Abstract
Gut microbiota play critical physiological roles in energy extraction from the intestine and in the control of systemic immunity, as well as local intestinal immunity. Disturbance of gut microbiota leads to the development of several diseases, such as colitis, inflammatory bowel diseases, metabolic disorders, cancer, etc. From a metabolic point of view, the gut is a large metabolic organ and one of the first to come into contact with dietary fats. Interestingly, excessive dietary fat has been incriminated as a primary culprit of metabolic syndrome and obesity. After intake of high-fat diet or Western diet, extensive changes in gut microbiota have been observed, which may be an underlying cause of alterations in whole body metabolism and nutrient homeostasis. Here, we summarize recent data on changes in the gut microbiota and immunity associated with dietary fat, as well as their relationships with the pathogenesis of metabolic syndrome. These findings may provide insight into the understanding of the complex pathophysiology related to the development of metabolic diseases and offer an opportunity to develop novel candidates for therapeutic agents.
Keywords: Gut microbiota, gut immunity, obesity, diabetes
INTRODUCTION
With greater industrialization, people have shown stronger preferences for Western-style diets of high fat content than traditional diets. This change in dietary preferences has contributed to a dramatic increase in metabolic diseases, such as obesity and type 2 diabetes, over the last decade, and these diseases now are a significant threat to the public health.1 In obesity and type 2 diabetes, inflammatory cells infiltrate adipose tissues, the liver, and pancreatic islets leading to the production of proinflammatory cytokines and chemokines; these metabolic diseases are now considered as chronic low-grade inflammatory diseases. Metabolic inflammation consequently causes insulin resistance, contributing to the development of metabolic syndrome.2 However, the underlying mechanisms of low-grade tissue inflammation inducing metabolic symptoms have still not been clearly elucidated. Recently, a modest increase in plasma contents of lipopolysaccharide (LPS) has been incriminated as an etiological event causing metabolic inflammation. The gut microbiota are a strong candidate as sources of the noted increases in plasma LPS.3 In this regard, gut microbiota seem to influence systemic immunity and local intestinal immunity. Moreover, gut microbiota are changed by obesity, which is followed by the altered intestinal immunity, contributing substantially to the pathogenesis of metabolic diseases. In this review, we summarize recent findings on changes in gut microbiota and intestinal immunity in association with diet-induced obesity and insulin resistance.
CHANGES IN GUT MICROBIOTA ASSOCIATED WITH METABOLIC SYNDROME
How does intestinal microbiota influence obesity and diabetes?
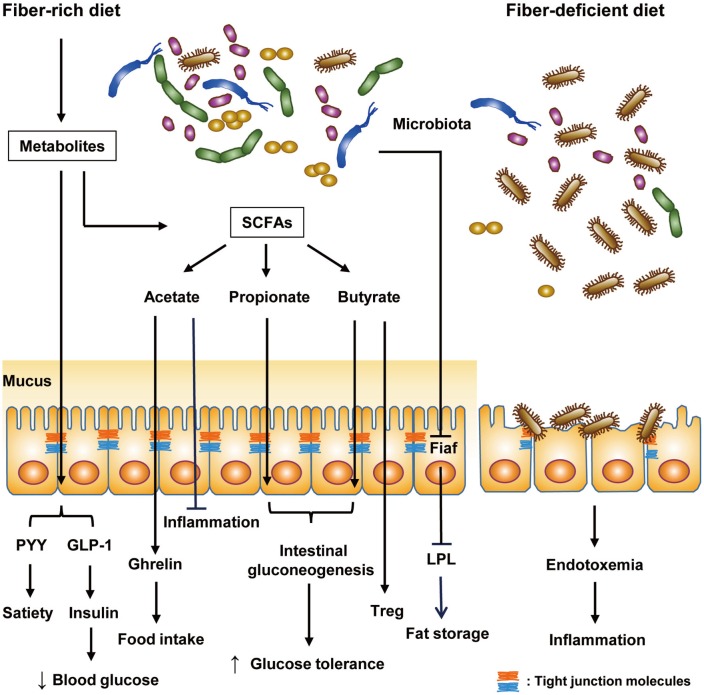
The gut microbiota in humans consist of 10-100 trillion microorganisms and outnumber all somatic and germ cells by 10,4 although it has recently been claimed that such number could be an overestimation. Further, the collective genomes of the gut microbiome contain 100-fold more genes than our own genome.5 Gut microbiota have coevolved with humans, eliciting profound effects on various physiological processes, such as nutrient metabolism and immunity. For instance, dietary fibers that the human host cannot digest are metabolized by gut microbiota into short-chain fatty acids (SCFAs), such as butyrate, acetate, and propionate. SCFAs act through G protein-coupled receptors GPR41 and GPR43 expressed in enteroendocrine cells, enteric neurons, and enteric leukocytes.6 Butyrate and propionate activate intestinal gluconeogenesis and exert beneficial effects on glucose and energy homeostasis: butyrate activates gluconeogenesis gene expression in a cAMP-dependent manner, whereas propionate does it through gut-brain neural circuits.7 Intestinal gluconeogenesis elicits beneficial effects on systemic glucose profiles through portal vein glucose sensors transmitting the signal to the brain.8 Butyrate also enhances gut barrier function and can protect enterocytes from injury or colitis,9 which might be related to the role of butyrate as a histone deacetylator inhibitor.10,11 On the immunological aspect, butyrate can promote Treg cell generation in the intestine.12 Acetate has anti-inflammatory activity against colitis and arthritis.13 Meanwhile, however, acetate may also increase glucose-stimulated insulin secretion and ghrelin secretion via parasympathetic activation, leading to increased food intake and obesity.14 In addition to these effects, SCFAs can contribute to improvement of metabolic syndrome by promoting secretions of peptide hormones, such as peptide YY and glucagon-like peptide-1, that decrease appetite and increase insulin release, respectively (Fig. 1).15
Fig. 1. Regulation of host metabolism and immunity by gut microbiota. Under a fiber-rich diet, gut microbiota metabolize undigested dietary fiber into SCFAs (acetate, propionate, and butyrate), affecting host metabolism and immunity. Microbial metabolites from this process improve host metabolism. In particular, the secretion of peptide hormones, such as PYY and GLP-1, is promoted by microbial metabolites: PYY decreases appetite and GLP-1 lowers blood glucose level via promotion of insulin secretion. Among SCFAs, butyrate and propionate activate intestinal gluconeogenesis and improve systemic glucose profiles. Meanwhile, acetate promotes secretion of ghrelin, a hunger hormone, and increases food intake, consequently causing hyperphagia and obesity. Nevertheless, acetate has anti-inflammatory function like butyrate. Butyrate enhances gut barrier function of intestinal epithelial cells and increases regulatory T (Treg) cells. In addition, gut microbiota suppress expression of fasting-induced adipose factor (Fiaf), an inhibitor of LPL, promoting fat storage in adipocytes. Under fiber-deficient diet, mucus-degrading bacteria expand and impair the integrity of the mucus layer. Thereby, endotoxemia-induced metabolic inflammation ensues. SCFAs, short-chain fatty acids; PYY, peptide YY; GLP-1, glucagon-like peptide-1; LPL, lipoprotein lipase.
Recently, many studies have shown that change in the gut microbiota is related to the development of obesity and diabetes: germ-free (GF) mice are resistant to high-fat diet (HFD)-induced obesity and glucose intolerance due to de-repressed expression of fasting-induced adipose factor (Fiaf) in the intestinal epithelium.16,17 Fiaf, which prevents fat storage in adipocytes via inhibition of lipoprotein lipase and is also called angiopoietin-like protein 4 (Angptl4), is suppressed by gut microbiota. Interestingly, transfer of gut microbiota from obese mice to recipient GF mice significantly increased body fat content and insulin resistance, compared to the transfer of gut microbiota from lean mice.18,19 These results suggest a crucial role of gut microbiota in nutrient homeostasis and also a possible etiological role of altered gut microbiota in the development of metabolic syndrome.
Phylum level changes and enterotype
To investigate the role of microbiota in nutrient uptake or in the development of metabolic syndrome, identification of individual microorganism is crucial. However, identification of gut microbiota has been extremely difficult since they are largely recalcitrant to in vitro culture. This technical obstacle can now be overcome with the advent of a revolutionary method of microorganism identification, sequencing of 16s rRNA genes that are ubiquitous and highly conserved among microorganisms.20 Combined with next-generation sequencing technology, 16s rRNA gene sequencing allows the identification of enormous complexity of enteric bacteria.
In the intestines of mouse and humans, >90% of bacterial species are composed of Bacteroidetes and Firmicutes phyla, while Actinobacteria, Proteobacteria, and Verrucomicrobia constitute relatively minor proportions. Compared with their lean counterparts, leptin-deficient ob/ob mice have a decreased abundance of Bacteroidetes and a correspondingly increased abundance of Firmicutes.21 Further studies have confirmed similar changes in mice with diet-induced obesity, a more physiological model of obesity than ob/ob mice, while overall diversity among Firmicutes is different from that of ob/ob mice.18 Similar changes in gut microbiota have also been observed in humans.19 Furthermore, there seems to be a causality between changes in gut microbiota and obesity, since transfer of gut microbiota from obese mice to recipient GF mice promotes fat deposition.18 The mechanism of increased Firmicutes abundance in obesity might be related to an enrichment of homoacetogens belonging to Firmicutes in obesity, which facilitates disposal of H2 produced by anaerobic bacteria during fermentation of nutrients.22 Among Bacteroidetes, a role for Bacteroides thetaiotaomicron, a glutamate-fermenting bacteria, in obesity was addressed in a recent study. Administration of Bacteroides thetaiotaomicron, the abundance of which was reduced in mice fed HFD, lowered fat mass and metabolic inflammation, which was associated decreased serum concentrations of glutamate and branched-chain amino acids.23
Although it has been generally accepted that the abundance of Bacteroidetes is decreased and that of Firmicutes are increased in obesity, it does not necessarily mean that the abundances of all bacteria belonging to Firmicutes phylum increase and those of all bacteria belonging to Bacteroidetes phylum decrease. Instead, at the genus level, the abundance of Gram-positive Lactobacillus belonging to Firmicutes phylum decreases and the abundances of Gram-negative Bacteroides and Prevotella belonging to Bacteroidetes phylum increase.24 Given that a major component of the outer membrane of Gram-negative bacteria is endotoxin or LPS, an increased abundance of Gram-negative Bacteroides and Prevotella may be linked to endotoxemia-induced metabolic inflammation.
It has also been reported that patients with diabetes have a reduced abundance of butyrate-producing Clostridiales belonging to Firmicutes (Roseburia and Faecalibacterium prauznitzii) and an increased abundance of non-butyrate-producing Clostridiales, suggesting differential regulation of the same Clostridiales, depending on the production of SCFAs.25,26 Faecalibacterium prauznitzii produces not only butyrate but also microbial anti-inflammatory molecules that can affect gut inflammation.27 Besides microbiota belonging to Bacteroidetes and Firmicutes phyla, abundance of Proteobacteria phylum has also been reported to be increased by HFD.28 Since Proteobacteria are Gram-negative bacteria, their increase may be related to endotoxemia-induced metabolic inflammation.
Gut microbiota is also important in the digestion of dietary fiber, which can modulate diverse aspects of gut immunity and metabolism. Dietary fiber-induced improvement of glucose profiles has been shown to be associated with an increased abundance of Prevotella species, such as P. copri, a Gram-negative bacteria belonging to Bacteroidetes phylum.29 Indeed, Prevotella has been reported to reflect long-term intake of carbohydrates, while Bacteroides reflects that of animal fat, among three bacteria characterizing enterotypes (Prevotella, Bacteroides, and Ruminococcus).30
These changes in microbiota composition in obesity may be able to work as early diagnostic markers in the clinic to better identify obese subjects who are prone to develop diabetes, and could be also novel therapeutic targets in the management of obesity or diabetes.
Changes in Akkermansia in metabolic syndrome
One of the prominent changes in gut microbiota associated with metabolic syndrome is that of Akkermansia muciniphila. Akkermansia muciniphila has recently been identified as a Gram-negative bacteria with mucolytic activity and the most abundant species of the Verrucomicrobia phylum. An abundance of Akkermansia muciniphila has been shown to be inversely correlated with body weight and reduced by HFD.31,32 Fish oil consumption has been found to increase the abundance of Akkermansia, which is associated with metabolic improvement.33 These results suggest that decreased abundance of Akkermansia may contribute to the metabolic deteriorations associated with HFD. Nevertheless, there is also a report that an abundance of Akkermansia increases in patients with diabetes,26 which might be related to patient selection, such as inclusion of patients under treatment with antidiabetic medicines affecting gut microbiota. Changes in Akkermansia abundance associated with HFD are opposite those in Bilophila wadsworthia,34 which is increased by milk-derived saturated fat and can aggravate colitis.35 The abundance of Akkermansia is decreased in older adults, although the significance of this finding is unclear.36
The in vivo effect of Akkermansia on systemic metabolism was demonstrated by oral administration of Akkermansia to mice with diet-induced obesity. Akkermansia administration improves glucose profiles and insulin sensitivity.37,38 Although the underlying mechanisms of the beneficial metabolic effects of Akkermansia are unclear, Akkermansia seems to decrease metabolic inflammation through Treg cell induction in adipose tissue.34,38 In addition to the indirect effect of Akkermansia on systemic metabolism through regulation of the immune system, Akkermansia may directly affect metabolic processes. The abundance of Akkermansia is reduced after prolonged cold exposure. Furthermore, adaptive changes maximizing caloric uptake, such as increased intestinal villi length during cold exposure, are attenuated by Akkermansia transfer, suggesting negative energy balance by Akkermansia.39 Akkermansia abundance is also correlated with the gene expression of fatty acid oxidation and fat browning.34
The beneficial metabolic effect of Akkermansia was also demonstrated in other experimental systems. For instance, the abundance of Akkermansia is increased in animals with gastric bypass showing reduced body weight, and is positively correlated with metabolic improvement after calorie restriction in experimental animals.40,41 Additionally, Akkermansia has a protective effect against the development of atherosclerosis.42
The metabolic action of metformin, the first-line anti-diabetic therapy recommended by the American Diabetes Association and the European Association for the Study of Diabetes, also appears to be related to changes in gut microbiota. Metformin administration increases the abundance of Akkermansia, which is accompanied by an increase in mucin-producing goblet cells. Moreover, administration of metformin ameliorates metabolic inflammation and restores Treg cells in adipose tissue, similar to the effect of Akkermansia administration.38,43 In a previous study, metformin action on gut microbiota of HFD-fed mice is analogous to the metformin action on gut microbiota of Caenorhabditis elegans, which results in alteration of folate and the methionine metabolism of E. coli in the intestine of the worm.44 Intriguingly, metformin administration greatly increases the abundance of Akkermansia in patients with type 2 diabetes as well.45,46
While these results suggest potential therapeutic value of Akkermansia or its components as a drug candidate against metabolic syndrome, such prospects have been hampered by the sensitivity of Akkermansia to oxygen, the presence of animal-derived compounds in its growth medium, and the absence of metabolic effects of killed bacteria.37,38 However, a recent paper showed that Akkermansia can be cultured successfully in a synthetic medium, which contains no compounds that are incompatible with human administration, and also that the pasteurized Akkermansia has stronger effects than live bacterium. Furthermore, Amun_1100, active component of Akkermansia that is resistant to pasteurization was identified, brightening the prospect for the development of novel therapeutics against metabolic syndrome and diabetes.47
CHANGES IN INNATE IMMUNITY OF THE INTESTINE IN METABOLIC SYNDROME
Role of gut innate immune receptors in metabolic syndrome
Several recent studies have suggested that disruption of the gut barrier function and the gut microbiota-derived LPS could contribute to the pathogenesis of metabolic syndrome and diabetes. HFD increases gut permeability and reduces the expression of tight junction proteins, such as occludin, in gut epithelium.48 Gut barrier disruption in HFD-fed mice increases gut permeability, resulting in the passage of LPS into the systemic circulation and low-grade metabolic inflammation.3,24 Consistent with these data, toll-like receptor 4 (TLR4)-knockout mice are resistant to diet-induced insulin resistance.49 NACHT, LRR, and PYD domains-containing protein 3 (NLRP3), a member of the Nod-like receptor (NLR) family, plays a crucial role in inflammasome activation and metabolic inflammation associated with metabolic syndrome and diabetes.50,51 Increased systemic LPS due to disrupted gut barrier function can activate not only TLR4 but also NLRP3, together with palmitic acid or other inflammasome activators that can be increased in obesity or metabolic syndrome.51 However, it is not clear whether such metabolic inflammation is associated with changes of innate immune system in the intestine. Since several TLRs and NLRs are expressed in diverse cells of the intestine,52,53 it is likely that these innate immune receptors in the intestine contribute to the development of metabolic syndrome. A direct role of intestinal TLR in metabolic syndrome has been demonstrated using mice with targeted disruption of MyD88, specifically in gut epithelial cells that are partially protected from diet-induced obesity and metabolic inflammation.54 In contrast, MyD88 deletion in myeloid cells does not improve the metabolic profile of mice fed HFD, indicating a more important role of innate immune receptors in intestinal epithelial cells than in myeloid cells in the development of metabolic syndrome.
An intriguing model of metabolic syndrome associated with the activation of innate immune receptors in the intestine is the occurrence of metabolic syndrome in TLR5-knockout mice. The development of metabolic syndrome in TLR5-knockout mice appears to be due to changes in gut microbiota associated with the absence of TLR5 in the intestine, since fecal microbial transplantation confers transfer of metabolic phenotype to wild-type mice.55 Following studies showed a crucial role of the absence of TLR5 in intestinal epithelial cells, but not that in dendritic cells (DCs), in the development of metabolic syndrome.56 Altered gut microbiota in TLR5-knockout mice can increase hepatic lipogenesis mediated by stearoyl CoA desaturase through production of cecal SCFAs.57
Changes in innate immune cells in the gut of metabolic syndrome
At the cell level, several changes were noted in obese subjects or HFD-fed mice. Recent investigations revealed a critical role of innate lymphoid cells (ILCs) that are derived from common lymphoid progenitors, but devoid of antigen receptors, in the development of metabolic syndrome. In adipose tissue, group 2 ILCs (ILC2s) producing Th2 cytokines, such as interleukin (IL)-4 and IL-13, play important roles in beige fat biogenesis, thermogenesis, and polarization of alternatively activated (M2) macrophages, and the proportion of ILC2s is reduced by HFD.58,59 In the intestine, NKp46+ group 3 ILCs (ILC3s) mainly producing IL-22 are decreased by HFD,60 which could be due to reduced expression of IL-23, an upstream regulator of IL-22. Since it has been reported that IL-22 improves insulin sensitivity, preserves intestinal barrier function, decreases chronic inflammation, and regulates lipid metabolism in liver and adipose tissues,61 NKp46+ ILC3s seem to alleviate metabolic syndrome via IL-22 production.
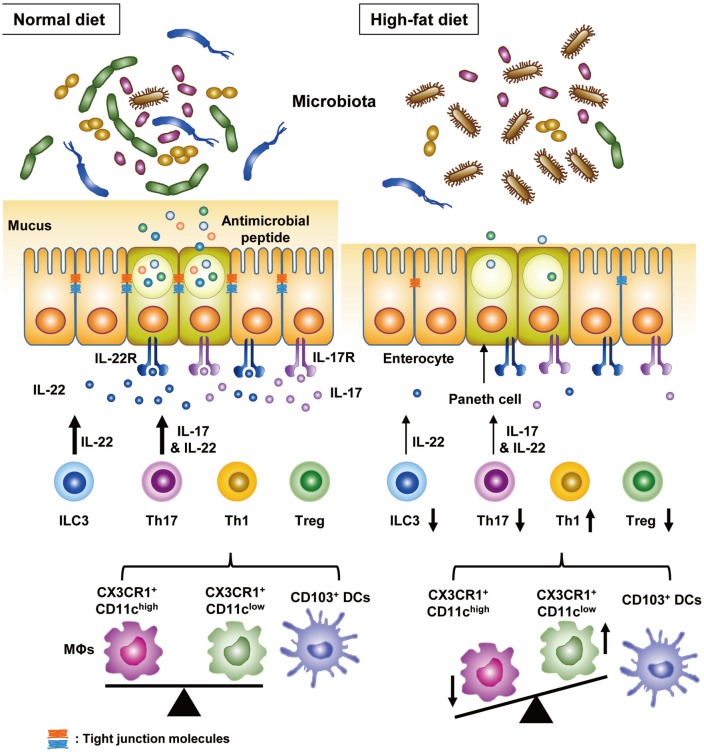
Macrophages and DCs are crucial members of innate immune cells belonging to antigen-presenting cells (APCs). In particular, macrophages and DCs in the intestine can be divided into several types with distinct functions.62 However, owing to the difficulty in preparing immune cells from the intestine of HFD-fed mice, there are only few reports studying the changes of APCs in the intestine of experimental animals with metabolic syndrome. One study showed that expressions of ICAM1, CD86 costimulatory molecule and certain cytokines (IL-6 and IL-12p40) in intestinal APCs are downregulated by HFD, which consequently affects intestinal adaptive immunity.63 Another study showed that intestinal CX3CR1+ macrophages can be divided into two subsets with different functions, and the frequencies of these two subsets are significantly affected by HFD: the proportion of a CD103−CX3CR1+CD11chigh subset increases, whereas that of a CD103−CX3CR1+CD11clow subset decreases (Fig. 2).64 In contrast, the proportion of CD103+ DCs is not affected by HFD. Given that CD103−CX3CR1+CD11chigh macrophages preferentially induce Th1 cells and CD103−CX3CR1+CD11clow macrophages preferentially induce Th17 cells, changes in their abundances may be linked to alternations of intestinal adaptive immunity (see below section regarding adaptive immunity of the gut).
Fig. 2. Changes in the intestinal immunity by HFD. HFD changes intestinal immunity, as well as gut microbiota composition. HFD, in particular, increases the frequency of Th1 cells among the CD4 T cells and decreases those of Th17 and Treg cells. HFD increases the frequency of a CX3CR1+CD11chigh macrophage (MΦ) subset, preferentially inducing Th1 cells, and decreases that of a CX3CR1+CD11clow MΦ subset, preferentially inducing Th17 cells without affecting that of CD103+ DCs. Changes in the proportions of two MΦ subsets lead to the changes in Th1 and Th17 cells after HFD feeding. Intriguingly, intestinal Th17 cells play an important role in improving metabolic diseases through IL-17 and IL-22. IL-22 is produced also by ILC3 and functions to improve metabolic profiles. Proportions of ILC3 are decreased by HFD. IL-17, mainly produced by Th17 cells, reverses decreased granules and antimicrobial peptide production of Paneth cells, leading to expansion of microbiota associated with lean phenotype. In addition, IL-17 enhances barrier function of intestinal epithelial cell by increasing expression of tight junction molecules. HFD, high-fat diet; DCs, dendritic cells; IL, interleukin, ILC3, group 3 innate lymphoid cells.
CHANGES IN THE ADAPTIVE IMMUNITY OF THE GUT IN METABOLIC SYNDROME
Recent investigations have shown a significant change in the adaptive immunity of the intestine in association with metabolic syndrome: Th1 cell increases and Treg cell decreases in the intestinal lamina propria of HFD-fed mice.60 In humans, CD8αβ+ intraepithelial lymphocytes (IELs) are increased by obesity, and these IELs impair insulin sensitivity of epithelial cells.65 In addition to cells traditionally associated with metabolic abnormalities, Th17 cells in the intestine may also play a role in the control of metabolic inflammation. According to two recent studies, although Th17 cells are well-known potent effector cells in autoimmune diseases, their reduction in the small intestine has been found to contribute to onset of HFD-induced metabolic changes.63 Supporting this contention, several studies have shown that Th17 cells do not always induce immune/inflammatory disorders and can be divided into pathogenic and non-pathogenic cells.66,67 In this regard, intestinal Th17 cells decreased by HFD seem to be non-pathogenic Th17 cells, rather than pathogen Th17 cells. The loss of Th17 cells in the small intestine of HFD-fed mice can be explained by the diminished ability of intestinal CX3CR1+ macrophages to induce Th17 cells and a significant reduction of CD103−CX3CR1+CD11clow macrophages, the most efficient Th17-inducing APC subset64 (Fig. 2). Transfer of Th17 cells, unlike that of Th1 cells, was shown to alleviate weight gain, increased fat mass, and glucose intolerance of HFD-fed Rag1-knockout mice. However, Th17 cells from integrin β7-knockout mice had no such metabolic effects, suggesting that gut homing of Th17 cells is crucial in the metabolic improvement by Th17 cells. LPAM-1 (α4β7) is a critical receptor for gut homing of T cells.68,69 In addition, IL-17 per se plays an important role in the improvement of metabolic profiles, since Th17 cells from IL-17-knockout mice have significantly less metabolic effects.64 Given that Th17 cells produce IL-22, as well as IL-17, Th17 cells may also improve metabolic syndrome via IL-22 production.61 Moreover, since Th17 cell transfer leads to expansion of microbiota associated with lean phenotype, such as Bacteriodetes or Akkermansia,64 the role of gut microbiota cannot be overlooked in regards to the metabolic improvement by Th17 cells. Alteration of gut microbiota by Th17 cells may be due to changes in antimicrobial peptides, such as Reg3γ produced by Paneth cells (Fig. 2). In support of this notion, reduced Paneth cell granules and Reg3γ expression in HFD-fed mice are reversed by IL-17 produced by Th17 cells.64 IL-17 can also enhance gut barrier function through upregulation of tight junction molecules, such as claudin, in intestinal epithelial cells.70 These data show an intriguing role of Th17 in metabolic regulation and the potential of guttrophic Th17 cell transfer as a new therapy for metabolic syndrome or diabetes.
MODULATION OF GUT MICROBIOTA OR IMMUNITY AS A NOVEL THERAPEUTIC STRATEGY AGAINST METABOLIC SYNBDROME AND DIABETES
Gut microbiota and immunity can be modulated by prebiotics or probiotics. Prebiotics are food components that induce expansion of beneficial microbiota. For example, oligofructose promotes the growth of Bifidobacterium that reduces endotoxin levels and enhances intestinal barrier function, improving metabolic parameters.71 In contrast to lard, fish oil rich in polyunsaturated fat promotes growth of beneficial bacteria, such as Akkermansia, Bifidobacterium, or Lactobacillus, and prevents metabolic inflammation in adipose tissue.33 In addition, a fiber-rich diet may contribute to amelioration of metabolic syndrome by inhibiting expansion and activity of mucus-degrading bacteria that are harmful to the intestinal barrier function (Fig. 1).72 Food enriched with inulin may have protective effects against gut injury associated with HFD and diet-induced obesity, since deficiency of inulin in HFD has been reported to a vital element in the loss of cecal and colonic mass due to HFD.73
Probiotics are live microorganisms that confer a beneficial effect on the host when administered properly. Some probiotic strains, especially those of the genera Lactobacillus and Bifiodobacterium, have been reported to ameliorate obesity and improve metabolic parameters. The suggested mechanisms thereof include inhibition of pathogen adherence to gut epithelium, stabilization of gut microbial community, and protection of mucosal integrity or barrier function.71,74 Enhanced gut barrier function may be due to SCFA production by bacterial fermentation. A recent study reported direct beneficial activity of Lactobacilli on intestinal epithelial cells and on the enteric nervous system regulating gut motility.75
In addition to prebiotics and probiotics, drugs can also be employed to modulate gut microbiota or immunity. As discussed above, the anti-diabetic drug metformin can exert beneficial metabolic effects through modulation of gut microbiota in mice and human patients.38,43,46 The action of metformin as an anti-aging or pro-longevity agent38,43 may also be related to its effect on gut microbiota.44 Anti-inflammatory agent 5-aminosalicylic acid can improve metabolic syndrome through suppression of inflammation as well.60 Active components of Akkermansia, such as Amuc_1100, and delivery of gut-tropic Th17 cells or agents that can boost non-pathogenic Th17 cells, specifically in the intestine, may also be able to open a new horizon in the development of next-generation therapies against metabolic syndrome or diabetes.
CONCLUSION
Changes in the gut microbiota and immunity are being accepted as important elements in the development of metabolic syndrome and diabetes. However, still numerous questions need to be elucidated to clearly understand the interaction between microbiota and gut immunity or disturbance therein associated with the diseases. Future studies addressing the complex interplay between gut microbiota, immunity, and host metabolism in a physiological and pathological context will pave the way for the development of innovative therapeutic agents against metabolic syndrome and diabetes.
ACKNOWLEDGEMENTS
This study was supported by a Global Research Laboratory Grant (K21004000003-12A0500-00310) and the Bio & Medical Technology Development Program (NRF-2015M3A9B6073846). M.S. Lee and K.Y. Lee are the recipients of a UNIST Fund (2014M3A9D8034459) and a Samsung Medical Center grant (#CRP1500058), respectively.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 3.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 4.Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 7.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Delaere F, Duchampt A, Mounien L, Seyer P, Duraffourd C, Zitoun C, et al. The role of sodium-coupled glucose co-transporter 3 in the satiety effect of portal glucose sensing. Mol Metab. 2012;2:47–53. doi: 10.1016/j.molmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plöger S, Stumpff F, Penner GB, Schulzke JD, Gäbel G, Martens H, et al. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- 10.Alenghat T, Osborne LC, Saenz SA, Kobuley D, Ziegler CG, Mullican SE, et al. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature. 2013;504:153–157. doi: 10.1038/nature12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978;14:115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- 12.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- 16.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 20.Woo PC, Ng KH, Lau SK, Yip KT, Fung AM, Leung KW, et al. Usefulness of the MicroSeq 500 16S ribosomal DNA-based bacterial identification system for identification of clinically significant bacterial isolates with ambiguous biochemical profiles. J Clin Microbiol. 2003;41:1996–2001. doi: 10.1128/JCM.41.5.1996-2001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krajmalnik-Brown R, Ilhan ZE, Kang DW, DiBaise JK. Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract. 2012;27:201–214. doi: 10.1177/0884533611436116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23:859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 24.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 26.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 27.Quévrain E, Maubert MA, Michon C, Chain F, Marquant R, Tailhades J, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease. Gut. 2016;65:415–425. doi: 10.1136/gutjnl-2014-307649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi Y, Kwon Y, Kim DK, Jeon J, Jang SC, Wang T, et al. Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Sci Rep. 2015;5:15878. doi: 10.1038/srep15878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlsson CL, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring) 2012;20:2257–2261. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 32.Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 33.Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015;22:658–668. doi: 10.1016/j.cmet.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73:7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 39.Chevalier C, Stojanović O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C, et al. Gut microbiota orchestrates energy homeostasis during cold. Cell. 2015;163:1360–1374. doi: 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 41.Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe-/- mice. Circulation. 2016;133:2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 43.Lee H, Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol. 2014;80:5935–5943. doi: 10.1128/AEM.01357-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cochemé HM, Noori T, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, et al. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40:54–62. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 46.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 47.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki T, Hara H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr Metab (Lond) 2010;7:19. doi: 10.1186/1743-7075-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 53.Chung H, Vilaysane A, Lau A, Stahl M, Morampudi V, Bondzi-Simpson A, et al. NLRP3 regulates a non-canonical platform for caspase-8 activation during epithelial cell apoptosis. Cell Death Differ. 2016;23:1331–1346. doi: 10.1038/cdd.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Everard A, Geurts L, Caesar R, Van Hul M, Matamoros S, Duparc T, et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat Commun. 2014;5:5648. doi: 10.1038/ncomms6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147:1363–1377. doi: 10.1053/j.gastro.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh V, Chassaing B, Zhang L, San Yeoh B, Xiao X, Kumar M, et al. Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab. 2015;22:983–996. doi: 10.1016/j.cmet.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21:527–542. doi: 10.1016/j.cmet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514:237–241. doi: 10.1038/nature13564. [DOI] [PubMed] [Google Scholar]
- 62.Gross M, Salame TM, Jung S. Guardians of the gut-murine intestinal macrophages and dendritic cells. Front Immunol. 2015;6:254. doi: 10.3389/fimmu.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garidou L, Pomié C, Klopp P, Waget A, Charpentier J, Aloulou M, et al. The gut microbiota regulates intestinal CD4 T cells expressing RORγt and controls metabolic disease. Cell Metab. 2015;22:100–112. doi: 10.1016/j.cmet.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 64.Hong CP, Park A, Yang BG, Yun CH, Kwak MJ, Lee GW, et al. Gut-specific delivery of T-helper 17 cells reduces obesity and insulin resistance in mice. Gastroenterology. 2017;152:1998–2010. doi: 10.1053/j.gastro.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 65.Monteiro-Sepulveda M, Touch S, Mendes-Sá C, André S, Poitou C, Allatif O, et al. Jejunal T cell inflammation in human obesity correlates with decreased enterocyte insulin signaling. Cell Metab. 2015;22:113–124. doi: 10.1016/j.cmet.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 66.Gaublomme JT, Yosef N, Lee Y, Gertner RS, Yang LV, Wu C, et al. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell. 2015;163:1400–1412. doi: 10.1016/j.cell.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagner N, Löhler J, Kunkel EJ, Ley K, Leung E, Krissansen G, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 69.Williams MB, Butcher EC. Homing of naive and memory T lymphocyte subsets to Peyer's patches, lymph nodes, and spleen. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]
- 70.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 71.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 72.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chassaing B, Miles-Brown J, Pellizzon M, Ulman E, Ricci M, Zhang L, et al. Lack of soluble fiber drives diet-induced adiposity in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G528–G541. doi: 10.1152/ajpgi.00172.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lakhan SE, Kirchgessner A. Gut microbiota and sirtuins in obesity-related inflammation and bowel dysfunction. J Transl Med. 2011;9:202. doi: 10.1186/1479-5876-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]