Abstract
Mitochondria serve a primary role in energy maintenance but also function to govern levels of mitochondria-derived reactive oxygen species (mROS). ROS have long been established to play a critical role in tumorigenesis and are now considered to be integral to the regulation of diverse signaling networks that drive proliferation, tumor cell survival and malignant progression. mROS can damage DNA, activate oncogenes, block the function of tumor suppressors and drive migratory signaling. The mitochondrion's oxidant scavenging systems including SOD2, Grx2, GPrx, Trx and TrxR are key of the cellular redox tone. These mitochondrial antioxidant systems serve to tightly control the levels of the primary ROS signaling species, H2O2. The coordinated control of mROS levels is also coupled to the activity of the primary H2O2 consuming enzymes of the mitochondria which are reliant on the epitranscriptomic control of selenocysteine incorporation. This review highlights the interplay between these many oncogenic signaling networks, mROS and the H2O2 emitting and consuming capacity of the mitochondria.
Keywords: reactive oxygen species, tRNA, antioxidants, tumorigenesis, signal transduction
Introduction
Mitochondria have emerged as integral participants in the regulation of cellular signaling, in part, through the generation and consumption of reactive oxygen species (ROS) under both physiologic and pathologic conditions. ROS are produced in many cellular compartments including phagosomes, peroxisomes, endoplasmic reticulum, cellular membranes, and mitochondria 1–3. Mitochondrial ROS (mROS), metabolic byproducts of normally and functionally active mitochondria as a result of electron leak during oxidative phosphorylation and reduction of molecular O24, have gained the attention of the cancer research community as their critical role in tumorigenesis continues to be unraveled.
mROS encompass a number of primary reactive species including superoxide anion (O2•-), hydroxyl radical (•OH), hydrogen peroxide (H2O2), and singlet oxygen (1O2) 5–9. Incomplete electron transfer through electron transport chain (ETC) complexes I and II results in O2•- production in the mitochondrial matrix, while electron leak at complex III generates O2•- in both matrix and intermembrane space. Intermembrane space O2•- can more readily travel to the cytosol and has been shown to modify DNA 10–13. The contribution of the different ETC complexes in the production of mROS varies when comparing healthy to pathological states as reviewed by Zorov et at (2014) 9. It is thought that under most pathologic conditions that complex I is the primary site of O2•- production while complex III generates O2•- as a result of hypoxic signaling and hypoxia inducible factor (HIF) activation in both pathological and physiological instances 14. Mitochondrial-localized NADPH-oxidase 4 (Nox4) also produces mROS predominantly in the form H2O215,16. Nox4 is implicated in the pathophysiology of numerous disease processes and its inhibition can induce mesothelioma cell apoptosis9,17; however the signaling events that drive the latter process are yet undefined. In addition, monoaminoxidase (MAO), an important flavoprotein resident of the outer mitochondrial membrane, is another H2O2 generator during ischemia/reperfusion injury in the brain and heart 18–20. As noted, there are multiples sources of mROS with the capacity to modulate cellular physiology in both beneficial and deleterious ways.
As both ROS production and scavenging are necessary to maintain cellular health, different antioxidant systems such as SOD2, Grx2, GPrx, Trx and TrxR, play coordinate roles in preserving redox balance. Disruption or overpowering of the antioxidant systems can lead to oxidative stress that induces damage to biomolecules that participate in numerous disease processes including cancer 21–25.
Historically, tumors were associated with high levels of ROS that induce tumorigenesis through DNA damage. In addition tumors are associated with a switch in the metabolic activity of the mitochondria called the Warburg effect (aerobic glycolysis with lactate production)26,27. While those associations remain true, new discoveries have increased the interest of the scientific community in unraveling the role of the mitochondria in cancer pathophysiology, as mROS production, redox regulation, and apoptotic signaling are all linked to cancer etiology 28. The exploration of mitochondria's beneficial and deleterious effects in cancer's pathophysiology through mROS regulation is an active research area. This review provides a summary of the mitochondria's role in the ROS regulation of cancer.
mROS in physiological cellular regulation
In physiological conditions mROS participate in the regulation of a diverse array of signaling networks. H2O2-mediated cysteine oxidation is the primary mode by which mROS participate in regulating proliferative and survival signals 29–32. Cellular proliferation can also be regulated by H2O2 through different mechanisms such as: phosphatase with sequence homology to tensin (PTEN) inactivation 33,34, activation of cyclin dependent kinase 1 (Cdk1) 35 (Lim 2015), inhibition of the protein tyrosine phosphatase (PTP1b) and mitogen-activated protein kinase (MAPK) phosphatases 36,37, propagation of growth factor cascades by activation of Lyn and Syk kinases 38, and positive regulation of the transcription factor activator protein 1 (AP-1) through c-Jun binding at the collagen production regulator CCN1 promoter site 39.
In addition to cellular proliferation, angiogenesis is induced by the upregulation of vascular endothelial growth factor (VEGF), by mROS transcriptionally and through mROS-induced HIF stabilization 40–42. mROS-induced HIF stabilization allows for its binding to hypoxia response element (HRE) to express hypoxic adaptation genes 43,44 in an adaptive response to low oxygen levels.
Therefore, while high levels of ROS have traditionally been considered harmful, evidence shows that the mitochondria's role in regulation of ROS production and homeostasis is crucial for maintaining normal cellular function.
mROS in cancer progression
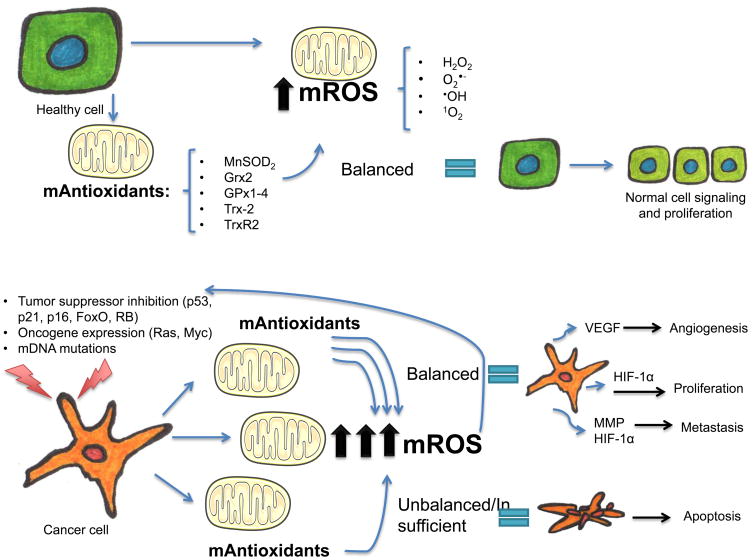
Oxidative stress, or the abnormal accumulation of ROS has long been associated with several disease processes including cancer 45–49. In fact the characteristic unrestricted growth pattern of tumor cells in response to ROS accumulation has been the focus of interest in several recent studies 50,51. It has also been established that while ROS may have a mitogenic effect in tumors50,51, at higher levels they can induce damage in cancer cells and induce apoptosis or necroptosis if not counteracted by antioxidant systems 27,52–55 (Figure 1). This shows that in terms of cellular growth and proliferation, just as healthy cells, cancer cells need to achieve a delicate redox balance to ensure survival.
Figure 1. Mitochondrial redox control in healthy and cancer cells.
ROS produced in the mitochondria, when balanced by the mitochondrial mediated antioxidant system, have several roles in healthy cell signaling pathways and cellular proliferation. An altered gene expression is seen in cancer cells, that have both an increased production of mROS, and an active antioxidant system to maintain a steady proliferative rate. In cancer cells a balanced production and scavenging of mROS allows the cell to perpetuate and induce more altered gene expression, and induce cellular mitosis, angiogenesis and metastasis through mROS mediated mechanisms, all in favor of cancer cell survival. In contrast, when the oxidant scavenging system is overpowered by mROS production in cancer cells, an oxidative-induced apoptosis occurs.
mROS-mitochondrial reactive oxygen species, H2O2—hydrogen peroxide, O2•- -superoxide, •OH-hydroxyl radical, 1O2-singlet oxygen, mAntioxidants-mitochondrial antioxidants, MnSOD2-manganese superoxide dismutase, Grx2-Glutaredoxin-2, GPX1-4-Glutathione peroxidases 1 and 4, Trx-2- thioredoxin 2, TrxR2-thioredoxin reductase, RB-retinoblastoma gene, mDNA-mitochondrial DNA, VEGF-vascular endothelial growth factor, HIF-1α-hypoxia inducible factor 1α, MMP- matrix metalloproteinase.
As cancer cells utilize the mitogenic effects of ROS to induce cellular proliferation, several mechanisms exist to ensure an adequate ROS supply. Deactivation of tumor suppressor genes 56–58, oncogene expression 59 and mutations in mitochondrial DNA (mDNA) 60 are some mechanisms that are ROS-regulated and that can in turn regulate tumorigenic ROS production to ensure such supply.
Tumor suppressor genes that regulate cell proliferation, differentiation, apoptosis, and other vital cellular activities respond to oxidative stress by regulating both antioxidant and pro-oxidant responses as reviewed by Vurusaner 2012. In particular, mROS-mediated regulation of the tumor suppressors p53, p21, p16, FoxO, retinoblastoma (RB) and breast cancer susceptibility genes 1 and 2 (BRCA1 and BRCA2) 56 has been shown. p53 for example can both promote and limit ROS production to induce apoptosis or restrict DNA damage, respectively 61,62. The FoxO family of transcription factors controls antioxidant levels and both regulates and is regulated by ROS and mROS through acetylation, phosphorylation, and ubiquitination 63. FoxOs can promote apoptosis in response to chemotherapy and can also induce cell quiescence 63. In breast cancer cells the inhibitory phosphorylation of FoxO is induced by ROS-dependent protein tyrosine phosphatase PTPN12 oxidation, promoting tumorigenesis 64. Also, Sirtuin 3 (SirT3), a mitochondrial localized tumor suppressor, 65 has been shown to decrease tumorigenesis in part by inhibiting mROS production and HIF-1α activation in fibroblast and colon carcinoma cell lines 66.
Oncogene expression may also affect mROS generation and tumor proliferation. The Ras and Myc oncogenes for instance, promote oxidant production in mitochondria and other organelles and can modulate tumorigenic and migratory signaling 57,67–69. Oncogene-driven ROS production induces mDNA mutations and mitochondrial dysfunction further enhancing ROS levels and apoptosis 70,71. In a murine model of lung cancer, complex III 57, and complex I 72 were shown to be the major source or ROS required for Kras mediated anchorage-independent cell growth 73.
Mitochondrial DNA instability is another mechanism that contributes to tumorigenesis in a canonical Wnt/β-catenin independent pathway that involves increased mROS production and oxidative mDNA damage as shown in a mouse model for intestinal cancer, 74. Further both mitochondria-generated O2•- and H2O2 have been shown to induce mutations in the gene of mitochondrial complex I's nicotinamide adenine dinucleotide dehydrogenase subunit 6 (ND6) in HepG2 cells75. This is consistent with the findings by Ishikawa et al. (2008) 72 where mutations in the gene encoding ND6a increased the metastatic potential of mouse tumor cell lines which was reversed by pretreatment with ROS scavengers. Moreover, inhibition of complex I in osteosarcoma cell lines results in increased mROS production and AKT activation which promotes cell survival 76.
Other ROS-mediated pathways in cancer include the activation of kinases, inhibition of phosphatases, and regulation of phosphoproteins and proteinases 77–81. Some other pathways are shared between healthy and cancer cells in term of cellular proliferation. As an example, the mechanisms by which mROS induce angiogenesis during hypoxia via mROS-induced HIF-1α stabilization, are also utilized by cancer cells to induce tumor growth and proliferation with deleterious effects to the host 66,82,83. HIF-1α has been associated with invasiveness of several types of cancer 25,84–87. In addition, matrix metalloproteinases (MMPs), a group of endopeptidases that hydrolyze extracellular matrix (ECM) components, have long been known to participate in tumor progression 88–90. As both MMPs and HIF-1α may play critical roles in metastatic disease progression, and mROS play critical roles in the regulation of both MMPs 91,92 and HIF-1α 93, it is very likely that their contribution to tumorigenesis may be controlled by redox-dependent programming under mitochondrial control.
Mitochondrial antioxidant systems
It is known that a precise regulation of ROS formation and scavenging is crucial for maintaining cellular and organismal homeostasis. For this purpose several enzymatic and non-enzymatic processes occur in order to coordinate the conversion of molecules from highly reactive into less reactive ones 9,24. Examples of these processes are the oxidant-scavenging activity that occurs through the superoxide dismutases (SOD), peroxidase, and catalase enzymatic systems at distinct cellular locations 94. For instance, reactive O2•- is converted into H2O2, by superoxide dismutase (SOD)1 in the cytosol, by SOD2 in the mitochondrial matrix, and by SOD3 in the extracellular space 24,95. Reduction of H2O2 into H2O restricts reactive •OH formation through fenton chemistry and is regulated by both the catalase and the peroxidase systems such as the thioredoxin/ thioredoxin reductase/peroxiredoxin (Trx/TrxR/Prx) and the glutathione/glutathione peroxidase (GSH/GPx) systems 94 (Figure 2). These redox systems seem to be independently regulated, and allow for the selective signaling regulation of the redox state 96. Moreover, the compartmentalization of some of these systems also denotes a localized effect of their regulating functions; for instance, the Trx2/TrxR2/Prx3 system in the mitochondria is an independent system from the Trx1/TrxR1/Prx system in the cytoplasm and the nucleus. The GSH system on the other hand is not exclusive of the mitochondria as it interacts with the cytosolic GSH system to enact its effects97.
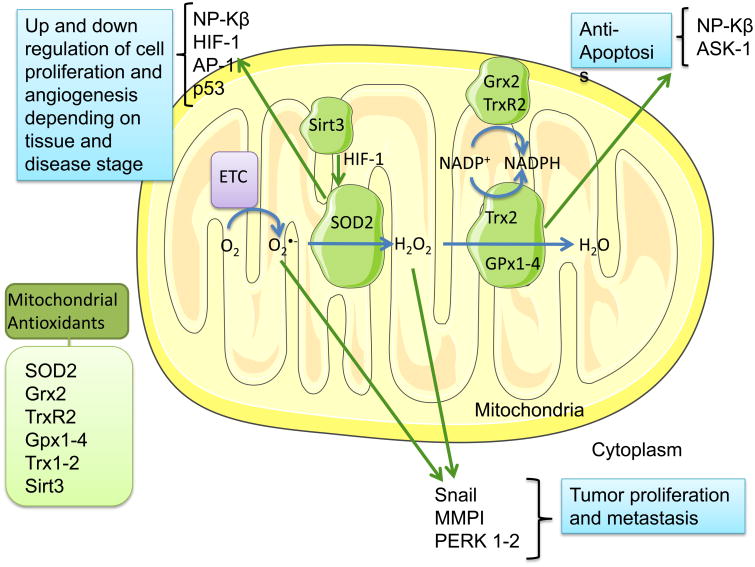
Figure 2. Mitochondrial detoxification systems in cancer.
The normal activity of the mitochondria's electron transport chain (ETC) produces reactive oxygen species (ROS), in particular O2-, whose conversion into the less reactive H2O2 is catalyzed by the mitochondrial superoxide dismutase 2 (SOD2). Both O2- and H2O2 can promote tumor proliferation and metastasis via Snail, MMPI and Perk1-2 regulation, thus other mitochondrial oxidant scavenger systems area activated to decrease the damaging effects of ROS. For this purpose, Gpx 1 and 4, and Trx 2, metabolize H2O2, while Grx2 prevents Trx2's oxidation, allowing its detox activity. Trx2 via NP-Kβ and ASK-1 can have an anti-apoptotic effect in cancer cells. SOD2's antioxidant activity is increased by Sirt3 via HIF-1 stabilization and lysine deacetylation. SOD2 is also involved in the regulation of cell proliferation, transformation, and angiogenesis by mediation of the transcriptional factors NP-Kβ, HIF-1, AP-1 and p53, which can have varying and contrasting effects depending on cancer type and stage of the disease.
The detoxification from ROS in the cytoplasm, or mROS in the mitochondria, is the main purpose of these antioxidant systems. When H2O2 is not cleared by these systems, it can produce protein thiol oxidation, which alters cellular signaling pathways for cellular division, differentiation and apoptosis 50. The protein thiol oxidation can be reversed by Trx and Grx, both of which depend on TrxR and GSH for reduction 98. In turn, both TrxR and GSH's reduction depends on NADPH oxidation to maintain a cellular redox balance. The thiol-reducing activity of GSH and Trx is then crucial for the other antioxidant elements to function properly. In addition to its role in the regulation of the cellular redox state, both Trx1 and Trx2 can activate an apoptotic response via forming a complex with apoptosis signal-regulating kinase 1 (ASK-1) in response to oxidative stress 99.
It is no surprise then, that while the mitochondrial production of ROS promotes tumor cell proliferation and metastasis, efficient ROS scavenging often inhibits cell proliferation in distinct cancer cells types and has been used to assign a tumor suppressor function to a number of ROS mitigation newtworks28. Thus, a special interest continues to develop in the mitochondria's antioxidant systems and their role in cancer pathophysiology as described below.
SOD2 / MnSOD2
Manganese superoxide dismutase (SOD2) is a mitochondrial antioxidant enzyme that catalyzes the conversion of O2•- to H2O2 100,101. SOD2 contributes to the regulation of cell proliferation, transformation, migration, invasion and angiogenesis primarily through the redox-dependent modulation of the transcription factors NF-KB, HIF-1, AP-1 and p53 100,102–104. SOD2 deficiency has been shown to have both pro- and antitumorigenic activity 102,105–107. High SOD2 expression can inhibit cell proliferation directly 108 or sensitize cells to the cytotoxicity of the anti-cancer drugs 109. In contrast, the SOD2-dependent production of H2O2 enhances the malignant properties of tongue squamous cell carcinoma cells by increasing Snail, MMP1, and pERK1/2 protein levels and repressing E-cadherin 110. SOD2 can also promote epithelial to mesenchymal transition (EMT) 111in breast cancer cells, which promotes tumor migration. Increased expression of SOD2 in tumor cells can also contribute to anoikis resistance 112, a type of apoptosis induced by disruption of cell-extracellular matrix contact 113, prolonging tumor cell's survival. As reviewed by Hempel et al (2011), a bimodal role for SOD2 during tumorigenesis is now considered. While SOD2 may have tumor suppressor activity during the initial stages of tumor progression, at later stages SOD2 levels appear to positively contribute to metastatic disease progression 108.
In a recent comprehensive meta-analysis a relation between SOD2 polymorphism and the development of non-Hodgkin lymphoma, lung cancer, and colorectal cancer was found 114 supporting the potential of SOD2 as a cancer biomarker 115. In addition, SOD2/catalase and SOD2/GPx1 ratios have been recently proposed as biomarkers for tumor progression and metastasis in several types of cancer 116. Considering the different roles of SOD2 in cancer progression, metastasis and inhibition according to different cancer types and stages108, careful assessment of SOD2 as a therapeutic target is indicated.
Grx2
Glutaredoxin-2 (Grx2) is another mitochondrial antioxidant system crucial for thiol/disulfide redox homeostasis 117. Grx2 system has been shown to be associated with anti-apoptotic signaling by protecting Trx2/1 from oxidation in HeLa cells118. Grx2 is also associated with regulation of angiogenesis in embryonic cells 119 and may have a similar function in tumor cells. Grx2 silencing sensitizes HeLa cells to death by anti-cancer drugs 120. Thus, the Grx2 system has an anti-apoptotic function via thiol redox modulation in several cancer cell models28.
GPx-1 and GPx-4
Glutathione peroxidases (GPx) are another group of isoenzymes capable of metabolizing H2O2, using reduced glutathione (GSH) as a cofactor. From this group, GPx-1 and GPx-4 are found in mitochondria 28. Both GPx-1 and GPx-4 are selenoproteins that use selenocysteine as a key active site amino acid 121. GPx-1 overexpression suppresses intracellular ROS122 which attenuates growth factor receptor activation mediated by oxidative stress 123, resulting in decreased cellular proliferation 122. Moreover, the loss of heterozygosity of the Gpx1 gene located on chromosome 3p is a prevalent event during early carcinogenesis in many types of cancers including lung 124, head and neck 125, breast 126 and colon cancer 127. In GPx4, a single nucleotide polymorphism within the 3'UTR has been linked to an increase risk of colorectal cancer 128. While in normal cells GPx4 prevents necroptosis 129, in cancer cells overexpression of GPx4 decreases the growth of fibrosarcoma and pancreatic cancer cells while having no effect on melanoma cell growth 130. Similar to other antioxidant systems, the mechanisms related to GPx and its effects on tumors, are not yet fully understood.
Trx2/TrxR2/Prx3
The mitochondrial thioredoxin/ thioredoxin reductase/peroxiredoxin (Trx2/TrxR2/Prx3) system, with detoxifying effects via inhibition of mROS131, consists of a unique thioredoxin (Trx2) that is reduced via NADPH by its unique corresponding thioredoxin reductase (TrxR2), and by a corresponding peroxiredoxin (Prx3) that depends on Trx2 for its reduction after the resulting oxidation from its H2O2-scavenging functions50,97. The Trx/TrxR thioredoxin systems modulate thiol-dependent thiol-disulfide exchange reactions that control cell growth, proliferation, and other cellular functions 132.
The importance of this axis as a H2O2 scavenging system in the mitochondria, has been shown by increased sensitivity to ROS-inducing toxins 133, and by the presence of lethal phenotypes in mice lacking any of the Trx2/TrxR2/Prx3 components 134. Besides having a key role in mitochondrial redox homeostasis, Trx2 also exerts a redox-dependent regulation of transcription and signaling factors that inhibit apoptosis through NF-Kβ 97 and ASK-1 pathways 135. In HeLa cells, Trx2 reduces TNFα-mediated mROS production and apoptosis, with inhibition of subsequent signaling pathways 97,135.
TrxRs are a group of selenoproteins that are important for maintaining cellular redox balance and eliminating ROS. TrxR2 is the mitochondrial isoform of the three known TrxRs found in mammals. TrxR2 catalyzes the NADPH-dependent reduction of Trx2, which in its reduced state protects against elevated levels of ROS within the mitochondria 136. It has been reported that TrxR2 expression is highly elevated in liver cancer 137, TrxR1 levels on the other hand, the cytosolic isoform, are upregulated in many different cancers including breast 138, thyroid 139, prostate 140, liver 141, melanomas 142 and colorectal, where strong overexpression of both TrxR and Trx may correlate with overall tumor aggressiveness 143, perhaps through the HIF-1 pathway144. The elevated level of the enzyme is an adaptation to the increased ROS production resulting from the higher metabolic activity of cancer cells 145. The TrxRs have become an important molecular target in cancer treatment, since its inhibition results in an increased susceptibility in regards to cytotoxicity and cell death 146. Single nucleotide polymorphism (SNP) in both TrxR1 and TrxR2 have been found to be associated with the risk of developing colorectal tumors 147,148.
Peroxiredoxins are a group of peroxidases that reduce peroxides with conserved cysteine residues 149. Of the 6 mammalian isoforms, Prx3 and Prx5 (also found in peroxisomes) localize in the mitochondria 150. Prx3, member of the Trx2/TrxR2/Prx3 axis, is the major target of the H2O2 generated in the mitochondrial matrix, and its inhibition has shown to sensitize cells to apoptosis 150. Prx3's expression is upregulated in prostate151, colon152, and cervical153 cancer, and some studies suggest it has an important role in the regulation of ROS-induced apoptosis in antiandrogen-resistant cells, which may convey its potential as a therapeutic target in prostate cancer151. Moreover, in a malignant mesothelioma cell line, it was established that Prx3 levels allowed cells to thrive in response to elevated mROS levels, and that any alteration in the redox activity of Prx3 impaired cell proliferation pausing the G2/M phase 154, showing that this important peroxidase allows for proper cell cycle dynamics in this particular cancer cell line.
Determination of ROS and antioxidant concentrations
As the balance between oxidative stress and the antioxidant systems play a crucial role in cellular homeostasis and cancer pathophysiology, the quantification of both ROS and antioxidant levels has become of increasing interest to scientists. As an example, in murine and human breast cancer models, cancer stem cells (CSC), unlike other cancer cell lines, display lower ROS levels than their corresponding non-cancerous cells. The low ROS burden in the CSC's is associated with increases in endogenous antioxidant systems and confers radiation-resistance that is reversed by pharmacologic depletion of antioxidants 155. In this case, the quantification of ROS and the antioxidant systems in CSC's and their non-tumor counterparts proved pivotal in defining strategies to reverse limit their therapeutic resistance.
As indicated above, the redox properties greatly vary between different cancer types, which makes the quantification of ROS and the antioxidant system's activity a potentially useful parameter to determine each cancer type's response to determined therapies. We are not aware of any broad quantitative ROS profiling of tumor cells but our own studies and those of other groups indicate that the concentrations of steady-state [H2O2] (SS-[H2O2]) in select tumor cell studies range anywhere from 5-50 picomolar156,157. Metastastic bladder tumor cells display a near 2-fold (18-31 pM) increase SS-[H2O2] when compared to their non-metastatic parental counterpart156. It is possible that chemotherapeutic strategies which both augment metabolic H2O2 production and limit ROS detoxification may allow for SS-[H2O2] to exceed these picomolar quantities and drive tumor cell death.
The ability to develop chemotherapeutic strategies based on the intrinsic redox-state of a particular cancer is reliant on precise monitoring of cellular ROS levels. For this purpose, several direct and indirect methods are used to measure oxidative stress, and their advantages and disadvantages have been reviewed by Poljsak et al 158. One of the disadvantages of direct quantification of free radicals is that the high reactivity and short half-life of such molecules, make it difficult to achieve steady concentrations of ROS in the micromolar range, limiting their accurate measurement with tools such electron spin resonance (ESR) in patients158.
Traditional indirect methods to quantify oxidative stress focus on detecting either more stable ROS intermediates or tracers of free radical damage in biomolecules158,159. Indirect methods include the measurement of total antioxidant status by colorimetric, enzymatic, fluorescent and immune methods, the measurement of endogenous enzymatic and non-enzymatic antioxidant systems160, and the fingerprinting methods through high performance liquid chromatography, gas-liquid chromatography and colorimetric tests that are able to measure markers of oxidative DNA damage, lipid peroxidation and protein damage 158. Markers of oxidative stress damage include 8-OHdG (8-hydroxyguanosine), double-strand DNA breaks, 4-HNE (4-Hydroxynonenal), MDA (Malondialdehyde), PCC (protein carbonyl content), 3-Nytrotyrosine, advanced glycation end products and others158,161.
A new generation of redox-sensors include genetically encoded probes or direct chemical sensors. These novel sensors can provide sensitivity to monitor near picomolar fluxes in SS-[H2O2] and we refer the reader to detailed reviews on this topic162,163.
The quantification of antioxidants and antioxidant systems in patients has its own challenges. The direct measurement of specific antioxidants is not only expensive but may fail to account for synergistic effects of antioxidants and leave many key antioxidants unmeasured158. Also, the changing values of oxidative stress markers over time and from patient to patient make it difficult to establish typical oxidative stress reference values 164,165. Therefore, while there is a continued effort to improve and expand the methods to quantify the oxidative stress status in an effective, efficient, cost permissive and accurate manner, to date, there is not a single established preferred method that would prove useful in clinical settings.
Targeting mROS and anti-oxidant systems in anticancer therapy
Strategies which target mitochondrial metabolism has been shown to be effective in a number of different clinical cancer studies and these findings are reviewed in 166,167. However, targeting mitochondria redox activity as a therapeutic cancer target is still in development. As discussed above, ROS can both assist or limit tumor cell proliferation. While tumor cell lines increase their production of ROS some cell lines engage antioxidant networks to ensure that ROS levels do not surpass a fatal threshold 27,52–55,168,169. Therefore, it is not surprising that treatment of cancer with dietary antioxidants has been successful in some studies while ineffective or detrimental in others. For example, while supplementation with carotenoids may increase mortality in breast cancer patients 170, supplementation with vitamin C and E in the same patients was associated with reduced recurrence rate 170,171. Vitamin C also potentiates the anti-proliferative effect of doxorubicin in breast cancer 172 while high vitamin D levels are associated with increased survival in colorectal cancer patients 173.
Despite these findings, when translated into human clinical trials, dietary antioxidants lack consistent beneficial effects 174–177, probably due to a generalized rather than a localized mROS targeting 166. Alternate strategies include the use of synthetic mitochondria-targeted antioxidants to inhibit tumor cell growth and promote apoptosis. For instance, inhibition of cell proliferation and induction of apoptosis was achieved in pancreatic cancer cells with the mitochondrial antioxidants Mito-CP, and Mito-CP-Ac, by altering mitochondrial and glycolytic functions, and intracellular citrate levels 178. In addition, Mito-Q and Mito-chromanol can selectively inhibit proliferation of different xenograft models of tumorigenesis as reported by Cheng et al 179. Moreover, cell growth inhibition and apoptosis was induced by decreased mitochondrial superoxide via the mitochondria superoxide scavenger MitoTEMPO in melanoma cells, while sparing healthy fibroblasts 180, allowing for enhanced tumor cell killing while limiting cytotoxicity in healthy tissue. Furthermore, the mitochondria deacetylase SirT3, mentioned previously in this review, which increases SOD antioxidant activity by lysine deacetylation and HIF-1α stabilization 181, can also attenuate tumorigenesis in cancer cell lines 66.
Considering that the targeted inhibition of mROS has shown to be beneficial as anti-cancer therapy, it would be reasonable to conclude that enhancing the activity of the natural antioxidant mechanisms in the mitochondria would convey the same results. However, the elevated expression of the natural antioxidants TrxR, in particular the mitochondrial TrxR2, has been encountered in several types of cancer, and in some instances has been correlated to tumor aggressiveness 138–143. Similarly, Grx2 has been shown to have an anti-apoptotic effect in tumor cells 28. Moreover it has been established that decreases in glutathione levels in murine breast cancer do not impede tumor development but increase Trx activity as a compensatory shift to buffer ROS levels 168. This latter observation suggests that cancer cells have the capacity to survive and adapt to glutathione inhibition by augmenting antioxidant function of the mitochondria. Targeting Trx2 has proven to be useful in inhibiting multiple myeloma growth by restricting proteasome function and promoting cytotoxic oxidative stress 182. In addition, Grx2 down regulation can sensitize cells to the cytotoxic effects of chemotherapy 120.
In general, while anti-oxidant cancer therapy is justified by ROS's role in cancer initiation, promotion and progression, pro-oxidant cancer therapy is also justified by ROS's role in inducing apoptosis and reversing chemo- and radio-resistance in tumors 183. This paradox has raised the concern for the use of ROS- and antioxidant- targeted therapies, especially since effectiveness of this treatment seems to be dependent on the specific environment in which the cell exists, including its base oxidative stress status183. Some authors propose the creation of a “redox signaling signature”, comprised of different parameters including redox status, expression of antioxidants, cell signaling and transcription factor activation profiles, as a reference to determine if anti-oxidant or pro-oxidant therapy would be effective in the treatment specific type of cancer183. This strategy would still prove challenging as ROS levels seem to vary even within the same type of cancer, and as previously discussed, the quantification methods still need to be improved183.
In conclusion, to inhibit growth and induce apoptosis, both targeting the tumor's mROS and ROS-scavenging systems can elicit anti-cancer effects. Reducing the levels of mROS impedes survival signaling, while truncating the cancer's cell antioxidant armature induces cell death 182. Therefore, in determining whether to take a pro-oxidant or anti-oxidant route for cancer therapy, the elucidation of a “redox signaling signature” may be critical in this decision making process. The development of accurate and specific reference parameters for determining the redox status of specific cancer types is still greatly needed.
Epitranscriptomic control of mROS
Recent work indicates that mitochondria are key to the regulation of cellular H2O2 consumption through Trx and glutathione dependent pathways, and that large changes in H2O2 efflux comes from altering the activity of mitochondrial matrix consumers185,186. The predominant matrix H2O2 consumers are the peroxiredoxins and glutathione peroxidases whose activity is indirectly or directly reliant on selenocysteine utilization, respectively186. Selenocysteine is the 21st amino acid and does not contain a dedicated codon. Selenocysteine incorporation during translation requires UGA-stop-codon recoding, which uses specifically modified tRNA for accurate decoding 187. Dynamic changes in tRNA modification are an epitranscriptomic signal because they regulate gene expression post-transcriptionally (i.e., during translation elongation), (Figure 3). It has been shown that the stress-induced translation of many selenocysteine containing ROS detoxifying enzymes is dependent on the Alkbh8 tRNA methyltransferase and the tRNA modification 5-methoxycarbonylmethyl-2′-O-methyluridine (mcm5Um) 188,189. Alkbh8 enzymatically methylates the uridine wobble base on tRNASelenocysteine to promote UGA-stop codon decoding. Also, Alkbh8 protein, stop-codon recoding and Alkbh8-dependent uridine wobble base modifications are increased in response to ROS stress (H2O2 or rotenone) to improve the translation of selenocysteine containing GPx and TrxR enzymes189. Thus it has been demonstrated that regulation of the ROS response is under epitranscriptomic control. Loss of Alkbh8-/- decreases the levels of many GSH metabolizing selenoproteins, promotes increased ROS and DNA damage levels, and confers enhanced sensitivity to oxidizing agents 189. Interestingly, over-expression of Alkbh8 has been identified in human bladder cancer models and invasive carcinomas, with in situ silencing of Alkbn8 suppressing invasion, angiogenesis and tumor growth in xenograft models 190. As Alkbh8 is a key node in the regulation of cytoplasmic and mitochondrial H2O2 via selenoprotein regulation, cancer cell addiction to increased selenoproteins may be a coping mechanism that could be exploited therapeutically.
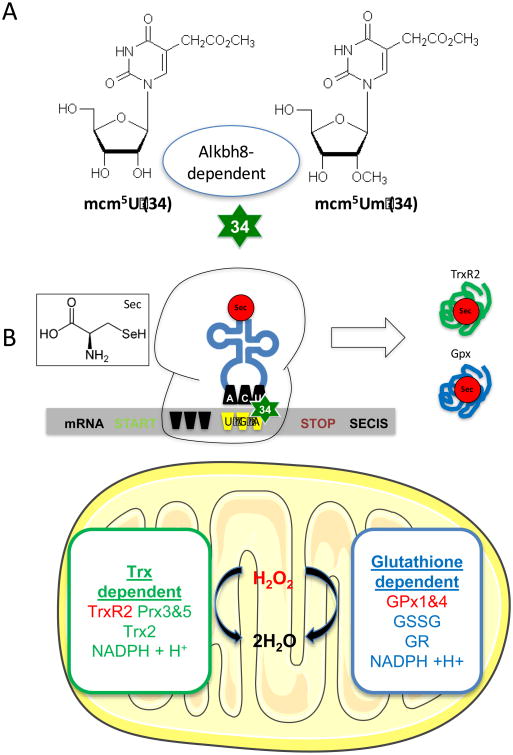
Figure 3. Epitranscriptomic control of mitochondrial ROS detoxification systems.
A. The mcm5U and mcm5Um modifications on the wobble position (34) of tRNASec are dependent on the methyltransferase activity of Alkbh8. B. Sec does not have a dedicated codon for use during translation, and its incorporation into a growing peptide utilizes the process of UGA stop codon recoding. The translation of selenoproteins requires transcripts with an internal UGA codon and a 3′ untranslated region (UTR) that contains a selenocysteine insertion sequence (SECIS). The Alkbh8 dependent mcm5Um modification has been shown to be increased in response to H2O2 exposure with mitochondrial specific TrxR2 and Gpx H2O2 detoxification protein levels dependent on Alkbh8 activity [171].
Preclinical studies suggest that drugs which affect glutathione metabolism can limit the most common renal malignancy, clear cell renal cell carcinoma 191. Moreover, GSH biosynthesis is significantly enhanced in patients with a mutation in fumarate hydratase which is associated with a highly malignant form of renal cancer192. Gottlieb and coworkers demonstrated that adducts formed between fumarate and glutathione that are observed as a result mutations in the TCA cycle enzyme fumarate hydratase (FH) disrupt glutathione metabolism leading to oxidative stress and cellular senescence 192. The FH mutation is commonly associated with a highly malignant form of renal cancer that was mimicked in mice dually deficient for FH and the senescence regulator p21, indicating that, in this model, senescence serves to restrict initiation of these renal cancers. Thus, it appears that disruption in mitochondrial GSH metabolism is met by engagement of the senescence which serves to restrict the emergence of cells with oncogenic potential. Future work will define whether epitranscriptomic defects in selenocysteine utilization leads to the engagement of the senescence and a shift in mitochondrial function, which would serve to restrict oncogenic activity and limit mROS production.
Conclusions
The metabolic state of the mitochondria has long been known to be altered in tumor cells relative to normal tissue because of the cancer cells limited access to both molecular oxygen and fuel sources. As outlined above it appears that the mitochondria also adapt to fluxes in ROS production which are either self-generated or from extra mitochondrial sources. It is not surprising that mitochondria through reactive thiols would serve as sentinels to any cellular redox changes as they are the primary sites for both generating and consuming the primary ROS signaling intermediate, H2O2. As a myriad of signaling networks have emerged as targets of ROS control it is very likely that in these many instances the mitochondrion is critical in signal regulation. The mitochondrion is a dynamic organelle and often juxtaposed intracellularly to regions of high energy demand. It is also likely that its cellular compartmentalization is dually purposed to engage coordinated redox-sensitive signaling nodules that are key for optimizing mitochondrial function. Under conditions where control of mitochondrial function and redox-signaling become discordant, as in response to an oncogenic or carcinogenic insult, the cell and mitochondria adapt by engaging protective response mechanisms that allow for cell survival and maintenance of cell function. The adaptive response often manifests itself as increases in antioxidant levels which confer a selective survival advantage that is often observed in aggressive metastatic cancers. Thus, it is not surprising that antioxidant-based cancer prevention strategies have shown poor therapeutic efficacy. Future therapeutic strategies might be directed at limiting global adaptions to mROS and ROS signaling. With the emergence of novel tumor mitochondria targeting strategies a new era in antioxidant based chemotherapeutic strategies is on the horizon.
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7:504–11. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandalio LM, Rodríguez-Serrano M, Romero-Puertas MC, del Río LA. Role of peroxisomes as a source of reactive oxygen species (ROS) signaling molecules. Subcell Biochem. 2013;69:231–55. doi: 10.1007/978-94-007-6889-5_13. [DOI] [PubMed] [Google Scholar]
- 3.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 4.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shadel GS, et al. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mailloux RJ. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015;4:381–98. doi: 10.1016/j.redox.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diebold L, Chandel NS. Mitochondrial ROS regulation of proliferating cells. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.04.198. [DOI] [PubMed] [Google Scholar]
- 8.Gammella E, Recalcati S, Cairo G. Dual Role of ROS as Signal and Stress Agents: Iron Tips the Balance in favor of Toxic Effects. Oxid Med Cell Longev. 2016;2016:8629024. doi: 10.1155/2016/8629024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–50. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemarie A, Huc L, Pazarentzos E, Mahul-Mellier AL, Grimm S. Specific disintegration of complex II succinate:ubiquinone oxidoreductase links pH changes to oxidative stress for apoptosis induction. Cell Death Differ. 2011;18:338–49. doi: 10.1038/cdd.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nickel A, Kohlhaas M, Maack C. Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol. 2014;73:26–33. doi: 10.1016/j.yjmcc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–63. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 14.Klimova T, Chandel NS. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008;15:660–666. doi: 10.1038/sj.cdd.4402307. [DOI] [PubMed] [Google Scholar]
- 15.Rivera J, Sobey CG, Walduck AK, Drummond GR. Nox isoforms in vascular pathophysiology: insights from transgenic and knockout mouse models. Redox Rep. 2010;15:50–63. doi: 10.1179/174329210X12650506623401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroder K, et al. Nox4 Is a Protective Reactive Oxygen Species Generating Vascular NADPH Oxidase. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M, et al. Inhibition of NADPH oxidase 4 induces apoptosis in malignant mesothelioma: Role of reactive oxygen species. Oncol Rep. 2015;34:1726–32. doi: 10.3892/or.2015.4155. [DOI] [PubMed] [Google Scholar]
- 18.Kaludercic N, Carpi A, Menabò R, Di Lisa F, Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta. 2011;1813:1323–32. doi: 10.1016/j.bbamcr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umbarkar P, et al. Monoamine oxidase-A is an important source of oxidative stress and promotes cardiac dysfunction, apoptosis, and fibrosis in diabetic cardiomyopathy. Free Radic Biol Med. 2015;87:263–273. doi: 10.1016/j.freeradbiomed.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YR, Zweier JL. Cardiac Mitochondria and Reactive Oxygen Species Generation. Circ Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan ALY, Forbes JM, Cooper ME. AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol. 2007;27:130–43. doi: 10.1016/j.semnephrol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 24.Tsang CK, et al. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat Commun. 2014;5:373–399. doi: 10.1038/ncomms4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu ZJ, Semenza GL, Zhang HF. Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang Univ Sci B. 2015;16:32–43. doi: 10.1631/jzus.B1400221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Zhang H, Zhou H, Ji W, Min W. Mitochondrial Redox Signaling and Tumor Progression. Cancers (Basel) 2016;8:40. doi: 10.3390/cancers8040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belikov AV, et al. T cells and reactive oxygen species. J Biomed Sci. 2015;22:85. doi: 10.1186/s12929-015-0194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill T, Levine AD. Mitochondria-derived hydrogen peroxide selectively enhances T cell receptor-initiated signal transduction. J Biol Chem. 2013;288:26246–55. doi: 10.1074/jbc.M113.476895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu CX, Li S, Whorton A. R Redox regulation of PTEN by S-nitrosothiols. Mol Pharmacol. 2005;68:847–54. doi: 10.1124/mol.104.010504. [DOI] [PubMed] [Google Scholar]
- 34.Lee SR, et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–42. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 35.Lim JM, Lee KS, Woo HA, Kang D, Rhee SG. Control of the pericentrosomal H2O2 level by peroxiredoxin I is critical for mitotic progression. J Cell Biol. 2015;210:23–33. doi: 10.1083/jcb.201412068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo Y, et al. Identification of MicroRNAs Involved in Growth Arrest and Apoptosis in Hydrogen Peroxide-Treated Human Hepatocellular Carcinoma Cell Line HepG2. Oxid Med Cell Longev. 2016;2016:7530853. doi: 10.1155/2016/7530853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostman A, Frijhoff J, Sandin A, Böhmer FD. Regulation of protein tyrosine phosphatases by reversible oxidation. J Biochem. 2011;150:345–56. doi: 10.1093/jb/mvr104. [DOI] [PubMed] [Google Scholar]
- 38.Patterson HC, et al. A respiratory chain controlled signal transduction cascade in the mitochondrial intermembrane space mediates hydrogen peroxide signaling. Proc Natl Acad Sci U S A. 2015;112:E5679–88. doi: 10.1073/pnas.1517932112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin Z, et al. Oxidant exposure induces cysteine-rich protein 61 (CCN1) via c-Jun/AP-1 to reduce collagen expression in human dermal fibroblasts. PLoS One. 2014;9:e115402. doi: 10.1371/journal.pone.0115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastukh V, et al. An oxidative DNA ‘damage’ and repair mechanism localized in the VEGF promoter is important for hypoxia-induced VEGF mRNA expression. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1367–75. doi: 10.1152/ajplung.00236.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Mehdi AB, et al. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal. 2012;5:ra47. doi: 10.1126/scisignal.2002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park H, et al. 3,3′-Diindolylmethane inhibits VEGF expression through the HIF-1α and NF-κB pathways in human retinal pigment epithelial cells under chemical hypoxic conditions. Int J Mol Med. 2015;36:301–8. doi: 10.3892/ijmm.2015.2202. [DOI] [PubMed] [Google Scholar]
- 43.Katavetin P, et al. High Glucose Blunts Vascular Endothelial Growth Factor Response to Hypoxia via the Oxidative Stress-Regulated Hypoxia-Inducible Factor/Hypoxia-Responsible Element Pathway. J Am Soc Nephrol. 2006;17:1405–1413. doi: 10.1681/ASN.2005090918. [DOI] [PubMed] [Google Scholar]
- 44.Storti F, et al. A novel distal upstream hypoxia response element regulating oxygen-dependent erythropoietin gene expression. Haematologica. 2014;99:e45–8. doi: 10.3324/haematol.2013.102707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, Yang X, Zou H, Li M. Oxidative Stress and Treg and Th17 Dysfunction in Systemic Lupus Erythematosus. Oxid Med Cell Longev. 2016;2016:2526174. doi: 10.1155/2016/2526174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wan C, Su H, Zhang C. Role of NADPH Oxidase in Metabolic Disease-Related Renal Injury: An Update. Oxid Med Cell Longev. 2016;2016:7813072. doi: 10.1155/2016/7813072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srikanthan K, Shapiro JI, Sodhi K. The Role of Na/K-ATPase Signaling in Oxidative Stress Related to Obesity and Cardiovascular Disease. Molecules. 2016;21 doi: 10.3390/molecules21091172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu D, Ke Z, Luo J. Thiamine Deficiency and Neurodegeneration: the Interplay Among Oxidative Stress, Endoplasmic Reticulum Stress, and Autophagy. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Udensi UK, Tchounwou PB. Oxidative stress in prostate hyperplasia and carcinogenesis. J Exp Clin Cancer Res. 2016;35:139. doi: 10.1186/s13046-016-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reczek CR, Chandel NS. The Two Faces of Reactive Oxygen Species in Cancer. Annu Rev Cancer Biol. 2017;1 annurev-cancerbio-041916-065808. [Google Scholar]
- 52.Chandel NS, Tuveson DA. The promise and perils of antioxidants for cancer patients. N Engl J Med. 2014;371:177–8. doi: 10.1056/NEJMcibr1405701. [DOI] [PubMed] [Google Scholar]
- 53.Dall’Acqua S, et al. Natural daucane esters induces apoptosis in leukaemic cells through ROS production. Phytochemistry. 2014;108:147–56. doi: 10.1016/j.phytochem.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Castaldo SA, Freitas JR, Conchinha NV, Madureira PA. The Tumorigenic Roles of the Cellular REDOX Regulatory Systems. Oxid Med Cell Longev. 2016;2016:8413032. doi: 10.1155/2016/8413032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schenk B, Fulda S. Reactive oxygen species regulate Smac mimetic/TNFα-induced necroptotic signaling and cell death. Oncogene. 2015;34:5796–806. doi: 10.1038/onc.2015.35. [DOI] [PubMed] [Google Scholar]
- 56.Vurusaner B, Poli G, Basaga H. Tumor suppressor genes and ROS: Complex networks of interactions. Free Radic Biol Med. 2012;52:7–18. doi: 10.1016/j.freeradbiomed.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 57.Weinberg F, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu Y, et al. SIRT3 and SIRT4 are mitochondrial tumor suppressor proteins that connect mitochondrial metabolism and carcinogenesis. Cancer Metab. 2014;2:15. doi: 10.1186/2049-3002-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogrunc M, et al. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014;21:998–1012. doi: 10.1038/cdd.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dasgupta S, et al. Mitochondrial DNA mutations in respiratory complex-I in never-smoker lung cancer patients contribute to lung cancer progression and associated with EGFR gene mutation. J Cell Physiol. 2012;227:2451–60. doi: 10.1002/jcp.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang SJ, Gu W. To be, or not to be: functional dilemma of p53 metabolic regulation. Curr Opin Oncol. 2014;26:78–85. doi: 10.1097/CCO.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kruiswijk F, Labuschagne CF, Vousden K. H p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16:393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 63.Klotz LO, et al. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris IS, et al. PTPN12 promotes resistance to oxidative stress and supports tumorigenesis by regulating FOXO signaling. Oncogene. 2014;33:1047–54. doi: 10.1038/onc.2013.24. [DOI] [PubMed] [Google Scholar]
- 65.Kim HS, et al. SIRT3 Is a Mitochondria-Localized Tumor Suppressor Required for Maintenance of Mitochondrial Integrity and Metabolism during Stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bell EL, Emerling BM, Ricoult SJH, Guarente L. SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986–96. doi: 10.1038/onc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maya-Mendoza A, et al. Myc and Ras oncogenes engage different energy metabolism programs and evoke distinct patterns of oxidative and DNA replication stress. Mol Oncol. 2015;9:601–616. doi: 10.1016/j.molonc.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236–41. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 69.Murphy DJ, et al. Distinct thresholds govern Myc's biological output in vivo. Cancer Cell. 2008;14:447–57. doi: 10.1016/j.ccr.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alberghina L, Gaglio D. Redox control of glutamine utilization in cancer. Cell Death Dis. 2014;5:e1561. doi: 10.1038/cddis.2014.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baracca A, et al. Mitochondrial Complex I decrease is responsible for bioenergetic dysfunction in K-ras transformed cells. Biochim Biophys Acta. 2010;1797:314–23. doi: 10.1016/j.bbabio.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Ishikawa K, et al. ROS-Generating Mitochondrial DNA Mutations Can Regulate Tumor Cell Metastasis. Science (80-) 2008:1156906. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 73.Mori S, et al. Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene. 2009;28:2796–805. doi: 10.1038/onc.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woo DK, et al. Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APC(Min/+) mice. Am J Pathol. 2012;180:24–31. doi: 10.1016/j.ajpath.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao W, Xu K, Li P, Tang B. Functional roles of superoxide and hydrogen peroxide generated by mitochondrial DNA mutation in regulating tumorigenicity of HepG2 cells. Cell Biochem Funct. 2011;29:400–7. doi: 10.1002/cbf.1764. [DOI] [PubMed] [Google Scholar]
- 76.Sharma LK, et al. Mitochondrial respiratory complex I dysfunction promotes tumorigenesis through ROS alteration and AKT activation. Hum Mol Genet. 2011;20:4605–16. doi: 10.1093/hmg/ddr395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Runchel C, Matsuzawa A, Ichijo H. Mitogen-Activated Protein Kinases in Mammalian Oxidative Stress Responses. Antioxid Redox Signal. 2011;15:205–218. doi: 10.1089/ars.2010.3733. [DOI] [PubMed] [Google Scholar]
- 78.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–39. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biasutto L, Chiechi A, Couch R, Liotta LA, Espina V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp Cell Res. 2013;319:2113–2123. doi: 10.1016/j.yexcr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lovett DH, et al. A Novel Intracellular Isoform of Matrix Metalloproteinase-2 Induced by Oxidative Stress Activates Innate Immunity. PLoS One. 2012;7:e34177. doi: 10.1371/journal.pone.0034177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao P, et al. HIF-Dependent Antitumorigenic Effect of Antioxidants In Vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim J, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–85. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 84.Horiuchi A, et al. Hypoxia upregulates ovarian cancer invasiveness via the binding of HIF-1α to a hypoxia-induced, methylation-free hypoxia response element of S100A4 gene. Int J cancer. 2012;131:1755–67. doi: 10.1002/ijc.27448. [DOI] [PubMed] [Google Scholar]
- 85.Chen S, et al. HIF-1α contributes to proliferation and invasiveness of neuroblastoma cells via SHH signaling. PLoS One. 2015;10:e0121115. doi: 10.1371/journal.pone.0121115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, et al. HIF-1α and HIF-2α correlate with migration and invasion in gastric cancer. Cancer Biol Ther. 2010;10:376–82. doi: 10.4161/cbt.10.4.12441. [DOI] [PubMed] [Google Scholar]
- 87.Baldewijns MM, et al. VHL and HIF signalling in renal cell carcinogenesis. J Pathol. 2010;221:125–138. doi: 10.1002/path.2689. [DOI] [PubMed] [Google Scholar]
- 88.Jiang W, et al. CD44 Regulates Pancreatic Cancer Invasion through MT1-MMP. Am Assoc Cancer Res. 2015;13 doi: 10.1158/1541-7786.MCR-14-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu G, Jiang C, Li D, Wang R, Wang W. MiRNA-34a inhibits EGFR-signaling-dependent MMP7 activation in gastric cancer. Tumor Biol. 2014;35:9801–9806. doi: 10.1007/s13277-014-2273-6. [DOI] [PubMed] [Google Scholar]
- 90.Liu H, et al. The role of MMP-1 in breast cancer growth and metastasis to the brain in a xenograft model. BMC Cancer. 2012;12:583. doi: 10.1186/1471-2407-12-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768–84. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 92.Kar S, Subbaram S, Carrico PM, Melendez JA. Redox-control of matrix metalloproteinase-1: a critical link between free radicals, matrix remodeling and degenerative disease. Respir Physiol Neurobiol. 2010;174:299–306. doi: 10.1016/j.resp.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nayak BK, et al. Stabilization of HIF-2α through redox regulation of mTORC2 activation and initiation of mRNA translation. Oncogene. 2013;32:3147–55. doi: 10.1038/onc.2012.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mohammedi K, et al. Plasma extracellular superoxide dismutase concentration, allelic variations in the SOD3 gene and risk of myocardial infarction and all-cause mortality in people with type 1 and type 2 diabetes. Cardiovasc Diabetol. 2015;14:845. doi: 10.1186/s12933-014-0163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hansen JM, Watson WH, Jones DP. Compartmentation of Nrf-2 Redox Control: Regulation of Cytoplasmic Activation by Glutathione and DNA Binding by Thioredoxin-1. Toxicol Sci. 2004;82:308–317. doi: 10.1093/toxsci/kfh231. [DOI] [PubMed] [Google Scholar]
- 97.Hansen JM, Zhang H, Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci. 2006;91:643–50. doi: 10.1093/toxsci/kfj175. [DOI] [PubMed] [Google Scholar]
- 98.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pober JS, Min W, Bradley JR. Mechanisms of Endothelial Dysfunction, Injury, and Death. Annu Rev Pathol Mech Dis. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 100.Dhar SK, St Clair DK. Manganese superoxide dismutase regulation and cancer. Free Radic Biol Med. 2012;52:2209–22. doi: 10.1016/j.freeradbiomed.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 101.Slot JW, Geuze HJ, Freeman BA, Crapo JD. Intracellular localization of the copper-zinc and manganese superoxide dismutases in rat liver parenchymal cells. Lab Invest. 1986;55:363–71. [PubMed] [Google Scholar]
- 102.Sun Y, K Holley A, K St Clair D. p53 Regulation of Energy Metabolism and Mitochondria Regulation of p53 in Cancer Cells: An Insight into the Role of Manganese Superoxide Dismutase. Curr Pharm Biotechnol. 2013;14:261–73. doi: 10.2174/1389201011314030003. [DOI] [PubMed] [Google Scholar]
- 103.Pani G, et al. Mitochondrial superoxide dismutase: a promising target for new anticancer therapies. Curr Med Chem. 2004;11:1299–308. doi: 10.2174/0929867043365297. [DOI] [PubMed] [Google Scholar]
- 104.Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 105.Wang R, et al. Reduced SOD2 expression is associated with mortality of hepatocellular carcinoma patients in a mutant p53-dependent manner. Aging (Albany NY) 2016;8:1184–200. doi: 10.18632/aging.100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Copin JC, Gasche Y, Chan PH. Overexpression of copper/zinc superoxide dismutase does not prevent neonatal lethality in mutant mice that lack manganese superoxide dismutase. Free Radic Biol Med. 2000;28:1571–6. doi: 10.1016/s0891-5849(00)00280-x. [DOI] [PubMed] [Google Scholar]
- 107.Hemachandra LPMP, et al. Mitochondrial Superoxide Dismutase Has a Protumorigenic Role in Ovarian Clear Cell Carcinoma. Cancer Res. 2015;75:4973–84. doi: 10.1158/0008-5472.CAN-14-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hempel N, Carrico PM, Melendez JA. Manganese superoxide dismutase (Sod2) and redox-control of signaling events that drive metastasis. Anticancer Agents Med Chem. 2011;11:191–201. doi: 10.2174/187152011795255911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oberley LW. Anticancer Therapy by Overexpression of Superoxide Dismutase. Antioxid Redox Signal. 2001;3:461–472. doi: 10.1089/15230860152409095. [DOI] [PubMed] [Google Scholar]
- 110.Liu Z, et al. Manganese superoxide dismutase induces migration and invasion of tongue squamous cell carcinoma via H2O2-dependent Snail signaling. Free Radic Biol Med. 2012;53:44–50. doi: 10.1016/j.freeradbiomed.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loo SY, et al. Manganese Superoxide Dismutase Expression Regulates the Switch Between an Epithelial and a Mesenchymal-Like Phenotype in Breast Carcinoma. Antioxid Redox Signal. 2016;25:283–99. doi: 10.1089/ars.2015.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kamarajugadda S, et al. Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death Dis. 2013;4:e504. doi: 10.1038/cddis.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kroemer G, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kang SW. Superoxide dismutase 2 gene and cancer risk: evidence from an updated meta-analysis. Int J Clin Exp Med. 2015;8:14647–55. [PMC free article] [PubMed] [Google Scholar]
- 115.Termini L, et al. SOD2 immunoexpression predicts lymph node metastasis in penile cancer. BMC Clin Pathol. 2015;15:3. doi: 10.1186/s12907-015-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miar A, et al. Manganese superoxide dismutase (SOD2/MnSOD)/catalase and SOD2/GPx1 ratios as biomarkers for tumor progression and metastasis in prostate, colon, and lung cancer. Free Radic Biol Med. 2015;85:45–55. doi: 10.1016/j.freeradbiomed.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 117.Fernando MR, Lechner JM, Löfgren S, Gladyshev VN, Lou MF. Mitochondrial thioltransferase (glutaredoxin 2) has GSH-dependent and thioredoxin reductase-dependent peroxidase activities in vitro and in lens epithelial cells. FASEB J. 2006;20:2645–7. doi: 10.1096/fj.06-5919fje. [DOI] [PubMed] [Google Scholar]
- 118.Zhang H, Du Y, Zhang X, Lu J, Holmgren A. Glutaredoxin 2 reduces both thioredoxin 2 and thioredoxin 1 and protects cells from apoptosis induced by auranofin and 4-hydroxynonenal. Antioxid Redox Signal. 2014;21:669–81. doi: 10.1089/ars.2013.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bräutigam L, et al. Vertebrate-specific glutaredoxin is essential for brain development. Proc Natl Acad Sci U S A. 2011;108:20532–7. doi: 10.1073/pnas.1110085108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lillig CH, Lönn ME, Enoksson M, Fernandes AP, Holmgren A. Short interfering RNA-mediated silencing of glutaredoxin 2 increases the sensitivity of HeLa cells toward doxorubicin and phenylarsine oxide. Proc Natl Acad Sci U S A. 2004;101:13227–32. doi: 10.1073/pnas.0401896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kryukov GV. Characterization of Mammalian Selenoproteomes. Science (80-) 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 122.Oberley LW. Mechanism of the tumor suppressive effect of MnSOD overexpression. Biomed Pharmacother. 2005;59:143–148. doi: 10.1016/j.biopha.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 123.Handy DE, et al. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem. 2009;284:11913–21. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moscow JA, et al. Loss of heterozygosity of the human cytosolic glutathione peroxidase I gene in lung cancer. Carcinogenesis. 1994;15:2769–73. doi: 10.1093/carcin/15.12.2769. [DOI] [PubMed] [Google Scholar]
- 125.Hu YJ, et al. Allelic Loss at the GPx-1 Locus in Cancer of the Head and Neck. Biol Trace Elem Res. 2004;101:097–106. doi: 10.1385/BTER:101:2:097. [DOI] [PubMed] [Google Scholar]
- 126.Hu YJ, Diamond AM. Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res. 2003;63:3347–51. [PubMed] [Google Scholar]
- 127.Hu Y, Benya R, V Carroll RE, Diamond AM. Allelic loss of the gene for the GPX1 selenium-containing protein is a common event in cancer. J Nutr. 2005;135:3021S–3024S. doi: 10.1093/jn/135.12.3021S. [DOI] [PubMed] [Google Scholar]
- 128.Bermano G, et al. Evidence that a polymorphism within the 3′UTR of glutathione peroxidase 4 is functional and is associated with susceptibility to colorectal cancer. Genes Nutr. 2007;2:225–232. doi: 10.1007/s12263-007-0052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Canli Ö, et al. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood. 2016;127:139–48. doi: 10.1182/blood-2015-06-654194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heirman I, et al. Blocking tumor cell eicosanoid synthesis by GP × 4 impedes tumor growth and malignancy. Free Radic Biol Med. 2006;40:285–94. doi: 10.1016/j.freeradbiomed.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 131.Huang Q, et al. Thioredoxin-2 Inhibits Mitochondrial Reactive Oxygen Species Generation and Apoptosis Stress Kinase-1 Activity to Maintain Cardiac Function. Circulation. 2015;131:1082–1097. doi: 10.1161/CIRCULATIONAHA.114.012725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arnér ESJ, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16:420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 133.Li L, et al. Increased susceptibility of MER5 (peroxiredoxin III) knockout mice to LPS-induced oxidative stress. Biochem Biophys Res Commun. 2007;355:715–721. doi: 10.1016/j.bbrc.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 134.Conrad M, et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–23. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang R, et al. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–91. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 136.Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000;346 Pt 1:1–8. [PMC free article] [PubMed] [Google Scholar]
- 137.Choi JH, et al. Overexpression of mitochondrial thioredoxin reductase and peroxiredoxin III in hepatocellular carcinomas. Anticancer Res. 2002;22:3331–5. [PubMed] [Google Scholar]
- 138.Cadenas C, et al. Role of thioredoxin reductase 1 and thioredoxin interacting protein in prognosis of breast cancer. Breast Cancer Res. 2010;12:R44. doi: 10.1186/bcr2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lincoln DT, et al. Thioredoxin and thioredoxin reductase expression in thyroid cancer depends on tumour aggressiveness. Anticancer Res. 2010;30:767–75. [PubMed] [Google Scholar]
- 140.Singh SS, et al. Thioredoxin Reductase 1 Expression and Castration-recurrent Growth of Prostate Cancer. Transl Oncol. 2008;1:153–7. doi: 10.1593/tlo.08145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tobe R, et al. Differences in Redox Regulatory Systems in Human Lung and Liver Tumors Suggest Different Avenues for Therapy. Cancers (Basel) 2015;7:2262–2276. doi: 10.3390/cancers7040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cassidy PB, et al. The role of thioredoxin reductase 1 in melanoma metabolism and metastasis. Pigment Cell Melanoma Res. 2015;28:685–695. doi: 10.1111/pcmr.12398. [DOI] [PubMed] [Google Scholar]
- 143.Lincoln DT, Ali Emadi EM, Tonissen KF, Clarke FM. The thioredoxin-thioredoxin reductase system: over-expression in human cancer. Anticancer Res. 2003;23:2425–33. [PubMed] [Google Scholar]
- 144.Hellfritsch J, et al. Knockout of Mitochondrial Thioredoxin Reductase Stabilizes Prolyl Hydroxylase 2 and Inhibits Tumor Growth and Tumor-Derived Angiogenesis. Antioxid Redox Signal. 2015;22:938–950. doi: 10.1089/ars.2014.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 146.Nguyen P, Awwad RT, Smart DDK, Spitz DR, Gius D. Thioredoxin reductase as a novel molecular target for cancer therapy. Cancer Lett. 2006;236:164–174. doi: 10.1016/j.canlet.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 147.Peters U, et al. Variation in the selenoenzyme genes and risk of advanced distal colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2008;17:1144–54. doi: 10.1158/1055-9965.EPI-07-2947. [DOI] [PubMed] [Google Scholar]
- 148.Slattery ML, Lundgreen A, Welbourn B, Corcoran C, Wolff RK. Genetic Variation in Selenoprotein Genes, Lifestyle, and Risk of Colon and Rectal Cancer. PLoS One. 2012;7:e37312. doi: 10.1371/journal.pone.0037312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Perkins A, Nelson KJ, Parsonage D, Poole LB, Karplus PA. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem Sci. 2015;40:435–445. doi: 10.1016/j.tibs.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cox AG, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J. 2010;425 doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 151.Whitaker H, et al. Peroxiredoxin-3 is overexpressed in prostate cancer and promotes cancer cell survival by protecting cells from oxidative stress. Br J Cancer. 2013;109:983–93. doi: 10.1038/bjc.2013.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Song IS, et al. FOXM1-Induced PRX3 Regulates Stemness and Survival of Colon Cancer Cells via Maintenance of Mitochondrial Function. Gastroenterology. 2015;149:1006–1016.e9. doi: 10.1053/j.gastro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 153.Kim K, et al. Expression of human peroxiredoxin isoforms in response to cervical carcinogenesis. Oncol Rep. 2009;21 [PubMed] [Google Scholar]
- 154.Cunniff B, Wozniak AN, Sweeney P, Decosta K, Heintz NH. Peroxiredoxin 3 levels regulate a mitochondrial redox setpoint in malignant mesothelioma cells. Redox Biol. 2014;3:79–87. doi: 10.1016/j.redox.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Diehn M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009 doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hempel N, Ye H, Abessi B, Mian B, Melendez JA. Altered redox status accompanies progression to metastatic human bladder cancer. Free Radic Biol Med. 2009;46:42–50. doi: 10.1016/j.freeradbiomed.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ahmad IM, et al. Mitochondrial O2*- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem. 2005;280:4254–4263. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 158.Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dikalov SI, Harrison DG. Methods for Detection of Mitochondrial and Cellular Reactive Oxygen Species. Antioxid Redox Signal. 2014;20:372–382. doi: 10.1089/ars.2012.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Poynton RA, Hampton MB. Peroxiredoxins as biomarkers of oxidative stress. Biochim Biophys Acta - Gen Subj. 2014;1840:906–912. doi: 10.1016/j.bbagen.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 161.Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brewer TF, Garcia FJ, Onak CS, Carroll KS, Chang CJ. Chemical Approaches to Discovery and Study of Sources and Targets of Hydrogen Peroxide Redox Signaling Through NADPH Oxidase Proteins. Annu Rev Biochem. 2015;84:765–790. doi: 10.1146/annurev-biochem-060614-034018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Van Laer K, Dick TP. Utilizing Natural and Engineered Peroxiredoxins As Intracellular Peroxide Reporters. Mol Cells. 2016;39:46–52. doi: 10.14348/molcells.2016.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Poljsak B, Jamik P. Methodology for oxidative state detection in biological systems,” in Handbook of Free Radicals: Formation, Types and Effects. Cell Biology Research Progress Series. 2010;Nova Scien [Google Scholar]
- 165.Argüelles S, Gómez A, Machado A, Ayala A. A Preliminary Analysis of Within-Subject Variation in Human Serum Oxidative Stress Parameters as a Function of Time. Rejuvenation Res. 2007;10:621–636. doi: 10.1089/rej.2006.0528. [DOI] [PubMed] [Google Scholar]
- 166.Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wen S, Zhu D, Huang P. Targeting cancer cell mitochondria as a therapeutic approach. Future Med Chem. 2013;5:53–67. doi: 10.4155/fmc.12.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Harris IS, et al. Glutathione and Thioredoxin Antioxidant Pathways Synergize to Drive Cancer Initiation and Progression. Cancer Cell. 2015;27:211–222. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 169.Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 170.Greenlee H, et al. Antioxidant supplement use after breast cancer diagnosis and mortality in the Life After Cancer Epidemiology (LACE) cohort. Cancer. 2012;118:2048–58. doi: 10.1002/cncr.26526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223–31. doi: 10.1016/j.ejca.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 172.Bober P, et al. Proteomic analysis of the vitamin C effect on the doxorubicin cytotoxicity in the MCF-7 breast cancer cell line. J Cancer Res Clin Oncol. 2016 doi: 10.1007/s00432-016-2259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Printz C. High vitamin D levels increase survival rates in patients with metastatic colorectal cancer. Cancer. 2015;121:2105. doi: 10.1002/cncr.29513. [DOI] [PubMed] [Google Scholar]
- 174.Bjelakovic G, Gluud C. Surviving antioxidant supplements. J Natl Cancer Inst. 2007;99:742–3. doi: 10.1093/jnci/djk211. [DOI] [PubMed] [Google Scholar]
- 175.Bjelakovic G, et al. Meta-analysis: antioxidant supplements for primary and secondary prevention of colorectal adenoma. Aliment Pharmacol Ther. 2006;24:281–91. doi: 10.1111/j.1365-2036.2006.02970.x. [DOI] [PubMed] [Google Scholar]
- 176.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet (London, England) 2004;364:1219–28. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 177.Caraballoso M, Sacristan M, Serra C, Bonfill X. Drugs for preventing lung cancer in healthy people. Cochrane database Syst Rev. 2003:CD002141. doi: 10.1002/14651858.CD002141. [DOI] [PubMed] [Google Scholar]
- 178.Cheng G, et al. Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: Role of mitochondrial bioenergetics and energy-sensing mechanism. Cancer Lett. 2015;365:96–106. doi: 10.1016/j.canlet.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cheng G, et al. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC Cancer. 2013;13:285. doi: 10.1186/1471-2407-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nazarewicz RR, et al. Does scavenging of mitochondrial superoxide attenuate cancer prosurvival signaling pathways? Antioxid Redox Signal. 2013;19:344–9. doi: 10.1089/ars.2013.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Finley LWS, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell. 2011;19:416–28. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fink EE, et al. Mitochondrial thioredoxin reductase regulates major cytotoxicity pathways of proteasome inhibitors in multiple myeloma cells. Leukemia. 2016;30:104–11. doi: 10.1038/leu.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang J, Yi J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol Ther. 2008;7:1875–1884. doi: 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]
- 184.Trachootham D, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by [beta]-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 185.Munro D, Banh S, Sotiri E, Tamanna N, Treberg JR. The thioredoxin and glutathione-dependent H2O2 consumption pathways in muscle mitochondria: Involvement in H2O2 metabolism and consequence to H2O2 efflux assays. Free Radic Biol Med. 2016;96:334–346. doi: 10.1016/j.freeradbiomed.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 186.Aon MA, et al. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: an experimental-computational study. J Gen Physiol. 2012;139:479–91. doi: 10.1085/jgp.201210772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN. Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci. 2014;39:112–20. doi: 10.1016/j.tibs.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fu D, et al. Human AlkB homolog ABH8 Is a tRNA methyltransferase required for wobble uridine modification and DNA damage survival. Mol Cell Biol. 2010;30:2449–59. doi: 10.1128/MCB.01604-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Endres L, et al. Alkbh8 Regulates Selenocysteine-Protein Expression to Protect against Reactive Oxygen Species Damage. PLoS One. 2015;10:e0131335. doi: 10.1371/journal.pone.0131335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shimada K, et al. A Novel Human AlkB Homologue, ALKBH8, Contributes to Human Bladder Cancer Progression. Cancer Res. 2009;69:3157–3164. doi: 10.1158/0008-5472.CAN-08-3530. [DOI] [PubMed] [Google Scholar]
- 191.Gameiro PA, et al. In Vivo HIF-Mediated Reductive Carboxylation Is Regulated by Citrate Levels and Sensitizes VHL-Deficient Cells to Glutamine Deprivation. Cell Metab. 2013;17:372–385. doi: 10.1016/j.cmet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zheng L, et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat Commun. 2015;6:6001. doi: 10.1038/ncomms7001. [DOI] [PMC free article] [PubMed] [Google Scholar]