Abstract
Background
There is little information on hospital and nursing facility stays during the transition from pre-dialysis kidney disease to end-stage renal disease treated with dialysis.
Objectives
To examine hospital and nursing facility stays in the years pre- and post-dialysis initiation, and to develop a novel method for visualizing these data.
Design
Observational study of patients in the US Renal Data System initiating dialysis from October 2011 to October 2012.
Participants
Patients aged ≥67 years with Medicare Part A/B coverage for 1 year pre-dialysis initiation.
Main Measures
Proportion of patients with ≥1 facility day, and among these, the mean number of days and the mean proportion of time spent in a facility in the first year post-dialysis initiation. We created “heat maps” to represent data visually.
Key Results
Among 28,049 patients, > 60% initiated dialysis in the hospital. Patients with at least 1 facility day spent 37–42 days in a facility in the year pre-dialysis initiation and 59–67 facility days in the year post-dialysis initiation. The duration of facility stay varied by age: patients aged 67–70 years spent 60 (95% CI 57–62) days or 25.8% of the first year post-dialysis initiation in a facility, while patients aged >80 years spent 67 (CI 65–69) days or 36.8% of the first year post-dialysis initiation in a facility. Patterns varied depending on the presence or absence of certain comorbid conditions, with dementia having a particularly large effect: patients with dementia spent approximately 50% of the first year post-dialysis initiation in a facility, regardless of age.
Conclusions
Older patients, particularly octogenarians and patients with dementia or other comorbidities, spend a large proportion of time in a facility during the first year after dialysis initiation. Our heat maps provide a novel and concise visual representation of a large amount of quantitative data regarding expected outcomes after initiation of dialysis.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-017-4151-6) contains supplementary material, which is available to authorized users.
KEY WORDS: dialysis, kidney failure, hospitalization, nursing facility
INTRODUCTION
In the United States, more than 100,000 patients with end-stage renal disease (ESRD) initiate maintenance dialysis each year. Older patients have the highest adjusted incidence rates of ESRD,1 and the initiation of maintenance dialysis in older patients is associated with declines in functional status2 and transfers to long-term care.3 Moreover, it is uncertain whether maintenance dialysis offers a survival advantage over conservative management in older patients with a high burden of comorbidities.4 – 7 Up to two-thirds of older adults with multiple comorbidities prioritize maintaining independence over extension of life.8 Despite indications that maintenance dialysis may not improve quality or quantity of life for some older patients with ESRD, a majority of these patients opt to initiate dialysis regardless of concomitant comorbidities.9
We sought to better understand older patients’ patterns of hospital and nursing facility stays (hereafter “facility stays”) during the transition from pre-dialysis chronic kidney disease CKD to ESRD treated with dialysis. We aimed to determine how patterns of facility stays differed depending on patient demographics and comorbidities. We examined differences in the proportion of patients who died during the 1-year period after initiation of dialysis. In addition to providing quantitative information, we sought to develop a visual representation of facility stay patterns and death during the transition period (a “heat map”) in order to convey, in a concise manner, a large amount of information about expected outcomes after initiation of dialysis.
METHODS
Data Sources and Cohort Assembly
We identified all patients in the US Renal Data System (USRDS) aged ≥67 years at the time of initiation of maintenance dialysis for ESRD between 3 October 2011 and 2 October 2012. The USRDS is a national registry of almost all patients receiving treatment for ESRD in the US and includes health care claims submitted to Medicare. Because we wanted to study the periods pre- and post-incident ESRD treated with dialysis (i.e., pre- and post-dialysis initiation), we required patients to have uninterrupted Medicare Part A and B coverage as primary payer for 1 year pre-dialysis initiation to ensure a uniform period in which to ascertain health care utilization. We excluded patients missing information on sex, race, ethnicity, modality, and cause of ESRD. We also excluded patients who received a kidney transplant within the first 90 days of dialysis initiation, since these patients may have had very different facility stay patterns from patients treated with maintenance dialysis (Appendix Fig. 1). Excluded patients were of similar mean age, but were more often of non-white race (29.5%) and Hispanic ethnicity (15.1%) than included participants.
The Stanford University Institutional Review Board approved the study.
Hospitalizations and Nursing Facility Stays
We examined the 1 year pre- and post-dialysis initiation for each patient. Days spent in a hospital or any type of nursing facility were considered “facility days.” We used Medicare Part A billing claims to determine whether a patient was in a hospital. To identify days spent in a nursing facility, we used Medicare Parts A and B claims. Because Part A covers only the first 100 days of skilled nursing facility care after a qualifying hospital stay, we used the place of service from Part B billing claims to identify additional days spent in a nursing facility (Appendix Table 1). Given the intermittent nature of Part B claims (which are generally for physician or laboratory services), we made the following assumptions: days between two consecutive Part B nursing facility claims (and without any other claims during that time) were categorized as nursing facility days (up to a maximum of 90 days); days between a Part B nursing facility claim and a hospitalization claim or death were categorized as nursing facility days (up to a maximum of 31 days). We defined patients as being at home if they had a Part A billing claim for home health services or a Part B claim with “home” as the place of service, or in the absence of any other nursing facility billing claims. We validated this approach by comparing it to the Minimum Data Set (MDS), a national registry of all patients residing in a Medicare- or Medicaid-certified nursing facility, and the “gold standard”10 , 11 for ascertaining nursing facility days (Appendix Table 2).
Outcomes
Our outcome of interest was the number of facility days in the 365 days post-dialysis initiation (absolute and relative to days alive). We censored follow-up when the patient died, received a kidney transplant, or lost Medicare Parts A and B coverage, or at 365 days post-dialysis initiation. We also examined the proportion of patients who died in the 365 days post-dialysis initiation.
Covariates
We ascertained the following patient characteristics from the USRDS patient and treatment history files: age, sex, race, Hispanic ethnicity, cause of ESRD (diabetes, hypertension, glomerulonephritis, other), and initial dialysis modality. Comorbidities were ascertained during the 1-year pre-dialysis initiation using a claims-based algorithm (Appendix Table 3).12 We ascertained nephrologist care pre-dialysis initiation from the Medical Evidence Report.
Statistical Analysis
All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC) and R version 3.3.1 (www.r-project.org) software programs. We considered age to be an effect modifier and thus stratified all analyses into four age categories at the time of incident ESRD (in years): 67–70, 71–75, 76–80, and >80. We were also particularly interested in the following six subgroups: female sex, black race, diabetes mellitus, cardiovascular disease, dementia, and depression.
For each age category overall and by the six specified subgroups, we calculated the proportion of patients with at least 1 facility day, and among these, the mean number of days and the mean proportion of time spent in a facility, in the first year post-dialysis initiation. We also calculated these values separately for patients who died and for patients who survived the first year post-dialysis initiation. All analyses presented are unadjusted, and 95% confidence intervals (CI) were computed using a generalized linear model (GLM) with binomial family and logit link for the proportions and GLM with binomial family and log link (negative binomial model) for the means.
Heat Maps
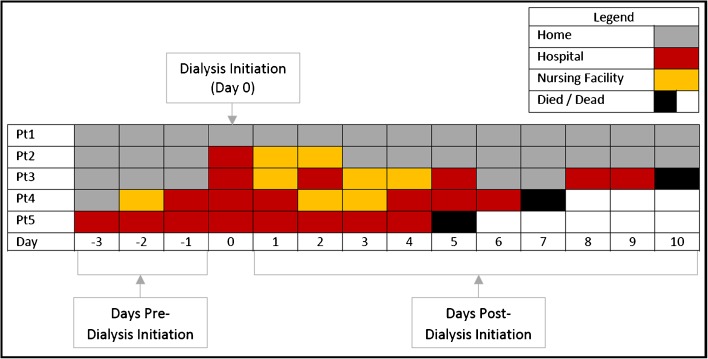
We developed a visual representation of facility stay patterns and death that we refer to as “heat maps” (Fig. 1). In the heat map, each patient is represented by one row, with each of the 120 days pre-dialysis initiation and the 365 days post-dialysis initiation represented by one box (for simplicity, we did not show all 365 days pre-dialysis initiation). Each day (box) is color-coded according to the status of the patient on that day. Red indicates that the patient is hospitalized, orange indicates a nursing facility, and gray indicates that the patient is presumed to be at home. A single black box indicates the day the patient died, and days thereafter are shown in white. Before plotting, the data are sorted as follows: 1) ascending by date of death in relation to the date of dialysis initiation; 2) descending number of facility days post-dialysis initiation; 3) descending number of total facility days pre- and post-dialysis initiation 4) by the first facility day post-dialysis initiation and 5) by the first facility day pre-dialysis initiation; and 6) descending number of days uncensored. This ordering facilitates the visual estimation of the percent of patients who die within the first year post-dialysis initiation, which can be made by looking at the proportion of white found in the right corner (i.e., more white color indicates that more patients died in the first year). This sorting also allows visual estimation of the proportion of patients alive at 365 days post-dialysis initiation with a large proportion of days in a facility, as indicated by the red/orange color above the white area in the figure. Finally, the sorting shows patients with no facility days pre- and post-dialysis initiation, who are represented by the band of gray at the top of each plot.
Figure 1.
Schematic representation of the “heat map.” For illustrative purposes, the schematic shows only 3 days pre-dialysis initiation and 10 days post-dialysis initiation for five hypothetical patients, while the actual heat maps extend to 120 days pre-dialysis and 365 days post-dialysis initiation. Each day is represented by a single box that is color-coded as indicated in the legend. Each patient is represented by one row in the heat map. For example, patient (Pt) 1 spent the 3 days pre- and 10 days post-dialysis initiation at home. Patient 2 had no facility days pre-dialysis initiation, was hospitalized at the time of dialysis initiation, and then spent 2 days in a nursing facility before going home. Patients 3 and 4 spent variable lengths of time in a facility before dying on days 10 and 7 post-dialysis initiation, respectively. Patient 5 was hospitalized from 3 days pre- through 4 days post-dialysis initiation before dying on day 5 post-dialysis initiation.
Due to the relatively small number of patients censored because of transplant or loss of Medicare Parts A and B coverage, we constructed the heat maps using a random sample of 1000 patients whose data were not censored for these reasons. If the plotted subgroup had <1000 patients, we plotted all patients. The annotated statistics displayed on the plots were computed using all patients in the subgroup. Heat maps for additional subgroups can be found at http://med.stanford.edu/kidneyresearch/clinical-trials/observational-studies.html.
RESULTS
We identified 28,049 patients in the final cohort (Appendix Fig. 1). Patients in the oldest age category were more often of white race and had a higher prevalence of cardiovascular disease than younger patients (Table 1). Older patients were also more likely than younger patients to have spent any time in a nursing facility pre-dialysis initiation. Among the four age groups, approximately 62–65% of patients initiated dialysis in the hospital, with a median of 3–4 days from hospital admission to the first dialysis session (Table 2). The primary diagnosis for 45% of these hospitalizations was kidney-related.
Table 1.
Baseline Characteristics of the Cohort, Stratified by Age Category (all values are counts [%] except where indicated)
| Variable | Age 67–70 (n = 5618) |
Age 71–75 (n = 7052) |
Age 76–80 (n = 6646) |
Age > 80 (n = 8733) |
|---|---|---|---|---|
| Age, mean (SD) | 68.5 (1.1) | 73.0 (1.4) | 77.9 (1.4) | 85.0 (3.7) |
| Male | 3006 (53.5) | 3873 (54.9) | 3565 (53.6) | 4770 (54.6) |
| Race | ||||
| White | 4046 (72.0) | 5312 (75.3) | 5158 (77.6) | 7209 (82.5) |
| Black | 1303 (23.2) | 1428 (20.2) | 1173 (17.6) | 1155 (13.2) |
| Other | 269 (4.8) | 312 (4.4) | 315 (4.7) | 369 (4.2) |
| Initial modality: in-center hemodialysis | 5188 (92.3) | 6564 (93.1) | 6214 (93.5) | 8335 (95.4) |
| Cause of end-stage renal disease | ||||
| Diabetes | 2857 (50.9) | 3218 (45.6) | 2678 (40.3) | 2594 (29.7) |
| Hypertension | 1461 (26.0) | 2204 (31.3) | 2434 (36.6) | 4214 (48.3) |
| Glomerulonephritis | 295 (5.3) | 367 (5.2) | 366 (5.5) | 334 (3.8) |
| Other | 1005 (17.9) | 1263 (17.9) | 1168 (17.6) | 1591 (18.2) |
| Cardiovascular comorbidities | ||||
| Coronary artery disease | 2931 (52.2) | 3978 (56.4) | 3918 (59.0) | 5311 (60.8) |
| Heart failure | 3100 (55.2) | 4082 (57.9) | 4066 (61.2) | 5715 (65.4) |
| Atrial fibrillation | 1402 (25.0) | 1982 (28.1) | 2296 (34.5) | 3577 (41.0) |
| Other arrhythmia | 1021 (18.2) | 1458 (20.7) | 1654 (24.9) | 2481 (28.4) |
| Stroke/Transient ischemic attack | 606 (10.8) | 785 (11.1) | 725 (10.9) | 999 (11.4) |
| Valvular disease | 1225 (21.8) | 1790 (25.4) | 1897 (28.5) | 2925 (33.5) |
| Peripheral arterial disease | 1568 (27.9) | 2099 (29.8) | 2012 (30.3) | 2725 (31.2) |
| Hypertension | 5276 (93.9) | 6677 (94.7) | 6313 (95.0) | 8366 (95.8) |
| Any of the above cardiovascular comorbidities | 5357 (95.4) | 6790 (96.3) | 6418 (96.6) | 8498 (97.3) |
| Other comorbid conditions | ||||
| Diabetes mellitus | 4025 (71.6) | 4861 (68.9) | 4272 (64.3) | 4632 (53.0) |
| Liver disease | 604 (10.8) | 649 (9.2) | 489 (7.4) | 496 (5.7) |
| Chronic lung disease | 2102 (37.4) | 2681 (38.0) | 2541 (38.2) | 3319 (38.0) |
| Dementia | 280 (5.0) | 407 (5.8) | 524 (7.9) | 905 (10.4) |
| Depression | 1088 (19.4) | 1256 (17.8) | 1079 (16.2) | 1341 (15.4) |
| Cancer | 839 (14.9) | 1231 (17.5) | 1197 (18.0) | 1659 (19.0) |
| Under care of a nephrologist | 3897 (69.4) | 5027 (71.3) | 4733 (71.2) | 6093 (69.8) |
| Had ≥1 day in an institution in 365 days pre-end-stage renal disease | 4560 (81.2) | 5816 (82.5) | 5534 (83.3) | 7524 (86.2) |
| Number of institutional days, mean (SD)* | 40 (73) | 37 (69) | 38 (70) | 42 (74) |
*Among those with >1 day in an institution pre-dialysis initiation
Table 2.
Days Between Hospital Admission and Dialysis Initiation and Primary Diagnosis Codes for Patients Hospitalized at the Time of Incident ESRD Treated with Dialysis
| Variable | Age 67–70 (n = 5618) |
Age 71–75 (n = 7052) |
Age 76–80 (n = 6646) |
Age > 80 (n = 8733) |
|---|---|---|---|---|
| Initiated dialysis as inpatient, % | 61.8 | 62.2 | 61.9 | 64.6 |
| Days from hospital admission to dialysis initiation | ||||
| Median (interquartile range) | 3 (1,8) | 3 (1,9) | 4 (1,9) | 4 (1,10) |
| Mean (SD) | 8 (15) | 8 (13) | 8 (18) | 8 (16) |
| Primary discharge diagnosis (%) | ||||
| Acute kidney injury | 22.2 | 22.9 | 23.4 | 25.0 |
| End-stage renal disease | 21.0 | 21.0 | 20.4 | 21.1 |
| Heart failure | 15.0 | 15.7 | 18.4 | 17.1 |
| Infection | 8.4 | 8.4 | 8.4 | 8.3 |
| Coronary artery disease | 4.5 | 4.0 | 4.4 | 3.5 |
| Diabetes mellitus | 3.5 | 3.8 | 2.8 | 2.0 |
| Respiratory failure | 3.3 | 2.7 | 2.3 | 2.3 |
| Bleed or anemia | 2.6 | 2.2 | 2.2 | 2.8 |
| Other cardiovascular condition | 2.5 | 3.0 | 2.3 | 3.1 |
| Cancer | 2.0 | 1.7 | 1.7 | 1.1 |
| Electrolyte or acid–base abnormality | 1.1 | 1.1 | 1.0 | 0.7 |
| Rehabilitation not otherwise specified | 1.0 | 0.8 | 1.5 | 1.3 |
| Lung disease | 0.8 | 0.9 | 0.9 | 1.3 |
| Other* | 12.0 | 11.8 | 10.5 | 10.5 |
*The “other” category includes 600 unique diagnosis codes, each accounting for <0.3% of the total cohort
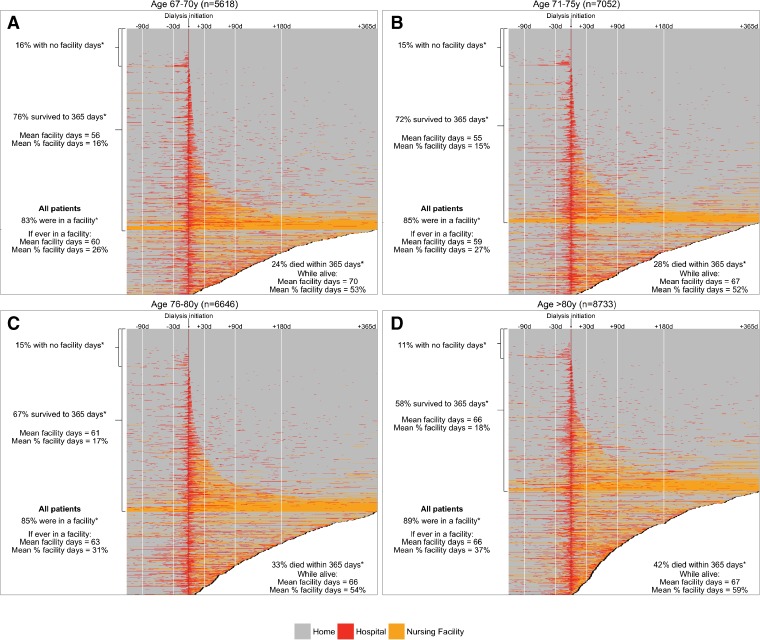
Patterns of facility stays and death in the first year post-dialysis initiation varied by age category (Fig. 2a–d). For example, patients aged 67–70 years (who had at least 1 facility day) spent an average of 60 days (CI 57–62 days) or 25.8% (CI 24.9–26.7%) of the first year post-dialysis initiation in a facility (Appendix Table 4A). In contrast, patients aged >80 years (who had at least 1 facility day) spent an average of 67 days (CI 65–69 day), which corresponded to 36.8% (CI 36.0–37.6) of the first year post-dialysis initiation, in a facility (Appendix Table 4D). Older patients were also more likely than younger patients to die in the first year post-dialysis initiation: 23.9% for patients aged 67–70 years and 42.1% for patients >80 years (Fig. 2a–d; Appendix Tables 4A–D). Consistent across all age groups, patients who died in the first year post-dialysis initiation spent 50%–60% of their remaining time in a hospital or nursing facility, while patients who survived the first year spent 15%–20% of the first year post-dialysis initiation in a facility (Appendix Tables 4A–D).
Figure 2.
Heat maps showing the status of patients aged a) 67–70 years, b) 71–75 years, c) 76–80 years, and d) >80 years during the 120 days pre- and 365 days post-dialysis initiation. *Post-dialysis initiation.
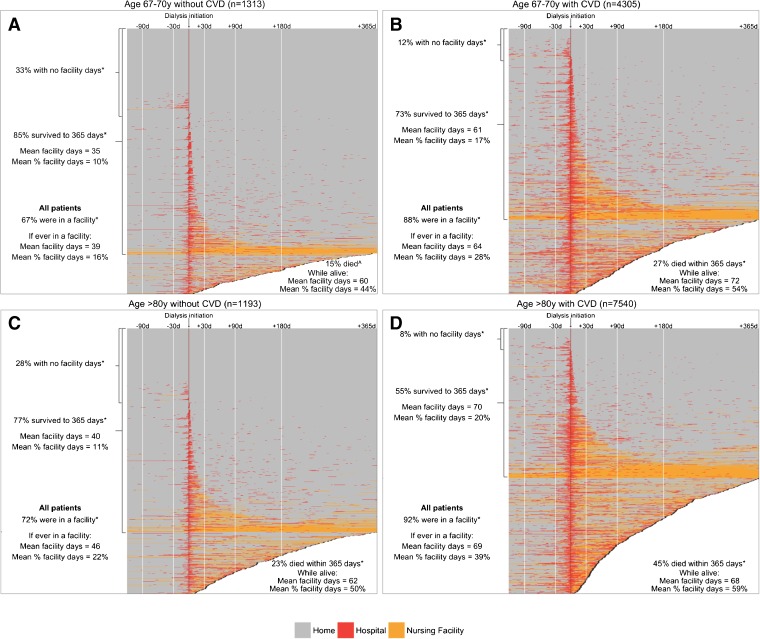
The presence or absence of comorbid conditions was associated with differences in facility stay patterns. For example, 67.1% of patients aged 67–70 years without cardiovascular disease had at least 1 facility day post-dialysis initiation, compared with 88.4% of patients in this age group with cardiovascular disease. Moreover, these patients without and with cardiovascular disease spent an average of 16% versus 28% of the first year post-dialysis initiation in a facility, respectively (Fig. 3a and b, Appendix Table 4A). These patterns were similar in the other age groups with respect to the proportion of patients with at least 1 facility day and the mean number of days these patients spent in a facility, among those without and with cardiovascular disease. However, because a larger proportion of patients in the older age categories died, the mean proportion of time spent in an institution during the first year was higher (e.g., patients aged >80 years without and with cardiovascular disease spent 22.1% and 38.6% of the first year in a facility, respectively; Fig. 3c and d, Appendix Tables 4A–D).
Figure 3.
Heat maps showing the status of patients aged 67–70 years a) without cardiovascular disease (CVD) and b) with CVD, and patients aged >80 years c) without CVD and d) with CVD, during the 120 days pre- and 365 days post-dialysis initiation. ^In the 365 days post-dialysis initiation. *Post-dialysis initiation.
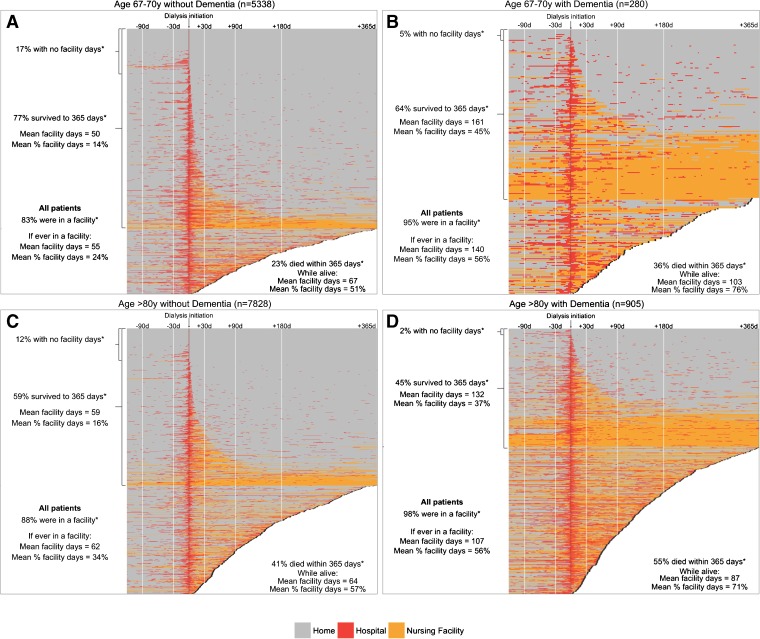
The presence of dementia had a particularly large effect on hospital and nursing facility stay patterns. Patients across all four age groups with dementia spent approximately 50% of the first year post-dialysis initiation in a facility (Fig. 4, Appendix Tables 3A–D). The proportion of patients who died was also very high among patients with dementia, ranging from 35.7% in patients aged 67–70 years to 54.9% in patients >80 years.
Figure 4.
Heat maps showing the status of patients aged 67–70 years a without dementia and b with dementia, and patients aged >80 years c without dementia and d with dementia, during the 120 days pre- and 365 days post-dialysis initiation. *Post-dialysis initiation.
DISCUSSION
We analyzed patterns of hospitalization and nursing facility stays among US patients aged ≥ 67 years during the transition from CKD to incident ESRD treated with dialysis. Patients with at least 1 facility day spent an average of about 1 month (37 to 42 days) in a facility pre-dialysis initiation. The 85.8% of patients who had at least 1 day in a facility post-dialysis initiation spent an average of approximately 2 months in a hospital or nursing home in the first year. This finding was consistent across all age groups. The presence of certain comorbid conditions was associated with a greater number of facility days post-dialysis initiation. For example, patients with cardiovascular disease had on average 60% more facility days than patients without cardiovascular disease, adjusted for age, and patients with dementia had approximately twice as many facility days compared to patients without dementia. As expected, the proportion of patients who died in the first year was higher in patients with cardiovascular disease, dementia, or depression than in patients without these conditions. Patients who died in the first year spent over 50% of their remaining time alive in a hospital or nursing facility, and patients who survived still spent 15–20% of the first year post-dialysis initiation in a facility.
Our findings are consistent with previous studies that have shown poorer outcomes among older patients with a greater number of comorbid conditions, particularly dementia. A study of incident patients started on dialysis in the mid-1990s showed that the average time to death for patients with versus without dementia was 1.1 versus 2.7 years 13 13; however, that study did not provide information on hospitalization or nursing facility stays. Our analysis provides additional clinically relevant information to patients and providers regarding not only mortality, but also hospitalization and nursing facility stays, post-dialysis initiation.
In our cohort of older patients with incident ESRD in 2011–2012, we found that over 60% initiated dialysis in the hospital, a proportion that is relatively unchanged from earlier studies.14 The reasons for hospitalization during dialysis initiation were largely related to kidney disease, with primary diagnosis codes for acute kidney injury, ESRD, and electrolyte/acid–base abnormalities accounting for 45% of hospitalizations. However, there was a relatively high proportion of patients hospitalized when initiating dialysis for other reasons, most notably heart failure and infection, which were similar across categories of age.
We showed that patients with cardiovascular disease or dementia spent approximately 30% and 50% of their first year post-dialysis initiation in a facility, respectively. Our results are consistent with a previous study of nursing facility residents, which showed that the initiation of dialysis was associated with a sharp decline in functional status.15 Similarly, in another study of older patients with ESRD, among those who survived to 6 months post-dialysis initiation, 37% required long-term care and 75% had some functional impairment.3 Although dialysis is associated with longer survival than conservative management,16 at least one study has shown that the survival advantage shrinks for patients with more comorbid conditions, with no significant survival advantage for patients >80 years old at the time of dialysis initiation.5
Despite these relatively poor outcomes, in the United States, older patients represent the fastest-growing population of patients initiating maintenance dialysis.1 A recent study of US veterans with advanced CKD9 found that only 14.5% of patients chose not to pursue dialysis, and that even among patients >85 years, more than half received or were preparing to receive dialysis. That study also showed no differences in the proportion of patients who opted for dialysis rather than conservative care among those with a high burden of comorbid conditions compared to healthier patients of similar age. One explanation for the propensity to pursue dialysis over conservative management could be a lack of shared decision making, where patients and providers together discuss the best available evidence prior to making a treatment decision.17 Systems-based barriers such as lack of time and fragmentation of care across settings and providers18 make it difficult to implement shared decision making. It is also possible that providers find that initiating dialysis is more convenient and is better remunerated, and clinicians have also cited difficulty in determining which patients might benefit from conservative management as a barrier to discussing conservative ESRD treatment options.19 , 20 As such, our visual heat maps, which provide qualitative and quantitative information in a concise way, could help providers and even patients gain a better understanding of expected outcomes after initiation of dialysis.
We acknowledge that our analysis has some limitations. First, our cohort only includes patients who developed incident ESRD who chose to initiate dialysis. We are thus unable to form conclusions about patterns of facility days among patients with advanced CKD who may have elected not to initiate dialysis. However, as patients who initiate dialysis are likely to be fundamentally different from those with advanced kidney disease who choose medical management, comparing these two patient groups would be challenging. Second, our cohort is restricted to older patients, and therefore our results may not be generalizable to younger patients. However, the oldest age groups have the highest adjusted ESRD incident rates1 and the smallest potential gains from dialysis initiation compared with comparative management,4 – 7 thus underscoring the importance of understanding facility stay patterns among this high-risk subgroup. Third, we relied on claims to ascertain comorbidities, which are known to be highly specific but less sensitive.21 Therefore, we may have misclassified some patients as lacking a condition/disease when it was present, and thus the true difference in facility days according to the presence of various comorbidities may be larger than what we have reported.
CONCLUSIONS
Older patients initiating dialysis in the United States experience high rates of hospitalization and nursing facility stays in the first year post-dialysis initiation, particularly those with cardiovascular disease and dementia. Our results underscore the clinically unstable nature of the transition from CKD to treated ESRD, not only with regard to the large proportion of patients who die within the first year of dialysis initiation, but also in the marked loss of independence, as illustrated by the large proportion of time spent in a hospital or nursing facility. Our novel heat maps provide a striking visual representation of large amounts of quantitative data, which could be tested in future studies as a tool to facilitate shared decision making among providers, patients, and their families, as they weigh different ESRD treatment options.
Electronic supplementary material
(DOCX 65.7 kb)
Contributors
None.
Disclaimer
This work was conducted under a data use agreement between Dr. Chang and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). An NIDDK officer reviewed this manuscript for research compliance and approved of its submission for publication. Data reported herein were supplied by the USRDS. Interpretation and reporting of these data are the responsibility of the authors and should in no way be seen as official policy or interpretation of the US government.
Funders
Dr. Chang is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; 5K23DK095914). Dr. Montez-Rath and Dr. Kurella Tamura are supported by grant U01DK102150 from the NIDDK.
Prior Presentations
A portion of this work was presented at the American Society of Nephrology Kidney Week 2016, Chicago, IL.
Conflict of Interest
MMR, YZ, and VG report no conflicts of interest.
MKT reports receiving honoraria from US Renal Care.
WCW has received consulting fees from AMAG, Akebia, Amgen, AstraZeneca, Bayer, Daichii Sankyo, Fibrogen, Medtronic, Relypsa, and Vifor FMC Renal Pharma.
TIC has received consulting fees from Janssen Research and Development, LLC.
References
- 1.United States Renal Data System. 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2016.
- 2.Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med. 2009;361(16):1612–3. doi: 10.1056/NEJMc0905289. [DOI] [PubMed] [Google Scholar]
- 3.Bowling CB, Plantinga L, Hall RK, Mirk A, Zhang R, Kutner N. Association of nondisease-specific problems with mortality, long-term care, and functional impairment among older adults who require skilled nursing care after dialysis initiation. Clin J Am Soc Nephrol. 2016;11(12):2218–24. doi: 10.2215/CJN.01260216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146(3):177–83. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Verberne WR, Geers AB, Jellema WT, Vincent HH, van Delden JJ, Bos WJ. Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. Clin J Am Soc Nephrol. 2016;11(4):633–40. doi: 10.2215/CJN.07510715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4(10):1611–9. doi: 10.2215/CJN.00510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain JA, Mooney A, Russon L. Comparison of survival analysis and palliative care involvement in patients aged over 70 years choosing conservative management or renal replacement therapy in advanced chronic kidney disease. Palliat Med. 2013;27(9):829–39. doi: 10.1177/0269216313484380. [DOI] [PubMed] [Google Scholar]
- 8.Fried TR, Tinetti ME, Iannone L, O'Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171(20):1854–6. doi: 10.1001/archinternmed.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong SP, Hebert PL, Laundry RJ, Hammond KW, Liu CF, Burrows NR, et al. Decisions about renal replacement therapy in patients with advanced kidney disease in the US Department of Veterans Affairs, 2000–2011. Clin J Am Soc Nephrol. 2016;11(10):1825–33. doi: 10.2215/CJN.03760416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei YJ, Simoni-Wastila L, Zuckerman IH, Brandt N, Lucas JA. Algorithm for identifying nursing home days using medicare claims and minimum data set assessment data. Med Care. 2016;54(11):e73–e7. doi: 10.1097/MLR.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 11.Intrator O, Hiris J, Berg K, Miller SC, Mor V. The residential history file: Studying nursing home residents' long-term care histories(*) Health Serv Res. 2011;46(1 Pt 1):120–37. doi: 10.1111/j.1475-6773.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang TI, Shilane D, Kazi DS, Montez-Rath ME, Hlatky MA, Winkelmayer WC. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in esrd. J Am Soc Nephrol. 2012;23(12):2042–9. doi: 10.1681/ASN.2012060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakowski DA, Caillard S, Agodoa LY, Abbott KC. Dementia as a predictor of mortality in dialysis patients. Clin J Am Soc Nephrol. 2006;1(5):1000–5. doi: 10.2215/CJN.00470705. [DOI] [PubMed] [Google Scholar]
- 14.Wong SP, Kreuter W, O'Hare AM. Healthcare intensity at initiation of chronic dialysis among older adults. J Am Soc Nephrol. 2014;25(1):143–9. doi: 10.1681/ASN.2013050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539–47. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joly D, Anglicheau D, Alberti C, Nguyen AT, Touam M, Grunfeld JP, et al. Octogenarians reaching end-stage renal disease: Cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14(4):1012–21. doi: 10.1097/01.ASN.0000054493.04151.80. [DOI] [PubMed] [Google Scholar]
- 17.Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: A model for clinical practice. J Gen Intern Med. 2012;27(10):1361–7. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Hare AM, Szarka J, McFarland LV, Taylor JS, Sudore RL, Trivedi R, et al. Provider perspectives on advance care planning for patients with kidney disease: Whose job is it anyway? Clin J Am Soc Nephrol. 2016;11(5):855–66. doi: 10.2215/CJN.11351015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parvez S, Abdel-Kader K, Pankratz VS, Song MK, Unruh M. Provider knowledge, attitudes, and practices surrounding conservative management for patients with advanced CKD. Clin J Am Soc Nephrol. 2016;11(5):812–20. doi: 10.2215/CJN.07180715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung E, Slesnick N, Kurella Tamura M, Schiller B. A survey of views and practice patterns of dialysis medical directors toward end-of-life decision making for patients with end-stage renal disease. Palliat Med. 2016;30(7):653–60. doi: 10.1177/0269216315625856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, et al. Assessing validity of CD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424-41. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 65.7 kb)