR signaling and between PR and PRL signaling, creating a continuum of overlapping and highly integrated signaling pathways. These same pathways are known to contribute to breast cancer biology and form the basis of approaches that employ molecular targeted therapies (i.e. endocrine therapies and combinations thereof with kinase inhibitors).
Hormones are drivers of luminal breast cancer
A majority (~70%) of breast tumors co-express ER and PR; these steroid hormone receptors (SRs) classically function as ligand activated transcription factors. SRs rapidly and dynamically shuttle between the nucleus and the cytoplasm, where they also bind membrane-associated and/or cytoplasmic protein kinases. Hormone-bound receptors rapidly activate signaling cascades that, in turn, function to regulate transcriptional activity and promoter selection via phosphorylation events [3]. The complexities regarding SR activation and regulation are discussed in other select reviews [4–8]. A growing list of selective estrogen receptor modulators, or SERMS (i.e. tamoxifen [Tam]), and aromatase inhibitors are used clinically to target ER in breast cancers [9]. Women with relatively high levels of endogenous hormones, or whose lifetime exposure to hormones is increased as a consequence of early menarche and/or late menopause, are at increased risk of developing breast cancer. Similarly, large-scale clinical trials showed that women taking hormone replacement therapies (HRT) that included estrogen and a progestin exhibited an increased risk of breast cancer [10–12]. Although estrogen treatment alone given to women who underwent a prior hysterectomy was protective, classical HRT typically contained equine estrogen and a synthetic progestin known as medroxyprogesterone acetate (MPA). The increased risk of developing breast cancer while taking HRT was fully reversible upon cessation of therapy, suggesting that combined HRT acts as a tumor-promoter (i.e. a stimulator of cell survival and/or proliferation) rather than as a mutagenic carcinogen. It is unclear whether MPA or other synthetic progestins (i.e. relative to progesterone) are the causative agents in HRT. While other large scale population studies support a role for synthetic progestins in increased breast cancer risk [13], the topic remains quite controversial [14].
Estrogen and progestins may act synergistically to promote the growth of early lesions [15–17]. Progesterone (and synthetic progestins) are proliferative in normal breast tissue and in breast cancer models [18] and progesterone, via PR-dependent paracrine signaling, is a mediator of mammary gland stem cell self-renewal and expansion [19–22]. These studies have been extended to include breast cancer stem cells [21, 22]. In addition, recent studies suggest that Her2 signaling, in cooperation with progesterone (via the action of progesterone-induced paracrine signals RANKL and Wnt), may drive the dissemination of cells from microscopic breast tumors to distant metastatic sites very early in tumor progression [23, 24]. Notably, ER and PR directly interact [25, 26] and participate in the same rapid signaling and transcriptional complexes at target genes relevant to breast cancer biology [25–27]. The presence of the B isoform of PR (PR-B) conferred estrogen-dependent gene regulation to numerous ER-target genes that required the scaffolding actions of both PR-B and PELP1 in ER-containing transcriptional complexes [26]. In estrogen treated cells, progestins dramatically altered global ER binding sites [27, 28]. These receptors also regulated up to 80% identical sets of target genes when cells were stimulated with estrogen or progestins alone [27]. While targeting PR in breast cancer is not currently standard of care, these studies and other emerging literature suggests that PR antagonists or selective PR modulators (SPRMs) that can disrupt ER signaling, may provide a viable second-line therapy for women who fail on ER targeted therapies. Such molecules could also be utilized in conjunction with current ER-directed strategies to delay or prevent endocrine resistance [29–31]. While high dose PR agonists that disrupt ER actions (i.e. including progesterone) have been suggested as useful breast cancer therapies [14], the effect of these agents on tumor cell fate (i.e. stem cell outgrowth, early dissemination, tumor heterogeneity) must be fully understood before they enter routine clinical use.
Prolactin (PRL) signaling is implicated in breast cancer
Prolactin (PRL) is a polypeptide hormone produced primarily by cells known as lactotrophs, which are located in the anterior pituitary gland of all vertebrates. In humans, PRL is also produced at multiple extra-pituitary sites and functions as a circulating cytokine hormone with both autocrine and paracrine actions [32]. The biological activities of PRL are mediated by membrane PRL receptors (PRLR), members of the cytokine receptor superfamily characterized by a non-tyrosine kinase single-pass transmembrane domain with conserved features of cytokine receptors within the extracellular domain [33, 34]. Ligand binding and activation of PRLR leads to downstream induction of the canonical Jak2/STAT5 or Jak1/STAT3 pathways, feeding into multiple signaling cascades including phosphoinositide3-kinase (PI3K)/Akt and Raf/Mek/Erk [35, 36]. Jak/Stat independent PRLR signaling can also be mediated through the Src kinases and focal adhesion kinases (FAK) [37, 38]. In the mammary gland, PRL plays a decisive role in epithelial cell proliferation and milk production [39]. The generation of PRL [39] and PRLR [40] gene knockout (KO) mice demonstrated that PRL and PRLR pathways are key regulators in mammary gland development (discussed above). In addition to its essential function in mammary gland biology, PRL has reproductive, metabolic, osmoregulatory, and immunoregulatory actions in diverse tissues [32].
The importance of PRL in breast cancer development and risk is less well-defined as compared to ovarian steroids. Similar to estrogen and progesterone, high PRL levels can augment mammary tumor development in mice [41, 42] and in women, elevated PRL levels are correlated with increased breast cancer risk and metastasis [43, 44]. In vitro studies have indicated a role for PRL in breast cancer proliferation and survival [45–49]. PRL also appears to significantly enhance the directed motility of breast cancer cells [47], in part through its activation of downstream effectors such as the NEK3 kinase, leading to cytoskeletal and focal adhesion reorganization [50, 51]. Interestingly, PRL and nuclear PRLR can enhance the expression of the estrogen (ERα) and progesterone (PR) receptors [52–54]. In contrast, studies have also shown that activation of PRLR can suppress the mesenchymal phenotype and reduce invasive behavior [55]. Loss of PRLR expression in breast cancer can be associated with poor differentiation and larger tumors [56] whereas the gene expression signatures of an activated PRL/PRLR pathway are associated with well-differentiated tumors, reduced metastasis and higher overall survival [57]. Such results indicate that PRL may have both a pro-oncogenic as well as a metastasis suppressor role in breast cancer [55]. They also support the idea of cross-talk between the actions of PRL and the steroid hormones [52].
Signaling interactions between progesterone, prolactin and STATs
As mentioned above, major downstream effectors of PR and PRLR signaling include the STAT (signal transducer and activator of transcription) family of latent cytoplasmic proteins. All members of this family possess similar protein structures including a DNA binding domain, SH2/SH3 domains and a C-terminal transactivating domain which confer functional properties [58]. Activation of STAT proteins results in phosphorylation on tyrosine and serine residues via signaling from upstream regulators like Janus Associated Kinases (JAKs; [58]) and Mitogen Activated Protein Kinases (MAPKs; [59, 60]). Tyrosine phosphorylation induces dimerization between two STAT molecules via their SH2 domains. Activated STAT dimers then translocate to the nucleus and bind to consensus promoter sequences to initiate transcription of their specific target genes.
The STAT proteins STAT1, STAT3, and STAT5 are involved in all stages of mammary gland development [61]. Genetic deletion experiments suggest that these proteins are most important in postnatal development where distinct expression and phosphorylation events are observed during gestation, lactation and involution. STAT1 has the most unique pattern of phosphorylation; highest in virgin mice and in late involuting glands. STAT1 may have a compensatory role, working in concert with STAT3, wherein STAT3 activation is essential for the regulation of cell death and inflammatory signaling during involution [62, 63]. The STAT5 protein isoforms, encoded by the genes STAT5A and STAT5B, are necessary and sufficient for alveologenesis and expression of milk protein genes during late pregnancy and lactation [64–66]. Both progesterone and PRL are important regulators of STAT proteins (Fig. 1). Activation of STAT5 through the PRLR-Jak2 pathway is critical for its functions [65, 67, 68]. Progesterone signaling activates JAK2 and increases STAT1, 3 and 5 at both the mRNA and protein level [69, 70]. PRL transcriptionally activates STAT 1, 3 and 5 (a and b) and progesterone enhances prolactin-mediated stimulation of STAT5 activation in part via amplification of convergent signaling pathway inputs to STAT phosphorylation [69, 70].
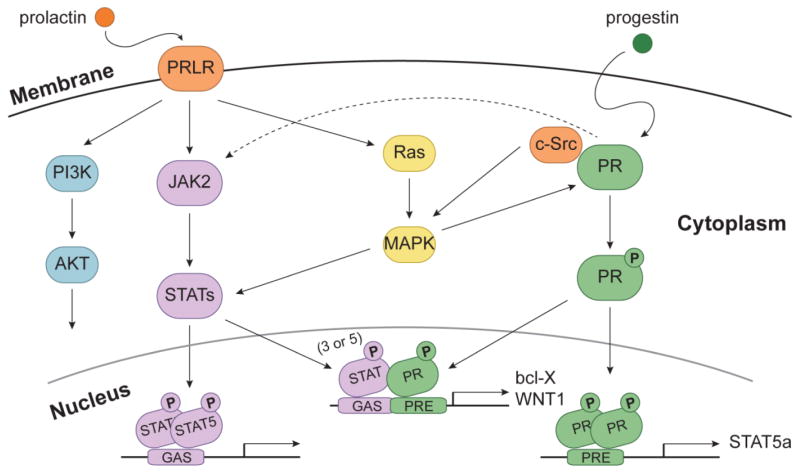
Figure 1. Convergence of PR and Prolactin Receptor (PRLR) Signaling Interactions.
Prolactin and progesterone are important regulators of STAT proteins. Prolactin-induced activation of PRLR triggers several signaling cascades. For example, signaling to JAK2 kinase activates STAT5. Activation of the MAPK pathway can also lead to activation of STATs through PRLR. Progestin-induced activation of PR leads to activation of PR genes that include the downstream components of the PRLR signaling pathway (e.g. STAT5a). In addition, progesterone enhances prolactin-mediated stimulation of STAT activation in part through the JAK2 and MAPK pathways. Progesterone-induced activation of c-Src also provides a hormone-induced input to PRLR (i.e. via STAT phosphorylation) signaling.
The functions of specific STAT proteins have important implications for their potential role in breast cancer progression. For example, STAT1 is believed to be a tumor suppressor that is often lost in ER+ tumors [61] although STAT1 knock-out mice spontaneously develop ER+ luminal mammary tumors [71]. Activation of STAT1, presumably through interferon signaling, inhibits tumor growth via upregulation of p27 [72] and via interaction with the DNA damage machinery p53 [73] and BRCA1 [74]. High levels of pSTAT1 in ER+ breast cancers from postmenopausal women are associated with greater disease-free survival [75, 76]. This suppressive role may be dependent on menopausal status since some clinical studies have observed poorer overall and disease-free survival in premenopausal women with elevated STAT1 [76, 77].
The STAT3 protein has also been found to be constitutively activated in breast cancers, especially in triple negative (ER-/PR-/HER2-) breast cancer [78], and is associated with invasive behavior and poor prognosis [79]. In vitro studies indicate that pSTAT3 induces the expression of genes that promote proliferation, survival, angiogenesis and stemness [80, 81]. Notably, in luminal breast cancer models, signaling via PR also drives similar functions of STAT3. For example, progestins activate STAT3 signaling and translocation to the nucleus [82]. Similar to earlier studies of PR cross talk with STAT5 [69, 70], progestin signaling was shown to induce expression of STAT3 and activate STAT3, Jak1, Jak2 and c-SRC. PR/STAT3 interaction drives expression of classical PR target genes (bcl-X) [83] and progestin-induced activation of JAK/STAT3 was required for progestin regulated growth of breast cancer cells and tumors in vivo. PR and STAT-containing complexes extensively cooperate with nuclear ErbB-2 receptors. An eloquent series of studies demonstrated that progestins activate an “enhanceosome” containing PR, AP1, STAT3 and ErbB-2 that translocates to the nucleus. Once there, this complex binds to DNA and induces expression of cyclin D1 to drive breast cancer cell growth in vitro and in vivo [84, 85]. In the presence of progestins, ErbB-2/PR/STAT3 transcriptional complexes also drive cell cycle progression in breast cancer cells by interaction with SP1 at Sp1 sites in the p21 promoter [83, 84].
The role of STAT5 in breast cancer is complex. Phosphorylation and nuclear localization of STAT5 in breast cancer is a positive predictor of response to endocrine therapy and patients with more activated STAT5 have decreased risk of disease recurrence and death [86]. However, STAT5 also appears to have a role in tumor formation, as TGF-in mice is delayed in the absence of the STAT5a protein [87]. This effect is also observed in an SV40-T model of mouse mammary cancer [88]. Additionally, over-expression of WT and the constitutively active form of STAT5a induced mammary tumors in mice [89] and this effect is dependent upon parity, as virgin mice do not develop tumors [90]. Interestingly, this is in contrast to the overall protective effect of parity on breast cancer prevention normally observed in mice and young (age <20) women [91]. The lack of an effect of activated STAT5a expression in virgin mice relative to parous animals may reflect the extensive cooperation between STAT5a and PR-B isoforms relative to PR-A isoforms [70, 92] (i.e. PR-A but not PR-B is primarily expressed in virgin mice). Additionally, this change is likely mediated by the increased number of luminal progenitor cells in the fully developed mammary gland due to increased PR and STAT5 signaling [93]. Similar to STAT3 (discussed above), STAT5 and PR interact and STAT5a is found with PR at PRE sites [69, 92]. PR-B but not PR-A enables STAT5a signaling. Phosphorylation of PR-B at serine 81 (a CK2 consensus site absent from PR-A) induces expression of STAT5a and in a feed forward signaling loop, STAT5a activation cooperates with pS81 PR-B to drive expression of a specific gene set co-regulated by STATs, including WNT1, thus linking this CK2-dependent PR-B signaling program to PRL/PRLR signaling [92]. Mutation of PR-B Ser81 to Ala confers PR-Alike behavior by preventing expression of numerous pS81 PR-B target genes that require STAT5a (GAS sites are significantly located near PRE sites in numerous PR-B target genes) for their increased expression [94].
Modulation of mammographic breast density via PR/PRLR signaling
In addition to hormone exposure as a major correlate of breast cancer risk, women in the highest quartile of mammographic breast density have a 1.8–6 fold increase in breast cancer risk [95, 96]. Mammographic density (MD) refers to the fibroglandular tissue of the mammary gland, composed of fibrous stroma and the epithelial cells that line the ducts. This is in contrast to the other main component of the gland, the adipose tissue. The fibroglandular tissue appears white or bright on a mammogram, whereas the adipose tissue appears dark. A higher percent MD reflects increased collagen, stroma and epithelial cells and a relative decrease in adipose cells, compared to the total breast tissue volume.
Hormone replacement therapy (HRT) and hormone therapy for breast cancer prevention and treatment can influence mammographic breast density [97, 98]. Notably, HRT that includes both estrogen and a progestin, more than estrogen alone, was associated with increased MD and a concurrent increased risk of breast cancer in both the Women’s Health Initiative (WHI) and Postmenopausal Estrogen/Progestin Intervention (PEPI) studies [99–101]. Discontinuation of HRT was associated with decreased MD [102, 103]. Similarly, endogenous hormone levels (namely, progesterone and prolactin) were reported to be associated with increased mammographic breast density [104–106]. Additionally, increased PR expression was observed in the mammary epithelial cells of women with increased MD relative to women whose MD was in the normal range [107]. Treatment with tamoxifen can cause a decrease in MD and a lower risk of breast cancer recurrence, possibly due to the loss of PR expression as an ER target gene and key downstream effector of estrogen signaling [108, 109]. Taken together, these studies suggest an important role for both PR and PRL signaling as mediators of increased MD, a reversible condition that is tightly linked to hormone action and breast cancer risk.
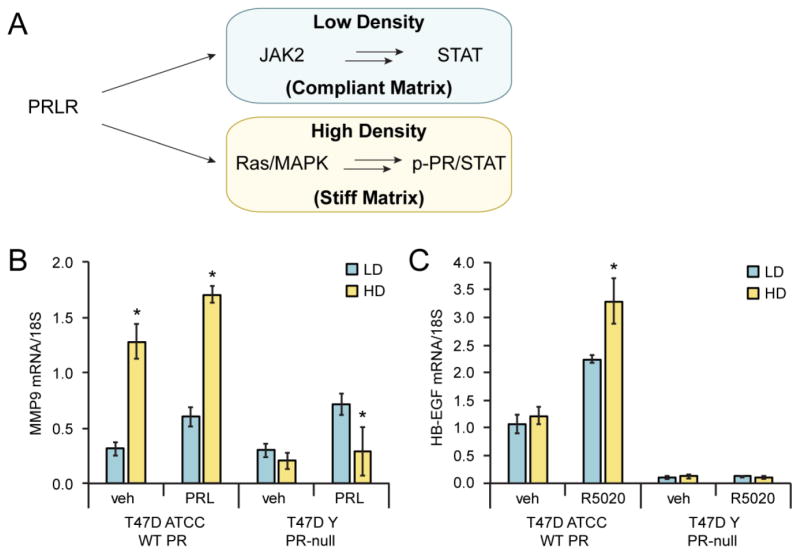
The mechanistic role of increased mammographic density and hormone signaling in breast cancer development have been investigated in in vivo and in vitro studies. Mice with defective collagen breakdown (ie. PyV MT. Col1a1tmJae) in the mammary gland have increased collagen density and three times the risk of tumor formation and metastasis [110]. In vitro modeling of mammographic density using differing concentrations of rat tail collagen I in free floating gels has been used to study the mechanisms contributing to increased tumorigenesis [110, 111]. Enhanced activation and use of distinct effector pathways downstream of PRL signaling was observed in breast cancer cell models in the presence or absence of stiff matrices [112]. For example, PRL signaling in a compliant matrix activated STAT5. However, if the same cells were instead maintained in a stiff matrix, PRL preferentially activated Src/FAK and ERK1/2 signaling. Interestingly, PRL signaling to STAT5 was inverse to its ability to activate AP1, a less well-studied PRL-induced pathway. Consistent with this finding, signaling through STAT5 reduced PRL signals to the transcription factor, AP-1; a protein complex made up of MAPK substrates (i.e. Fos/Jun) which is also known to interact with and mediate both ER and PR signaling [113].
Prolactin has also been implicated in cell migration in the context of varying matrix microenvironments (Fig. 2A). In stiff matrices, PRL activates the expression of MMPs including MMP9 in T47D ATCC cells that express PRLR, ER and PR [112]. MMP9 is a type IV collagenase that contributes to tumor invasiveness in vitro by degradation of basement membrane proteins, and elevated MMP9 expression is associated with breast cancer tumor invasiveness and metastasis [114–116]. Other studies have shown that MMP9 levels are inversely correlated with pSTAT5 levels [117]. In breast cancer cells, we discovered that PRL-induced expression of MMP9 is dependent on co-expression of PR-B. Notably, when T47D ATTC cells expressing PR-B were grown in high density collagen (HD; stiff matrix) relative to low density collagen (LD; compliant matrix), PRL treatment induced increased MMP9 expression (Fig. 2B; left). In contrast, T47D ATTC cells naturally lacking PR expression (T47D Y cells) failed to induce MMP9 expression upon exposure to PRL in stiff matrix (Fig. 2B; right). This finding suggests that PR-B (but not progesterone) is required to enable an alternative signaling pathway specifically activated by PRL in stiff matrices, such as ERK1/2 MAPKs [112]. Notably, HB-EGF is a PR-B target gene product whose progesterone-induced expression requires rapid activation of ERK1/2 MAPKs [118]. In the presence of the synthetic progestin, R5020, T47D ATCC cells cultured in LD compliant matrix showed increased expression of HB-EGF mRNA levels relative to vehicle treated controls. However, progestin-induced HB-EGF expression was further increased by exposure to a stiff matrix (Fig. 2C), consistent with the concept that ERK1/2 MAPK signaling is elevated in the context of HD stiff matrix [110]. These data illustrate a cooperative role for progesterone and prolactin in pro-tumorigenic signaling relevant to altered hormone action in a model of mammographically dense breast tissue. Understanding the details of cross-talk between these two signaling pathways may provide further insight into the role of hormonal regulation in the context of increased MD, thereby opening new avenues of research into a potential means of blocking the relevant interactions and signaling pathways in order to prevent breast cancer development in high risk women.
Figure 2. Progestin- and prolactin-induced signaling is dependent on PR expression and matrix microenvironment.
(A) PRLR signaling favors JAK2/STAT5 signaling in low density (compliant matrix), whereas high density (stiff matrix) enhances PR/STAT mediated signaling through c-SRC and MAPK pathways [112]. mRNA expression levels of matrix metalloproteinase-9 (B; MMP-9) and heparin-binding epidermal growth factor-like (C; HB-EGF) are pictured. T47D ATCC (WT; PR expressing) and T47D-Y (PR-null) cells were grown in MEM supplemented with 5% FBS, Pen/Strep, NEAA, and insulin (6 ng/ml). Cells were plated in low density (LD; 1.2 mg/ml) or high density (HD; 2.8 mg/ml) type I rat tail collagen as previously described [110]. Collagen gels were released from wells 24h after plating and serum-starved for an additional 24h. Cells were then treated with vehicle (veh), R5020 (10 nM) or PRL (8 nM) for 24h. RNA was extracted from collagen gels using Trizol and qRT-PCR analysis was performed to determine mRNA expression of MMP-9 and HB-EGF. Data shown is representative of three individual experiments (performed in triplicate).
Conclusions
Herein we have focused on PRLR and PR as inter-dependent signaling pathways relevant to both mammary gland development and breast cancer biology, whose downstream biological effects can be modulated by major determinants of increased breast cancer risk (i.e. lifetime hormone exposure, mammographic breast density, etc.). Both PR and STAT5a are key transcription factors in these pathways and have been shown to be mediators of breast cancer stem cell outgrowth [119]. This evidence, coupled with their established function in the same transcriptional complexes at phospho-PR-target genes with high cancer relevance, supports the importance of this PR-PRLR cross-talk [5]. Targeting this cooperative PRLR and PR signaling (i.e. at the level of STATs or as part of combination therapies) may provide an effective means of blocking early tumor progression by limiting the breast cancer stem cell compartment. Additionally, a deeper understanding of the details of cross-talk between these mediators and the modulation of this by matrix interactions will be critical to the development of breast cancer prevention strategies for high risk women, such as BRCA1/2 mutation carriers, where mammographic breast density is a relevant risk factor.
Highlights.
PR and PRLR signaling cooperate in breast cancer
PR/PRLR signaling may play a role in modulating mammographic breast density.
PR/PRLR pathways could offer alternative targets for breast cancer prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clarke RB, et al. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57(22):4987–91. [PubMed] [Google Scholar]
- 2.Russo J, et al. Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat. 1999;53(3):217–27. doi: 10.1023/a:1006186719322. [DOI] [PubMed] [Google Scholar]
- 3.Hammes SR, Levin ER. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152(12):4489–95. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dressing GE, et al. Progesterone receptors act as sensors for mitogenic protein kinases in breast cancer models. Endocr Relat Cancer. 2009;16(2):351–61. doi: 10.1677/ERC-08-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagan CR, et al. Role of phosphorylation in progesterone receptor signaling and specificity. Mol Cell Endocrinol. 2012;357(1–2):43–9. doi: 10.1016/j.mce.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knutson TP, Lange CA. Dynamic regulation of steroid hormone receptor transcriptional activity by reversible SUMOylation. Vitam Horm. 2013;93:227–61. doi: 10.1016/B978-0-12-416673-8.00008-3. [DOI] [PubMed] [Google Scholar]
- 7.Heldring N, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–31. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 8.Thomas C, Gustafsson JA. Progesterone receptor-estrogen receptor crosstalk: a novel insight. Trends Endocrinol Metab. 2015;26(9):453–4. doi: 10.1016/j.tem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Lim E, et al. Pushing estrogen receptor around in breast cancer. Endocr Relat Cancer. 2016;23(12):T227–T241. doi: 10.1530/ERC-16-0427. [DOI] [PubMed] [Google Scholar]
- 10.Beral V C. Million Women Study. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 11.Chlebowski RT, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573–87. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chlebowski RT, Anderson GL. Changing concepts: Menopausal hormone therapy and breast cancer. J Natl Cancer Inst. 2012;104(7):517–27. doi: 10.1093/jnci/djs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soini T, et al. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 Pt 1):292–9. doi: 10.1097/AOG.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 14.Lim E, Palmieri C, Tilley WD. Renewed interest in the progesterone receptor in breast cancer. Br J Cancer. 2016;115(8):909–911. doi: 10.1038/bjc.2016.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allred DC, Mohsin SK, Fuqua SA. Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer. 2001;8(1):47–61. doi: 10.1677/erc.0.0080047. [DOI] [PubMed] [Google Scholar]
- 16.Mote PA, et al. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72(2):163–72. doi: 10.1023/a:1014820500738. [DOI] [PubMed] [Google Scholar]
- 17.Boyle DP, Mullan P, Salto-Tellez M. Molecular mapping the presence of druggable targets in preinvasive and precursor breast lesions: a comprehensive review of biomarkers related to therapeutic interventions. Biochim Biophys Acta. 2013;1835(2):230–42. doi: 10.1016/j.bbcan.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Brisken C. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nat Rev Cancer. 2013;13(6):385–96. doi: 10.1038/nrc3518. [DOI] [PubMed] [Google Scholar]
- 19.Asselin-Labat ML, et al. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst. 2006;98(14):1011–4. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]
- 20.Asselin-Labat ML, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465(7299):798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz KB, Sartorius CA. Progestins in hormone replacement therapies reactivate cancer stem cells in women with preexisting breast cancers: a hypothesis. J Clin Endocrinol Metab. 2008;93(9):3295–8. doi: 10.1210/jc.2008-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi PA, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–7. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 23.Harper KL, et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature. 2016;540:588–592. doi: 10.1038/nature20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseini H, et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540:552–558. doi: 10.1038/nature20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giulianelli S, et al. Estrogen receptor alpha mediates progestin-induced mammary tumor growth by interacting with progesterone receptors at the cyclin D1/MYC promoters. Cancer Res. 2012;72(9):2416–27. doi: 10.1158/0008-5472.CAN-11-3290. [DOI] [PubMed] [Google Scholar]
- 26.Daniel AR, et al. Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1- and estrogen receptor-containing transcription complexes. Oncogene. 2015;34(4):506–15. doi: 10.1038/onc.2013.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singhal H, et al. Genomic agonism and phenotypic antagonism between estrogen and progesterone receptors in breast cancer. Sci Adv. 2016;2(6):e1501924. doi: 10.1126/sciadv.1501924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammed H, et al. Progesterone receptor modulates ERalpha action in breast cancer. Nature. 2015;523(7560):313–7. doi: 10.1038/nature14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutson TP, Lange CA. Tracking progesterone receptor-mediated actions in breast cancer. Pharmacol Ther. 2014;142(1):114–25. doi: 10.1016/j.pharmthera.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrieu T, et al. Detection and functional portrayal of a novel class of dihydrotestosterone derived selective progesterone receptor modulators (SPRM) J Steroid Biochem Mol Biol. 2015;147:111–23. doi: 10.1016/j.jsbmb.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Li W, et al. Current Progresses and Trends in the Development of Progesterone Receptor Modulators. Curr Med Chem. 2016;23(23):2507–54. doi: 10.2174/0929867323666160428105310. [DOI] [PubMed] [Google Scholar]
- 32.Freeman ME, et al. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523–631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 33.Kelly PA, et al. The prolactin/growth hormone receptor family. Endocr Rev. 1991;12(3):235–51. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- 34.Bachelot A, Binart N. Reproductive role of prolactin. Reproduction. 2007;133(2):361–9. doi: 10.1530/REP-06-0299. [DOI] [PubMed] [Google Scholar]
- 35.Neilson LM, et al. Coactivation of janus tyrosine kinase (Jak)1 positively modulates prolactin-Jak2 signaling in breast cancer: recruitment of ERK and signal transducer and activator of transcription (Stat)3 and enhancement of Akt and Stat5a/b pathways. Mol Endocrinol. 2007;21(9):2218–32. doi: 10.1210/me.2007-0173. [DOI] [PubMed] [Google Scholar]
- 36.Damiano JS, Wasserman E. Molecular pathways: blockade of the PRLR signaling pathway as a novel antihormonal approach for the treatment of breast and prostate cancer. Clin Cancer Res. 2013;19(7):1644–50. doi: 10.1158/1078-0432.CCR-12-0138. [DOI] [PubMed] [Google Scholar]
- 37.Canbay E, et al. Prolactin stimulates the JAK2 and focal adhesion kinase pathways in human breast carcinoma T47-D cells. Biochem J. 1997;324( Pt 1):231–6. doi: 10.1042/bj3240231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acosta JJ, et al. Src mediates prolactin-dependent proliferation of T47D and MCF7 cells via the activation of focal adhesion kinase/Erk1/2 and phosphatidylinositol 3-kinase pathways. Mol Endocrinol. 2003;17(11):2268–82. doi: 10.1210/me.2002-0422. [DOI] [PubMed] [Google Scholar]
- 39.Horseman ND, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16(23):6926–35. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ormandy CJ, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11(2):167–78. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 41.Wennbo H, et al. Activation of the prolactin receptor but not the growth hormone receptor is important for induction of mammary tumors in transgenic mice. J Clin Invest. 1997;100(11):2744–51. doi: 10.1172/JCI119820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose-Hellekant TA, et al. Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene. 2003;22(30):4664–74. doi: 10.1038/sj.onc.1206619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tworoger SS, Hankinson SE. Prolactin and breast cancer etiology: an epidemiologic perspective. J Mammary Gland Biol Neoplasia. 2008;13(1):41–53. doi: 10.1007/s10911-008-9063-y. [DOI] [PubMed] [Google Scholar]
- 44.Tworoger SS, et al. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res. 2013;73(15):4810–9. doi: 10.1158/0008-5472.CAN-13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuh G, Wells JA. Prolactin receptor antagonists that inhibit the growth of breast cancer cell lines. J Biol Chem. 1995;270(22):13133–7. doi: 10.1074/jbc.270.22.13133. [DOI] [PubMed] [Google Scholar]
- 46.Chen WY, et al. A human prolactin antagonist, hPRL-G129R, inhibits breast cancer cell proliferation through induction of apoptosis. Clin Cancer Res. 1999;5(11):3583–93. [PubMed] [Google Scholar]
- 47.Maus MV, Reilly SC, Clevenger CV. Prolactin as a chemoattractant for human breast carcinoma. Endocrinology. 1999;140(11):5447–50. doi: 10.1210/endo.140.11.7245. [DOI] [PubMed] [Google Scholar]
- 48.Schroeder MD, et al. Inhibition of prolactin (PRL)-induced proliferative signals in breast cancer cells by a molecular mimic of phosphorylated PRL, S179D-PRL. Endocrinology. 2003;144(12):5300–7. doi: 10.1210/en.2003-0826. [DOI] [PubMed] [Google Scholar]
- 49.Perks CM, et al. Prolactin acts as a potent survival factor for human breast cancer cell lines. Br J Cancer. 2004;91(2):305–11. doi: 10.1038/sj.bjc.6601947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller SL, et al. Nek3 kinase regulates prolactin-mediated cytoskeletal reorganization and motility of breast cancer cells. Oncogene. 2007;26(32):4668–78. doi: 10.1038/sj.onc.1210264. [DOI] [PubMed] [Google Scholar]
- 51.Harrington KM, Clevenger CV. Identification of NEK3 Kinase Threonine 165 as a Novel Regulatory Phosphorylation Site That Modulates Focal Adhesion Remodeling Necessary for Breast Cancer Cell Migration. J Biol Chem. 2016;291(41):21388–21406. doi: 10.1074/jbc.M116.726190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiorillo AA, et al. The prolactin receptor transactivation domain is associated with steroid hormone receptor expression and malignant progression of breast cancer. Am J Pathol. 2013;182(1):217–33. doi: 10.1016/j.ajpath.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shafie S, Brooks SC. Effect of prolactin on growth and the estrogen receptor level of human breast cancer cells (MCF-7) Cancer Res. 1977;37(3):792–9. [PubMed] [Google Scholar]
- 54.Gutzman JH, Miller KK, Schuler LA. Endogenous human prolactin and not exogenous human prolactin induces estrogen receptor alpha and prolactin receptor expression and increases estrogen responsiveness in breast cancer cells. J Steroid Biochem Mol Biol. 2004;88(1):69–77. doi: 10.1016/j.jsbmb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Nouhi Z, et al. Defining the role of prolactin as an invasion suppressor hormone in breast cancer cells. Cancer Res. 2006;66(3):1824–32. doi: 10.1158/0008-5472.CAN-05-2292. [DOI] [PubMed] [Google Scholar]
- 56.Faupel-Badger JM, et al. Prolactin receptor expression and breast cancer: relationships with tumor characteristics among pre- and post-menopausal women in a population-based case-control study from Poland. Horm Cancer. 2014;5(1):42–50. doi: 10.1007/s12672-013-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hachim IY, et al. A favorable role of prolactin in human breast cancer reveals novel pathway-based gene signatures indicative of tumor differentiation and favorable patient outcome. Hum Pathol. 2016;53:142–52. doi: 10.1016/j.humpath.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity. 2012;36(4):503–14. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord. 2006;7(4):225–35. doi: 10.1007/s11154-007-9025-5. [DOI] [PubMed] [Google Scholar]
- 60.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 61.Haricharan S, Li Y. STAT signaling in mammary gland differentiation, cell survival and tumorigenesis. Mol Cell Endocrinol. 2014;382(1):560–9. doi: 10.1016/j.mce.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapman RS, et al. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 1999;13(19):2604–16. doi: 10.1101/gad.13.19.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chapman RS, et al. A novel role for IRF-1 as a suppressor of apoptosis. Oncogene. 2000;19(54):6386–91. doi: 10.1038/sj.onc.1204016. [DOI] [PubMed] [Google Scholar]
- 64.Liu X, et al. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci U S A. 1995;92(19):8831–5. doi: 10.1073/pnas.92.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X, et al. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11(2):179–86. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 66.Teglund S, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93(5):841–50. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 67.Gallego MI, et al. Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol. 2001;229(1):163–75. doi: 10.1006/dbio.2000.9961. [DOI] [PubMed] [Google Scholar]
- 68.Wagner KU, et al. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol. 2004;24(12):5510–20. doi: 10.1128/MCB.24.12.5510-5520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richer JK, et al. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273(47):31317–26. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- 70.Lange CA, et al. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J Biol Chem. 1998;273(47):31308–16. doi: 10.1074/jbc.273.47.31308. [DOI] [PubMed] [Google Scholar]
- 71.Chan SR, et al. STAT1-deficient mice spontaneously develop estrogen receptor alpha-positive luminal mammary carcinomas. Breast Cancer Res. 2012;14(1):R16. doi: 10.1186/bcr3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang S, et al. Stat1 phosphorylation determines Ras oncogenicity by regulating p27 kip1. PLoS One. 2008;3(10):e3476. doi: 10.1371/journal.pone.0003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Townsend PA, et al. STAT-1 facilitates the ATM activated checkpoint pathway following DNA damage. J Cell Sci. 2005;118(Pt 8):1629–39. doi: 10.1242/jcs.01728. [DOI] [PubMed] [Google Scholar]
- 74.Ouchi T, et al. Collaboration of signal transducer and activator of transcription 1 (STAT1) and BRCA1 in differential regulation of IFN-gamma target genes. Proc Natl Acad Sci U S A. 2000;97(10):5208–13. doi: 10.1073/pnas.080469697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Widschwendter A, et al. Prognostic significance of signal transducer and activator of transcription 1 activation in breast cancer. Clin Cancer Res. 2002;8(10):3065–74. [PubMed] [Google Scholar]
- 76.Magkou C, et al. Prognostic significance of phosphorylated STAT-1 expression in premenopausal and postmenopausal patients with invasive breast cancer. Histopathology. 2012;60(7):1125–32. doi: 10.1111/j.1365-2559.2011.04143.x. [DOI] [PubMed] [Google Scholar]
- 77.Khodarev NN, Roizman B, Weichselbaum RR. Molecular pathways: interferon/stat1 pathway: role in the tumor resistance to genotoxic stress and aggressive growth. Clin Cancer Res. 2012;18(11):3015–21. doi: 10.1158/1078-0432.CCR-11-3225. [DOI] [PubMed] [Google Scholar]
- 78.Marotta LL, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(-) stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121(7):2723–35. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diaz N, et al. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin Cancer Res. 2006;12(1):20–8. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- 80.Bromberg JF, et al. Stat3 as an oncogene. Cell. 1999;98(3):295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 81.Barbieri I, et al. Constitutively active Stat3 enhances neu-mediated migration and metastasis in mammary tumors via upregulation of Cten. Cancer Res. 2010;70(6):2558–67. doi: 10.1158/0008-5472.CAN-09-2840. [DOI] [PubMed] [Google Scholar]
- 82.Proietti C, et al. Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src-dependent mechanism in breast cancer cells. Mol Cell Biol. 2005;25(12):4826–40. doi: 10.1128/MCB.25.12.4826-4840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Proietti CJ, et al. Novel role of signal transducer and activator of transcription 3 as a progesterone receptor coactivator in breast cancer. Steroids. 2011;76(4):381–92. doi: 10.1016/j.steroids.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 84.Diaz Flaque MC, et al. Progestin drives breast cancer growth by inducing p21(CIP1) expression through the assembly of a transcriptional complex among Stat3, progesterone receptor and ErbB-2. Steroids. 2013;78(6):559–67. doi: 10.1016/j.steroids.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 85.Beguelin W, et al. Progesterone receptor induces ErbB-2 nuclear translocation to promote breast cancer growth via a novel transcriptional effect: ErbB-2 function as a coactivator of Stat3. Mol Cell Biol. 2010;30(23):5456–72. doi: 10.1128/MCB.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peck AR, et al. Loss of nuclear localized and tyrosine phosphorylated Stat5 in breast cancer predicts poor clinical outcome and increased risk of antiestrogen therapy failure. J Clin Oncol. 2011;29(18):2448–58. doi: 10.1200/JCO.2010.30.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Humphreys RC, Hennighausen L. Transforming growth factor alpha and mouse models of human breast cancer. Oncogene. 2000;19(8):1085–91. doi: 10.1038/sj.onc.1203278. [DOI] [PubMed] [Google Scholar]
- 88.Ren S, et al. Loss of Stat5a delays mammary cancer progression in a mouse model. Oncogene. 2002;21(27):4335–9. doi: 10.1038/sj.onc.1205484. [DOI] [PubMed] [Google Scholar]
- 89.Iavnilovitch E, et al. Deregulation of Stat5 expression and activation causes mammary tumors in transgenic mice. Int J Cancer. 2004;112(4):607–19. doi: 10.1002/ijc.20484. [DOI] [PubMed] [Google Scholar]
- 90.Eilon T, Groner B, Barash I. Tumors caused by overexpression and forced activation of Stat5 in mammary epithelial cells of transgenic mice are parity-dependent and developed in aged, postestropausal females. Int J Cancer. 2007;121(9):1892–902. doi: 10.1002/ijc.22954. [DOI] [PubMed] [Google Scholar]
- 91.Merrill RM, et al. Cancer risk associated with early and late maternal age at first birth. Gynecol Oncol. 2005;96(3):583–93. doi: 10.1016/j.ygyno.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 92.Hagan CR, Knutson TP, Lange CA. A Common Docking Domain in Progesterone Receptor-B links DUSP6 and CK2 signaling to proliferative transcriptional programs in breast cancer cells. Nucleic Acids Res. 2013;41(19):8926–42. doi: 10.1093/nar/gkt706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamaji D, et al. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev. 2009;23(20):2382–7. doi: 10.1101/gad.1840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hagan CR, et al. ck2-dependent phosphorylation of progesterone receptors (PR) on Ser81 regulates PR-B isoform-specific target gene expression in breast cancer cells. Mol Cell Biol. 2011;31(12):2439–52. doi: 10.1128/MCB.01246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boyd NF, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 96.Vachon CM, et al. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(1):43–9. doi: 10.1158/1055-9965.EPI-06-0738. [DOI] [PubMed] [Google Scholar]
- 97.Boyd N, et al. Mammographic density and breast cancer risk: evaluation of a novel method of measuring breast tissue volumes. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1754–62. doi: 10.1158/1055-9965.EPI-09-0107. [DOI] [PubMed] [Google Scholar]
- 98.Boyd NF, et al. Mammographic density, response to hormones, and breast cancer risk. J Clin Oncol. 2011;29(22):2985–92. doi: 10.1200/JCO.2010.33.7964. [DOI] [PubMed] [Google Scholar]
- 99.McTiernan A, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J Natl Cancer Inst. 2005;97(18):1366–76. doi: 10.1093/jnci/dji279. [DOI] [PubMed] [Google Scholar]
- 100.Greendale GA, et al. Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Postmenopausal Estrogen/Progestin Interventions (PEPI) Investigators. Ann Intern Med. 1999;130(4 Pt 1):262–9. doi: 10.7326/0003-4819-130-4_part_1-199902160-00003. [DOI] [PubMed] [Google Scholar]
- 101.Greendale GA, et al. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95(1):30–7. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- 102.Rutter CM, et al. Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. JAMA. 2001;285(2):171–6. doi: 10.1001/jama.285.2.171. [DOI] [PubMed] [Google Scholar]
- 103.Buist DS, et al. Short-term hormone therapy suspension and mammography recall: a randomized trial. Ann Intern Med. 2009;150(11):752–65. doi: 10.7326/0003-4819-150-11-200906020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ursin G, et al. Post-treatment change in serum estrone predicts mammographic percent density changes in women who received combination estrogen and progestin in the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. J Clin Oncol. 2004;22(14):2842–8. doi: 10.1200/JCO.2004.03.120. [DOI] [PubMed] [Google Scholar]
- 105.Greendale GA, et al. Serum prolactin levels are positively associated with mammographic density in postmenopausal women. Breast Cancer Res Treat. 2007;105(3):337–46. doi: 10.1007/s10549-006-9454-y. [DOI] [PubMed] [Google Scholar]
- 106.Lee E, et al. Progestogen levels, progesterone receptor gene polymorphisms, and mammographic density changes: results from the Postmenopausal Estrogen/Progestin Interventions Mammographic Density Study. Menopause. 2012;19(3):302–10. doi: 10.1097/gme.0b013e3182310f9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heusinger K, et al. Association of mammographic density with hormone receptors in invasive breast cancers: results from a case-only study. Int J Cancer. 2012;131(11):2643–9. doi: 10.1002/ijc.27515. [DOI] [PubMed] [Google Scholar]
- 108.Cuzick J, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103(9):744–52. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 109.Ko KL, et al. Adjuvant tamoxifen-induced mammographic breast density reduction as a predictor for recurrence in estrogen receptor-positive premenopausal breast cancer patients. Breast Cancer Res Treat. 2013;142(3):559–67. doi: 10.1007/s10549-013-2726-4. [DOI] [PubMed] [Google Scholar]
- 110.Provenzano PP, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wozniak MA, Keely PJ. Use of three-dimensional collagen gels to study mechanotransduction in T47D breast epithelial cells. Biol Proced Online. 2005;7:144–61. doi: 10.1251/bpo112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barcus CE, et al. Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells. J Biol Chem. 2013;288(18):12722–32. doi: 10.1074/jbc.M112.447631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gutzman JH, et al. Stat5 activation inhibits prolactin-induced AP-1 activity: distinct prolactin-initiated signals in tumorigenesis dependent on cell context. Oncogene. 2007;26(43):6341–8. doi: 10.1038/sj.onc.1210454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ballin M, et al. Ras oncogene mediated induction of a 92 kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem Biophys Res Commun. 1988;154(3):832–8. doi: 10.1016/0006-291x(88)90215-x. [DOI] [PubMed] [Google Scholar]
- 115.Zucker S, et al. M(r) 92,000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res. 1993;53(1):140–6. [PubMed] [Google Scholar]
- 116.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755(1):37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 117.Arendt LM, et al. Prolactin-induced mouse mammary carcinomas model estrogen resistant luminal breast cancer. Breast Cancer Res. 2011;13(1):R11. doi: 10.1186/bcr2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27(2):466–80. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guy MS, et al. Progestin treatment decreases CD133+ cancer stem cell populations in endometrial cancer. Gynecol Oncol. 2016;140(3):518–26. doi: 10.1016/j.ygyno.2015.12.022. [DOI] [PubMed] [Google Scholar]