Abstract
Background
Pulmonary metastasectomy is an established treatment modality for patients with soft as well as bone tissue sarcomas. Aim of this study is to describe the Essen experience in the surgical management of patients with pulmonary sarcoma metastases.
Methods
This is a retrospective single center analysis of perioperative outcome of patients undergoing pulmonary metastasectomy for sarcoma metastases from 1997-2017 and a summary of published papers on this topic.
Results
During the observation period 327 patients (49.23% female) underwent pulmonary metastasectomy for metastases of extrathoracic sarcomas in curative intent. The number of resected metastases was 1–3 in 283 cases (86.54%), 4–9 in 31 cases (9.48%) and 10 or more lesions in 14 cases (4.28%). Wedge resections or precision excisions with laser or electrocautery were performed in 278 cases (85.02%), anatomical segmental resections in 16 patients (4.89%) and lobectomies in 33 patients (10.09%). Bilateral procedures were performed in 98 cases (29.96%). Lymphadenectomy was performed in 122 patients. Positive lymph nodes were found only in 6 cases. All of these cases were patients with soft tissue sarcoma as primary tumor. Preoperative neoadjuvant treatment was performed in 79 patients (24.15%) with chemotherapy, in 54 patients (16.51%) with radiochemotherapy and in 10 patients (3.05%) with radiotherapy. Major postoperative complications were observed in 2.75% of all patients. Thirty-day mortality was 0%.
Conclusions
Pulmonary metastasectomy in sarcoma patients is a feasible and safe treatment strategy even in patients with bilateral metastases and multiple lesions. Thoracic lymph node metastases are rare and did not influence survival in our cohort.
Keywords: Pulmonary metastasectomy, sarcoma metastases, lung metastases
Introduction
Pulmonary metastasectomy is an established treatment modality for patients with soft as well as bone tissue sarcomas. There are considerable advances in multimodality management of sarcoma patients, however pulmonary metastatic disease still remains the leading cause of death. Thus optimal management is crucial in these patients. Due to high recurrence rates of more than 50% selection of the subset of patients who will gain a survival benefit from surgical resection is crucial. Well established criteria and prerequisite to pulmonary surgery are local control of the primary tumor, absence of extrathoracic disease, functional operability and radical resectability of all lesions. Previously identified clinical prognostic factors include histology of the primary tumor, number of nodules, disease free interval, and completeness of the resection. Due to the low overall incidence of sarcoma treatment in dedicated centers is of crucial importance. University Medicine Essen is a tertiary referral center for sarcoma patients. More than 200 patients with newly diagnosed sarcoma are treated annually. All treatment decisions are made in a dedicated interdisciplinary tumor conference with participation of surgeons, oncologists, radiation oncologists, pulmonologists, pathologists and radiologists. Aim of this study is to describe the Essen experience in the surgical management of patients with pulmonary sarcoma metastases.
Methods
This is a retrospective single center analysis of perioperative outcome of patients undergoing pulmonary metastasectomy for sarcoma metastases from 1997–2017 and a summary of published papers on this topic. Our institutional review board approved this study.
Results
During the observation period 327 patients (49.23% female) underwent pulmonary metastasectomy for metastases of extrathoracic sarcomas in curative intent. The majority of patients were operated in the recent decade (Figure 1). Mean age at the time of operation was 50.57±16.21 years. 86.23% of patients were diagnosed with soft tissue sarcoma 10.70% with osteosarcoma and 3.05% with chondrosarcoma as primary tumor (Figure 2). The number of resected metastases was 1–3 in 283 cases (86.54%), 4–9 in 31 cases (9.48%) and 10 or more lesions in 14 cases (4.28%) (Figure 3). Development of metastases was synchronous to the primary tumor in 89 cases and metachronous in 238 patients (Figure 4). Mean disease free interval was 35.72±47.7 months. Mean diameter of metastatic lesions was 2.17 cm. Wedge resections or precision excisions with laser or electrocautery were performed in 278 cases (85.02%), anatomical segmental resections in 16 patients (4.89%) and lobectomies in 33 patients (10.09%). Bilateral procedures were performed in 98 cases (29.96%). Lymphadenectomy was performed in 122 patients. Positive lymph nodes were found only in 6 cases. All of these cases were patients with soft tissue sarcoma as primary tumor. Preoperative neoadjuvant treatment was performed in 79 patients (24.15%) with chemotherapy, in 54 patients (16.51%) with radiochemotherapy and in 10 patients (3.05%) with radiotherapy. Major postoperative complications were observed in 2.75% of all patients. Thirty-day mortality was 0%.
Figure 1.
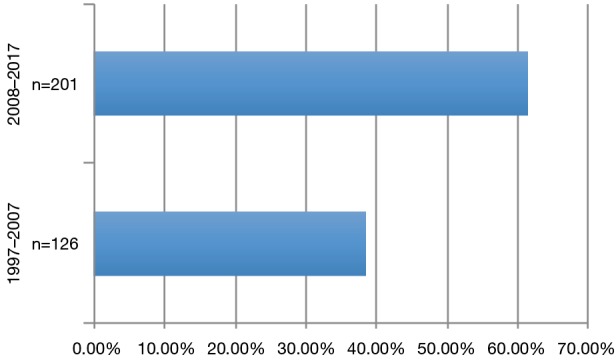
Distribution of patients during the observation period.
Figure 2.
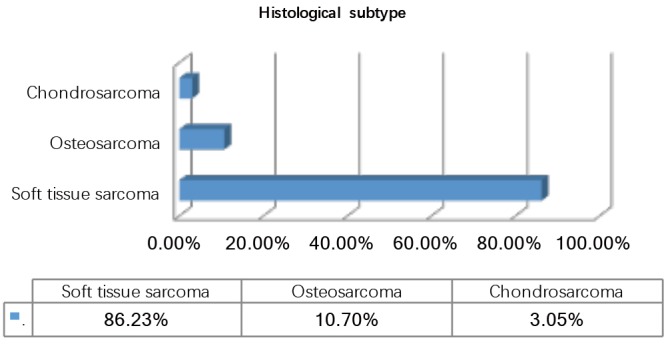
Histological subtype.
Figure 3.
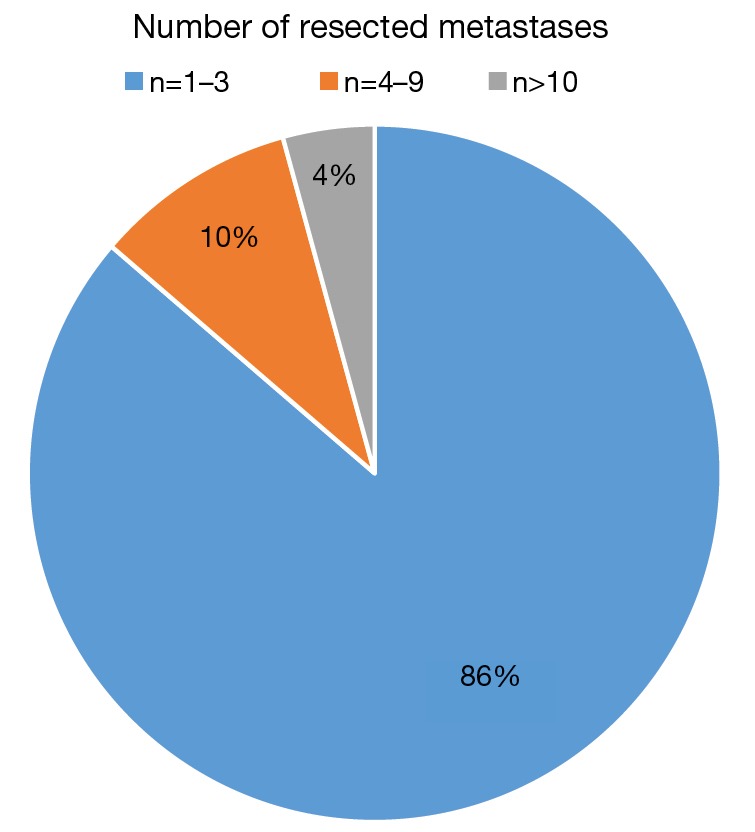
Number of resected metastases.
Figure 4.
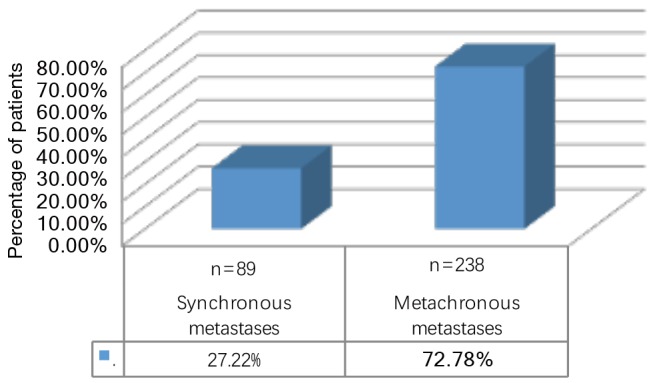
Timing of metastatic development.
Discussion
Sarcomas frequently and often exclusively metastasize to the lung. Pulmonary metastasectomy is an important part of the treatment strategy in patients as long as a radical resection is feasible. Investigations of histologic growth patterns might provide further information on tumor aggressiveness, necessary resection margins and might serve a prognostic parameter. Sarcoma metastases in the lung typically present as high-grade malignancies. Half of them are ill defined by aggressive patterns of local spread like interstitial growth, tumour satellites, vascular infiltration, lymphangitic spread and growth along vascular and bronchial structures. Fibrous pseudo-capsules are rare, and reactive zones are completely absent. However, chondrosarcoma metastases seem to present pseudo-capsules frequently (90%) (1). Several histological growth patterns were identified in a patient cohort operated between 2006 and 2009. Median survival in this cohort was 50.3 months and 5-year survival rate was 44.7%. ‘Interstitial growth’ was defined as a diffuse spread of the sarcoma cells into the alveolar septa at the transition of the metastasis to the normal lung tissue and was found to be a prognostic parameter. ‘Pleural penetration’ was defined as the infiltration and destruction of all visceral pleural layers by the tumour and was found to be a risk factor for local recurrence together with a metastatic size of >5 mm. Besides interstitial growth an age of >55 years at metastasectomy, a diameter >35 mm of the largest lesion and recurrence of disease were significant risk factors for mortality (2). Thus it seems reasonable—while trying to preserve as much functional lung tissue as possible—to ensure a radical resection taking growth patterns into account (3).
Pulmonary resection usually leads to a loss of lung function, even though a pure calculation based on the number of resected segments usually overestimates the actual functional loss. In patients with hyperinflation due to concomitant pulmonary emphysema a lung volume reduction might be present, even though this is a rather infrequent situation in the setting of sarcoma patients. A prospective observational study of 117 cases demonstrated a significantly reduced lung function 3 months postoperative with a mean loss of 10% in FEV1 and DLCO values. KCO and blood gases however remained unchanged. A risk factor for an increased loss of FEV1 was bilateral metastasectomy due to bilateral chest wall impairment. Adjuvant chemotherapy was found to be a risk factor for an increased deterioration in gas exchange. Further long term follow up measurements were not reported, even though particularly FEV1 values might still be impaired 3 months postoperatively due to postoperative chest wall restriction (4). These findings were confirmed in a more recent analysis of bilateral metastasectomies leading to a loss of 15% of spirometry values and 10% of DLCO after 3 months. However the immediate postoperative loss can be up to 40% after the second operation if the procedure is performed in a staged way by bilateral thoracotomies (5).
A prospective analysis of quality of life after metastasectomy performed on a subcohort of our patients from 2008–2010 demonstrated no significant changes between preoperative and postoperative quality of life values for emotional, cognitive and social functioning. However moderate deterioration in physical functioning, pain and dyspnea was observed after a mean follow up of 3–6 months. Since there is no change in global health-related quality of life the paper concluded that metastasectomy should be offered to all patients with a potential survival benefit (6).
No high quality evidence regarding the optimal investigations and intervals for follow-up after pulmonary metastasectomy is available. Computed tomography of the chest is the standard diagnostic test used by most centers including ours to follow up patients and screen for pulmonary metastases. Once metastasectomy has been performed patients are routinely followed up in 3 months intervals. The presentation of metastases on CT scan may vary depending on the underlying sarcoma histology. Osteosarcoma metastases are frequently calcified (7).
Median overall survival of patients undergoing pulmonary metastasectomy for sarcoma metastases is in the range of 40–50%. A paper focusing exclusively on soft tissue sarcoma metastases reported on a 45.3 months median overall survival (8).
Recently a single center retrospective review of 155 metastasectomies of soft tissue and bone sarcomas identified seven risk factors associated with poor overall survival in a multivariate analysis: (I) age more than 45 years; (II) disease-free interval less than 1 year; (III) thoracotomy, (IV) synchronous disease; (V) location and (VI) type of sarcoma (soft tissue vs. bone sarcoma); and (VII) performance of a lobectomy (9).
Frequently re-metastasectomy has to be performed and similar outcomes can be expected as long as a radical resection is feasible (10). In our patient collective 26.91% of all patients underwent re-metastasectomy.
In summary pulmonary metastasectomy in sarcoma patients is a feasible and safe treatment strategy. Thoracic lymph node metastases are rare. Metastasectomy should be considered even in patients with bilateral metastases and multiple lesions as long as radical resection is possible.
Acknowledgements
None.
Ethical Statement: The study was approved by the institutional review board of the Medical Faculty of the University Duisburg-Essen.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Welter S, Grabellus F, Bauer S, et al. Growth patterns of lung metastases from sarcomas. Virchows Arch 2011;459:213-9. 10.1007/s00428-011-1116-8 [DOI] [PubMed] [Google Scholar]
- 2.Welter S, Grabellus F, Bauer S, et al. Growth patterns of lung metastases from sarcoma: prognostic and surgical implications from histology. Interact Cardiovasc Thorac Surg 2012;15:612-7. 10.1093/icvts/ivs269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welter S, Arfanis E, Christoph D, et al. Growth patterns of pulmonary metastases: should we adjust resection techniques to primary histology and size?†. Eur J Cardiothorac Surg 2017. [epub ahead of print]. 10.1093/ejcts/ezx063 [DOI] [PubMed] [Google Scholar]
- 4.Welter S, Cheufou D, Ketscher C, et al. Risk factors for impaired lung function after pulmonary metastasectomy: a prospective observational study of 117 cases. Eur J Cardiothorac Surg 2012;42:e22-7. 10.1093/ejcts/ezs293 [DOI] [PubMed] [Google Scholar]
- 5.Welter S, Cheufou D, Zahin M, et al. Short- and Mid-Term Changes in Lung Function after Bilateral Pulmonary Metastasectomy. Thorac Cardiovasc Surg 2016;64:139-45. [DOI] [PubMed] [Google Scholar]
- 6.Welter S, Schwan A, Cheufou D, et al. Midterm changes in quality of life: a prospective evaluation after open pulmonary metastasectomy. Ann Thorac Surg 2013;95:1006-11. 10.1016/j.athoracsur.2012.11.059 [DOI] [PubMed] [Google Scholar]
- 7.Ciccarese F, Bazzocchi A, Ciminari R, et al. The many faces of pulmonary metastases of osteosarcoma: Retrospective study on 283 lesions submitted to surgery. Eur J Radiol 2015;84:2679-85. 10.1016/j.ejrad.2015.09.022 [DOI] [PubMed] [Google Scholar]
- 8.Schur S, Hoetzenecker K, Lamm W, et al. Pulmonary metastasectomy for soft tissue sarcoma--report from a dual institution experience at the Medical University of Vienna. Eur J Cancer 2014;50:2289-97. 10.1016/j.ejca.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 9.Lin AY, Kotova S, Yanagawa J, et al. Risk stratification of patients undergoing pulmonary metastasectomy for soft tissue and bone sarcomas. J Thorac Cardiovasc Surg 2015;149:85-92. 10.1016/j.jtcvs.2014.09.039 [DOI] [PubMed] [Google Scholar]
- 10.Hamaji M, Chen F, Miyamoto E, et al. Surgical and non-surgical management of repeat pulmonary metastasis from sarcoma following first pulmonary metastasectomy. Surg Today 2016;46:1296-300. 10.1007/s00595-016-1312-x [DOI] [PubMed] [Google Scholar]


