Abstract
Distinguishing multiple primary lung cancers from intrapulmonary metastases in patients with synchronous multifocal lung adenocarcinomas can be challenging. The most recent 8th edition American Joint Committee on Cancer staging manual (AJCC staging manual) distinguishes four disease patterns in patients with multiple lung nodules: (I) two or more distinct and histologically different masses (considered unrelated and staged as individual cancers); (II) multiple ground-glass or part-solid nodules, histologically of with lepidic growth pattern (considered separate tumors, T staged based on highest T stage lesion); (III) patchy areas of ground-glass and consolidations, histologically often invasive mucinous adenocarcinomas (considered single tumor with diffuse “pneumonic-type” involvement); and (IV) separate nodules with the same histologic features based on comprehensive histologic subtyping (considered intrapulmonary metastases). Histologic and molecular features, in conjunction with clinical and radiological information, can all be tools to assist with staging of multiple nodules. Histologic features of adenocarcinomas are best characterized using comprehensive histologic subtyping (percentage of lepidic, acinar, solid, papillary and micropapillary pattern). Genomic alterations are commonly assessed using fluorescence in-situ hybridization and next generation sequencing (NGS). The AJCC considers exactly matching breakpoints by comparative genomic hybridization (CGH) as the only evidence for intrapulmonary metastases, and clearly different histologic types or subtypes as the only evidence for separate primary tumors. Similar histologic subtypes or the same biomarker pattern are considered merely relative arguments in favor of a single tumor source. When assessing multifocal lung cancer, pathologists should consider, and carefully weigh the importance of, molecular testing results in addition to the tumor’s histologic features. For many cases encountered in routine clinical practice, absolute certainty cannot be reached as to whether they represent multiple primary cancers or intrapulmonary metastases. Classification of difficult cases often benefits from multidisciplinary discussion.
Keywords: Lung cancer, staging, primary, intrapulmonary metastasis
Introduction
The reported incidence of lung carcinoma patients presenting with multiple nodules ranges from 0.2% to 20% (1-3). The incidence has been increasing because of improved imaging techniques, lung cancer screening programs and surveillance of patients with previously treated cancers. Classification of multiple lung nodules as either multiple primary tumors or intrapulmonary metastases can be challenging. Martini and Melamed proposed the most frequently used clinical and pathological criteria in 1975 (1). The main idea behind their classification was that the morphology of metastases should match that of the primary tumor, while different morphology supports classification of tumors as unrelated separate primaries. Although these criteria sound simple and straight forward, there has been variability in their application among clinicians and pathologists. The resulting inadequate classification of tumors failed to predict the clinical course or outcome. Distinction between multiple primary tumors and intrapulmonary metastases is important because treatments and outcomes are different. Therefore, the 7th and, even more so, the current 8th edition of the American Joint Committee on Cancer staging manual (AJCC staging manual) attempt to classify multiple tumors more precisely by not only using location criteria, but also by incorporating the most recent histologic classification criteria and molecular characteristics of the tumors (4,5). This review summarizes clinical, pathological and molecular approach to classification and staging of multiple lung nodules.
Staging of multifocal lung cancer
Staging of multiple lung tumor nodules has been challenging and has undergone significant changes in the past two editions of the AJCC staging manual. Staging of multifocal lung cancers in the 7th edition of AJCC staging manual was mostly based on the Martini and Melamed criteria established in 1975 (1,5). The AJCC staging manual has since recognized that these criteria may not be clinically optimal; some new proposals were put forward but without detailed guideline for their implementation. For example, pathologists were given the option to include their morphologic impressions, immunohistochemistry results and any available molecular studies into pathological staging. However, no detailed recommendations were given as to which histological criteria or molecular studies should be used. Furthermore, it was not defined how to incorporate molecular studies into the staging of multifocal lung cancers.
The 8th edition of the AJCC staging manual categorized multifocal lung cancer into four disease patterns (Table 1) (4,6). These patterns are based on clinical presentation (including radiologic impression and distribution of disease), histological assessment, and outcomes.
Table 1. Patterns of multifocal lung cancers recognized by the 8th edition of the American Joint Committee on Cancer staging manual (4,6).
| Disease pattern (relationship of the tumors) | Imaging findings | Histology | TNM classification |
|---|---|---|---|
| Second primary cancer | Two or more distinct masses typical for lung cancer | Different histologic type or subtype | Separate T, N and M for each tumor |
| Separate tumor nodules of same cancer (intrapulmonary metastases) | Typical lung cancer (solid, spiculated) with separate solid nodule | Same histologic type or subtype | T3 if in the same lobe; T4 if ipsilateral lobe; M1a if contralateral lobe; single N and M for all |
| Multifocal ground glass/lepidic adenocarcinoma | Multiple ground glass or part-solid nodules | Adenocarcinoma with lepidic growth pattern (including AIS, MIA, LPA) | T based on highest T lesion with (m) for multiplicity; single N and M |
| Pneumonic-type adenocarcinoma | Patchy areas of ground glass and consolidation | Same histology (often invasive mucinous adenocarcinoma) | Same as for separate tumor nodules |
AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; LPA, lepidic-predominant adenocarcinoma.
The first pattern is defined by multiple primary tumors. Clinical characteristics of each separate tumor nodule are similar to those of a single individual lung cancer according to its respective stage and histology. Each tumor should be assigned a separate T, N and M stage as if it were a single tumor.
The second pattern is defined as separate tumor nodules of single origin (intrapulmonary metastases), usually represented by multiple tumor nodules of the same histologic type and/or molecular profile. Overall survival of these patients is primarily determined by the treatment. Staging of these tumors follows the same rules as those in the 7th edition of the AJCC staging manual. A separate tumor nodule in the same lobe is staged as T3, in the ipsilateral lobe as T4 and in the contralateral lobe as M1a.
The third pattern is defined by multifocal lung adenocarcinoma with ground glass features radiologically or lepidic features histologically. Patients with multifocal lepidic adenocarcinoma are highly unlikely to develop nodal or distant metastases, but they do have an increased risk of developing additional ground glass/subsolid lung cancers. Overall, this pattern of lung cancer often exhibits a more indolent behavior. The largest lesion, appended with an “m” in parenthesis to indicate multiplicity, determines the pathologic T stage.
The fourth pattern is so-called “pneumonic type” lung adenocarcinoma. It is characterized by a diffuse consolidative pattern by chest imaging without well-demarcated nodules. Histologically this pattern typically correlates with invasive mucinous adenocarcinoma. Progression of these tumors is typically slow, but overall survival is worse than for ground glass/lepidic tumors. Pathologic T staging follows the same as those for intrapulmonary metastases.
Morphological approach to multifocal lung cancer
Pathologic assessment of multiple lung nodules plays a significant role in distinguishing multiple primary tumors from intrapulmonary metastases. The utility of Martini and Melamed pathologic criteria has been limited because they consider only the major histological tumor type (adenocarcinoma versus squamous cell carcinoma). They do not account for the vast morphological heterogeneity seen in lung adenocarcinomas, the most common histological type of non-small cell carcinoma in patients with isolated as well as multiple nodules. Comprehensive histologic subtyping of this heterogeneous morphology was formally described in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) classification of lung adenocarcinoma and subsequently adopted by the World Health Organization (WHO) classification of lung tumors in 2015 (7,8). Briefly, there are five main morphologically distinct subtypes of invasive lung adenocarcinoma including acinar, papillary, micropapillary, solid and lepidic. A spectrum of mucinous tumors is considered to be variants of lung adenocarcinoma. In contrast, squamous cell carcinoma is morphologically less heterogenous with currently three recognized subtypes (keratinizing, non-keratinizing and basaloid).
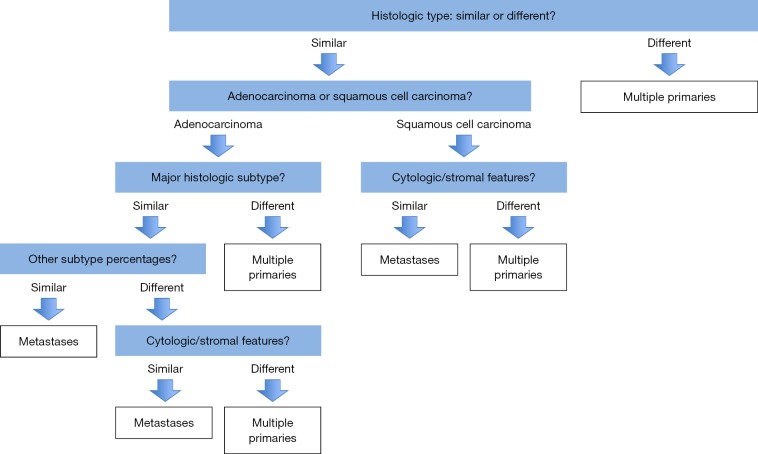
The prognostic significance of histological subtyping of lung adenocarcinoma has been demonstrated in several studies (9-11). Solid and micropapillary subtypes are associated with poor prognosis, while lepidic subtype usually indicates favorable outcomes (12,13). Due to the prognostic significance of various histological subtypes, comprehensive histological assessment of the surgically resected lung adenocarcinoma in 5% increments has been recommended by the IASLC/ATS/ERS classification of lung adenocarcinoma and by the 2015 WHO classification of the lung tumors. Comprehensive histological assessment has also been shown to be extremely valuable in the classification and staging of multiple lung tumor nodules (14-16). An algorithmic approach to surgical resection specimens with multiple tumor nodules was proposed by Girard et al. (Figure 1) (14). Pathologists should decide whether two tumors are morphologically similar or not. It is easier to establish that two cancers are separate primary tumors than that they are intrapulmonary metastases, but since major differences in histologic subtype could be due to more aggressive subtypes being overrepresented in metastatic foci, this is method is not perfect. Histological evaluation should include the relative percentage of each histologic subtype. Adenocarcinomas may also be assessed for variants (mucinous, enteric, colloid, fetal) and cytological changes such as clear cell or signet ring features. In addition to histological subtypes, other tumor elements such as appearance of the stroma, and presence of inflammation or necrosis may be helpful distinguishing features. Distinguishing multiple primary squamous cell carcinomas from intrapulmonary metastases is much more difficult. For squamous cell carcinomas, the initial histological assessment should consider the amount of keratinization, presence of basaloid cytology, stromal appearance (e.g., myxoid, hyalinized) and necrosis. The presence of additional features such as clear cell change or sarcomatoid morphology might also be helpful. Although there is no universally accepted grading scheme for non-small cell carcinoma, detailed cytological features such as cell size, nuclear contours and the presence or absence of nucleoli may need to be assessed in difficult cases. An IASLC reproducibility study of poorly differentiated non-small cell carcinoma demonstrated great agreement among pulmonary pathologists in recognizing features of squamous cell carcinoma such as keratinization, keratin pearl formation and intercellular bridges (17). This suggests a potential role of this simple approach in staging of multifocal squamous cell carcinoma. In contrast, consistent comprehensive histological subtyping of adenocarcinoma is more challenging. An IASLC reproducibility study of adenocarcinoma found agreement between pulmonary pathologists to be only fair to moderate (2). In this study, papillary, micropapillary and lepidic subtypes were responsible for most disagreement among pathologists. The reason for discrepancies in the interpretation may lie in the inconsistent application of existing diagnostic criteria.
Figure 1.
Algorithmic approach to multiple lung nodules using comprehensive histology subtyping (14).
Noguchi et al. demonstrated that pathologist education of diagnostic criteria should ultimately lead to significant improvement in diagnostic reproducibility (3). One could argue that this study did not reflect daily routine practice because the interpretation was based on the review of a representative static image of a particular histological subtype rather than review of the entire tumor section. Another study conducted by the same group of thoracic pathologists, assessing the morphology of synchronous lung nodules, further supports this hypothesis (18). Pathologists reviewed whole slide images of 126 tumors from 48 patients and evaluated numerous histological and cytological features. The overall agreement for tumor classification as either primary tumor of intrapulmonary metastasis reached kappa score of 0.60, indicating that pathologist in most of the cases can perform very well in the overall morphological characterization of multiple lung nodules and subsequent staging. The most useful morphological features to distinguish between multiple primary tumors and metastases were main tumor type, predominant histological patterns, acinus formation, nuclear pleomorphism, cell and nucleolar size and mitotic rate (18).
Requests to assess during intraoperative frozen section consultations whether two tumors as separate primaries or intrapulmonary metastases remains a challenge. Frozen sections usually have many processing artifacts that can make a reliable comprehensive histological assessment difficult. A few studies reported moderate agreement for histological subtyping of lung adenocarcinoma on frozen sections (19,20). Similar to studies on permanent sections, micropapillary and lepidic subtypes were the most challenging, while acinar and solid patterns were most likely to be correctly identified. In addition to the poor quality of frozen sections, sampling error may contribute significantly to suboptimal histological assessment because only a small portion and not the entire tumor can be evaluated in the intraoperative setting.
A dilemma poses the morphological assessment of multiple nodules on small biopsies and cytology specimens that are frequently the only tissue specimens available in patients with lung carcinomas. Precise classification into specific main types such as adenocarcinoma and squamous cell carcinoma is possible in the majority of the cases based on morphology or with the help of immunohistochemistry. Therefore, in cases of separate primary tumors of a different histologic type, small biopsy and cytology specimens can be sufficient (21-25). However, morphologic subclassification of lung adenocarcinoma on cytology specimen is difficult and largely depends on the procedure type and tumor cellularity of the sample (26,27). The acinar pattern appears to be more readily classified on cytology specimens, while recognition of micropapillary and solid patterns can be extremely difficult and unreliable (28).
The 2015 WHO classification of lung tumor introduced the term and concept of adenocarcinoma in situ (AIS), replacing with it the term bronchioloalveolar carcinoma (BAC) (8). It is not uncommon to identify multiple nodules in the lung that would be classified as AIS. According to the WHO these tumors are defined by gross size of up to 3 cm and characterized by growth of malignant cells, typically showing type II pneumocyte or Clara cell differentiation, along the alveolar septa without evidence of stromal, vascular, air space or pleural invasion. On imaging studies, these tumors appear as multiple ground glass opacities. They are considered to represent separate primary adenocarcinoma rather than intrapulmonary metastases. Similarly, minimally invasive adenocarcinoma (MIA) and invasive adenocarcinoma with predominantly lepidic growth pattern are considered to represent separate primary tumors. This distinction is important because the entire spectrum of adenocarcinoma with lepidic component (AIS, MIA or lepidic predominant adenocarcinoma) have good clinical outcomes (29-31). This rule is applicable to non-mucinous adenocarcinomas only. Mucinous adenocarcinomas are uncommon. With adequate sampling, one can find invasive foci in the vast majority of mucinous tumors showing a lepidic growth pattern. Separate foci of pure mucinous AIS can be considered separate primary tumors. However, invasive mucinous adenocarcinomas have a tendency for intrapulmonary metastatic spread, and, therefore, multiple mucinous tumors are often best considered to represent metastatic foci (8). Furthermore, invasive mucinous adenocarcinoma tends to have worse outcome than its non-mucinous counterparts (32).
In summary, published studies demonstrate feasibility of morphological subtyping of lung carcinoma that predicts patients’ stage and outcome better than Martini and Melamed criteria.
Molecular approach to multifocal lung cancer
Over the past decade multiple studies using different molecular approaches to analysis of synchronous lung tumor nodules have emerged including DNA microsatellite analysis, comparative genomic hybridization (CGH), array CGH and, most recently, next generation sequencing (NGS) (15,33-39). In general, tumors with largely concordant molecular results were considered clonal in origin (metastases), and those with discordant findings were considered to be independent primary tumors. Discrepancy between clinical and molecular classification of originally presumed cases of multiple primary lung cancers ranged from 18% to 30% in different series (14,15).
The earliest molecular studies used clonality assays such as a panel of variable number of polymorphic microsatellite markers and X-chromosome inactivation analysis (37,38,40). Data generated by those assays had to be interpreted with caution since similar patterns of allelic losses may be present throughout the respiratory epithelium of smokers with or without lung cancer. The main shortcomings of the first molecular studies were the small number of analyzed cases and the lack of standardized methodology and interpretation criteria. The data from published reports indicate a highly variable percentage of multifocal tumors identified as clonally related (up to 70%), and all reports agree that multifocal tumors may arise either as metastases from a single tumor or as independent tumors (16,33-35,41).
Advances in molecular techniques have led to more comprehensive approaches to analyzing genomic alterations such as gene mutations, amplifications, deletions and rearrangements. Many studies assessed particular mutations with the assumption that the matched driver mutations define tumors as clonally related (16,33,42). However, one needs to be very careful with the interpretation because the same mutations may occur in the morphologically different tumors. Furthermore, driver mutations can also be detected in normal appearing lung in patients with lung cancer and may represent just the most prevalent mutation (43). The possibility of an EGFR germline mutation can also further complicate the interpretation of clonal relationship of multiple lung adenocarcinoma (44,45) Therefore, identification of the same mutation in two tumors does not necessarily indicate a clonal relationship. Such result should always be correlated with tumor morphology and imaging findings. Another issue that has been extensively reported in the literature is heterogeneity of mutations, particularly for EGFR and KRAS, between primary tumors and metastatic sites with discordance rate ranging from 0–45% (46-50). However, variations in these earlier studies likely due to technical factors such as low assay sensitivity, amount of tumor in the sample and DNA/RNA quality. Most recent studies using next-generation sequencing demonstrated a high concordance rate (94%) for driver somatic alterations between primary lung tumors and matched metastases (51). Yatabe et al. showed that the co-founding factors in the interpretation of targeted PCR-based mutation assays are related to the co-existence of gene amplification, contamination with normal tissue, tumor cell content and assay sensitivity rather than heterogeneity of somatic mutations (52).
Taken all together, molecular testing for oncogenic mutations in advanced stage lung adenocarcinoma has become standard of care. Therefore, information about gene mutation status can often be incorporated along with histological assessment of the tumor in the staging of multiple lung tumors. The 8th edition of the AJCC staging manual considers a similar biomarker pattern (usually genomic alterations) only a relative argument in favor of a single tumor source, and instead endorses exactly matching breakpoints identified by CGH as the only evidence of clonality. The number of studies using CGH is very limited (15,39). Interestingly, in addition to CGH, these studies also employed mutation studies and morphological criteria to classify tumors as either separate primary tumors or metastases. There are several limitations to routine use of CGH. Firstly, CGH is not a standard method used for genotyping of solid tumors in clinical practice, mainly because large amounts of DNA are needed and no information on potentially actionable targets is provided. Secondly, NGS platforms have been implemented in many clinical laboratories that provide simultaneous detection of gene mutations, copy number changes and gene rearrangements, rendering CGH an even less desirable assay for routine clinical practice. Murphy et al. proposed that the assessment of DNA rearrangements by next-generation DNA sequencing might be a better approach as identifier of lineage than single-nucleotide mutations (53).
In contrast to lung adenocarcinoma, molecular profiling of squamous cell carcinoma is not routinely performed. Published studies that used limited gene panels mostly failed to demonstrate significant improvement in staging of multifocal squamous cell carcinoma (16).
Clinical management and outcome of multifocal lung cancer
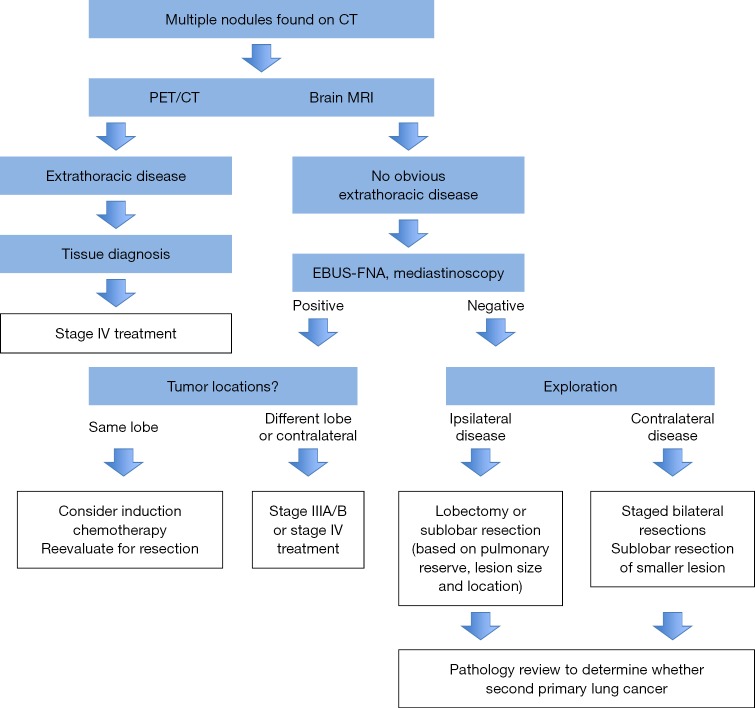
The American College of Chest Physicians Clinical Guideline recommends imaging and minimally invasive needle techniques as the first choice for detection of occult mediastinal and distant metastases in patients with synchronous separate primary lung cancers as well as additional tumor nodules (intrapulmonary metastases) (54,55). Treatment is determined based on the histological classification of tumors and staging (Figure 2). Patients with synchronous independent primary lung carcinomas without evidence of metastatic disease will most likely be considered for surgical resection. The IASLC database of the multiple tumor nodules showed that almost all same-lobe nodules were surgically resected, whereas contralateral nodules were not (57). Surgical treatment of patients with synchronous primary lung carcinoma can result in survival that is comparable with patients with stage matched single lung cancers. The 5-year overall survival for surgically resected tumors in the same lobe and without lymph node metastases is about 50% (56,58). Overall, it seems that the number of tumor nodules does not affect the survival, although Rao et al. reported a trend towards better survival in patients with solitary additional nodules (59). Fewer survival data are available for surgically resected synchronous nodules occurring in the ipsilateral different or contralateral lobes. Nagai et al. reported no difference in survival of patients with multiple nodules occurring in the same or in the different lobes (60). Cumulative data from published studies indicate that the 5-year overall survival for stage N0 patients with tumor nodules in a different ipsilateral lobe is about 40% (57). Survival data for patients with contralateral tumor nodules have to be interpreted with caution since not all contralateral tumors are resected. In one study, limited resection of a second contralateral primary lung cancer nodule had no significant effect on 5-year-survival (61). These patients are usually thought to have poor survival, but they are often not treated with curative intent and, therefore, the impact of surgery on survival is uncertain. Analysis of surgically resected synchronous lung cancers occurring in multiple lobes found that older age, male gender, lymph node metastases and unilateral tumor location are poor prognostic factors (62). Other reported prognostic factor includes size of the tumor with smaller tumors being associated with better survival (63-65). One should note that for all these data to be considered reliable one must assume that classification as multiple primary cancers or intrapulmonary metastases was accurate.
Figure 2.
Algorithmic approach to the management of multiple lung nodules (56).
Conclusions
Distinguishing multiple biologically unrelated primary lung cancers tumors from intrapulmonary metastatic disease of single source can help predict outcome and guide therapy. Pathologists are at the forefront of making that distinction. Multifocal lung cancers should first undergo histologic subtyping to gather relative arguments in favor or against a single tumor source. A different pattern of biomarkers and lack of nodal or systemic metastases favor biologically unrelated tumors (separate primaries). Conversely, the same biomarker pattern or presence of significant nodal or systemic metastases provides relative arguments in favor of biologically related tumors (intrapulmonary metastases). Practitioners need to weigh carefully the importance of each of these features in each individual patient. Absolute certainty as to whether two tumors represent separate primary cancers or an intrapulmonary metastasis can often not be reached. Classification of difficult cases often benefits from multidisciplinary discussion amongst radiologists, oncologists, surgeons and pathologists.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed] [Google Scholar]
- 2.Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012;25:1574-83. 10.1038/modpathol.2012.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noguchi M, Minami Y, Iijima T, et al. Reproducibility of the diagnosis of small adenocarcinoma of the lung and usefulness of an educational program for the diagnostic criteria. Pathol Int 2005;55:8-13. 10.1111/j.1440-1827.2005.01782.x [DOI] [PubMed] [Google Scholar]
- 4.AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2017. [Google Scholar]
- 5.AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010. [Google Scholar]
- 6.Detterbeck FC, Nicholson AG, Franklin WA, et al. The IASLC Lung Cancer Staging Project: Summary of Proposals for Revisions of the Classification of Lung Cancers with Multiple Pulmonary Sites of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J Thorac Oncol 2016;11:639-50. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. 10.1513/pats.201107-042ST [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Burke AP, et al. editors. World Health Organization Classification of Tumours. Lyon: International Agency for Research on Cancer, 2015. [Google Scholar]
- 9.Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. 10.1200/JCO.2011.37.2185 [DOI] [PubMed] [Google Scholar]
- 10.Okada M. Subtyping lung adenocarcinoma according to the novel 2011 IASLC/ATS/ERS classification: correlation with patient prognosis. Thorac Surg Clin 2013;23:179-86. 10.1016/j.thorsurg.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 11.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. 10.1038/modpathol.2010.232 [DOI] [PubMed] [Google Scholar]
- 12.Ohtaki Y, Yoshida J, Ishii G, et al. Prognostic significance of a solid component in pulmonary adenocarcinoma. Ann Thorac Surg 2011;91:1051-7. 10.1016/j.athoracsur.2010.11.071 [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Mora N, Presmanes MC, Monroy V, et al. Micropapillary lung adenocarcinoma: a distinctive histologic subtype with prognostic significance. Case series. Hum Pathol 2008;39:324-30. 10.1016/j.humpath.2007.05.029 [DOI] [PubMed] [Google Scholar]
- 14.Girard N, Deshpande C, Lau C, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am J Surg Pathol 2009;33:1752-64. 10.1097/PAS.0b013e3181b8cf03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard N, Ostrovnaya I, Lau C, et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers. Clin Cancer Res 2009;15:5184-90. 10.1158/1078-0432.CCR-09-0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider F, Derrick V, Davison JM, et al. Morphological and molecular approach to synchronous non-small cell lung carcinomas: impact on staging. Mod Pathol 2016;29:735-42. 10.1038/modpathol.2016.66 [DOI] [PubMed] [Google Scholar]
- 17.Thunnissen E, Noguchi M, Aisner S, et al. Reproducibility of histopathological diagnosis in poorly differentiated NSCLC: an international multiobserver study. J Thorac Oncol 2014;9:1354-62. 10.1097/JTO.0000000000000264 [DOI] [PubMed] [Google Scholar]
- 18.Nicholson A, Viola P, Torkko K, et al. Reproducibility of Comprehensive Histologic Assessment and Refining Histologic Criteria in P Staging of Multiple Tumor Nodules. J Thorac Oncol 2017;12:S1131-2. 10.1016/j.jtho.2016.11.1587 [DOI] [Google Scholar]
- 19.Trejo Bittar HE, Incharoen P, Althouse AD, et al. Accuracy of the IASLC/ATS/ERS histological subtyping of stage I lung adenocarcinoma on intraoperative frozen sections. Mod Pathol 2015;28:1058-63. 10.1038/modpathol.2015.71 [DOI] [PubMed] [Google Scholar]
- 20.Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of </= 3 cm: accuracy and interobserver agreement. Histopathology 2015;66:922-38. 10.1111/his.12468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zakowski MF, Rekhtman N, Auger M, et al. Morphologic Accuracy in Differentiating Primary Lung Adenocarcinoma From Squamous Cell Carcinoma in Cytology Specimens. Arch Pathol Lab Med 2016;140:1116-20. 10.5858/arpa.2015-0316-OA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omland SH, Henrik H, Olsen EK, et al. Subtyping of nonsmall cell lung cancer on cytology specimens: reproducibility of cytopathologic diagnoses on sparse material. Diagn Cytopathol 2014;42:105-10. 10.1002/dc.22995 [DOI] [PubMed] [Google Scholar]
- 23.Pelosi G, Sonzogni A, Viale G. The classification of lung carcinoma: time to change the morphology-based approach? Int J Surg Pathol 2010;18:161-72. 10.1177/1066896910361736 [DOI] [PubMed] [Google Scholar]
- 24.Ocque R, Tochigi N, Ohori NP, et al. Usefulness of immunohistochemical and histochemical studies in the classification of lung adenocarcinoma and squamous cell carcinoma in cytologic specimens. Am J Clin Pathol 2011;136:81-7. 10.1309/AJCPFKOLGL6PMOF3 [DOI] [PubMed] [Google Scholar]
- 25.Rossi G, Pelosi G, Graziano P, et al. A reevaluation of the clinical significance of histological subtyping of non--small-cell lung carcinoma: diagnostic algorithms in the era of personalized treatments. Int J Surg Pathol 2009;17:206-18. 10.1177/1066896909336178 [DOI] [PubMed] [Google Scholar]
- 26.Coghlin CL, Smith LJ, Bakar S, et al. Quantitative analysis of tumor in bronchial biopsy specimens. J Thorac Oncol 2010;5:448-52. 10.1097/JTO.0b013e3181ca12c4 [DOI] [PubMed] [Google Scholar]
- 27.Tochigi N, Dacic S, Ohori NP. Bronchoscopic and transthoracic cytology and biopsy for pulmonary nonsmall cell carcinomas: performance characteristics by procedure and tumor type. Diagn Cytopathol 2012;40:659-63. 10.1002/dc.21588 [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez EF, Monaco SE, Dacic S. Cytologic subtyping of lung adenocarcinoma by using the proposed International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) adenocarcinoma classification. Cancer Cytopathol 2013;121:629-37. 10.1002/cncy.21314 [DOI] [PubMed] [Google Scholar]
- 29.Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. 10.1097/PAS.0000000000000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsutsui S, Ashizawa K, Minami K, et al. Multiple focal pure ground-glass opacities on high-resolution CT images: Clinical significance in patients with lung cancer. AJR Am J Roentgenol 2010;195:W131-8. 10.2214/AJR.09.3828 [DOI] [PubMed] [Google Scholar]
- 31.Gu B, Burt BM, Merritt RE, et al. A dominant adenocarcinoma with multifocal ground glass lesions does not behave as advanced disease. Ann Thorac Surg 2013;96:411-8. 10.1016/j.athoracsur.2013.04.048 [DOI] [PubMed] [Google Scholar]
- 32.Shim HS, Kenudson M, Zheng Z, et al. Unique Genetic and Survival Characteristics of Invasive Mucinous Adenocarcinoma of the Lung. J Thorac Oncol 2015;10:1156-62. 10.1097/JTO.0000000000000579 [DOI] [PubMed] [Google Scholar]
- 33.Warth A, Macher-Goeppinger S, Muley T, et al. Clonality of multifocal nonsmall cell lung cancer: implications for staging and therapy. Eur Respir J 2012;39:1437-42. 10.1183/09031936.00105911 [DOI] [PubMed] [Google Scholar]
- 34.Wu C, Zhao C, Yang Y, et al. High Discrepancy of Driver Mutations in Patients with NSCLC and Synchronous Multiple Lung Ground-Glass Nodules. J Thorac Oncol 2015;10:778-83. 10.1097/JTO.0000000000000487 [DOI] [PubMed] [Google Scholar]
- 35.Takamochi K, Oh S, Matsuoka J, et al. Clonality status of multifocal lung adenocarcinomas based on the mutation patterns of EGFR and K-ras. Lung Cancer 2012;75:313-20. 10.1016/j.lungcan.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 36.Kalikaki A, Koutsopoulos A, Trypaki M, et al. Comparison of EGFR and K-RAS gene status between primary tumours and corresponding metastases in NSCLC. Br J Cancer 2008;99:923-9. 10.1038/sj.bjc.6604629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J, Behrens C, Wistuba I, et al. Molecular analysis of synchronous and metachronous tumors of the lung: impact on management and prognosis. Ann Diagn Pathol 2001;5:321-9. 10.1053/adpa.2001.29338 [DOI] [PubMed] [Google Scholar]
- 38.Dacic S, Ionescu DN, Finkelstein S, et al. Patterns of allelic loss of synchronous adenocarcinomas of the lung. Am J Surg Pathol 2005;29:897-902. 10.1097/01.pas.0000164367.96379.66 [DOI] [PubMed] [Google Scholar]
- 39.Arai J, Tsuchiya T, Oikawa M, et al. Clinical and molecular analysis of synchronous double lung cancers. Lung Cancer 2012;77:281-7. 10.1016/j.lungcan.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Wang M, MacLennan GT, et al. Evidence for common clonal origin of multifocal lung cancers. J Natl Cancer Inst 2009;101:560-70. 10.1093/jnci/djp054 [DOI] [PubMed] [Google Scholar]
- 41.Lin MW, Wu CT, Kuo SW, et al. Clinicopathology and genetic profile of synchronous multiple small adenocarcinomas: implication for surgical treatment of an uncommon lung malignancy. Ann Surg Oncol 2014;21:2555-62. 10.1245/s10434-014-3642-5 [DOI] [PubMed] [Google Scholar]
- 42.Girard N, Deshpande C, Azzoli CG, et al. Use of epidermal growth factor receptor/Kirsten rat sarcoma 2 viral oncogene homolog mutation testing to define clonal relationships among multiple lung adenocarcinomas: comparison with clinical guidelines. Chest 2010;137:46-52. 10.1378/chest.09-0325 [DOI] [PubMed] [Google Scholar]
- 43.Tang X, Shigematsu H, Bekele BN, et al. EGFR tyrosine kinase domain mutations are detected in histologically normal respiratory epithelium in lung cancer patients. Cancer Res 2005;65:7568-72. 10.1158/0008-5472.CAN-05-1705 [DOI] [PubMed] [Google Scholar]
- 44.Gazdar A, Robinson L, Oliver D, et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. J Thorac Oncol 2014;9:456-63. 10.1097/JTO.0000000000000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikeda K, Nomori H, Mori T, et al. Novel germline mutation: EGFR V843I in patient with multiple lung adenocarcinomas and family members with lung cancer. Ann Thorac Surg 2008;85:1430-2. 10.1016/j.athoracsur.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 46.Taniguchi K, Okami J, Kodama K, et al. Intratumor heterogeneity of epidermal growth factor receptor mutations in lung cancer and its correlation to the response to gefitinib. Cancer Sci 2008;99:929-35. 10.1111/j.1349-7006.2008.00782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Italiano A, Cortot AB, Ilie M, et al. EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: implications for anti-EGFR treatment of a rare lung malignancy. Int J Cancer 2009;125:2479-82. 10.1002/ijc.24610 [DOI] [PubMed] [Google Scholar]
- 48.Bozzetti C, Tiseo M, Lagrasta C, et al. Comparison between epidermal growth factor receptor (EGFR) gene expression in primary non-small cell lung cancer (NSCLC) and in fine-needle aspirates from distant metastatic sites. J Thorac Oncol 2008;3:18-22. 10.1097/JTO.0b013e31815e8ba2 [DOI] [PubMed] [Google Scholar]
- 49.Han HS, Eom DW, Kim JH, et al. EGFR mutation status in primary lung adenocarcinomas and corresponding metastatic lesions: discordance in pleural metastases. Clin Lung Cancer 2011;12:380-6. 10.1016/j.cllc.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 50.Schmid K, Oehl N, Wrba F, et al. EGFR/KRAS/BRAF mutations in primary lung adenocarcinomas and corresponding locoregional lymph node metastases. Clin Cancer Res 2009;15:4554-60. 10.1158/1078-0432.CCR-09-0089 [DOI] [PubMed] [Google Scholar]
- 51.Vignot S, Frampton GM, Soria JC, et al. Next-generation sequencing reveals high concordance of recurrent somatic alterations between primary tumor and metastases from patients with non-small-cell lung cancer. J Clin Oncol 2013;31:2167-72. 10.1200/JCO.2012.47.7737 [DOI] [PubMed] [Google Scholar]
- 52.Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol 2011;29:2972-7. 10.1200/JCO.2010.33.3906 [DOI] [PubMed] [Google Scholar]
- 53.Murphy SJ, Aubry MC, Harris FR, et al. Identification of independent primary tumors and intrapulmonary metastases using DNA rearrangements in non-small-cell lung cancer. J Clin Oncol 2014;32:4050-8. 10.1200/JCO.2014.56.7644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer. 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S. [DOI] [PubMed] [Google Scholar]
- 55.Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S. [DOI] [PubMed] [Google Scholar]
- 56.Finley DJ, Yoshizawa A, Travis W, et al. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol 2010;5:197-205. 10.1097/JTO.0b013e3181c814c5 [DOI] [PubMed] [Google Scholar]
- 57.Detterbeck FC, Bolejack V, Arenberg DA, et al. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Classification of Lung Cancer with Separate Tumor Nodules in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:681-92. [DOI] [PubMed] [Google Scholar]
- 58.Nakata M, Sawada S, Yamashita M, et al. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg 2004;78:1194-9. 10.1016/j.athoracsur.2004.03.102 [DOI] [PubMed] [Google Scholar]
- 59.Rao J, Sayeed RA, Tomaszek S, et al. Prognostic factors in resected satellite-nodule T4 non-small cell lung cancer. Ann Thorac Surg 2007;84:934-8. 10.1016/j.athoracsur.2007.04.097 [DOI] [PubMed] [Google Scholar]
- 60.Nagai K, Sohara Y, Tsuchiya R, et al. Prognosis of resected non-small cell lung cancer patients with intrapulmonary metastases. J Thorac Oncol 2007;2:282-6. 10.1097/01.JTO.0000263709.15955.8a [DOI] [PubMed] [Google Scholar]
- 61.Yang H, Sun Y, Yao F, et al. Surgical Therapy for Bilateral Multiple Primary Lung Cancer. Ann Thorac Surg 2016;101:1145-52. 10.1016/j.athoracsur.2015.09.028 [DOI] [PubMed] [Google Scholar]
- 62.Tanvetyanon T, Finley DJ, Fabian T, et al. Prognostic factors for survival after complete resections of synchronous lung cancers in multiple lobes: pooled analysis based on individual patient data. Ann Oncol 2013;24:889-94. 10.1093/annonc/mds495 [DOI] [PubMed] [Google Scholar]
- 63.Terzi A, Falezza G, Benato C, et al. Survival following complete resection of multifocal T4 node-negative NSCLC: a retrospective study. Thorac Cardiovasc Surg 2007;55:44-7. 10.1055/s-2006-924441 [DOI] [PubMed] [Google Scholar]
- 64.Pennathur A, Lindeman B, Ferson P, et al. Surgical resection is justified in non-small cell lung cancer patients with node negative T4 satellite lesions. Ann Thorac Surg 2009;87:893-9. 10.1016/j.athoracsur.2008.11.073 [DOI] [PubMed] [Google Scholar]
- 65.Okamoto T, Iwata T, Mizobuchi T, et al. Surgical treatment for non-small cell lung cancer with ipsilateral pulmonary metastases. Surg Today 2013;43:1123-8. 10.1007/s00595-012-0452-x [DOI] [PubMed] [Google Scholar]